Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amberlite-15 promoted an unprecedented aza Michael rearrangement for one pot synthesis of dihydroquinazolinone compounds†
V. Narayana Murthyab,
Satish P. Nikumbha,
Krishnaji Tadiparthic,
M. V. Madhubabua,
Subba Rao Jammulaa,
L. Vaikunta Rao*b and
Akula Raghunadh *a
*a
aTechnology Development Centre, Custom Pharmaceutical Services, Dr Reddy's Laboratories Ltd, Hyderabad 500049, India. E-mail: raghunadha@drreddys.com
bDepartment of Chemistry, GIS, Gitam University, Visakhapatnam 530045, India
cDepartment of Chemistry, Christ University, Hosur road, Bangalore 560029, India
First published on 19th June 2018
Abstract
A new one pot multicomponent annulation strategy for the synthesis of various dihydroquinazolinone compounds has been developed using Amberlite-15 as a catalyst, giving good to moderate yields. In this reaction the substrate scope for amines and aldehydes was also investigated. The reaction has been checked on a large scale and the possible reaction mechanism has also been proposed.
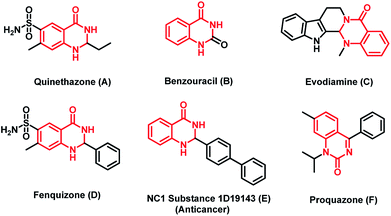
Functionalized dihydroquinazolinone frameworks are present in many biologically active molecules like quinethazone (A), fenquizone (D), benzouracil (B), evodiamine (C), NC1 substance1D19143 (E) and proquazone (F) (Fig. 1). Among the various dihydroquinazolinone compounds, some of them show various potential therapeutic effects, such as antidiabetic,1 anticancer,2 antihypertension,3 anticonvulsant,4 antibacterial,5 anti-inflammatory,6 and antianxietic activities.7 Moreover these compounds have also been used as antihistamine, antidepressant and vasodilating agents. Owing to their application in the medicinal and agro industries,8,9 many researchers around the world have put substantial efforts into the preparation of these compounds by various synthetic approaches.
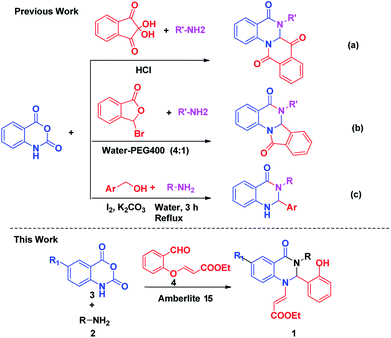
Indeed from the last few years various synthetic methods have been reported for the construction of various dihydroquinazolinones derivatives (Scheme 1).10,11 Bunce et al. reported the synthesis of substituted quinazolinones using a dissolving metal reduction–condensative cyclization strategy,12 Pal et al. synthesized 6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives by employing a three component reaction of isatoic anhydride, an amine and 2-formyl benzoic acid, using montmorillonite K10 as the catalyst,13 and the Sashidhara team also reported the synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione by using acetic acid.14 However among all the methods, the investigation of a practical and efficient multicomponent reaction for the synthesis of isoquinoline units with different nucleophiles has attracted many researchers because of their broad functional groups and simple construction of molecular architectures. Our group previously reported numerous protocols for the synthesis of quinazolinone based biologically active natural products and their derivatives. In continuation of our earlier efforts15 for the development of novel synthetic methods, herein we would like to report a new elegant one pot strategy for the construction of novel functionalized dihydroquinazolinones by the condensation of isatoic anhydride, amine and aldehyde.
