Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environment-friendly magnetic Fe–Ce–W catalyst for the selective catalytic reduction of NOx with NH3: influence of citric acid content on its activity-structure relationship†
Zhi-bo Xiong *a,
Xing Ninga,
Fei Zhouab,
Bin Yanga,
Yan-wu Tua,
Jing Jina,
Wei Lua and
Zong-hao Liuc
*a,
Xing Ninga,
Fei Zhouab,
Bin Yanga,
Yan-wu Tua,
Jing Jina,
Wei Lua and
Zong-hao Liuc
aSchool of Energy and Power Engineering, University of Shanghai for Science & Technology, Shanghai 200093, China. E-mail: xzb328@163.com; Tel: +86 21 55270508
bJiangsu Guoxin Jingjiang Power LTD, Jingjiang 214500, China
cShandong Province Environmental Protection Technology Service Center, Jinan 250100, China
First published on 14th June 2018
Abstract
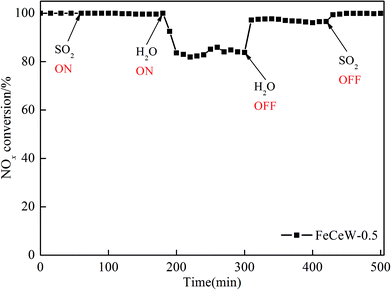
The influence of the citric acid content on the structural and redox properties of a magnetic iron–cerium–tungsten mixed oxide catalyst prepared through a microwave-assisted citric acid sol–gel method is investigated via TG–DTG–DSC, XRD, N2 adsorption–desorption, XPS, H2-TPR and NH3-TPD. Additionally, the NH3-SCR activity of the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts are also studied. The results indicate that an increase in citric acid content strengthens the sol–gel reaction between citric acid and metal ions and promotes the formation of the γ-Fe2O3 crystallite not α-Fe2O3. Meanwhile, it decreases the BET surface area and pore volume of the catalyst. Furthermore, the surface concentration of iron species on the catalyst is enhanced when the molar ratio of citric acid/(Fe + Ce + W) increases from 0.25 to 1.0, but its surface absorbed oxygen and total oxygen concentration decrease. The magnetic FeCeW-0.5 catalyst shows the best reducibility at temperatures below 790 °C. The increase in the citric acid content inhibits the formation of acid sites in the catalyst, thus the magnetic FeCeW-0.25 catalyst possesses the most Lewis acid sites and Brønsted acid sites among the catalysts. The enhancement in citric acid content is beneficial to improve the SCR reaction rates normalized by the surface area of the catalyst. This catalyst exhibits high anti-SO2 and H2O poisoning, and the molar ratio of citric acid/(Fe + Ce + W) affects the adsorption of NOx species on its surface.
1. Introduction
Nitrogen oxide (NOx) emitted from the combustion of fossil fuel in coal-fired power plants or automobile engines is a typical environmental pollutant, which causes serious problems to the environment and human health, such as acid rain, photochemical smog, pulmonary edema and tissue hypoxia.1–6 Therefore, many technologies have been developed to reduce the emission of NOx from coal-fired power plants.7–9 Compared with other de-nitrogen technologies, the selective catalytic reduction of NOx by NH3 (NH3-SCR) has drawn increasing attention due to its high efficiency.7 V2O5–WO3(MoO3)/TiO2 is widely used as an NH3-SCR catalyst due to its high NOx conversion and high anti-SO2 poisoning. However, it has some limitations, such as a relatively narrow temperature window and the toxicity of vanadium species. Therefore, it is necessary to develop novel non-vanadium catalysts with high deNOx performances to replace the commercial V2O5–WO3(MoO3)/TiO2 catalyst.10–16Due to their relatively high NH3-SCR activity, low cost and non-toxicity, iron-based catalysts have been receiving significant attention by many researchers.17–25 Cerium or/and tungsten are widely used additives to optimize the NH3-SCR activity of iron-based catalysts owing to the high oxygen storage capacity and high redox ability of the Ce species by shifting between Ce4+ and Ce3+, and the high surface acidity and excellent thermal stability of W species.26–30 In our previous research, a novel magnetic iron–cerium–tungsten mixed oxide catalyst was proposed through a microwave-assisted citric acid sol–gel method with both Ce and W as additives, and the synergistic promotional effect of Ce and W on the NH3-SCR activity of iron oxide was also investigated.15,16 Meanwhile, many researches have indicated that the amount of citric acid plays an important role in the sol–gel reaction between citric acid and metal ions, thereby influencing the structural and redox properties of the powder obtained by the citric acid sol–gel method.31–35 However, these properties are usually thought to be the important factors in the NH3-SCR activity of iron-based mixed oxide catalysts.16,17 Therefore, herein, to reveal the effect of the physical structure of magnetic iron–cerium–tungsten mixed oxide catalysts on their NH3-SCR activity, three types of catalysts are obtained by changing the content of citric acid, where the molar ratios of citric acid/(Fe + Ce + W) are 0.25, 0.5 and 1.0. Thermogravimetric analysis (TG–DTG–DSC), X-ray diffraction (XRD), N2 adsorption–desorption, X-ray photoelectron spectroscopy (XPS), temperature-programmed reduction (H2-TPR) and temperature-programmed desorption (NH3-TPD) are used to characterize the physical structural properties of the catalysts. The influence of the citric acid/(Fe + Ce + W) molar ratio on the NH3-SCR mechanism over the catalyst at 200 °C is obtained using in situ diffuse reflection infrared Fourier transform spectroscopy (in situ DRIFTS).
