Open Access Article
Open Access ArticleLiMg0.1Co0.9BO3 as a positive electrode material for Li-ion batteries†
Ceren Zor a,
Mehmet Somera and
Semih Afyon
a,
Mehmet Somera and
Semih Afyon *b
*b
aKoc University, Department of Chemistry, 34450 Sariyer, Istanbul, Turkey. E-mail: msomer@ku.edu.tr; cezor@ku.edu.tr
bETH Zurich, Department of Materials, CH-8093 Zurich, Switzerland. E-mail: semih.afyon@alumni.ethz.ch
First published on 26th April 2018
Abstract
LiCoBO3 could be a promising cathode material given the electronic and ionic conductivity problems are addressed. Here, Mg substitution in LiCoBO3 is employed to stabilise the structure and improve the electrochemical performance. LiMg0.1Co0.9BO3 is synthesised for the first time via sol–gel method and Mg substitution in the structure is verified by X-ray powder diffraction and energy dispersive X-ray analyses. The electrochemical properties are investigated by galvanostatic cycling and cyclic voltammetry tests. The composite electrode with conductive carbon (reduced graphite oxide and carbon black) delivers a first discharge capacity of 32 mA h g−1 within a 4.7–1.7 voltage window at a rate of 10 mA g−1. The cycling is relatively stable compared to the unsubstituted LiCoBO3. Mg substitution may enhance the electrochemical performance of borate-based electrode materials when combined with suitable electrode design techniques.
1. Introduction
Li-ion batteries have been widely investigated and commercially used in many electronic applications including everyday portable devices since their commercialisation by Sony in 1990.1 These batteries need to be developed further to meet the requirements of new portable devices, all-electric vehicles, stationary electric energy storage in a grid2 and to be implemented in energy harvesting via wind, solar, and thermal sources.3 Rechargeable Li-ion batteries with non-aqueous electrolytes enable higher operating voltages compared to conventional lead-acid and nickel-based batteries and are also suitable for novel electrode designs. One of the main drawbacks of Li-ion batteries is cathode materials with limited energy densities. Various cathodes including conversion type materials, organic molecules mimicking bioenergetics,4 intercalation compounds of transition metal oxides, metal chalcogenides, and polyanion-based materials have been investigated to solve this problem and improve energy densities.Currently, LiFePO4, an olivine type polyanion cathode material, which is safer and cheaper than LiCoO2 is also used as a popular cathode material in commercial applications.5 Several other olivine type materials including LiNiPO4,6 LiCoPO4,6 and LiMnPO4,7 have been investigated as potential cathode materials as well. Moreover, other polyanions such as (SO4)2−,8 (BO3)3−,9 and (SiO4)4–10 can be used in cathodes as promising frameworks with suitable transition metal cations. On a more practical note, due to their better stability regarding oxygen loss, polyanion cathode batteries could be safer than layered oxide cathode materials.11 (BO3)3−-based cathode materials function similar to (PO4)3−-based ones and can be better alternatives owing to their lower weight and thus higher specific capacities. (BO3)3−-based cathode materials also offer the highest theoretical specific capacity among one-electron per formula unit polyanion systems11 and they have not been extensively studied. First electrochemical investigation on borate-based Li-ion cathode materials was conducted by Legagneur et al.9 on LiFeBO3, LiMnBO3, and LiCoBO3 and they found that only 4%, 2%, and 1.5% Li per formula unit were extracted, respectively. The main reason hindering the practical specific capacities of (BO3)3−-based and other polyanion-based cathode materials is their limited ionic and electronic conductivities. Conductive coatings,12,13 utilising interconnected nano-sized particles14 or attaching