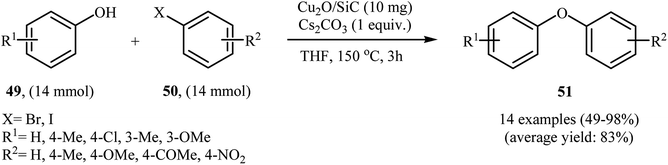Open Access Article
Open Access ArticleDiaryl ethers synthesis: nano-catalysts in carbon-oxygen cross-coupling reactions
Kamellia Nejati
*a,
Sheida Ahmadi
a,
Mohammad Nikpassand
b,
Parvaneh Delir Kheirollahi Nezhad
a and
Esmail Vessally
 a
a
aDepartment of Chemistry, Payame Noor University, P. O. Box 19395-1697, Tehran, Iran. E-mail: k_nejati@pnu.ac.ir; nejati_k@yahoo.com
bDepartment of Chemistry, Rasht Branch, Islamic Azad University, Rasht, Iran
First published on 23rd May 2018
Abstract
The diaryl ether moiety is not only prevalent in a significant number of natural products and synthetic pharmaceuticals but also widely found in many pesticides, polymers, and ligands. Ullmann-type cross-coupling reactions between phenols and aryl halides are regarded as one of the most important methods for the synthesis of this important and versatile structural motif. In recent years, the use of nano-sized metal catalysts in this coupling reaction has attracted a lot of attention because of these catalysts with their high surface-to-volume ratio, high surface energy, and reactive morphology allows for rapid C–O bond formation under mild and ligand-free conditions. In this review we will highlight the power of these catalysts in Ullmann-type C–O cross-coupling reactions.
1. Introduction
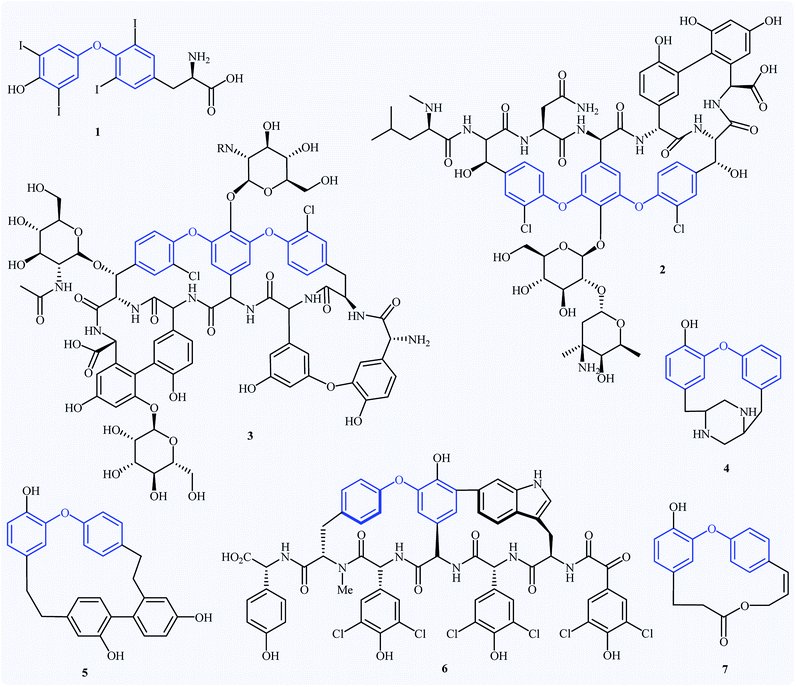
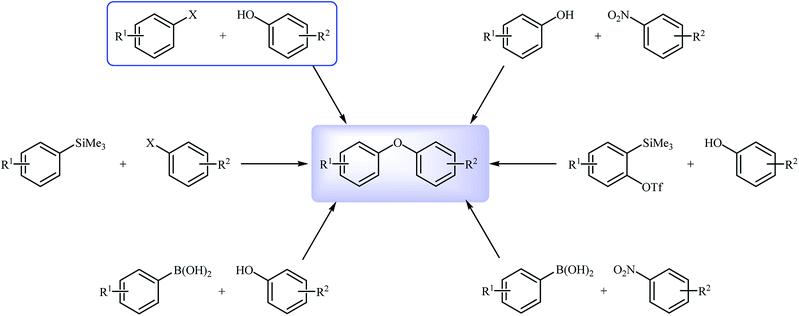
Diaryl ether derivatives have attracted considerable interest as they are a common structural motif encountered in numerous natural and synthetic pharmaceutically important compounds (Fig. 1), such as the antithyroid levothyroxine 1,1 the antibacterial vancomycin 2,2 the antibiotic teicoplanin 3,3 the antifungal piperazinomycin 4,4 the antitumor riccardin C 5,5 the anti-HIV chloropeptin II 5,6 and the antineoplastic combretastatin 7.7 This motif is also found in many pesticides (e.g., cypermethrin and deltamethrin),8 polymers,9 and ligands.10 Due to their immense biological properties, the synthesis of diaryl ethers received tremendous attention from synthetic organic chemists.Transition metal-catalyzed cross-coupling reactions are among the most powerful and versatile tools for the construction of various carbon-carbon11 and carbon-heteroatom12 bonds and have experienced considerable growth over the past decades. In this domain, C–O cross-coupling reactions have been considered as the most popular routes to diaryl ethers. Among the various C–O cross-coupling reactions for the construction of this biologically and synthetically important structural motif (Fig. 2),13–15 Ullmann coupling reaction between easily available and low-cost phenols and aryl halide find maximum application in industrial processes.16
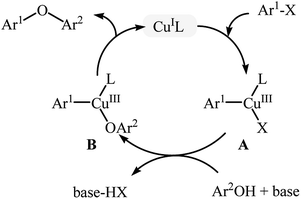
The traditional Ullmann C–O coupling reaction is the coupling of aryl halides (most often aryl iodides) with electron-rich phenols mediated by copper at elevated temperature (>200 °C).17 The major drawbacks of the classical Ullmann reaction, i.e., harsh reaction conditions along with long reaction time, stoichiometric or greater amount of single-use copper reagents, poor functional group tolerance and low yield of products had limited the applications of this reaction for a long time. Over the years, many research groups have studied the mechanism of this coupling reaction. The most widely accepted mechanism for Ullmann C–O cross-coupling involves formation of the organocopper halide A through the oxidative addition of organic halide to the copper catalyst (this step is often the rate-determining step in the catalytic cycle), followed by transmetallation with phenol under the basic conditions to give the diorganocopper complex B, which after a reductive elimination delivers the coupling product (Fig. 3).17b,18 This mechanism indicate that the reaction is faster when the catalyst is more electron rich, because it accelerates oxidative addition of the aryl halide. For instance, it has been proved that in this reaction copper alkoxide or amide complex is much more reactive than the copper halide.19 By increasing the understanding of the mechanistic aspects of the Ullmann reaction, various ligands and efficient catalyst systems have been developed and most of them allowed this reaction to be conducted under mild conditions with desirable yields with excellent functional group tolerance.
During the past few years, significant progress has been made in modifying this coupling reaction by using several nano-sized metal catalysts. The high surface-to-volume ratio, high surface energy, and reactive morphology of metal nanoparticles20 made them very successful catalysts in Ullmann-type cross-coupling reactions. They allow for rapid C–O bond formation under mild and ligand-free conditions, with the benefits of excellent yield of desired product coupled with the ease of catalyst separation and recovery. Despite great popularity of nanocatalysts in Ullmann-type cross-coupling reactions, no review in the literature is available covering features, advantages, and limitations associated with the use of these catalysts in the aforementioned coupling reaction. Thus, considering the lack of such a review in the literature and in continuation of our recent works,21 here we will highlight the power of nano-sized metals as catalysts in Ullmann-type cross-coupling reactions. Literature has been surveyed until March 2018.