2. Material and methods
2.1 Catalyst preparation and activity test
The magnetic iron–cerium–tungsten mixed oxide catalyst was prepared through a microwave-assisted citric acid sol–gel method according to ref. 15 and 16. Fe(NO3)3·9H2O, Ce(NO3)3·6H2O, (NH4)6H2W12O40·nH2O were used as the precursors and citric acid as the complexing agent. The precursors were successively dissolved in 10 mL water to obtain a mixed solution by controlling the molar ratio of Fe/Ce/W to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The mixed solution was then stirred for about 10 min at ambient temperature to ensure all the precursors were completely dissolved. A certain amount of citric acid (2.9472, 5.8944 and 11.7888 g) was added to this mixed solution according to the citric acid/(Fe + Ce + W) molar ratio of 0.25, 0.5 and 1.0, respectively. After stirring for about 10 min, the mixed solution was placed in a household microwave oven (EG8MEA6-NR, 2.45 GHz, 800 W) irradiated for 10 min at 36.4% power (microwave irradiation 8 s, 14 s suspended for a cycle with full power), and a pale red dry gel was obtained. The dry gel was calcined at 500 °C for 5 h under an air atmosphere (at a heating rate of 5 °C min−1), and then it was crushed and sieved to 40–60 mesh for the NH3-SCR activity tests. The catalyst was denoted as FeCeW-m, where m represents the molar ratio of citric acid/(Fe + Ce + W). For example, FeCeW-0.5 contained the molar ratio of citric acid/(Fe + Ce + W) of 0.5. Meanwhile, it should be mentioned that there existed a weak spreading combustion phenomenon for the FeCeW-1.0 sol–gel during microwave irradiation.
5. The mixed solution was then stirred for about 10 min at ambient temperature to ensure all the precursors were completely dissolved. A certain amount of citric acid (2.9472, 5.8944 and 11.7888 g) was added to this mixed solution according to the citric acid/(Fe + Ce + W) molar ratio of 0.25, 0.5 and 1.0, respectively. After stirring for about 10 min, the mixed solution was placed in a household microwave oven (EG8MEA6-NR, 2.45 GHz, 800 W) irradiated for 10 min at 36.4% power (microwave irradiation 8 s, 14 s suspended for a cycle with full power), and a pale red dry gel was obtained. The dry gel was calcined at 500 °C for 5 h under an air atmosphere (at a heating rate of 5 °C min−1), and then it was crushed and sieved to 40–60 mesh for the NH3-SCR activity tests. The catalyst was denoted as FeCeW-m, where m represents the molar ratio of citric acid/(Fe + Ce + W). For example, FeCeW-0.5 contained the molar ratio of citric acid/(Fe + Ce + W) of 0.5. Meanwhile, it should be mentioned that there existed a weak spreading combustion phenomenon for the FeCeW-1.0 sol–gel during microwave irradiation.
The selective catalytic reduction of NOx with NH3 was carried out in a fixed-bed continuous flow quartz reactor at atmospheric pressure.15,16 The concentration of the reactants was controlled as follows: 1000 ppm NO, 1000 ppm NH3, 3 vol% O2, 100 ppm SO2 (when used), 5 vol% H2O (when used) and balance N2. The volume of sample used in each experiment was 2 mL (0.512 g for FeCeW-0.25, 1.049 g for FeCeW-0.5 and 0.989 g for FeCeW-1.0) with a gas hourly space velocity (GHSV) of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The downstream concentrations of NO and NO2 at the inlet and outlet of the reactor were measured using a flue gas analyzer (Model 60i, Thermo Fisher Scientific Co. Ltd, USA). NO conversion (XNOx) was calculated as follows: XNOx = (1 − [NOx]out/[NOx]in) × 100% with [NOx] = [NO] + [NO2]. The different bulk densities of the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts might be mainly attributed to the influence of the citric acid/(Fe + Ce + Ti) molar ratio on the sol reaction between citric acid and the ions, and the burning characteristic of the formed sol–gel during the calcination process. Citric acid is the burning fuel in addition the complexing agent, and the nitrate ion is the oxidizer for the spreading combustion during the calcination process. The enhancement of the citric acid/(Fe + Ce + W) molar ratio improved both the sol reaction among the reactants and the formation of NO2 due to the decomposition of nitric acid during the process of microwave irradiation (a type of brown gas was formed). In addition, a high molar ratio of citric acid/(Fe + Ce + W) might cause the agglomeration of the sol–gel particles.37