the active material particles to conductive polymers15 help overcoming the low electronic conductivity of these materials. One of the main strategies to improve electrochemical performance of poorly conducting electrode materials is decreasing the particle size to shorten the distance for Li+ to travel upon charging/discharging.12,16–18 Further investigations employing these strategies show that specific capacities over 100 mA h g−1 could be achieved for LiMnBO3 which has a theoretical capacity of 222 mA h g−1,16,19,20 and a capacity of 190 mA h g−1 could be achieved for LiFeBO3 which has a theoretical capacity of 220 mA h g−1.21
LiCoBO3 is another interesting borate-based cathode candidate for rechargeable Li-ion batteries with a theoretical capacity of ∼215 mA h g−1 and with a high potential for redox couple Co2+/Co3+ operating at potentials above ∼4.0 V vs. Li/Li+.22–24 However, at the current state of the art, the theoretical promise of this cathode material has not been achieved practically. The aforementioned strategies for polyanion-based cathodes such as the use of nano-sized particles, conductive coatings, composites, and several synthesis methods including solid-state,9 sol–gel,23,25 molten salt,24 hydrothermal,26 and polyacrylamide-gel methods26 were also employed to improve the electrochemical properties LiCoBO3. Afyon et al.23 showed that reduced graphite oxide/nano-LiCoBO3 composite delivers a first charge capacity of 55 mA h g−1 at C/20 rate, and by decreasing the particle size further Ragupathi et al.25 claimed to obtain higher capacities. LiCoBO3 was also prepared as a thin film via reactive magnetron sputtering and its electrochemical properties were investigated, where Khalifah and co-workers recorded no significant electrochemical response and reported a very low conductivity (∼10−12 S cm−1) value for LiCoBO3.27 The substitution of Co in LiFeBO3 and LiMnBO3 were also reported with Li(Mn1−xCox)BO3 (ref. 28) and LiFe0.5Co0.5BO3 (ref. 29) delivering a capacity of 60 mA h g−1 at 1.8–4.7 V window and a capacity of 120 mA h g−1 at 1.5–4.7 V window, respectively.
Another strategy to improve the electrochemical properties of poorly performing polyanion-based cathode materials is doping of metal atoms at the transition metal site. The effect of Mg substitution in such cathode materials has been studied in various investigations.11,30–33 Delmas et al.34 showed that Mg doping increases the electronic conductivity and practical capacity in LiMgxCoyO2 by creating defects in the structure. In another report, Mg was shown to decrease the charge transfer resistance in Li2FeSiO4/C.31 Specifically for borate-based cathodes, Ceder and co-workers suggested Mg substitution in LiMnBO3.30 LiMgBO3 has the similar monoclinic structure with LiMnBO3 and Mg could be a candidate for structure stabilisation in LiMBO3 materials.30 They reported that phase decomposition of LiMnBO3 decreases upon Mg substitution and the capacity retention over multiple cycles is improved. This effect was further investigated in a system where the material was partially substituted by Fe as well.11 98% of the theoretical capacity was achieved for LiMn0.5Fe0.4Mg0.1BO3 and the stability of the phase below 1.8 V upon discharge was enhanced by the prevention of conversion type reactions for LiMBO3 (M = Fe and Mn).11 The Li+ ion transport mechanism in Fe and Mg substituted borate was also suggested to be different from the unsubstituted LiMnBO3 resulting improved electrochemical properties.
In line with these earlier reports for various cathode materials, we adapt the Mg substitution in LiCoBO3 and report on LiMg0.1Co0.9BO3 here for the first time. The sol–gel synthesised LiMg0.1Co0.9BO3 is electrochemically active delivering a first discharge capacity of 32 mA h g−1 at a rate of 10 mA g−1 within 4.7–1.7 V, and largely maintains this capacity over multiple cycles.