2. Copper nanocatalysts
Copper nanoparticles (Cu-NPs) have attracted great interest in recent years because of their availability, versatility, and unique physical properties.22 They have proven to be very efficient catalysts for a wide variety of organic reactions.23 Recently, the extensive attention devoted to the employing copper nanocatalysts in C–O cross-coupling reactions of phenols with aryl halides. In this section, we describe the current literature on Cu-NPs catalyzed coupling reactions between phenols and aryl halides. The reactions have been classified based on the type of catalysts (e.g., single element CuNPs, copper oxide NPs, supported CuNPs).2.1. Single element Cu nanoparticles
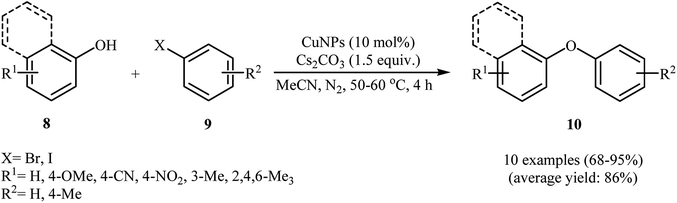
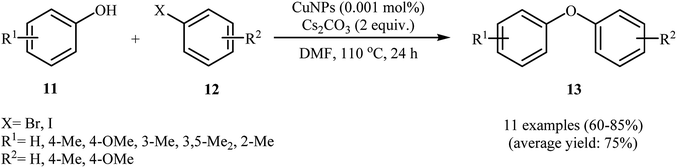
In 2007, Kidwai and co-workers reported the first Cu-nanoparticle catalyzed Ullmann cross-coupling of phenols 8 with aryl halides 9 into corresponding diaryl ethers 10 (Scheme 1). These reactions proceeded rapidly in the presence of 10 mol% of CuNPs (18 ± 2 nm) as a catalyst, 1.5 equiv. of Cs2CO3 as a base in MeCN at 50–60 °C. Cross-coupling with a broad range of electrophilic substituents at both the phenols and the aryl halide component occurred with high selectivity. Interestingly, the reaction was equally efficient for aryl bromides and aryl iodides. Moreover, the cross-coupling of sterically hindered 2,2′-disubstituted and bicyclic phenols was possible, affording diaryl ether products in good to high yields. It should be noted that the catalytic action of the CuNPs was highly dependent on the nanoparticle size. The maximum reaction rate was observed for a particle of an average diameter of about 20 nm. With a decrease or increase in average particle size, the reaction rate decreased.24 Three years later, Schouten and Wheatley along with their co-workers showed that the reaction could be catalyzed by CuNPs (9.6 nm) under microwave irradiation, allowing base-free Ullmann etherification with high yields.25In a closely related investigation, Obora's group also showed that functionalized diaryl ethers 13 were formed from the corresponding phenols 11 and aryl halides 12 through Ullmann cross-coupling in a simple process employing single-nano-sized colloidal CuNPs (2 nm) as catalyst and Cs2CO3 as a base, in DMF as solvent and at 110 °C (Scheme 2). It is noted that the catalyst was prepared by a simple thermal heating of CuCl2 in DMF at 140 °C for 8 h. To identify the solvent potentially suitable for the coupling, the authors first chose MeCN, DMF, and NMP. For this cross-coupling reaction, DMF was the most effective solvent, giving the expected diaryl ether product in high yields. The results showed that aryl bromides gave the target products in lower yields than aryl halides. In this study, the authors found some limitations in their methodology when they used 2-iodothiophene as a coupling partner. In this case, no formation of target product was observed.26
2.2. Copper oxide nanoparticles
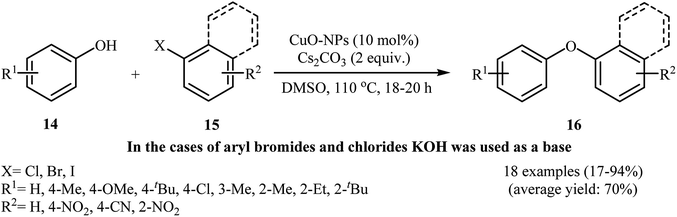
With the objective of designing a milder procedure to diaryl ether derivatives through Ullmann coupling of phenols with aryl halides, Babu and Karvembu were able to demonstrate that a variety of functionalized diaryl ethers 19 could be obtained from the reaction of corresponding phenols 17 with aryl bromides/chlorides 18 at room temperature employing 3 mol% of CuO nanoparticles as the catalyst and 2 equiv. of KOH as the base (Scheme 4). These reactions were carried out in N,N-dimethyl acetamide (DMAc) under nitrogen atmosphere and generally provided the highly substituted diaryl ethers 19 in moderate to excellent yields. In addition, the CuO-NPs catalyzed C–O coupling reaction of phenols with heteroaryl bromides was also carried out smoothly to afford the corresponding heteroaryl aryl ethers in high yields.29
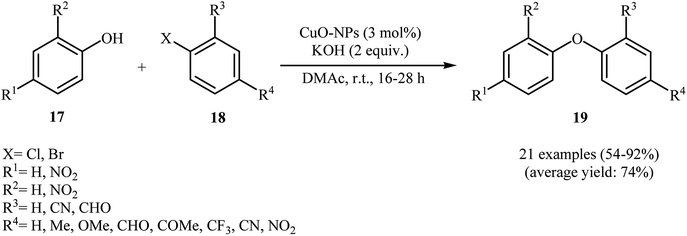 | ||
| Scheme 4 Synthesis of diaryl ethers 19 through nano-CuO catalyzed coupling of phenols 17 with aryl bromides/chlorides 18. | ||
Similarly, Khalilzadeh and co-workers reported the synthesis of a diverse range of diaryl ethers in high yields (up to 86%) through CuO NPs-catalyzed coupling of corresponding phenols with aryl iodides using KF/clinoptilolite as an effective solid base.30
Four years later, Zhang and co-workers improved the efficiency of this coupling in terms of yield and reaction temperature by performing the process in the presence of CuO2/Cu-CNTs as the heterogeneous reusable catalyst in DMF at 140 °C.32
2.3. CuI nanoparticles
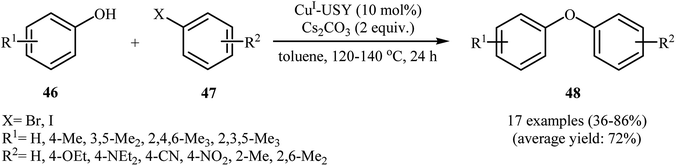
In 2009, Sreedhar and his team demonstrated that the cross-coupling reaction of phenols 23 with less reactive aryl chlorides 24 was efficiently performed when using low-cost commercially available CuI nanoparticles and K2CO3 under ligand-free conditions. This was the first report of Ullmann-type C–O cross-coupling reactions using nano-sized copper salts. A series of functionalized diaryl ethers 25 were prepared in almost quantitative yields under heating (110 °C) in DMF (Scheme 6a). However, 1-naphthol failed to participate in the reaction. This protocol was also successfully applied to the C–N cross-coupling reaction of various N-heterocycles with aryl chlorides, and the corresponding coupling products were obtained in good to excellent yields with the same catalyst. The mechanism shown in Scheme 6b was proposed for this O-arylation. It consists of the following key steps: (i) stabilization of the well-dispersed CuI nanoparticles by DMF and phenol 23 forms the active cluster intermediate A, (ii) oxidative addition of this intermediate with aryl halide 24 produces the intermediate B, and (iii) reductive elimination of intermediate B gives the expected diaryl ether 25 followed by the removal of hydrogen chloride with base.33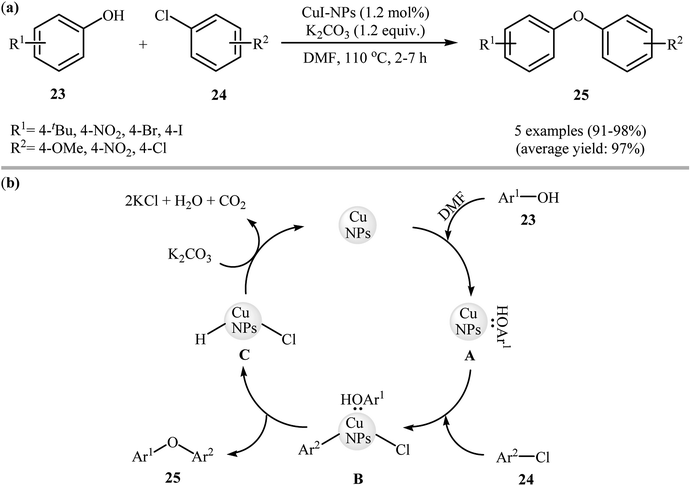 | ||
| Scheme 6 (a) CuI-NPs catalyzed synthesis of diaryl ethers 25 from corresponding phenols 23 and aryl chlorides 24; (b) proposed mechanism for the formation of diaryl ethers 25. | ||
2.4. CuFe2O4 nanoparticles