000 h−1. The downstream concentrations of NO and NO2 at the inlet and outlet of the reactor were measured using a flue gas analyzer (Model 60i, Thermo Fisher Scientific Co. Ltd, USA). NO conversion (XNOx) was calculated as follows: XNOx = (1 − [NOx]out/[NOx]in) × 100% with [NOx] = [NO] + [NO2]. The different bulk densities of the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts might be mainly attributed to the influence of the citric acid/(Fe + Ce + Ti) molar ratio on the sol reaction between citric acid and the ions, and the burning characteristic of the formed sol–gel during the calcination process. Citric acid is the burning fuel in addition the complexing agent, and the nitrate ion is the oxidizer for the spreading combustion during the calcination process. The enhancement of the citric acid/(Fe + Ce + W) molar ratio improved both the sol reaction among the reactants and the formation of NO2 due to the decomposition of nitric acid during the process of microwave irradiation (a type of brown gas was formed). In addition, a high molar ratio of citric acid/(Fe + Ce + W) might cause the agglomeration of the sol–gel particles.37
2.2 Catalyst characterization
The thermal decomposition properties of the citric acid crystallite and the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) sol–gels were determined on a thermal gravimetric analyzer (Netzsch, STA449 F3) under an air atmosphere. In addition, the physicochemical properties and the NH3-SCR mechanism of the samples were also characterized via XRD, N2 adsorption–desorption, XPS, H2-TPR, NH3-TPD and in situ DRIFTS according to ref. 15, 16 and 38. The detailed information is listed in the ESI.†3. Results and discussion
3.1 TG–DTG–DSC
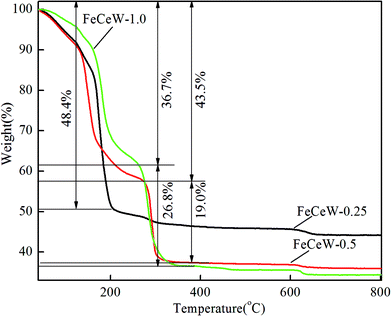
Thermo-gravimetric analysis is an important characterization method for investigating the relationship between catalyst weight and temperature or differential thermal analysis. The TG–DTG–DSC curves of the FeCeW-m (m = 0.25, 0.5 and 1.0) sol–gels were measured after microwave irradiation, and the thermal decomposition property of the citric acid crystallite was also studied for comparison (Fig. 1, Fig. S1 and S2†).From Fig. S1,† two obvious weight loss peaks are observed in the TG curve of the citric acid crystallite. The low-temperature weight loss peak is assigned to its rapid decomposition with about 84.9% weight loss, and the high-temperature weight loss peak is attributed to the slow oxidation of the residual carbon after its rapid decomposition. Different from the TG curve of the citric acid crystallite, three weight loss peaks appear for the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalyst sol–gels after microwave irradiation (Fig. 1). The temperature of the first weight loss peak for the FeCeW-0.25 sol–gel is lower than that of the citric acid crystallite, which is mainly attributed to the introduction of nitrate ions from iron or/and the cerium precursors. Meanwhile, the decomposition of nitric acid gradually improved during the process of microwave irradiation due to the enhancement of the sol–gel reaction between citric acid and metal ions when the molar ratio of citric acid/(Fe + Ce + W) was increased from 0.25 to 1.0. Similar to the thermal decomposition properties of the citric acid crystallite, the FeCeW-0.25 sol–gel almost completely decomposed at the first weight loss peak, although its starting decomposition temperature decreased due to the introduction of nitrate ions. Meanwhile, a second peak with a larger weight loss is observed for the FeCeW-0.5 and FeCeW-1.0 sol–gels, and the FeCeW-1.0 sol–gel showed a larger weight loss than the FeCeW-0.5 sol–gel. This indicates that the sol–gel reaction between citric acid and metal ions becomes stronger when the molar ratio of citric acid/(Fe + Ce + W) is increased from 0.25 to 1.0. The DSC curves also show the presence of two exothermic peaks for the FeCeW-m (m = 0.5 and 1.0) sol–gels compared to one exothermic peak for the FeCeW-0.25 sol–gel (Fig. S2†). Meanwhile, the first exothermic peak of the FeCeW-1.0 sol–gel almost disappeared and was smaller than that of the FeCeW-0.5 sol–gel. This is mainly attributed to the occurrence of a weak spreading combustion phenomenon for the FeCeW-1.0 sol–gel during the microwave irradiation process. Therefore, it can be concluded that the citric acid content plays an important role in the sol–gel process between citric acid and Fe/Ce/W ions, and the complex reaction between them is fully completed with the molar ratio of citric acid/(Fe + Ce + W) increasing from 0.25 to 1.0. Thus, it influences the structural properties and NH3-SCR activity of the magnetic iron–cerium–tungsten mixed oxide catalysts prepared through the microwave-assisted citric acid sol–gel method.
3.2 Structure and redox properties
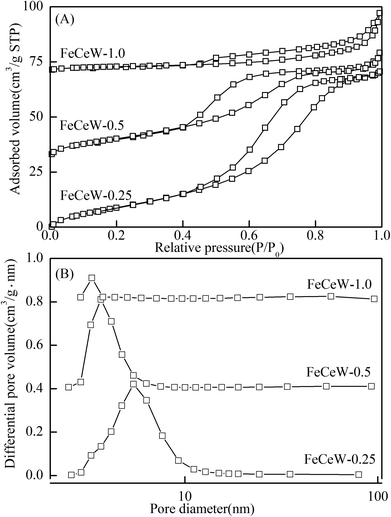 | ||
| Fig. 3 N2 adsorption–desorption of the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts (A) N2 adsorption–desorption isotherms and (B) pore size distributions. | ||
| Sample | SBETa (m2 g−1) | Pore volumeb (cm3 g−1) | Pore diameterc (nm) | Rsd × 109 (mol (s−1 m−2)) | ||
|---|---|---|---|---|---|---|
| 150 °C | 175 °C | 200 °C | ||||
| a BET surface area.b BJH desorption pore volume.c BJH desorption pore diameter.d SCR reaction rates normalized by the catalyst surface area. | ||||||
| FeCeW-0.25 | 66.30 | 0.127 | 5.6 | 0.93 | 2.77 | 8.64 |
| FeCeW-0.5 | 53.34 | 0.086 | 4.5 | 2.55 | 7.07 | 21.3 |
| FeCeW-1.0 | 10.10 | 0.042 | 9.1 | 4.73 | 19.23 | 65.7 |