2. Experimental section
2.1. Synthesis
The gel-powder precursors were obtained via sol–gel method by dissolving stoichiometric amounts of LiNO3, Mg(NO3)2·6H2O, Co(NO3)2·6H2O, and B(OMe)3 in propionic acid. 0.01 mol LiNO3 (≥99%, Alfa Aesar), 0.01 mol Mg(NO3)2·6H2O (≥99%, Merck) and 0.01 mol Co(NO3)2·6H2O (≥99%, Merck) were dissolved in 12 ml propionic acid (≥99%, Merck) at 80 °C for 3 h. Similarly, 0.01 mol B(OMe)3 (≥99%, Merck) was added to 30 ml propionic acid and 50 ml ethanol (≥99.9%, Merck) mixture and heated following the same procedure. These two solutions were mixed together and heated at 80 °C for 1 h. 1 ml of distilled water was added to the final solution for hydrolysis and the heating continued until the evaporation was complete. The resulting gel-powder was annealed at ∼750 °C for 10–15 h under ambient conditions for the preparation of LiMg0.1Co0.9BO3. LiCoBO3 was also synthesised following the same sol–gel protocol to compare the electrochemical performances.2.2. Characterisation
The phase and the structure of the samples were determined by X-ray powder diffraction (XRD) method using a Bruker D8 Advance X-ray diffractometer and CuKα1 radiation (operated at 40 mA, 40 kV). The homogeneous distribution of the Mg content was confirmed by energy-dispersive X-ray spectroscopy (EDX) performed with Zeiss Ultra Plus field emission GeminiSEM equipped with 123 eV resolution Bruker XFlash® 5010 EDS detector under 15 kV EHT. The particle size and the morphology of the samples were investigated by scanning electron microscopy (SEM) using the same field emission scanning electron microscope.2.3. Electrochemical tests
For the preparation of the cathode material, initially, 70 wt% active material LiMg0.1Co0.9BO3 (or LiCoBO3), 10 wt% conductive carbon (Super P), 10 wt% reduced graphite oxide (rGO), and 10 wt% PVDF (Sigma-Aldrich) were ground in an agate mortar. Then, a slurry of this powder was ultrasonically dispersed in toluene (≥99.9%, Merck)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF (≥99%, Merck) (1
THF (≥99%, Merck) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) solution. For the fabrication of cathodes, this slurry was cast on Ti current collectors and dried at 80 °C for 3 h. The weight of active material on Ti current collectors was ∼2 mg cm−2 after drying. For the fabrication of anodes, Li metal disks cut from Li rods were used. 1 M Li[(C2F5)3PF3] in EC