In 2011, Zhang et al. reported the synthesis of magnetically recoverable CuFe2O4 nanoparticles through simple heating (90 °C) of Fe(NO3)2·9H2O and Cu(NO3)2·2H2O in the presence of citric acid in water and then decomposition of citric acid at 300 °C. Transmission electron microscopy (TEM) image of the synthesized catalyst showed that the average size of the CuFe2O4 particles was about 5–10 nm. The saturation magnetization is as high as 33.8 emu g−1 at room temperature. This makes possible a very fast magnetic separation of nanoparticles by simply applying an external magnetic field. The catalytic activity of CuFe2O4 was tested for O-arylation of various phenols with substituted aryl halides in the presence of Cs2CO3 as a base in DMF at 135 °C. Some important information of the reactions are listed below: (i) phenols with electron-withdrawing substituents compare to phenols bearing electron-donating substituents gave lower yield of desired products; (ii) aryl iodides gave a higher yield of products than aryl bromides; and (iii) aryl chlorides were shown to be completely unreactive.34 Subsequently, the group of Yang–Xu improved the efficiency of this protocol by performing the process in NMF employing 2,2,6,6-tetramethylheptane-3,5-dione(L) as an efficient ligand. Both electron-rich and electron-poor phenols 26 and all the three kinds of substituted aryl halides 27 (aryl iodides, bromides, and chlorides) worked well under optimized conditions [CuFe2O4-NPs (5 mol%), L (10 mol%), Cs2CO3 (2 equiv.), NMP, 135 °C, 24 h] as provided corresponding diaryl ethers 28 in high to excellent yields (Scheme 7). However, unsubstituted bromobenzene resulted in a poor yield of the desired product and iodobenzene failed to participate in this reaction.35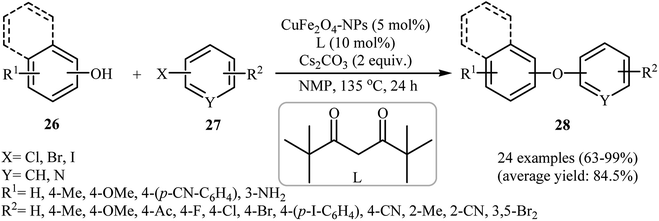 | ||
| Scheme 7 C–O cross-coupling reaction between phenols 26 and aryl halides 27 using CuFe2O4-NPs as catalyst. | ||
The comparison of the catalytic activity of CuFe2O4 nanoparticles with a variety of nanosized metal oxides such as Co3O4, SnO2, Y2O3, YFe2O4, Sb2O3, and Bi2O3 NPs established its superior comparability with them in terms of product yield.36 Very recently it is found that the use of cheaper and more readily available K2CO3 as a base instead of Cs2CO3 in this protocol produced similar yields of products.37
2.5. Catalyst Supports
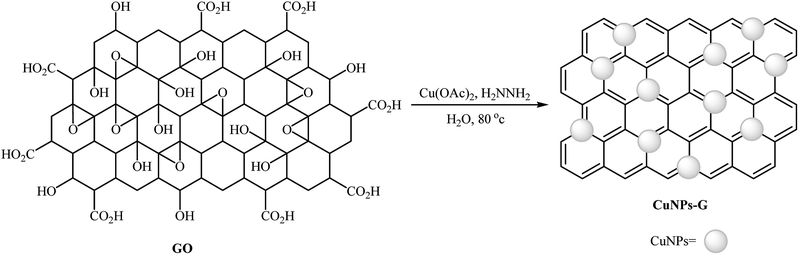
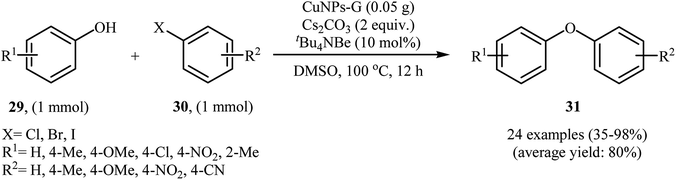
2.5.1.1. Graphene supported CuNPs. Graphene has excellent mechanical and thermal stability, and outstanding electronic properties. It has been widely used as valuable support in various heterogeneous catalyst system.38 In 2013, Mondal and co-workers developed an efficient Cu(0)NPs–graphene-based composite by simple heating (80 °C) of reduced graphene oxide with Cu(OAc)2 in the presence of hydrazine as a reducing agent in water (Scheme 8). The precipitated product was easily separated by filtration. The hydrophobic nature of the copper nanoparticle-activated carbon (CuNPs–C) composite indicated that the Cu(II) ions were converted to Cu(0). The authors characterized the nanocomposite by using various analyses such as TEM, AFM, Raman and XPS. The results show the composite nature of Cu–G. The size of the copper nanoparticles was found to be 2–3 nm by TEM technique. The catalytic utility of the composite was investigated for O-arylation of phenols 29 using aryl halides 30 (Scheme 9). The results established excellent catalytic activity of the nanocomposite (yield up to 98%) which was as reusable and could be recovered and reused for 7 runs with negligible loss of catalytic activity. For a comparative study, the authors tested these reactions using various catalytic systems such as Cu(OAc)2·H2O, Cu(bpy)2BF4, BINAM-Cu(OTf)2, CuFAP, Cu2O, nano-CuO, Cu-FAP, and PANI-Cu. The best result was obtained by using CuNPs–G catalyst in terms of conversions and isolated yields of the coupling products. High catalytic activity of this composite could be explained by interaction between highly dispersed Cu species and graphene as well as the role of as well as the role of carbon vacancies and the defects of graphene surface.39
Shortly afterwards, the group of Guo reported the successful preparation of a highly active and reusable graphene-supported Cu2O nanoparticles with a mean diameter of about 8 nm. The synthesized nanocatalyst (Cu2ONPs/graphene) exhibited a high catalytic activity in the Ullmann C–O cross-coupling of phenols with aryl iodides. The results showed that the desired diaryl ethers were readily formed in high to quantitative yields (82–99%) after 3 h.40
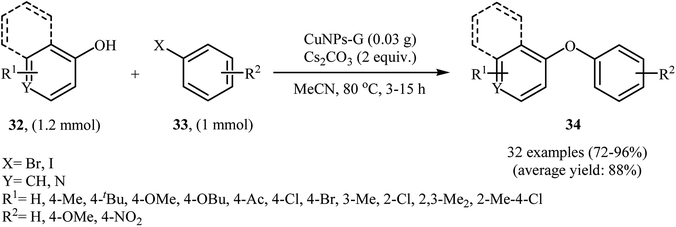
In 2014, Singh, Shendage, and Nagarkar synthesized copper nanoparticles (<25 nm) by electrochemical deposition on nafion-graphene nanoribbons (average width – 100 nm). The prepared catalyst was successfully applied for C–O cross-coupling reactions of phenols 32 with aryl iodides and bromides 33 in the presence of Cs2CO3 as the base in MeCN (Scheme 10). In this investigation, the authors found some limitation in their protocol, when they attempted to react 2-iodothiophene. In this case, the expected product was not observed. In addition, aryl chlorides and phenol bearing a strong electron-withdrawing group (e.g., NO2) did not work well under this reaction conditions.41
Inspired by these works, Nasrollahzadeh and co-workers designed and synthesized a novel copper-based heterogeneous magnetic catalyst by immobilizing Cu NPs onto reduced graphene oxide (RGO)/Fe3O4 nanocomposite via reduction of Cu2+ to Cu(0) by using barberry fruit extract. The magnetically separable, Cu NPs/RGO/Fe3O4 system was used for promoting O-arylation of phenols with aryl halides (Ar–I, A–Br, Ar–Cl) in the presence of Cs2CO3 as a base in DMSO. The desired products were slowly obtained in good to excellent yields under open air conditions. The catalyst could be freely recycled and reused six times for the same reaction, with very low yield decrease (from 98% in the first run to 95% in the sixth run).42
2.5.1.2. Carbon nanofiber/tube supported CuNPs. In 2014, Li and co-workers reported a carbon nanofiber supported copper nanoparticle (CuNP/CNF) catalyst for Ullmann-type C–O and C–N bond formation couplings. The catalytic activity of synthesized Cu/C nanocatalyst was explored for O-arylation of differently substituted phenols with aryl iodides and bromides using Cs2CO3 in DMAc at 140 °C under nitrogen atmosphere and achieved fair to excellent yields of diaryl ether products with good recyclability up to five runs. However, aryl chlorides showed very low reactivity under this reaction conditions. CuNP/CNF was also successfully employed for N-arylation of a small series of N-heterocycles with iodobenzene.43,44
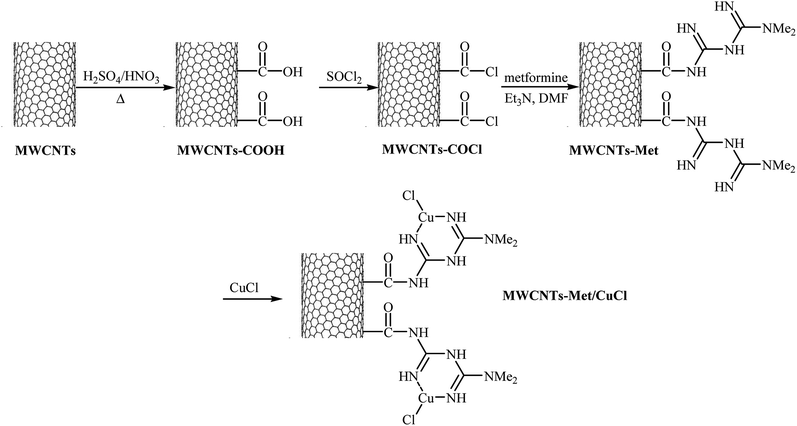
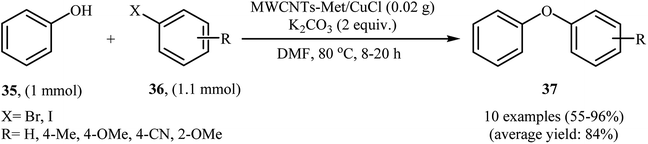