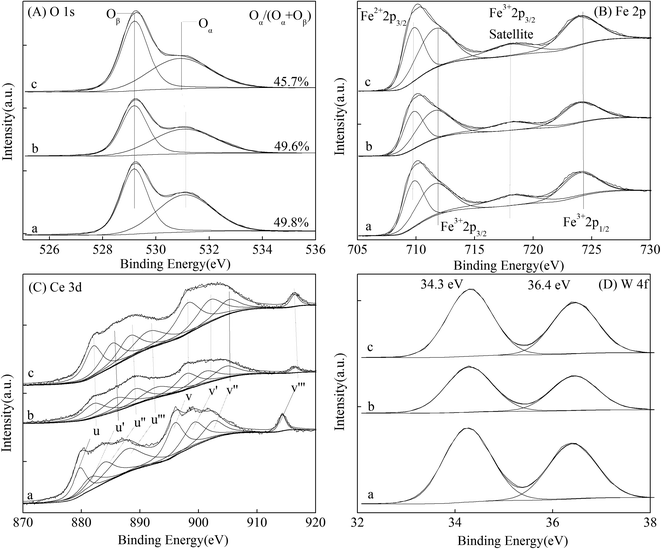 | ||
| Fig. 5 XPS spectra of the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts ((A) O 1s, (B) Fe 2p, (C) Ce 3d, and (D) W 4f). | ||
| Sample | Surface atomic concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe2+ | Fe3+ | Fetotal | Ce | W | Oα | Oβ | Ototal | |
| FeCeW-0.25 | 4.41 | 12.38 | 16.79 | 6.52 | 1.64 | 37.66 | 37.39 | 75.05 |
| FeCeW-0.5 | 4.67 | 13.86 | 18.53 | 5.96 | 1.33 | 36.78 | 37.40 | 74.18 |
| FeCeW-1.0 | 5.00 | 14.69 | 19.69 | 7.10 | 1.40 | 32.84 | 38.97 | 71.81 |
3.3 Catalytic performance
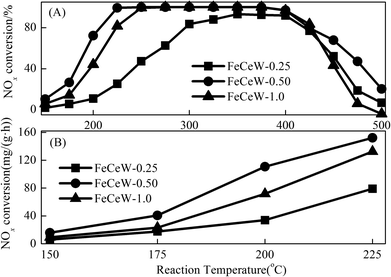
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, respectively. Fig. 7(B) shows the calculated NOx conversion per gram of magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalyst at 150–225 °C in one hour. The NOx conversion at 150–225 °C per gram catalyst also decreased as follows: FeCeW-0.5 > FeCeW-1.0 > FeCeW-0.25. Therefore, the citric acid content unquestionably influenced the NH3-SCR activity of the magnetic iron-cerium–tungsten mixed oxide catalyst prepared through the microwave-assisted citric acid sol–gel method.
000 h−1, respectively. Fig. 7(B) shows the calculated NOx conversion per gram of magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalyst at 150–225 °C in one hour. The NOx conversion at 150–225 °C per gram catalyst also decreased as follows: FeCeW-0.5 > FeCeW-1.0 > FeCeW-0.25. Therefore, the citric acid content unquestionably influenced the NH3-SCR activity of the magnetic iron-cerium–tungsten mixed oxide catalyst prepared through the microwave-assisted citric acid sol–gel method.
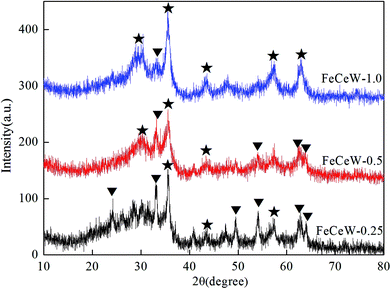
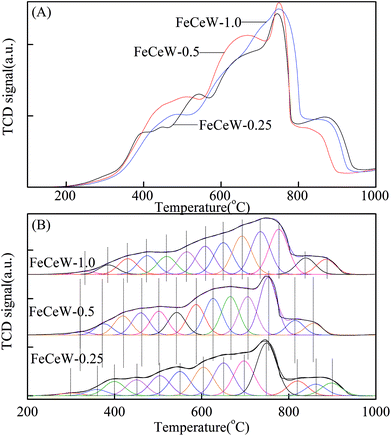
The results in Fig. 1 demonstrate that the enhancement in citric acid content strengthened the sol–gel reaction between citric acid and metal ions, and the sol–gel reaction between them was fully completed when the molar ratio of citric acid/(Fe + Ce + W) was increased from 0.25 to 1.0. This depressed the formation of α-Fe2O3 crystallite and caused the average pore size of the catalyst to become bigger (Fig. 2 and 3, respectively), thereby decreasing its BET surface area and pore volume (Table 1). In addition, the enhancement of the citric acid/(Fe + Ce + W) molar ratio from 0.5 to 1.0 promoted the formation of the γ-Fe2O3 crystallite, and magnetic FeCeW-1.0 shows the smallest BET surface area and pore volume among the catalysts. Furthermore, the SCR reaction rates normalized by the catalyst surface area over the magnetic FeCeW-1.0 catalyst was the fastest among the catalysts, as shown in Table 1. Therefore, it might be concluded that the formation of metal species crystallite is an important factor in the NH3-SCR activity of the magnetic iron–cerium–tungsten mixed oxide catalyst, and the formation of γ-Fe2O3 crystallite under a higher molar ratio of citric acid/(Fe + Ce + W) might be beneficial to NOx conversion over the unit area of catalyst. The H2-TPR results also demonstrate that the citric acid content affects the formation of metal species crystallite in the catalyst, and the magnetic FeCeW-0.5 catalyst shows the highest H2 consumption at the temperature range of 400–790 °C due to the formation of amorphous iron and cerium species on its surface. Meanwhile, the enhancement in the citric acid/(Fe + Ce + W) molar ratio improved the surface concentration of Fe element, and decreased the surface concentrations of both the absorbed oxygen (Oα) and total oxygen (Oα + Oβ), as shown in Fig. 5 and Table 2. Li42 et al. reported that the presence and quantity of Fe2+ are important to create charge imbalance, vacancies, interactions and unsaturated chemical bonds on the surface of catalysts, which are beneficial to promote their NH3-SCR activity. Compared with lattice oxygen (Oβ), absorbed oxygen (Oα) is often thought to be more reactive in oxidizing NO to NO2 due to its higher mobility, and a higher Oα/(Oα + Oβ) ratio can facilitate a fast SCR reaction owing to the higher oxidation of NO to NO2 in the NH3-SCR reaction at low temperature.43,44 Interestingly, the low-temperature NOx conversion (150–225 °C) at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 over the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts decreased as follows: FeCeW-0.5 > FeCeW-1.0 > FeCeW-0.25. Therefore, the concentration of absorbed oxygen (Oα) might not play a primary role in the low-temperature NOx conversion by the catalyst. The enhancement in acid sites is known as an important factor in the NH3-SCR activity of catalysts, and is usually thought to be beneficial to promote their NOx conversion. However, the quantity of both Lewis acid sites and Brønsted acid sites in the catalyst decreased when the molar ratio of citric acid/(Fe + Ce + W) increased from 0.25 to 1.0 (Fig. 6), although FeCeW-0.25 showed the worst NH3-SCR activity and the lowest SCR reaction rates normalized by the catalyst surface area.