4) solution. For the fabrication of cathodes, this slurry was cast on Ti current collectors and dried at 80 °C for 3 h. The weight of active material on Ti current collectors was ∼2 mg cm−2 after drying. For the fabrication of anodes, Li metal disks cut from Li rods were used. 1 M Li[(C2F5)3PF3] in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Merck, LF-30 SelectiLyte™) solution was used as the electrolyte in galvanostatic and cyclic voltammetry tests. Swagelok type of cells were put together in a glove-box under argon atmosphere using the described cathode, anode, and electrolyte, and glass and polypropylene/polyethylene/polypropylene separators. The galvanostatic cycling and cyclic voltammetry tests were carried out using a Metrohm Autolab PGSTAT30 potentiostat/galvanostat.
1) (Merck, LF-30 SelectiLyte™) solution was used as the electrolyte in galvanostatic and cyclic voltammetry tests. Swagelok type of cells were put together in a glove-box under argon atmosphere using the described cathode, anode, and electrolyte, and glass and polypropylene/polyethylene/polypropylene separators. The galvanostatic cycling and cyclic voltammetry tests were carried out using a Metrohm Autolab PGSTAT30 potentiostat/galvanostat.
3. Results and discussion
The active material, LiMg0.1Co0.9BO3, was synthesised through a sol–gel method. The gel-powder obtained from the stable sols of the homogenous mixture of nitrate salt precursors was annealed to reach the desired phase, as depicted in Fig. 1a. The X-ray powder diffraction (XRD) pattern of LiMg0.1Co0.9BO3 is displayed in Fig. 1b. The reflections in the pattern can be matched to the calculated pattern for LiCoBO3 (ICSD 59346)35 and no major residual diffraction peaks are observed confirming the phase purity of the sample (Fig. 1b). However, a residual impurity peak around 42° is observed that is thought to stem from CoO, which is also majorly present at lower annealing temperatures (see ESI†). The effect of Mg doping in the lattice parameters was investigated via Rietveld method with GSAS-II36 and according to the refinement results (see ESI† for further details), the purple LiMg0.1Co0.9BO3 crystals have a monoclinic symmetry (space group: C12/c1) and a smaller unit cell (a = 5.1101(6) Å, b = 8.7962(8) Å, c = 10.0351(9) Å, β = 91.44(1)°, and V = 450.93(6) Å3) compared to the unsubstituted LiCoBO3 (a = 5.129(1) Å, b = 8.840(2) Å, c = 10.100(2) Å, β = 91.36(3)°, and V = 457.81(37) Å3)35 (Fig. 1d). The crystal structure of LiMBO3 (M = Mg, Co) is shown in Fig. 1c. In the crystal system, Li atoms are coordinated by 4 O atoms and Co atoms are coordinated by 5 O atoms forming tetrahedra and trigonal-bipyramids, respectively. These two different polyhedra are connected to each other via corner and edge sharing and condensed to a polyhedral chain along [−1 0 1]. Two polyhedral chains are further interconnected through trigonal planar BO3 units (Fig. 1c).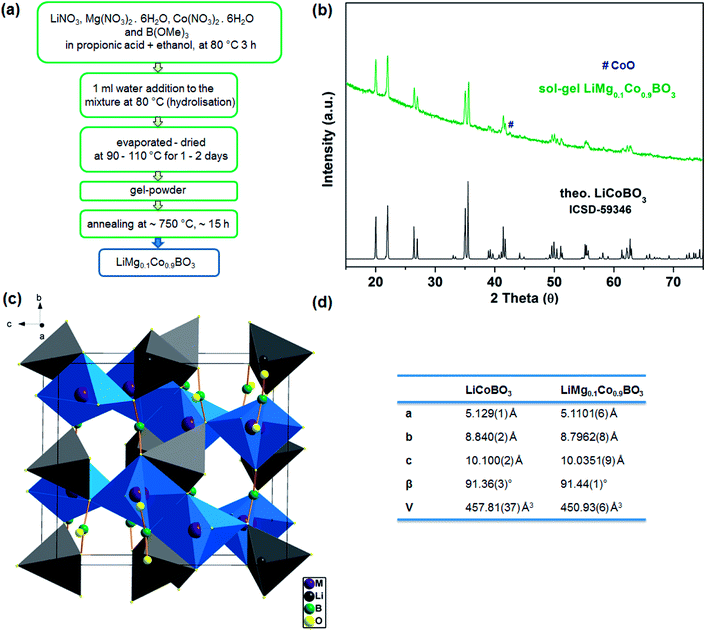 | ||