 | ||
| Scheme 1 Various pathways for the construction of different dihydroquinazolinones moieties from isatoic anhydride. | ||
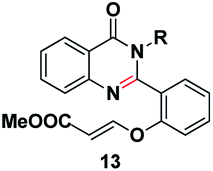
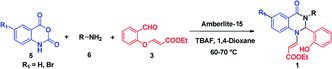
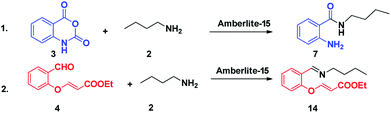
We commenced our investigation with the model substrate by taking isatoic anhydride (3), butyl amine and aldehyde (4) as depicted in Table 1. In the first step of optimization, the reaction was screened with various catalysts like Wang resin, glacial acetic acid, BF3·OEt2, TiCl4, FeCl3 and Amberlite-15 in the presence of 1,4-dioxane as a solvent. Gratifyingly by using Amberlite-15 as a catalyst, we found a new functionalized dihydroquinazolinones compound. But using the other acid catalysts, the product with one double bond compound 13 was obtained as depicted in Fig. 2 along with the product 1. To optimize the molar ratio of the Amberlite-15 catalyst, initially we screened the reaction with 0.05 (w/w%) of Amberlite-15 and reaction was completed within 7–8 h and whereas with 0.10 (w/w%) of the catalyst, the reaction was completed within 3–4 h. In the next stage, the reaction was investigated in different solvents like DMSO, DMF, 1,4-dioxane, ethanol, methanol, THF, toluene and acetonitrile. Among all the solvents, the reaction was not progressed well with polar protic solvents like ethanol and methanol, whereas in polar aprotic solvents DMSO and DMF nearly 30% of the product formation was observed. Finally, we found that the reaction providing promising yields in 1,4-dioxane as solvent (Table 1). Indeed, the reaction rate is slow at room temperature and is fast under reflux conditions. Further the reaction was also checked in 10 and 20 vol but there is no difference in terms of yield and purity. The aza Michael addition step was attempted with 1 eq. of TBAF but only 15% of the product was observed whereas with 2 equivalent of TBAF, 60% of the product was observed whereas with3 equivalent of TBAF the reaction was completed.
| S. no. | Solvent | Isolated yield (%) |
|---|---|---|
| 1 | DMSO | 28 |
| 2 | DMF | 30 |
| 3 | 1,4-Dioxane | 64 |
| 4 | Methanol | Trace |
| 5 | Ethanol | Trace |
| 6 | THF | 48 |
| 7 | Toluene | 40 |
| 8 | Acetonitrile | 53 |
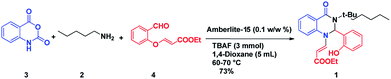
After optimizing the reaction conditions, we extended our studies for substrate scope of amines, aldehydes with isatoic anhydride and bromoisatoic anhydride. The substrate scope with various aliphatic, cyclic and aryl amines were studied and to our delight there is no abnormality in terms of yield and purity. This clearly states that all the substrates are well tolerated under these conditions to get the reasonable good yields (Table 2). Furthermore in order to elaborate the synthetic utility of this methodology we have extended our studies by performing a reaction on gram scale (Scheme 2) and gratefully we could replicate the yield and purity which clearly indicating that the reaction can be carried out on large scale.
| a Reaction conditions: isatoic anhydride (1 mmol), amine (1 mmol), aldehyde (1 mmol), Amberlite-15 (0.1 w/w%), 1,4-dioxane (5 mL). |
|---|
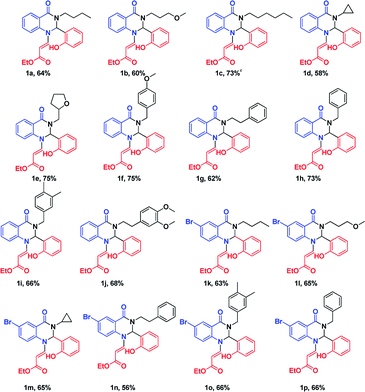 |
In order to gain insight into the mechanism, few control experiments have also been carried out. In the first experiment, the reaction with isatoic anhydride and amine without the aldehyde was carried out which provided the ring opening of the anhydride to get the anthranilide. In the second experiment, aldehyde with amine was carried out and the corresponding imine was obtained. In the third experiment, isatoic anhydride with aldehyde was carried out in the presence of the catalyst but the starting materials were intact (Scheme 3).
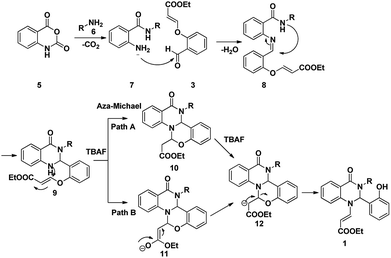
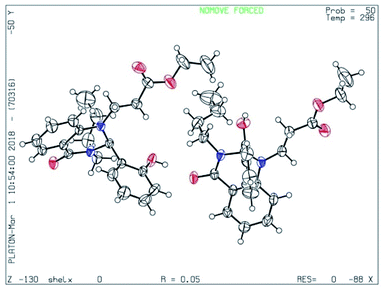
From the above experimental data and literature reports, we have postulated a new possible reaction mechanism (Scheme 4). In addition to the spectroscopic evidence, the chemical structure of dihydroquinazolinone 1 was further confirmed from the single crystal X-ray diffraction analysis (Fig. 3).