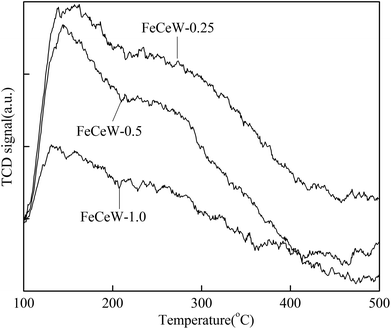
000 h−1 over the magnetic FeCeW-m (m = 0.25, 0.5 and 1.0) catalysts decreased as follows: FeCeW-0.5 > FeCeW-1.0 > FeCeW-0.25. Therefore, the concentration of absorbed oxygen (Oα) might not play a primary role in the low-temperature NOx conversion by the catalyst. The enhancement in acid sites is known as an important factor in the NH3-SCR activity of catalysts, and is usually thought to be beneficial to promote their NOx conversion. However, the quantity of both Lewis acid sites and Brønsted acid sites in the catalyst decreased when the molar ratio of citric acid/(Fe + Ce + W) increased from 0.25 to 1.0 (Fig. 6), although FeCeW-0.25 showed the worst NH3-SCR activity and the lowest SCR reaction rates normalized by the catalyst surface area.
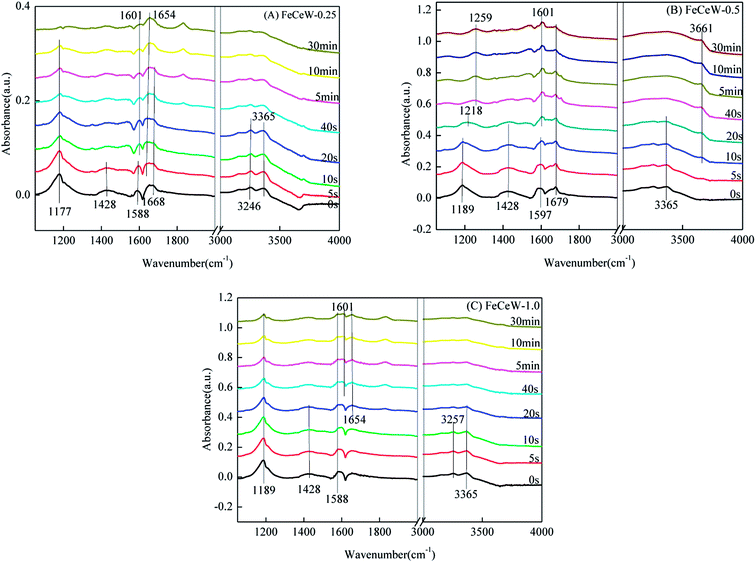
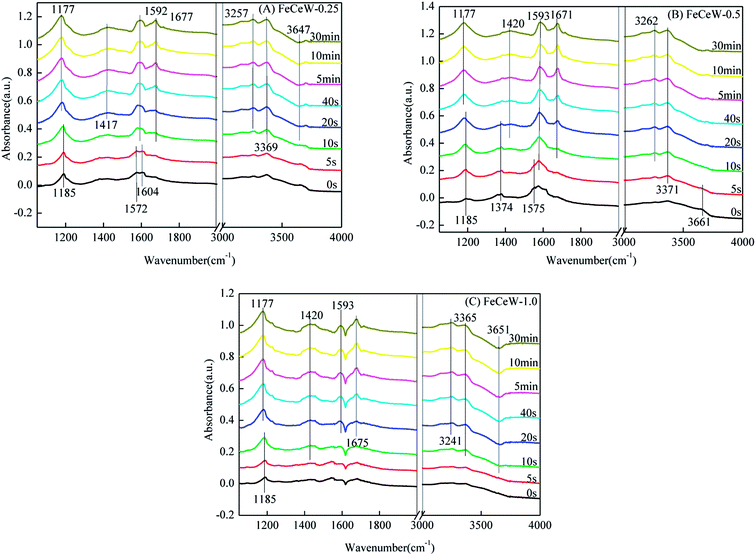
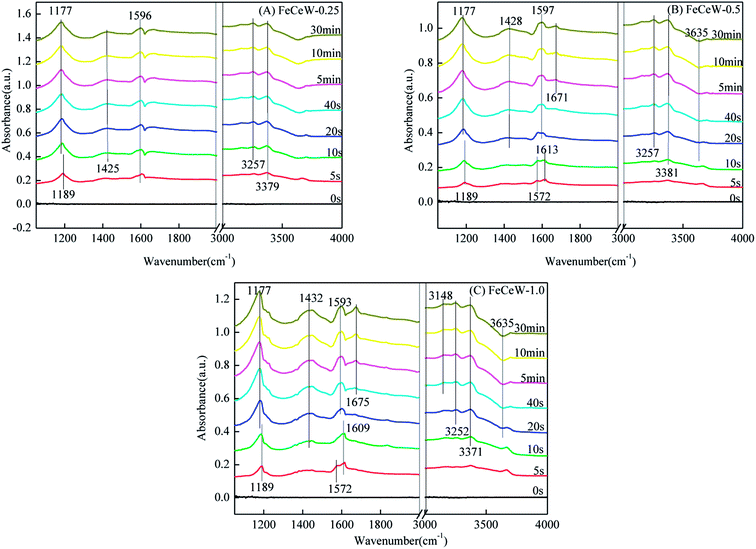
3.4 In situ DRIFTS
4. Conclusions
The influence of citric acid content on the NH3-SCR activity, structure and redox properties of a magnetic iron–cerium–tungsten mixed oxide catalyst prepared through the microwave-assisted citric acid sol–gel method was investigated. The enhancement in citric acid/(Fe + Ce + W) molar ratio is beneficial to the formation of γ-Fe2O3 crystallite and promotion of the SCR reaction rates normalized by the surface area of the magnetic catalyst, although this decreased its BET surface area and pore volume. The concentrations of both Fe3+ and Fe2+ on the surface of the catalyst were enhanced when the molar ratio of citric acid/(Fe + Ce + W) increased from 0.25 to 1.0, but it decreased the concentration of absorbed oxygen and total oxygen. Also, the magnetic FeCeW-0.5 catalyst showed the best reducibility at temperatures below 790 °C. Simultaneously, the enhanced citric acid content inhibited the formation of acid sites in the magnetic iron–cerium–tungsten mixed oxide catalyst, and FeCeW-0.25 showed the most Lewis acid sites and Brønsted acid sites among the catalysts. The molar ratio of citric acid/(Fe + Ce + W) exhibited almost no effect on the adsorption of NH3 species over the catalyst. Meanwhile, it affected the adsorption of NOx species. The main reaction occurs between the adsorbed NH3 species and gaseous NO + O2 over FeCeW-0.25 at 200 °C, which follows an E–R mechanism. Meanwhile, both E–R and L–H mechanisms exist over FeCeW-0.5 and FeCeW-1.0 at 200 °C.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2016YFB0600601), the National Science Foundation of China (No. 51406118), Program of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. QD2015017).References