| Fig. 1 (a) The synthesis scheme for LiMg0.1Co0.9BO3, (b) XRD powder patterns of sol–gel synthesised novel LiMg0.1Co0.9BO3 (green) (# represents CoO impurity) and calculated (ICSD 59346) LiCoBO3 (black),35 (c) crystal structure of LiMg0.1Co0.9BO3 (skew [100] view of the phase, M = Co or Mg), and (d) lattice parameters for LiCoBO3 (ref. 35) and LiMg0.1Co0.9BO3. | ||
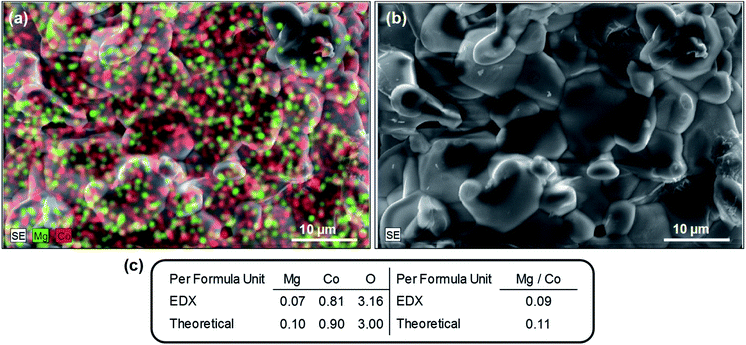
The substitution of Mg in the crystal lattice that was evidenced by the refinement results is further investigated by energy dispersive X-ray spectroscopy (EDX) analysis. The SEM-EDX micrographs indicate that Mg is homogenously distributed in LiMg0.1Co0.9BO3 crystallites (Fig. 2 and ESI Fig. S2†). The elemental analysis within the limits of this technique shows that Mg/Co ratio is 0.09 per formula unit, which is close to the expected theoretical ratio of 0.11. SEM micrographs were used to investigate the morphology of LiMg0.1Co0.9BO3 particles before and after the electrode preparation with reduced graphite oxide (rGO) and Super P carbon. The SEM micrographs in Fig. 3 show that the micron and submicron-sized crystallites of LiMg0.1Co0.9BO3 form larger agglomerates, 15 μm to 50 μm in size. Relatively higher synthesis temperatures are considered to cause the larger agglomerates, as the gel-powder could only yield the phase pure active material when annealed at temperatures above 700–750 °C and other residual oxide impurities are found to exist when annealed at lower temperatures (ESI Fig. S3†). Nevertheless, these agglomerates could be ground to the submicron range particles that were completely coated with conductive carbon after mixing with rGO and carbon black forming a textured conducting composite electrode, see ESI Fig. S4.†
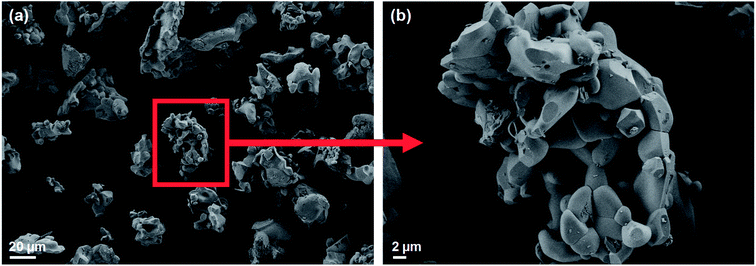 | ||
| Fig. 3 SEM micrographs displaying (a) general view of LiMg0.1Co0.9BO3 agglomerates and (b) magnified region in (a). | ||
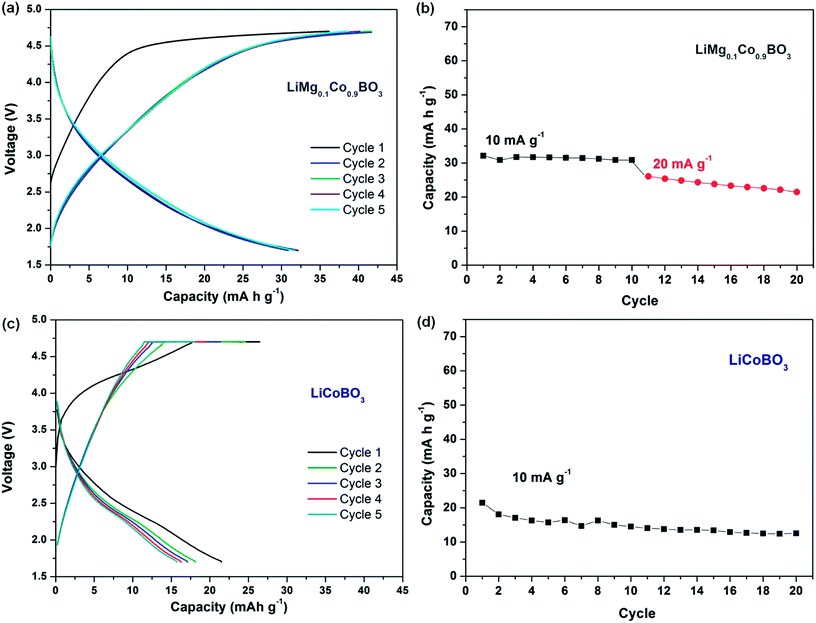
The electrochemical properties of LiMg0.1Co0.9BO3 were investigated through galvanostatic cycling and cyclic voltammetry measurements. The cyclic voltammetry analysis for LiMg0.1Co0.9BO3 was conducted at 0.05 mV s−1 rate between 4.7–1.7 V for four cycles (Fig. 4). The analysis shows broad oxidation and reduction processes indicating apparent polarisation in the system. The whole oxidation process extends from ∼3.0 V till 4.7 V with a peak position at ∼4.1 V that is in line with the previous findings based on plain LiCoBO3.23,26,29 A reduction peak can be observed at ∼2.5 V and the whole process again spreads to a large potential window ∼4.0–1.7 V.