The Scheme 4 describes a plausible mechanism for the three component reaction leading to the compound 1. The nucleophilic attack of primary amine on carbonyl group of isatoic anhydride followed by ring opening and subsequent decarboxylation will yield to compound 7. Nucleophilic attack of amine to the aldehyde will yield imine intermediate 8; which on subsequent cyclization lead to the formation of 9. Nucleophilic attack of amine on the double bond via aza Michael reaction will yield the tetracyclic intermediate 10 or 11 which on further rearrangement leads to the formation of dihydroquinazolinones 1.
Conclusion
In summary we have demonstrated the synthesis of novel functionalized dihydroquinazolinones using Amberlite-15 as a catalyst. It is a one pot multicomponent annulation strategy with broad substrate scope and the products obtained were in moderate to good yield. Moreover, the reaction is atom economic, metal-free and mild reaction conditions. Furthermore, these reactions are scalable and simple workup procedures. Further investigation of these types of model substrates is underway in our laboratory.Conflicts of interest
We confirm that this manuscript has not been published elsewhere and is not under consideration by any other journal. All of the authors agreed with submission to RSC Advance. We have no conflicts of interest to declare.Acknowledgements
The authors would like to thank Dr Rama Mohan, Dr Reddy's Laboratories for the support to carry out this work and Dr Shubu M. Eappen, Kochi University, Kerala for single crystal data.References
- M. S. Malamas and J. Millen, J. Med. Chem., 1991, 34, 1492 CrossRef PubMed.
- (a) J. B. Jiang, D. Hesson, B. Dusak, D. Dexter, G. Kang and E. Hamel, J. Med. Chem., 1990, 33, 1721 CrossRef PubMed; (b) S. L. Cao, Y. P. Feng, Y. Y. Jiang, S. Y. Liu, G. Y. Ding and R. T. Li, Bioorg. Med. Chem. Lett., 2005, 15, 1915 CrossRef PubMed.
- J. W. Chern, P. L. Tao, K. C. Wang, A. Gutcait, S. W. Liu, M. H. Yen, S. L. Chien and J. K. Rong, J. Med. Chem., 1998, 41, 3128 CrossRef PubMed.
- J. F. Wolfe, T. L. Rathman, M. C. Sleevi, J. A. Campbell and T. D. Greenwood, J. Med. Chem., 1990, 33, 161 CrossRef PubMed.
- (a) W. Pendergast, J. V. Johnson, S. H. Dickerson, I. K. Dev, D. S. Duch, R. Ferone, W. R. Hall, J. Humphreys, J. M. Kelly and D. C. Wilson, J. Med. Chem., 1993, 36, 227 Search PubMed; (b) P. P. Kung, M. D. Casper, M. D. Cook, L. L. Wilson, L. M. Risen, T. A. Vickers, R. Ranken, L. B. Blyn, J. R. Wyatt and P. D. Cook, J. Med. Chem., 1999, 42, 4705 CrossRef PubMed.
- (a) F. Rorsch, E. Buscato, K. Deckmann, G. Schneider, M. S. Zsilavecz, G. Geisslinger, E. Proschak and S. Grosch, J. Med. Chem., 2012, 55, 3792 CrossRef PubMed; (b) S. E. De Laszlo, C. S. Quagliato, W. J. Greenlee, A. A. Patchett, R. S. L. Chang, V. J. Lotti, T. B. Chen, S. A. Scheck and K. A. Faust, J. Med. Chem., 1993, 36, 3207 CrossRef PubMed.
- C. Mustazza, A. Borioni, I. Sestili, M. Sbraccia, A. Rodomonte, R. Ferretti and M. R. D. Giudice, Chem. Pharm. Bull., 2006, 54, 611 CrossRef PubMed.
- (a) Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, Oxford, UK, 2008 Search PubMed; (b) D. A. Horton, G. T. Bourne and M. L. Smyth, Chem. Rev., 2003, 103, 893 CrossRef PubMed.
- (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787 CrossRef; (b) S. H. Lee, J.-K. Son, B. S. Jeong, T.-C. Jeong, H. W. Chang, E.-S. Lee and Y. Jahng, Molecules, 2008, 13, 272 CrossRef PubMed.