- L. B. Duan, Y. Q. Duan, C. S. Zhao and J. W. Anthony, NO emission during co-firing coal and biomass in an oxy-fuel circulate in fluidized bed combustor, Fuel, 2015, 150, 8–13 CrossRef.
- D. Husain, Chemistry of Atmospheres: An Introduction to the Chemistry of Atmospheres of Earth, the Planets and their Satellites By R. P. Wayne, J. Photochem. Photobiol., A, 1992, 63(2), 253–254 CrossRef.
- L. B. Duan, Z. X. Jiang, X. P. Chen and C. S. Zhao, Investigation on water vapor effect on direct sulfation during wet-recycle oxy-coal combustion, Appl. Energy, 2013, 108, 121–127 CrossRef.
- L. B. Duan, D. Y. Liu, X. P. Chen and C. S. Zhao, Fly ash recirculation by bottom feeding on a circulating fluidized bed boiler co-burning sludge and coal, Appl. Energy, 2012, 95, 295–299 CrossRef.
- R. Kurose, H. Makino and A. Suzuki, Numerical analysis of pulverized coal combustion characteristics using advanced low-NOx burner, Fuel, 2004, 83, 693–703 CrossRef.
- S. J. Zhao, L. Wang, Y. Wang and X. Li, Hierarchically porous LaFeO3 perovskite prepared from the pomelo peel bio-template for catalytic oxidation of NO, J. Phys. Chem. Solids, 2018, 116, 43–49 CrossRef.
- Y. Y. Wang, H. Y. Fang, D. Zhou, H. C. Han and J. Chen, Characterization of nitrous oxide and nitric oxide emissions from a full-scale biological aerated filter for secondary nitrification, Chem. Eng. J., 2016, 299, 304–313 CrossRef.
- K. Zhao, W. L. Han, G. X. Lu, J. Y. Lu, Z. C. Tang and X. P. Zhen, Promotion of redox and stability features of doped Ce–W–Ti for NH3-SCR reaction over a wide temperature range, Appl. Surf. Sci., 2016, 379, 316–322 CrossRef.
- M. Kong, Q. C. Liu, X. Q. Wang, S. Ren, J. Yang, D. Zhao, W. C. Xi and L. Yao, Performance impact and poisoning mechanism of arsenic over commercial V2O5–WO3/TiO2 SCR catalyst, Catal. Commun., 2015, 72, 121–126 CrossRef.
- L. Qiu, Y. Wang, D. D. Pang, F. Ouyang and C. L. Zhang, SO42−–Mn–Co–Ce supported on TiO2/SiO2 with high sulfur durability for low-temperature SCR of NO with NH3, Catal. Commun., 2016, 78, 22–25 CrossRef.