 | ||
| Fig. 4 Cyclic voltammogram (cycles #1–4) of rGO/C/LiMg0.1Co0.9BO3 between 4.7–1.7 V recorded at 0.05 mV s−1 rate. | ||
The first five charge/discharge curves for LiMg0.1Co0.9BO3 are displayed in Fig. 4a. The working electrode consisted of 70% active material, 10% conductive carbon, 10% reduced graphite oxide, and 10% PVDF with an active material loading of ∼2 mg cm−2. The measurement conducted within a 4.7–1.7 V voltage window at a rate of 10 mA g−1 and the active material delivers a first charge capacity of 36 mA h g−1 and a first discharge capacity of 32 mA h g−1. The electrolyte chosen (Li[(C2F5)3PF3] in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Merck, LF-30 SelectiLyte™) is expected to be stable at the chosen voltage window,37 thus we do not expect any major contribution from the electrolyte oxidation or decomposition. The fifth discharge capacity is found to be 31.5 mA h g−1 indicating that the material is fairly stable upon cycling compared to the similar cathode systems.16,24,29 The cycling properties of LiMg0.1Co0.9BO3 within 4.7–1.7 V is presented in Fig. 4b for the first 10 cycles at a rate of 10 mA g−1 and at a rate of 20 mA g−1 for the next 10 subsequent cycles, respectively. The average discharge capacity drop at 10 mA g−1 current rate is found to be ∼1.3%, and a discharge capacity of ∼25 mA h g−1 is still obtained at the 15th cycle when the current rate is increased to 20 mA g−1. In order to evaluate the effects of Mg substitution on the electrochemical performance, unsubstituted micron-sized LiCoBO3 (see ESI Fig. S5 and S6† for further details) was also obtained via sol–gel synthesis and tested under a similar protocol. To give a further edge for the Li+ extraction in this kinetically limited system, a potentiostatic step at 4.7 V was applied till the current drops below 2 mA g−1. The charge and discharge rates were kept same with the Mg substituted system at 10 mA g−1. For the sol–gel synthesised micron-sized LiCoBO3, a first charge capacity of ∼17 mA h g−1 is obtained till the potentiostatic step, which reaches to ∼27 mA h g−1 at the end of the potentiostatic step (Fig. 5c). The coulombic efficiency is lower than that of LiMg0.1Co0.9BO3, as the capacity stays at ∼21 mA h g−1 at the first discharge step (Fig. 5c). The cycling stability for the micron-sized LiCoBO3 is also poorer compared to LiMg0.1Co0.9BO3, as the discharge capacity drops to ∼14 mA h g−1 at the 10th cycle with an average of loss ∼3.25% at each cycle (Fig. 5d).
1), Merck, LF-30 SelectiLyte™) is expected to be stable at the chosen voltage window,37 thus we do not expect any major contribution from the electrolyte oxidation or decomposition. The fifth discharge capacity is found to be 31.5 mA h g−1 indicating that the material is fairly stable upon cycling compared to the similar cathode systems.16,24,29 The cycling properties of LiMg0.1Co0.9BO3 within 4.7–1.7 V is presented in Fig. 4b for the first 10 cycles at a rate of 10 mA g−1 and at a rate of 20 mA g−1 for the next 10 subsequent cycles, respectively. The average discharge capacity drop at 10 mA g−1 current rate is found to be ∼1.3%, and a discharge capacity of ∼25 mA h g−1 is still obtained at the 15th cycle when the current rate is increased to 20 mA g−1. In order to evaluate the effects of Mg substitution on the electrochemical performance, unsubstituted micron-sized LiCoBO3 (see ESI Fig. S5 and S6† for further details) was also obtained via sol–gel synthesis and tested under a similar protocol. To give a further edge for the Li+ extraction in this kinetically limited system, a potentiostatic step at 4.7 V was applied till the current drops below 2 mA g−1. The charge and discharge rates were kept same with the Mg substituted system at 10 mA g−1. For the sol–gel synthesised micron-sized LiCoBO3, a first charge capacity of ∼17 mA h g−1 is obtained till the potentiostatic step, which reaches to ∼27 mA h g−1 at the end of the potentiostatic step (Fig. 5c). The coulombic efficiency is lower than that of LiMg0.1Co0.9BO3, as the capacity stays at ∼21 mA h g−1 at the first discharge step (Fig. 5c). The cycling stability for the micron-sized LiCoBO3 is also poorer compared to LiMg0.1Co0.9BO3, as the discharge capacity drops to ∼14 mA h g−1 at the 10th cycle with an average of loss ∼3.25% at each cycle (Fig. 5d).