Very recently, the group of Veisi developed a novel catalyst (MWCNTs-Met/CuCl) via a four-step procedure through carboxylation of multi-walled carbon nanotubes (MWCNTs) with a mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) HNO3 and H2SO4 and subsequent acylation of resulted MWCNTs–COOH with SOCl2 followed by functionalization with metformine and finally coordination of produced MWCNTs-Met with CuCl catalyst (Fig. 4). The catalyst, MWCNTs-Met/CuCl was found to be an efficient catalyst in the synthesis of diaryl ethers 37 through Ullmann-type C–O cross-coupling of phenol 35 with aryl halides 36 in the presence of K2CO3 as a base in DMF (Scheme 11). The nanocatalyst was readily separated by centrifuge and washed with water and ethanol, dried, and then reused seven times without observable loss of its catalytic activity and yield.45 It is noted that magnetically separable carbon nanotube-supported Fe2O3@CuO was also synthesized and successfully applied as an efficient catalyst in this coupling reaction.46
3 (v/v) HNO3 and H2SO4 and subsequent acylation of resulted MWCNTs–COOH with SOCl2 followed by functionalization with metformine and finally coordination of produced MWCNTs-Met with CuCl catalyst (Fig. 4). The catalyst, MWCNTs-Met/CuCl was found to be an efficient catalyst in the synthesis of diaryl ethers 37 through Ullmann-type C–O cross-coupling of phenol 35 with aryl halides 36 in the presence of K2CO3 as a base in DMF (Scheme 11). The nanocatalyst was readily separated by centrifuge and washed with water and ethanol, dried, and then reused seven times without observable loss of its catalytic activity and yield.45 It is noted that magnetically separable carbon nanotube-supported Fe2O3@CuO was also synthesized and successfully applied as an efficient catalyst in this coupling reaction.46
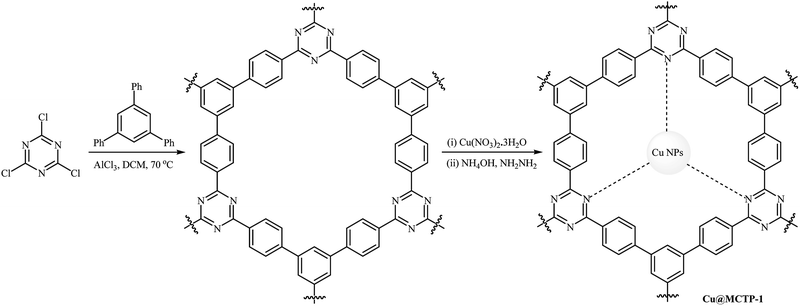
Recently, Puthiaraj and Ahn prepared a microporous covalent triazine polymer (MCTP-1) by the Friedel–Crafts reaction of readily available cyanuric chloride with tri-/dual site-reactive 1,3,5-triphenylbenzene and subsequently used as a solid support for immobilization of copper nanoparticles (Scheme 13). Cu nanoparticles supported on the MCTP-1 were applied as efficient catalysts for coupling reactions of a series of phenols with aryl bromides and chlorides under air; the corresponding diaryl ethers were produced in good to excellent yields. The recycling test established that the catalyst could be recovered and reused for five consecutive reaction runs. Notably, the ICP-OES analysis indicated the leaching of the active catalytic species was negligible.48
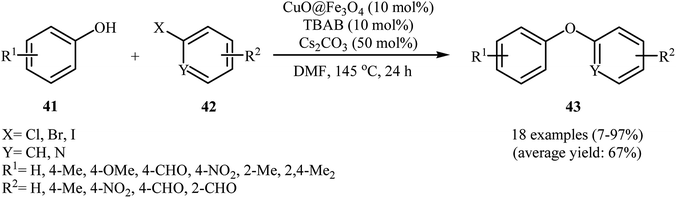
In 2014, Zhang and co-workers explored the application of Fe3O4-encapsulated CuO nanoparticle (CuO@Fe3O4) with an average particle size of 15 nm in O-arylation of phenols 41 with aryl halides 42 in the presence of TBAB as a phase transfer catalysis and Cs2CO3 as a base in DMF at 145 °C (Scheme 15). Moderate to excellent yields of coupling products 43 were achieved using aryl iodides or bromides. Whereas, poor yields were obtained using aryl chlorides as reagents. Just like previous works, electron-poor phenols did not work well in this coupling reactions. This spherical shape catalyst can be recycled by magnetic separation and can be reused three times with no remarkable loss in activity. Although the activity of the catalyst decreased notably after the 3rd cycle, the spherical shape remained almost unaltered. For comparative study, the authors studied this reaction using Fe3O4. However, when Fe3O4 was used as catalyst, the reaction failed.53 Shortly afterwards, magnetic (γ-Fe2O3)-supported copper nanoparticles have been used as a catalyst for the carbon-heteroatom coupling reactions by Sharma and co-workers. The catalyst has been tested in a series of coupling reactions, including the C–O coupling of phenols with aryl halides. With a low catalyst loading, various phenols and aryl iodides can be converted into the corresponding diaryl ethers in yields of 87–99%, but phenols bearing a methyl group in ortho- or meta- position of aromatic ring give low yields after long reaction times.54
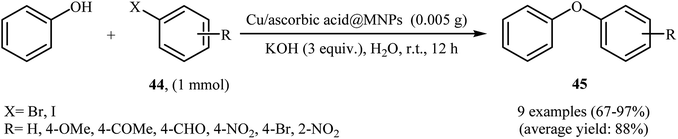
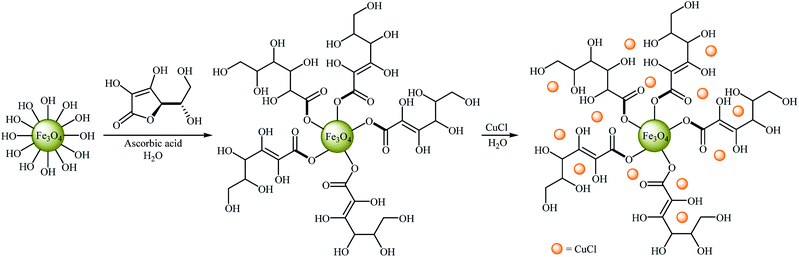
Very recently, Hajipour, Check, and Khorsandi reported a mild, efficient, and green procedure for etherification of aryl halides with phenols employing Cu/ascorbic acid@MNPs as a novel magnetic catalyst. Thus, a variety of functionalized diaryl ethers 45 were synthesized in good to almost quantitative yields through the coupling of corresponding aryl halides 44 with phenol using the combination of Cu-ascorbic acid@MNPs/KOH as a catalytic system in the most environmentally benign solvent, water, at room temperature (Scheme 16). The protocol also successfully applied in the N-arylation of various amines and N-heterocycles with aryl halides. It has been shown that the catalyst can be reused over six reaction cycles with little but noticeable loss in catalytic activity (from 96% in the first run to 73% in the sixth run). Synthesis route for the preparation of Cu/ascorbic acid@MNPs is described in Scheme 17.55
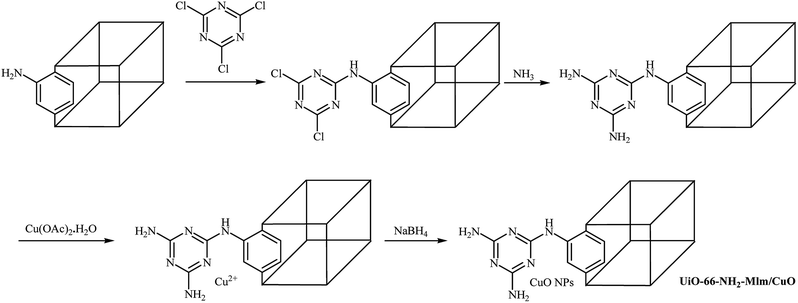
Recently, Sadeghi and co-workers introduced a novel catalyst (UiO-66-NH2-Mlm/CuO) for O-arylation of phenols with aryl halides. The catalyst was prepared by functionalization of as-prepared UiO-66-NH2 with cyanuric chloride and subsequent reaction with ammonia followed by incorporation of Cu through reaction with copper(II) acetate monohydrate and finally reduction of Cu2+ by NaBH4 to Cu(0) (Scheme 19). Various diaryl ethers were successfully synthesized employing this heterogeneous catalyst. When compared with other aryl halides, aryl iodides were found to be the best halide for this O-arylation reaction. Good to excellent yields of coupling products were obtained in the presence of aryl iodides (92–95%) and bromides (65–93%). The yields of the final products were significantly decreased when aryl chlorides were used as aryl halide (30–87%). It is noted that this catalyst can be easily separated from the final reaction mixture by centrifugation, and then be reused five times without significant losses in the catalytic activity.61
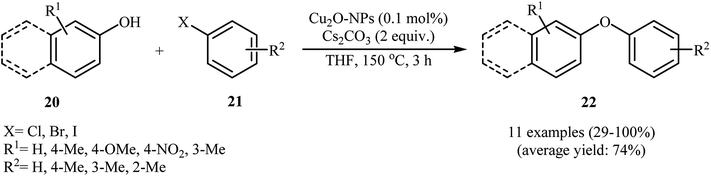
In another synthetic methodology, a variety of functionalized diaryl ethers 51 have been synthesized by Wang's research team in 2017 in moderate to very excellent yields through the C–O cross-coupling reactions of phenols 49 with aryl iodides and bromides 50 using SiC-supported copper nanoparticle as a recyclable and efficient heterogeneous catalyst and Cs2CO3 as base in THF under argon atmosphere (Scheme 20). However, the reaction did not work with aryl chlorides.62
3. Palladium Nanocatalysts
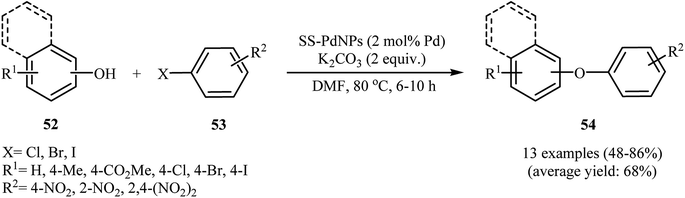