- S. Lai, D. Meng, W. Zhan, Y. Guo and Y. Guo, The promotional role of Ce in Cu/ZSM-5 and in situ surface reaction for selective catalytic reduction of NOx with NH3, RSC Adv., 2015, 5(110), 90235–90244 RSC.
- Q. Xu, R. Su, L. Cao, Y. Li and C. Yang, Facile preparation of high-performance Fe-doped Ce-Mn/TiO2 catalysts for the low-temperature selective catalytic reduction of NOx with NH3, RSC Adv., 2017, 7(77), 48785–48792 RSC.
- P. Lu, H. Li, H. Liu, Y. Chen and Z. Zhang, Influence of tungsten on the NH3-SCR activity of MnWOx/TiO2, RSC Adv., 2017, 7(32), 19771–19779 RSC.
- C. Liu, G. Gao, J. W. Shi, C. He, G. D. Li, N. Bai and C. M. Niu, MnOx-CeO2 shell-in-shell microspheres for NH3-SCR de-NOx at low temperature, Catal. Commun., 2016, 86, 36–40 CrossRef.
- Z. B. Xiong, B. Peng, F. Zhou, C. Wu and W. Lu, Magnetic iron–cerium–tungsten mixed oxide pellets prepared through citric acid sol–gel process assisted by microwave irradiation for selective catalytic reduction of NOx with NH3, Powder Technol., 2017, 319, 19–25 CrossRef.
- Z. B. Xiong, J. Liu, F. Zhou, D. Y. Liu and W. Lu, Selective catalytic reduction of NOx with NH3 over ironcerium–tungsten mixed oxide catalyst prepared by different methods, Appl. Surf. Sci., 2017, 406, 218–225 CrossRef.
- F. D. Liu, H. He, C. B. Zhang, Z. C. Feng, L. R. Zheng, Y. N. Xie and T. D. Hu, Selective catalytic reduction of NO with NH3 over iron titanate catalyst: catalytic performance and characterization, Appl. Catal., B, 2010, 96, 408–420 CrossRef.
- Y. Xin, N. N. Zhang, Q. Li, Z. L. Zhang, X. M. Gao, L. R. Zheng, Y. W. Zeng and J. A. Anderson, Selective catalytic reduction of NOx with NH3 over short-range ordered W–O–Fe structures with high thermal stability, Appl. Catal., B, 2018, 229, 81–87 CrossRef.
- G. H. Yao, F. Wang, X. B. Wang and K. T. Gui, Magnetic field effects on selective catalytic reduction of NO by NH3 over Fe2O3 catalyst in a magnetically fluidized bed, Energy, 2010, 35, 2295–2300 CrossRef.
- J. Grzybek, B. Gil, W. J. Roth, M. Skozej, A. Kowalczyk and L. Chmielarz, Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: an in situ FTIR study, Spectrochim. Acta A, 2018, 196, 281–288 CrossRef PubMed.
- Z. B. Xie, F. Wang, J. S. Liang, Z. S. Wang, N. Hui and Y. Ding, Enhanced catalytic efficiency of FeMnTiOx SCR catalysts through adding tourmaline nanopowders during the one-step sol–gel process, Mater. Lett., 2018, 217, 60–63 CrossRef.
- C. Z. Shao, X. F. Liu, D. M. Meng, Q. Xu, Y. Guo, W. Z. Zhan, L. Wang and G. Z. Lu, Catalytic performance of Co–Fe mixed oxide for NH3-SCR reaction and the promotional role of cobalt, RSC Adv., 2016, 70, 66169–66179 RSC.
- J. Liu, L. Kang, P. Maitarad, J. P. Zhang, L. Y. Shi and D. S. Zhang, Mn–Fe bi-metal oxides in situ created on metal wire mesh as monolith catalysts for selective catalytic reduction of NO with NH3, RSC Adv., 2017, 7, 40444–40451 RSC.
- Z. B. Xiong, C. Wu, Q. Hu, Y. Z. Wang, J. Jin, C. M. Lu and D. X. Guo, Promotional effect of microwave hydrothermal treatment on the low-temperature NH3-SCR activity over iron-based catalyst, Chem. Eng. J., 2016, 286, 459–466 CrossRef.
- Z. B. Xiong, Q. Hu, D. Y. Liu, C. Wu, F. Zhou, Y. Z. Wang, J. Jin and C. M. Lu, Influence of partial substitution of iron oxide by titanium oxide on the structure and activity of iron–cerium mixed oxide catalyst for selective catalytic reduction of NOx with NH3, Fuel, 2016, 165, 432–439 CrossRef.
- K. Zhao, W. L. Han, Z. C. Tang, J. Y. Lu and X. Hu, High-Efficiency Environmental-Friendly Fe–W–Ti Catalyst for Selective Catalytic Reduction of NO with NH3: The Structure-Activity Relationship, Catal. Surv. Asia, 2018, 22, 20–30 CrossRef.
- A. Stahl, Z. Wang, T. Schwaemmle, J. Ke and X. B. Li, Novel Fe–W–Ce Mixed Oxide for the Selective Catalytic Reduction of NOx with NH3 at Low Temperatures, Catalysis, 2017, 7(2), 1–12 Search PubMed.
- H. Wang, Z. P. Qu, S. C. Dong, H. B. Xie and C. Tang, Superior Performance of Fe1−xWxO delta for the Selective Catalytic Reduction of NOx with NH3: Interaction between Fe and W, Environ. Sci. Technol., 2016, 50(24), 13511–13519 CrossRef PubMed.