Though the electrochemical activity of LiMg0.1Co0.9BO3 is shown here, the practical capacity obtained is still a fraction of the theoretical promise. The effect Mg substitution on the electrochemical performance of LiCoBO3 is considered to be beneficial as a slight improvement in the capacity and cycling properties can be achieved (e.g. ∼30 mA h g−1 for micron-sized LiMg0.1Co0.9BO3 particles vs. ∼1–6 mA h g−1 for micron-sized LiCoBO3 particles9,23) compared to the similar size borate-based cathodes in the literature. Although the inertness of LiCoBO3 as an electrode material was reported in some investigations,27 we believe that the main reason for the poor electrochemical activity is the limited ionic and electronic conductivity in the system. The time for intercalation in nanomaterials is 106 times less than micron-sized materials;1 hence, the utilisation of nano-particles has been tried in various reports12,16–18 with some success to overcome the conductivity issue as well as the kinetic polarisation problem. Here, we tried to apply both the substitution of the transition metal and the low dimensional composite electrode employment, however relatively large particles still exist in the electrode due to the processing method and draw the capacity of the synthesised material away from the theoretical capacity. Development of different conductive coatings and employment of nano-sized active material in the electrode fabrication could still improve the electrochemical performance of LiMg0.1Co0.9BO3, as they do in previously reported polyanion cathodes.1,12,23,25 Another approach could be the use of micron-sized active materials with mesopores rather than nano-sized materials17 to overcome the disconnection problem between particles. Nevertheless, Mg substitution in LiCoBO3 has been realised here through a sol–gel method and could be a viable way to further improve the electrochemical characteristics in the system when combined with other electrode enhancement techniques.
4. Conclusion
LiMg0.1Co0.9BO3 was synthesised following a sol–gel method for the first time. The crystal structure and pertaining lattice parameters were investigated by X-ray powder diffraction and the homogenous distribution of Mg in the phase was confirmed by EDX analysis. An electrode mixture using the active material and conductive carbon (reduced graphite oxide and Super P carbon) was used in electrochemical tests including galvanostatic and cyclic voltammetry analyses. A first discharge capacity of 32 mA h g−1 was observed in galvanostatic cycling tests conducted within a 4.7–1.7 V voltage window at a rate of 10 mA g−1. The realised electrochemical properties are relatively better compared to the similar sized LiCoBO3. The obtained results suggest that Mg substitution may contribute positively towards the electrochemical properties of the material. Decreasing the particle size of the Mg substituted LiCoBO3 and meticulous electrode engineering can further enhance the electrochemical performance of this system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Koc University Surface Science and Technology Center (KUYTAM) for the use of characterisation facilities.References
- P. G. Bruce, Solid State Ionics, 2008, 179, 752–760 CrossRef CAS.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- M. Lee, J. Hong, D. H. Seo, D. H. Nam, K. T. Nam, K. Kang and C. B. Park, Angew. Chem., Int. Ed., 2013, 52, 8322–8328 CrossRef CAS PubMed.
- S. Y. Chung, J. T. Bloking and Y. M. Chiang, Nat. Mater., 2002, 1, 123–128 CrossRef CAS PubMed.
- J. Wolfenstine and J. Allen, J. Power Sources, 2004, 136, 150–153 CrossRef CAS.
- G. H. Li, H. Azuma and M. Tohda, Electrochem. Solid State, 2002, 5, A135–A137 CrossRef CAS.
- A. Manthiram and J. B. Goodenough, J. Power Sources, 1989, 26, 403–408 CrossRef CAS.
- V. Legagneur, Y. An, A. Mosbah, R. Portal, A. L. La Salle, A. Verbaere, D. Guyomard and Y. Piffard, Solid State Ionics, 2001, 139, 37–46 CrossRef CAS.
- A. Nyten, A. Abouimrane, M. Armand, T. Gustafsson and J. O. Thomas, Electrochem. Commun., 2005, 7, 156–160 CrossRef CAS.
- J. C. Kim, D. H. Seo and G. Ceder, Energy Environ. Sci., 2015, 8, 1790–1798 CAS.
- H. Huang, S. C. Yin, T. Kerr, N. Taylor and L. F. Nazar, Adv. Mater., 2002, 14, 1525–1528 CrossRef CAS.
- Y. B. Lin, Y. Lin, T. Zhou, G. Y. Zhao, Y. D. Huang and Z. G. Huang, J. Power Sources, 2013, 226, 20–26 CrossRef CAS.
- Y. S. Hu, Y. G. Guo, R. Dominko, M. Gaberscek, J. Jamnik and J. Maier, Adv. Mater., 2007, 19, 1963–1966 CrossRef CAS.