In 2012, Bandna63 and co-workers reported an efficient C–O cross-coupling of phenols 52 with nitro-substituted aryl halides 53 using solid supported Pd(0) nanoparticles (SS)–Pd as catalyst under relatively mild basic conditions. These O-arylation reactions were successfully carried out using readily available K2CO3 as a base in DMF and generally provided corresponding diaryl ethers 54 in moderate to high yields (Scheme 21). The catalytic system was also found to be very effective for the cross-coupling of nitro-substituted aryl halides with sulfur and nitrogen nucleophiles. Moreover, the catalyst can be reused at least seven times without a significant loss in its activity.63 Following this work, Joshi et al. have reported high catalytic activity of the graphene oxide grafted with Pd17Se15 nanoparticles (GO-Pd17Se15) in C–O cross-coupling between phenol and (het)aryl chlorides/bromides at room temperature. In this methodology, a series of solvents and bases were used by the authors for a comparative study. The best result was obtained when DMF and K2CO3 were used as a solvent and a base, respectively.64Iranpoor, Firouzabadi, and Rostami designed novel supported Pd nanoparticles based on the synthesis of silica diphenylphosphinite (SDPP), through direct phosphorylation of silica gel with chlorodiphenylphosphine (ClPPh2) using solvent-free conditions and subsequent reaction with PdCl2 in an aqueous solution of nBu4NOH to afford nano Pd(0)/SDPP. The supported PdNPs was used as an efficient catalyst for the synthesis of a wide range of diaryl ethers through C–O arylation of different aryl iodides, bromides and chloride with corresponding phenols under basic conditions at 70 °C. Interestingly, this coupling reaction can be performed either in the presence of the pre-prepared Pd(0)/SDDP or using the in situ generated catalyst.65
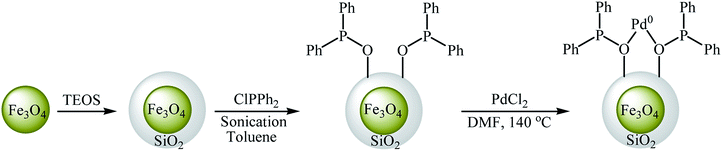
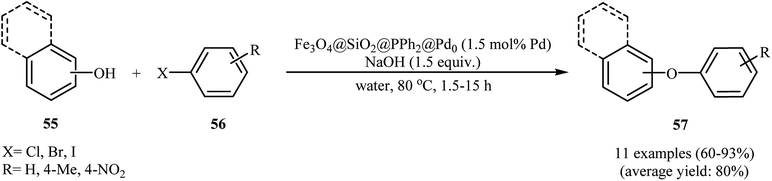
In an initiative research, the group of Zolfigol–Luque designed and synthesized a novel highly stable and active magnetically separable Pd nanocatalyst (Fe3O4@SiO2@PPh2@Pd0). The synthetic approach included preparation of silica-coated magnetite particles (Fe3O4@SiO2) through reaction of Fe3O4 with tetraethyl orthosilicate (TEOS) in aqueous solution of ammonia and subsequent phosphorylation of hydroxyl groups on the surface of the support (SiO2) with ClPPh2 followed by incorporation of Pd through reaction with PdCl2 (Scheme 22). The Fe3O4@SiO2@PPh2@Pd0 was employed as an efficient catalyst for C–O coupling of phenols 55 with aryl halides 56 in water. Various aryl iodides, aryl bromides as well as aryl chlorides were effectively used to synthesize functionalized diaryl ethers 57 in good to excellent yields (Scheme 23). These nanocomposites were also successfully utilized in Sonogashira reaction of aryl halides with phenylacetylene. Moreover, the catalyst could be readily separated from the reaction medium by using a simple external magnet and reused six times with no significant loss in activity.66 In 2013, the group of Nagarkar reported that palladium supported on zinc ferrite (Pd–ZnFe2O4) is a highly efficient superparamagnetic solid catalyst not only for C–O cross-coupling reactions of phenols with aryl halides but also for various C–C cross-coupling reactions including Sonogashira, Suzuki, and Heck–Matsuda reactions. Cyanation of aryl halides was also successfully done using K4[Fe(CN)6] as the cyanide source over this catalyst.67,68
Following these works, the use of AT-nano-CP-Pd0 (Pd NPs supported on activated natural nanozeolite clinoptilolite) catalyst for the O-arylation of phenols with aryl halides was reported by Baghbanian et al. Under optimized reaction conditions [AT-nano-CP-Pd0 (0.06 mol% Pd), K2CO3 (1.5 equiv.), water] several diaryl ethers were obtained in good to almost quantitative yields at 60 °C independently of the nature of the aryl iodide employed. Nevertheless, the reaction became sluggish when aryl bromides and chlorides were employed, being necessary longer reaction times.69
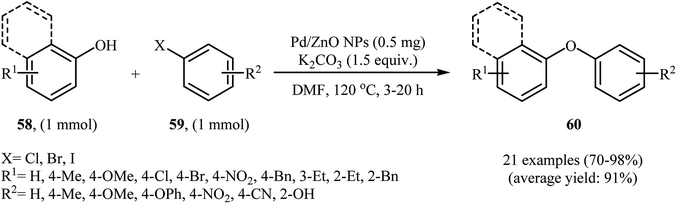
Recently, Sarvari and Razmi reported the preparation of nanosized Pd/ZnO (with a Pd loading of 9.84 wt%) catalyst by co-precipitation (CP) method. The catalyst has been fully characterized by various techniques, including XRD, TEM, SEM, FT-IR, XPS, TGA, ICP, and AAS, and its activity in the C–O cross-coupling of phenols 58 with a wide range of aryl halides 59 has been tested by employing K2CO3/DMF as the base/solvent at 120 °C under air and the corresponding diaryl ethers 60 were obtained in good to very excellent yields (Scheme 24). It has been shown that after recycling over five successive runs, without a significant decrease in the coupling of phenol with 1-(4-bromophenyl)ethanone, the catalyst shows 0.02% leaching.70
4. Nickel nanocatalysts
In 2014, Ghatak and co-workers reported the applicability of alumina-supported nickel nanoparticles (Ni–Al2O3) as an efficient catalysts for the coupling of phenols with aryl halides. This etherification reaction afforded the optimum yield in aqueous solution of potassium carbonate, while the addition of 8 mol% of SDS as a surfactant to the reaction mixture gave excellent results. Various phenols 61 and (het)aryl halides 62 were used to establish the general applicability of the method. The results showed that all the three kinds of aryl halides were applicable to this reaction (Scheme 25). A variety of sensitive functional groups are compatible with the reaction conditions, including nitro, amino, chloro, bromo, formyl, allyl, ketone, and ester functionalities. This made possible the further derivatization of the diaryl ether products. A special feature of this procedure is the simple removal of the catalyst by filtration after completion of the reaction. The separated catalyst could be reused seven times with almost no loss of activity.71 Nickel ferrite nanoparticles (NiFe2O4 NPs)72 and cobalt ferrite nanoparticles (CoFe2O4 NPs)73 were also excellent catalysts for this transformation.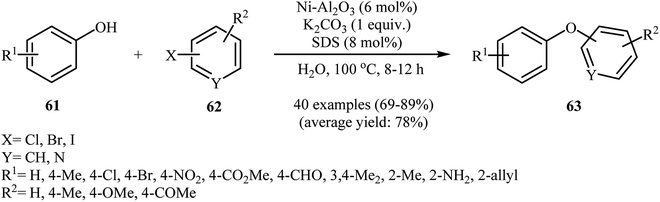 | ||
| Scheme 25 Cross-coupling reaction of phenols 61 and (het)aryl halides 62 catalyzed by Ni nanoparticles in water. | ||
5. Iron nanocatalysts
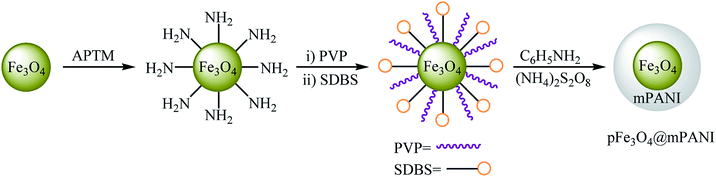
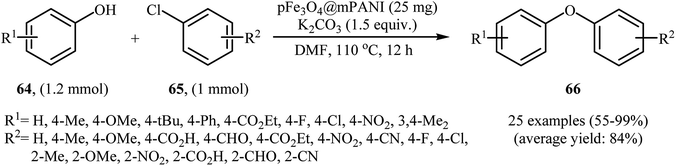
In 2011, the catalytic activity of magnetic Fe3O4@mesoporouspolyaniline core–shell nanocomposite (pFe3O4@mPANI) in the O-arylation of phenols 64 with less reactive aryl chlorides 65 was studied by Arundhathi et al. The nanocomposite was synthesized through the encapsulation of as-prepared porous magnetite Fe3O4 nanoparticles with the mesoporous polyaniline shell by in situ surface polymerization of aniline with the use of ammonium persulfate [(NH4)2S2O8] in the presence of polyvinylpyrrolidone (PVP) as a linker and sodium dodecylbenzenesulfonate (SDBS) as the structure-directing agent (Scheme 26). The coupling reactions were carried out in DMF as the solvent at 110 °C and in the presence of K2CO3 as the base. The expected diaryl ethers 66 were obtained in good to very excellent yields (Scheme 27). This catalytic system has also been successfully used in the cross-coupling of benzylic and aliphatic alcohols with aryl chlorides under the same conditions. The catalyst was recovered by easy decantation of the reaction mixture in the presence of an external magnet and reused at least five times without a loss in its activity.74Later, nano-sized unsupported Fe3O4 was found to be an efficient catalyst for the etherification of aryl halides with phenols under solvent-free conditions at 130 °C. Yields were fair to excellent (43–98% for 5 examples).75