- Y. Sun, Y. Guo, W. Su and Y. J. Wei, Low-Temperature Selective Catalytic Reduction of NO with NH3 over Fe–Ce–Ox Catalysts, Trans. Tianjin Univ., 2017, 23(1), 35–42 CrossRef.
- X. B. Wang, L. Zhang, S. G. Wu, W. X. Zou, S. H. Yu, Y. Shao and L. Dong, Promotional Effect of Ce on Iron-Based Catalysts for Selective Catalytic Reduction of NO with NH3, Catalysts, 2016, 6(8), 1–15 CrossRef.
- A. Mali and A. Ataie, Influence of the metal nitrates to citric acid molar ratio on the combustion process and phase constitution of barium hexaferrite particles prepared by sol–gel combustion method, Ceram. Int., 2004, 30, 1979–1983 CrossRef.
- W. D. Yang, Y. H. Chang and S. H. Huang, Influence of molar ratio of citric acid to metal ions on preparation of La0.67Sr0.33MnO3 materials via polymerizable complex process, J. Eur. Ceram. Soc., 2005, 25, 3611–3618 CrossRef.
- C. Cannas, A. Musinu, D. Peddis and G. Piccaluga, New synthesis of ferrite-silica nanocomposites by a sol–gel auto-combustion, J. Nanopart. Res., 2004, 6, 223–232 CrossRef.
- R. Ran, X. D. Wu and D. A. Weng, Effect of complexing species in a sol–gel synthesis on the physicochemical properties of La0.7Sr0.3Mn0.7Cu0.3O3 + λ catalyst, J. Alloys Compd., 2006, 414(1–2), 169–174 CrossRef.
- Z. X. Yue, W. Y. Guo, J. Zhou, Z. L. Gui and L. T. Li, Synthesis of nanocrystilline ferrites by sol–gel combustion process: the influence of pH value of solution, J. Magn. Magn. Mater., 2004, 270, 216–223 CrossRef.
- F. D. Liu, H. He, C. B. Zhang, Z. C. Feng, L. R. Zheng, Y. N. Xie and T. D. Hu, Selective catalytic reduction of NO with NH3 over iron titanate catalyst: catalytic performance and characterization, Appl. Catal., B, 2010, 96, 408–420 CrossRef.
- Z. X. Yue, J. Zhou, H. G. Zhang, Z. L. Gui and L. T. Li, Auto-combustion behavior of nitrate-citrate gels and synthesis of ferrite nano-particles, J. Chin. Ceram. Soc., 1999, 27(4), 466–470 Search PubMed.
- F. Zhou, Z. B. Xiong, W. Lu, J. Jin and X. C. Ding, Microwave-pyrolysis assisted preparation of magnetic iron–titanium mixed oxide catalyst for Selective catalytic reduction of NOx with NH3, Clean: Soil, Air, Water, 2017, 45(11), 1–9 Search PubMed.
- Y. K. Yu, J. S. Chen, J. X. Wang and Y. T. Chen, Performances of CuSO4/TiO2 catalysts in selective catalytic reduction of NOx by NH3, Chin. J. Catal., 2016, 37, 281–287 CrossRef.
- M. P. Ruggeri, A. Grossale, I. Nova, E. Tronconi, H. Jirglova and Z. Sobalik, FTIR in situ mechanistic study of the NH3–NO/NO2 “Fast SCR” reaction over a commercial Fe-ZSM-5 catalyst, Catal. Today, 2012, 184(1), 107–114 CrossRef.
- G. Busca, L. Lietti, G. Ramis and F. Berti, Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review, Appl. Catal., B, 1998, 18, 1–36 CrossRef.
- X. Li, J. H. Li, Y. Peng, T. Zhang, S. Liu and J. M. Hao, Selective catalytic reduction of NO with NH3 over novel iron–tungsten mixed oxide catalyst in a broad temperature range, Catal. Sci. Technol., 2015, 5, 4556–4564 Search PubMed.
- F. He, Y. G. Wei, H. B. Li and H. Wang, Synthesis Gas Generation by Chemical-Looping Reforming using Ce-Based Oxygen Carriers Modified with Fe, Cu, and Mn oxides, Energy Fuels, 2009, 23, 2095–2102 CrossRef.
- H. He, H. X. Dai and C. T. Au, Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions, Catal. Today, 2004, 90, 245–254 CrossRef.
- M. P. Ruggeri, A. Grossale, I. Nova, E. Tronconi, H. Jirglova and Z. Sobalik, FTIR in situ mechanistic study of the NH3–NO/NO2 “Fast SCR” reaction over a commercial Fe-ZSM-5 catalyst, Catal. Today, 2012, 184(1), 107–114 CrossRef.
- Z. M. Liu, Y. Yi, J. H. Li, S. I. Woo, B. Y. Wang, X. Z. Cao and Z. X. Li, A superior catalyst with dual redox cycles for the selective reduction of NOx by ammonia, Chem. Commun., 2013, 70, 726–7728 Search PubMed.
- Y. Q. Zeng, S. L. Zhang, Y. N. Wang and Q. Zhong, CeO2 supported on reduced TiO2 for selective catalytic reduction of NO by NH3, J. Colloid Interface Sci., 2017, 496, 487–495 CrossRef PubMed.
- J. Liu, X. Y. Li, Q. D. Zhao, J. Ke, H. N. Xiao, X. J. Lv, S. M. Liu, M. Tadé and S. B. Wang, Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce–Ti mixed oxide: In situ FTIR analysis for structure-activity correlation, Appl. Catal., B, 2017, 200, 297–308 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03131b |
| This journal is © The Royal Society of Chemistry 2018 |