- (a) C. Jagan mohan Reddy, K. P. V. Kumar, G. S. Reddy, S. Mohanty, J. Kumar, B. V. Rao, T. Krishnaji and A. Raghunadh, Synth. Commun., 2018, 48, 168–174 CrossRef; (b) V. N. Murthy, S. P. Nikumbh, S. P. Kumar, Y. Chiranjeevi, L. V. Rao and A. Raghunadh, Synlett, 2016, 27, 2362 CrossRef; (c) S. B. Azimi and J. Azizian, Tetrahedron Lett., 2016, 57, 181 CrossRef; (d) J. Zhang, J. Zhao, L. Wang, J. Liu, D. Ren and Y. Ma, Tetrahedron, 2016, 72, 936 CrossRef; (e) S. B. Azimi and J. Azizian, Synlett, 2016, 27, 1836 CrossRef; (f) T. Fatemeh, K. Varnamkhasti and M. Taghi, Synlett, 2016, 27, 2510 CrossRef; (g) M. V. M. babu, R. Shankar, G. R. Reddy, T. S. Rao, M. V. B. Rao and A. Raghunadh, Tetrahedron Lett., 2016, 57, 5033 CrossRef; (h) J. Yang, S. Sun, B. Zhang, J. Zhang and Y. Gu, Tetrahedron, 2014, 70, 9214–9223 CrossRef; (i) S. Sun, C. Cheng, J. Yang, A. Taheri, D. Jiang, B. Zhang and Y. Gu, Org. Lett., 2014, 16, 4520–4523 CrossRef PubMed; (j) M. Mahdavi, N. Foroughi, M. Saeedi, M. Karimi, H. Alinezhad, A. Foroumadi, A. Shafiee and T. Akbarzadeh, Synlett, 2014, 25, 385–388 Search PubMed; (k) T. Kalai, B. Bognar, D. Zsolnai, Z. Berente and K. Hideg, Synthesis, 2012, 44, 3655–3660 CrossRef.
- (a) P. V. N. S. Murthy and D. Rambabu, Tetrahedron Lett., 2012, 53, 863 CrossRef; (b) S. Kiaee, A. Masoumnia and M. Maghsoodlou, Res. Pharm. Sci., 2012, 7 Search PubMed; (c) J. Chen, D. Wu, F. He, M. Liu, H. Wu, J. Ding and W. Su, Tetrahedron Lett., 2008, 49, 3814 CrossRef; (d) J. X. Chen, W. K. Su, H. Y. Wu, M. C. Liu and C. Jin, Green Chem., 2007, 9, 972 RSC; (e) M. Rueping, A. P. Antonchick, E. Sugiono and K. Grenader, Angew. Chem., Int. Ed., 2009, 48, 908 CrossRef PubMed; (f) R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475 CrossRef; (g) Z. Jianguang and F. Jie, J. Org. Chem., 2011, 142, 631 Search PubMed; (h) X. Cheng, S. K. Vellalath, R. Goddard and B. List, J. Am. Chem. Soc., 2008, 130, 15786 CrossRef PubMed.
- R. A. Bunce and B. Nammalwar, J. Heterocycl. Chem., 2011, 48, 991 CrossRef.
- K. S. Kumar, P. M. Kumar, M. A. Reddy, M. Ferozuddin, M. Sreenivasulu, A. A. Jafar, G. R. Krishna, C. M. Reddy, D. Rambabu, K. Shiva Kumar, S. Pal and M. Pal, Chem. Commun., 2011, 47, 10263–10265 RSC.
- K. V. Sashidhara, G. R. Palnati, S. R. Avula and A. Kumar, Synlett, 2012, 23, 611 CrossRef.
- (a) M. V. Madhubabu, R. Shankar, S. M. Satish, M. V. Basaveswara Rao, U. K. Syam Kumar and A. Raghunadh, RSC Adv., 2016, 6, 36599 RSC; (b) R. K. Raghavendra, M. Ramamohan, A. Raghunadh, B. M. Suresh, K. S. Praveen, D. Kalita, E. Laxminarayana, B. Prasad and M. Pal, RSC Adv., 2015, 5, 61575 RSC; (c) A. V. D. Rao, B. P. Vykunteswara rao, K. T. Bhaskar, R. J. Nivrutti, K. Dipak, J. D. L. Kumar, V. Siddaiah, S. P. Douglas and A. Raghunadh, Tetrahedron Lett., 2015, 56, 4714 CrossRef; (d) N. V. Murthy, S. P. Nikumbh, S. Praveen Kumar, L. Vaikunta Rao and A. Raghunadh, Tetrahedron Lett., 2015, 56, 5767 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03308k |
| This journal is © The Royal Society of Chemistry 2018 |