6. Cerium nanocatalysts
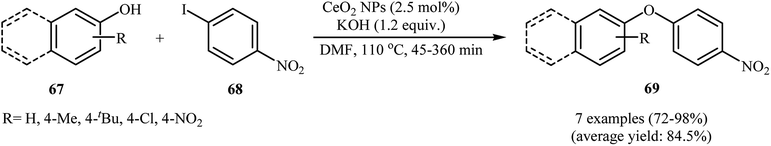
In 2011, Agawane and Nagarkar reported for the first time the usefulness of magnetically separable nano cerium oxide for the C–O cross-coupling reactions of phenols and aryl halides. Thus, in the presence of 2.5 mol% of CeO2 nanoparticles as a recyclable catalyst and 1.2 equiv. of KOH as a base in DMSO under air atmosphere, O-arylation reactions of phenols 67 with 4-nitroiodobenzene 68 furnished the corresponding diaryl ethers 69 in good to excellent yields (Scheme 28), whereas other catalytic systems such ZnO, Al2O3, TiO2, MnO2, La2O3, and bulk CeO2 give incomparable results under the same reaction conditions. It is noted that this catalytic system was also able to promote the coupling of 4-nitroiodobenzene with various aromatic and aliphatic amines.76 Similarly, ceria supported gold nanoparticles (Au–CeO2 NPs) furnished the formation of C–O bonds in a reaction involving phenols and electron-poor aryl chlorides. The corresponding coupling products were obtained in fair to almost quantitative yields (41–99%).777. Conclusion
Diaryl ether moiety play important role in medicinal and agrochemical chemistry, being present in a wide range of natural products and biologically active compounds. Several commercially available drugs, including levothyroxine, vancomycin, and teicoplanin, are derived from this structural motif. Consequently, numerous efforts have been undertaken toward efficient and convenient strategies for the synthesis of this skeleton. One of the most common processes for the synthesis of diaryl ether involves the Ullmann cross-coupling reaction of phenols with aryl halides. However, the utility of classical Ullmann coupling has been invariably limited by its harsh reaction conditions, the stoichiometric use of copper reagents and the use of expensive ligands. A great deal of effort has been devoted to developing more convenient methods of Ullmann-type C–O cross-coupling reaction, mainly focusing on catalysts. As illustrated, the use of nanosized metal catalysts in Ullmann-type O-arylation allows for rapid transformations under relatively mild and ligand-free conditions, with the benefits of excellent yield of products (Table 1) with the ease of catalyst separation and recovery. Even with these recent improvements, there is still a need for the discovery of new catalytic systems, which can allow the coupling under milder conditions. We hope that this review will stimulate further thinking and growth in the topic.| Entry | Catalyst | Conditions | X= I/Br/Cl | Number of examples | Yield (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Range | Average | ||||||
| 1 | CuNPs | MeCN, Cs2O3, 50–60 °C, 4 h | +/+/− | 10 | 68–95 | 86 | 24 |
| 2 | CuNPs | DMF, Cs2CO3, 110 °C, 24 h | +/+/− | 11 | 60–85 | 75 | 26 |
| 3 | CuONPs | DMSO, Cs2CO3, 110 °C, 18–20 h | +/+/+ | 18 | 17–94 | 70 | 27 |
| 4 | CuONPs | DMSO, KOH, 110–120 °C, 14–30 h | +/+/− | 28 | 5–98 | 61 | 28 |
| 5 | CuONPs | DMAc, KOH, r.t., 16–28 h | +/+/+ | 21 | 54–92 | 74 | 29 |
| 6 | CuONPs | DMF, KF.CP, 120 °C, 18–30 h | +/+/− | 17 | 42–87 | 72 | 30 |
| 7 | Cu2ONPs | THF, Cs2CO3, 150 °C, 3 h | +/+/+ | 11 | 29–100 | 74 | 31 |
| 8 | CuINPs | DMF, K2CO3, 110 °C, 2–7 h | +/+/+ | 5 | 91–98 | 97 | 33 |
| 9 | CuFe2O4NPs | DMF, Cs2CO3, 135 °C, 0.5–24 h | +/+/− | 17 | 14–99 | 70 | 34 |
| 10 | CuFe2O4NPs | NMP, ligand, Cs2CO3, 135 °C, 24 h | +/+/+ | 24 | 63–99 | 84.5 | 35 |
| 11 | CuNPs–G | DMSO, Cs2CO3, Bu4NBr, 100 °C, 12 h | +/+/+ | 24 | 35–98 | 80 | 39 |
| 12 | Cu2ONPs–G | THF, Cs2CO3, 150 °C, 3 h | +/+/− | 14 | 20–99 | 79 | 40 |
| 13 | CuNPs–G | MeCN, Cs2CO3, 80 °C, 3–15 | +/+/− | 32 | 72–96 | 88 | 41 |
| 14 | CuNPs/RGO/Fe3O4 NPs | DMSO, Cs2CO3, 120 °C, 12 h | +/+/+ | 19 | 56–98 | 85 | 42 |
| 15 | MWCNTs/Met/CuClNPs | DMF, K2CO3, 80 °C, 8–20 h | +/+/− | 10 | 55–96 | 84 | 46 |
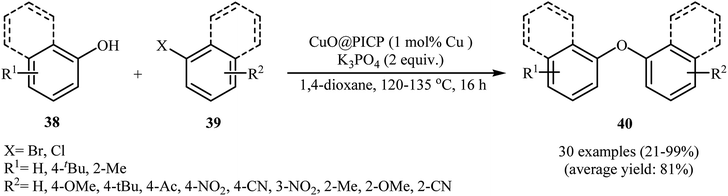
| 16 | CuO@PICP | 1,4-Dioxane, K3PO4, 120–135 °C, 16 h | +/+/+ | 30 | 21–99 | 81 | 47 |
| 17 | Cu@MCTP-1 | DMF, Cs2CO3, 130 °C, 24 h | +/+/− | 29 | 71–96 | 87 | 48 |
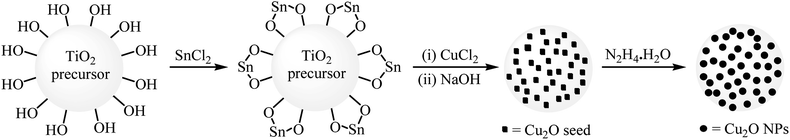
| 18 | TiO2–Cu2ONPs | DMF, KOH, 150 °C, 6 h | +/+/− | 2 | 56–90 | 73 | 50 |
| 19 | CuO@Fe3O4 | DMF, TBAB, Cs2CO3, 145 °C, 24 h | +/+/+ | 18 | 7–97 | 67 | 51 |
| 20 | Cu@Fe2O3 | DMF, Cs2CO3, 130 °C, 24 h | +/−/− | 12 | 13–99 | 83.5 | 54 |
| 21 | Cu/ascorbic acid@MNPs | H2O, KOH, r.t., 12 h | +/+/− | 9 | 67–97 | 88 | 55 |
| 22 | CuI-USY | Toluene, Cs2CO3, 120–140 °C, 24 h | +/+/− | 17 | 36–86 | 72 | 60 |
| 23 | UiO-66-NH2-Mlm/CuONPs | DMSO, KOH, 110 °C, 18–24 h | +/+/+ | 9 | 30–95 | 74 | 61 |
| 24 | SiC@Cu2O | THF, Cs2CO3, 150 °C, 3 h | +/+/− | 14 | 49–98 | 83 | 62 |
| 25 | SS-PdNPs | DMF, K2CO3, 80 °C, 6–10 h | +/+/+ | 13 | 48–86 | 68 | 63 |
| 26 | Pd17Se15–GO | DMSO, K2CO3, r.t., 1–3 h | +/+/+ | 22 | 59–94 | 80 | 64 |
| 27 | PdCl2@SDPP | Bu4NOH aq, NaOH, 70 °C, 1.5–10 h | +/+/+ | 14 | 70–99 | 88 | 65 |
| 28 | Fe3O4@SiO2@PPh2@Pd0 | H2O, NaOH, 80 °C, 1.5–15 h | +/+/+ | 11 | 60–93 | 80 | 66 |
| 29 | Pd@ZnFe2O4 | DMSO, K3PO4, 110 °C, 3.5–5 h | +/+/+ | 11 | 81–92 | 87 | 67 |
| 30 | AT-CP PdNPs | H2O, K2CO3, 60 °C, 1–14 h | +/+/+ | 17 | 60–98 | 84.5 | 69 |
| 31 | Pd/ZnONPs | DMF, K2CO3, 120 °C, 3–20 h | +/+/+ | 21 | 70–98 | 91 | 70 |
| 32 | Ni–Al2O3 | H2O, SDS, K2CO3, 100 °C, 8–12 | +/+/+ | 40 | 69–89 | 78 | 71 |
| 33 | NiFe2O4NPs | Dioxane, Cs2CO3, reflux, 10 h | +/+/+ | 5 | 88–95 | 90 | 72 |
| 34 | CoFe2O4NPs | DMF, K2CO3, 80 °C, 1–7.5 h | +/+/+ | 25 | 81–96 | 88 | 73 |
| 35 | pFe3O4@mPAMI | DMF, K2CO3, 110 °C, 12 h | −/−/+ | 25 | 55–99 | 84 | 74 |
| 36 | Fe3O4NPs | Solvent-free, K2CO3, 130 °C, 48 h | +/+/+ | 5 | 43–98 | 68 | 75 |
| 37 | CeO2NPs | DMF, KOH, 110 °C, 45–360 min | +/−/− | 7 | 72–98 | 84.5 | 76 |
| 38 | Au–CeO2NPs | DMSO, KOH, 110 °C, 2–24 h | −/+/+ | 16 | 3–99 | 65 | 77 |
Conflicts of interest
There are no conflicts to declare.References
- R. Negro, G. Formoso, T. Mangieri, A. Pezzarossa, D. Dazzi and H. Hassan, J. Clin. Endocrinol. Metab., 2006, 91, 2587–2591 CrossRef PubMed
.