- Y. H. Huang, K. S. Park and J. B. Goodenough, J. Electrochem. Soc., 2006, 153, A2282–A2286 CrossRef CAS.
- S. Afyon, D. Kundu, F. Krumeich and R. Nesper, J. Power Sources, 2013, 224, 145–151 CrossRef CAS.
- F. Jiao, K. M. Shaju and P. G. Bruce, Angew. Chem., Int. Ed., 2005, 44, 6550–6553 CrossRef CAS PubMed.
- S. L. Yang, X. F. Zhou, J. G. Zhang and Z. P. Liu, J. Mater. Chem., 2010, 20, 8086–8091 RSC.
- M. Moradi, J. C. Kim, J. F. Qi, K. Xu, X. Li, G. Ceder and A. M. Belcher, Green Chem., 2016, 18, 2619–2624 RSC.
- Y. S. Lee and H. Lee, Electron. Mater. Lett., 2014, 10, 253–258 CrossRef CAS.
- A. Yamada, N. Iwane, Y. Harada, S. Nishimura, Y. Koyama and I. Tanaka, Adv. Mater., 2010, 22, 3583–3587 CrossRef CAS PubMed.
- Y. Yamashita, P. Barpanda, Y. Yamada and A. Yamada, ECS Electrochem. Lett., 2013, 2, A75–A77 CrossRef CAS.
- S. Afyon, C. Mensing, F. Krumeich and R. Nesper, Solid State Ionics, 2014, 256, 103–108 CrossRef CAS.
- A. P. Tang, Q. W. Zhong, G. R. Xu and H. S. Song, RSC Adv., 2016, 6, 84439–84444 RSC.
- V. Ragupathi, S. Krishnaswamy, S. Raman, P. Panigrahi, J. Lee and G. S. Nagarajan, Appl. Surf. Sci., 2017 DOI:10.1016/j.apsusc.2017.11.087.
- O. A. Drozhzhin, I. V. Tereshchenko and E. V. Antipov, Ceram. Int., 2017, 43, 4670–4673 CrossRef CAS.
- S. H. Bo, G. M. Veith, M. R. Saccomanno, H. F. Huang, P. V. Burmistrova, A. C. Malingowski, R. L. Sacci, K. R. Kittilstved, C. P. Grey and P. G. Khalifah, ACS Appl. Mater. Interfaces, 2014, 6, 10840–10848 CAS.
- B. Le Roux, C. Bourbon, O. I. Lebedev, J. F. Colin and V. Pralong, Inorg. Chem., 2015, 54, 5273–5279 CrossRef CAS PubMed.
- B. Le Roux, C. Bourbon, J. F. Colin and V. Pralong, RSC Adv., 2015, 5, 72801–72804 RSC.
- J. C. Kim, X. Li, C. J. Moore, S. H. Bo, P. G. Khalifah, C. P. Grey and G. Ceder, Chem. Mater., 2014, 26, 4200–4206 CrossRef CAS.
- L. Qu, D. Luo, S. H. Fang, Y. Liu, L. Yang, S. Hirano and C. C. Yang, J. Power Sources, 2016, 307, 69–76 CrossRef CAS.
- M. V. Reddy, T. W. Jie, C. J. Jafta, K. I. Ozoemena, M. K. Mathe, A. S. Nair, S. S. Peng, M. S. Idris, G. Balakrishna, F. I. Ezema and B. V. R. Chowdari, Electrochim. Acta, 2014, 128, 192–197 CrossRef CAS.
- J. H. Shim, J. Lee, S. Y. Han and S. Lee, Electrochim. Acta, 2015, 186, 201–208 CrossRef CAS.
- S. Levasseur, M. Menetrier and C. Delmas, Chem. Mater., 2002, 14, 3584–3590 CrossRef CAS.
- Y. Piffard, K. K. Rangan, Y. L. An, D. Guyomard and M. Tournoux, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 1561–1563 Search PubMed.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- M. Schmidt, U. Heider, A. Kuehner, R. Oesten, M. Jungnitz, N. Ignat'ev and P. Sartori, J. Power Sources, 2001, 97–8, 557–560 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02745e |
| This journal is © The Royal Society of Chemistry 2018 |