- R. Nagarajan, Antimicrob. Agents Chemother., 1991, 35, 605–609 CrossRef PubMed
.
- P. E. Reynolds, Eur. J. Clin. Microbiol. Infect. Dis., 1989, 8, 943–950 CrossRef PubMed
.
- S. Ghosh, A. S. Kumar, G. Mehta, R. Soundararajan and S. Sen, ARKIVOC, 2009, 7, 72–78 Search PubMed
.
- Y. S. Kosenkova, M. Polovinka, N. Komarova, D. Korchagina, N. Y. Kurochkina, V. Cheremushkina and N. Salakhutdinov, Chem. Nat. Compd., 2007, 43, 712–713 CrossRef
.
- H. Deng, J.-K. Jung, T. Liu, K. W. Kuntz, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2003, 125, 9032–9034 CrossRef PubMed
.
-
(a) G. R. Pettit, S. B. Singh, M. R. Boyd, E. Hamel, R. K. Pettit, J. M. Schmidt and F. Hogan, J. Med. Chem., 1995, 38, 1666–1672 CrossRef PubMed
; (b) G. R. Pettit, M. R. Rhodes, D. L. Herald, E. Hamel, J. M. Schmidt and R. K. Pettit, J. Med. Chem., 2005, 48, 4087–4099 CrossRef PubMed
.
- C. Tomlin, A World Compendium, The Pesticide Manual. Incorporating the Agrochemicals Handbook, Crop Protection Publications, Bungay, Suffolk, UK, 10th edn, 1994 Search PubMed
.
-
(a) P. M. Hergenrother, Angew. Chem., Int. Ed., 1990, 29, 1262–1268 CrossRef
; (b) M. Strukelj, F. Papadimitrakopoulos, T. M. Miller and L. J. Rothberg, Science, 1995, 267, 1969–1972 Search PubMed
.
- X.-L. Jiang, H.-L. Zhang and J. Wu, Heterocycles, 2006, 68, 2153–2160 CrossRef
.
-
(a) A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC
; (b) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC
; (c) C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef PubMed
.
-
(a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596–1636 CrossRef PubMed
; (b) F. M. Tappe, V. T. Trepohl and M. Oestreich, Synthesis, 2010, 3037–3062 Search PubMed
; (c) M. Abdoli, Z. Mirjafary, H. Saeidian and A. Kakanejadifard, RSC Adv., 2015, 5, 44371–44389 RSC
.
- M. Mondal, S. K. Bharadwaj and U. Bora, New J. Chem., 2015, 39, 31–37 RSC
.
- D. Kikelj, Synthesis, 2006, 2271–2285 CrossRef
.
- S. A. Mulla, S. M. Inamdar, M. Y. Pathan and S. S. Chavan, RSC Adv., 2012, 2, 12818–12823 RSC
.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef PubMed
.
-
(a) F. Ullmann, Eur. J. Inorg. Chem., 1904, 37, 853–854 Search PubMed
; (b) C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525–3550 RSC
; (c) D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799–3802 CrossRef PubMed
; (d) Y.-J. Chen and H.-H. Chen, Org. Lett., 2006, 8, 5609–5612 CrossRef PubMed
.
- E. Sperotto, G. P. van Klink, G. van Koten and J. G. de Vries, Dalton Trans., 2010, 39, 10338–10351 RSC
.
-
(a) J. W. Tye, Z. Weng, R. Giri and J. F. Hartwig, Angew. Chem., Int. Ed. Engl., 2010, 49, 2185–2189 CrossRef PubMed
; (b) S. Sung, D. C. Braddock, A. Armstrong, C. Brennan, D. Sale, A. J. White and R. P. Davies, Chem.–Eur. J., 2015, 21, 7179–7192 CrossRef PubMed
.
-
(a) F. Zaera, ChemSusChem, 2013, 6, 1797–1820 CrossRef PubMed
; (b) B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736–1789 CrossRef
; (c) T. Cheng, D. Zhang, H. Li and G. Liu, Green Chem., 2014, 16, 3401–3427 RSC
; (d) M.-A. Neouze, J. Mater. Sci., 2013, 48, 7321–7349 CrossRef
.
-
(a) E. Vessally, M. Babazadeh, A. Hosseinian, S. Arshadi and L. Edjlali, J. CO2 Util., 2017, 21, 491–502 CrossRef
; (b) K. Didehban, E. Vessally, A. Hosseinian, L. Edjlali and E. S. Khosroshahi, RSC Adv., 2018, 8, 291–301 RSC
; (c) E. Vessally, K. Didehban, R. Mohammadi, A. Hosseinian and M. Babazadeh, J. Sulfur Chem., 2018 DOI:10.1080/17415993.2018.1436711
; (d) E. Vessally, R. Mohammadi, A. Hosseinian, K. Didehban and L. Edjlali, J. Sulfur Chem., 2018 DOI:10.1080/17415993.2018.1436712
; (e) E. Vessally, A. Hosseinian, L. Edjlali, E. Ghorbani-Kalhor and R. Hosseinzadeh-Khanmiri, J. Iran. Chem. Soc., 2017, 14, 2339–2353 CrossRef
; (f) E. Vessally, K. Didehban, M. Babazadeh, A. Hosseinian and L. Edjlali, J. CO2 Util., 2017, 21, 480–490 CrossRef
; (g) M. Babazadeh, S. Soleimani-Amiri, E. Vessally, A. Hosseinian and L. Edjlali, RSC Adv., 2017, 7, 43716–43736 RSC
; (h) S. Farshbaf, L. Zare Fekri, M. Nikpassand, R. Mohammadi and E. Vessally, J. CO2 Util., 2018, 25, 194–204 CrossRef
; (i) E. Vessally, R. Mohammadi, A. Hosseinian, L. Edjlali and M. Babazadeh, J. CO2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Util., 2018, 24, 361–368 Search PubMed
Util., 2018, 24, 361–368 Search PubMed ; (j) K. Didehban, E. Vessally, M. Salary, L. Edjlali and M. Babazadeh, J. CO2 Util., 2018, 23, 42–50 CrossRef
; (k) E. Vessally, A. Hosseinian, L. Edjlali, M. Babazadeh and R. Hosseinzadeh-Khanmiri, Iran. J. Chem. Chem. Eng., 2017, 36, 1–13 Search PubMed
.
- M. B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 CrossRef PubMed
.
-
(a) R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569–573 RSC
; (b) H. Saeidian, M. Abdoli and R. Salimi, C. R. Chim., 2013, 16, 1063–1070 CrossRef
; (c) M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni and M. Khalaj, J. Colloid Interface Sci., 2015, 453, 237–243 CrossRef PubMed
; (d) H. Xu, X. F. Liu, C. S. Cao, B. Zhao, P. Cheng and L. N. He, Adv. Sci., 2016, 3, 1600048–1600054 CrossRef PubMed
; (e) S. Roy, T. Chatterjee and S. M. Islam, Tetrahedron Lett., 2015, 56, 779–783 CrossRef
.
- M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2007, 48, 8883–8887 CrossRef
.
-
(a) V. Engels, F. Benaskar, N. Patil, E. V. Rebrov, V. Hessel, L. A. Hulshof, D. A. Jefferson, J. A. Vekemans, S. Karwal and J. C. Schouten, Org. Process Res. Dev., 2010, 14, 644–649 CrossRef
; (b) F. Benaskar, V. Engels, N. Patil, E. V. Rebrov, J. Meuldijk, V. Hessel, L. A. Hulshof, D. A. Jefferson, J. C. Schouten and A. E. H. Wheatley, Tetrahedron Lett., 2010, 51, 248–251 CrossRef
.
- Y. Isomura, T. Narushima, H. Kawasaki, T. Yonezawa and Y. Obora, Chem. Commun., 2012, 48, 3784–3786 RSC
.
- J. Zhang, Z. Zhang, Y. Wang, X. Zheng and Z. Wang, Eur. J. Org. Chem., 2008, 5112–5116 CrossRef
.
- S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971–1976 CrossRef PubMed
.
- S. G. Babu and R. Karvembu, Tetrahedron Lett., 2013, 54, 1677–1680 CrossRef
.
- M. A. Khalilzadeh, H. Keipour, A. Hosseini and D. Zareyee, New J. Chem., 2014, 38, 42–45 RSC
.
- J. Y. Kim, J. C. Park, A. Kim, A. Y. Kim, H. J. Lee, H. Song and K. H. Park, Eur. J. Inorg. Chem., 2009, 4219–4223 CrossRef
.
- Y.-P. Zhang, Y.-C. Jiao, Y.-S. Yang and C.-L. Li, Tetrahedron Lett., 2013, 54, 6494–6497 CrossRef
.
- B. Sreedhar, R. Arundhathi, P. L. Reddy and M. L. Kantam, J. Org. Chem., 2009, 74, 7951–7954 CrossRef PubMed
.
- R. Zhang, J. Liu, S. Wang, J. Niu, C. Xia and W. Sun, ChemCatChem, 2011, 3, 146–149 CrossRef
.
- S. Yang, C. Wu, H. Zhou, Y. Yang, Y. Zhao, C. Wang, W. Yang and J. Xu, Adv. Synth. Catal., 2013, 355, 53–58 CrossRef
.
- V. Avudoddi, V. K. G. Palle and V. R. Pallapothula, Eur. J. Chem., 2012, 3, 298–304 CrossRef
.
- N. Kazemi and M. M. Shahri, J. Inorg. Organomet. Polym., 2017, 27, 1264–1273 CrossRef
.
- N. M. Julkapli and S. Bagheri, Int. J. Hydrogen Energy, 2015, 40, 948–979 CrossRef
.
- P. Mondal, A. Sinha, N. Salam, A. S. Roy, N. R. Jana and S. Islam, RSC Adv., 2013, 3, 5615–5623 RSC
.
- Z. Zhai, X. Guo, Z. Jiao, G. Jin and X.-Y. Guo, Catal. Sci. Technol., 2014, 4, 4196–4199 Search PubMed
.
- A. S. Singh, S. S. Shendage and J. M. Nagarkar, Tetrahedron Lett., 2014, 55, 4917–4922 CrossRef
.
- M. Nasrollahzadeh, M. Maham, A. Rostami-Vartooni, M. Bagherzadeh and S. M. Sajadi, RSC Adv., 2015, 5, 64769–64780 RSC
.
- H. Li, C. Li, J. Bai, C. Zhang and W. Sun, RSC Adv., 2014, 4, 48362–48367 RSC
.
- C. Zhang, C. Li, J. Bai and H. Li, Catal. Lett., 2015, 145, 1764–1770 CrossRef
.
- M. Akhavan, S. Hemmati, M. Hekmati and H. Veisi, New J. Chem., 2018, 42, 2782–2789 RSC
.
- D. Saberi, M. Sheykhan, K. Niknam and A. Heydari, Catal. Sci. Technol., 2013, 3, 2025–2031 Search PubMed
.
- T. Hu, X. Chen, J. Wang and J. Huang, ChemCatChem, 2011, 3, 661–665 CrossRef
.
- P. Puthiaraj and W.-S. Ahn, Catal. Sci. Technol., 2016, 6, 1701–1709 Search PubMed
.
- E. A. Monyoncho, S. Ntais, N. Brazeau, J. J. Wu, C. L. Sun and E. A. Baranova, ChemElectroChem, 2016, 3, 218–227 CrossRef
.
- F. Niu, Y. Jiang and W. Song, Nano Res., 2010, 3, 757–763 CrossRef
.
- F. Benaskar, V. Engels, E. V. Rebrov, N. G. Patil, J. Meuldijk, P. C. Thüne, P. C. Magusin, B. Mezari, V. Hessel and L. A. Hulshof, Chem.–Eur. J., 2012, 18, 1800–1810 CrossRef PubMed
.
- P. Ling, D. Li and X. Wang, J. Mol. Catal. A: Chem., 2012, 357, 112–116 CrossRef
.
- Y.-P. Zhang, A.-H. Shi, Y.-S. Yang and C.-L. Li, Chin. Chem. Lett., 2014, 25, 141–145 CrossRef
.
- R. K. Sharma, R. Gaur, M. Yadav, A. K. Rathi, J. Pechousek, M. Petr, R. Zboril and M. B. Gawande, ChemCatChem, 2015, 7, 3495–3502 CrossRef
.
- A. R. Hajipour, M. Check and Z. Khorsandi, Appl. Organomet. Chem., 2017, 31, e3769–e3778 CrossRef
.
- S. Chassaing, M. Kumarraja, A. Sani Souna Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883–886 CrossRef PubMed
.
- A. Alix, S. Chassaing, P. Pale and J. Sommer, Tetrahedron, 2008, 64, 8922–8929 CrossRef
.
- V. Bénéteau, A. Olmos, T. Boningari, J. Sommer and P. Pale, Tetrahedron Lett., 2010, 51, 3673–3677 CrossRef
.
- M. A. Khalilzadeh, A. Hosseini and A. Pilevar, Eur. J. Org. Chem., 2011, 2011, 1587–1592 CrossRef
.
- V. Magné, T. Garnier, M. Danel, P. Pale and S. Chassaing, Org. Lett., 2015, 17, 4494–4497 CrossRef PubMed
.
- S. Sadeghi, M. Jafarzadeh, A. R. Abbasi and K. Daasbjerg, New J. Chem., 2017, 41, 12014–12027 RSC
.
- Y. Wang, X. Guo, M. Lü, Z. Zhai, Y. Wang and X. Guo, Chin. J. Catal., 2017, 38, 658–664 CrossRef
.
- Bandna, N. R. Guha, A. K. Shil, D. Sharma and P. Das, Tetrahedron Lett., 2012, 53, 5318–5322 CrossRef
.
- H. Joshi, K. N. Sharma, A. K. Sharma, O. Prakash and A. K. Singh, Chem. Commun., 2013, 49, 7483–7485 RSC
.
- N. Iranpoor, H. Firouzabadi and A. Rostami, Appl. Organomet. Chem., 2013, 27, 501–506 CrossRef
.
- M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M. H. Beyzavi and R. Luque, Green Chem., 2013, 15, 2132–2140 RSC
.
- A. S. Singh, S. S. Shendage and J. M. Nagarkar, Tetrahedron Lett., 2013, 54, 6319–6323 CrossRef
.
- A. S. Singh, U. B. Patil and J. M. Nagarkar, Catal. Commun., 2013, 35, 11–16 CrossRef
.
- S. M. Baghbanian, H. Yadollahy, M. Tajbakhsh, M. Farhang and P. Biparva, RSC Adv., 2014, 4, 62532–62543 RSC
.
- M. Hosseini-Sarvari and Z. Razmi, RSC Adv., 2014, 4, 44105–44116 RSC
.
- A. Ghatak, S. Khan, R. Roy and S. Bhar, Tetrahedron Lett., 2014, 55, 7082–7088 CrossRef
.
- S. Paul, K. Pradhan, S. Ghosh, S. De and A. R. Das, Adv. Synth. Catal., 2014, 356, 1301–1316 CrossRef
.
- F. M. Moghaddam, G. Tavakoli and A. Aliabadi, RSC Adv., 2015, 5, 59142–59153 RSC
.
- R. Arundhathi, D. Damodara, P. R. Likhar, M. L. Kantam, P. Saravanan, T. Magdaleno and S. H. Kwon, Adv. Synth. Catal., 2011, 353, 1591–1600 CrossRef
.
- X. Haiyun, L. Lantao, W. Jing and L. Ying, Chin. J. Appl. Chem., 2014, 31, 536–540 Search PubMed
.
- S. M. Agawane and J. M. Nagarkar, Tetrahedron Lett., 2011, 52, 5220–5223 CrossRef
.
- P. Emayavaramban, S. Ganesh Babu, R. Karvembu and N. Dharmaraj, J. Nanosci. Nanotechnol., 2015, 15, 9358–9368 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |