Open Access Article
Open Access ArticleOne-step green approach for functional printing and finishing of textiles using silver and gold NPs
T. Abou Elmaaty *a,
Kh. El-Nagareb,
S. Raoufa,
Kh. Abdelfattahc,
S. El-Kadi
*a,
Kh. El-Nagareb,
S. Raoufa,
Kh. Abdelfattahc,
S. El-Kadi c and
E. Abdelazizd
c and
E. Abdelazizd
aDepartment of Textile Printing, Dyeing and Finishing, Faculty of Applied Arts, Damietta University, Damietta, Egypt. E-mail: tasaid@du.edu.eg; soola.a@hotmail.com
bNational Institute for Standards, Cairo, Egypt. E-mail: khnagare@hotmail.com
cDepartment of Agricultural Chemistry and Department of Agricultural Microbiology, Faculty of Agriculture, Damietta University, Egypt. E-mail: drkhaled_du@yahoo.com; sherifelkadi@du.edu.eg
dDepartment of textile printing, dyeing & finishing, Faculty of Applied Arts, Benha University, Benha, Egypt. E-mail: emanabdelaziz@hotmail.com
First published on 17th July 2018
Abstract
In this study, we present a successful simple method for printing and finishing of polyester and cotton fabrics using gold and silver nanoparticles (Au-NPs and Ag-NPs, respectively) as stable, fast colorants and functional components. The surface plasmon resonance (SPR) bands of the colloidal gold and silver NPs were observed at λmax 520 nm and 450 nm, respectively, indicating the presence of spherical Au-NPs and Ag-NPs, which was further confirmed by TEM analysis. The printed samples were subjected to SEM, XRD and EDX analyses. The SEM images and EDX spectra unequivocally confirmed the existence of embedded NPs on the fabric surfaces. Both the cotton and polyester samples possessed excellent color fastness, as indicated from the color fastness test. The functional properties of the printed fabrics indicated that the incorporation of Au-NPs and Ag-NPs into the fabrics simultaneously imparted multifunctional properties such as stable brilliant colors, highly durable antimicrobial activity and very good UV-protection properties.
1. Introduction
Textile printing is the most versatile and important method used to introduce color and design in textile fabrics. It is also known as localized application of dye or pigment in a thickened form to a substrate to create an attractive design with well-defined boundaries. In the textile industry, screen-printing is the most frequently applied method for textile prints, and it accounts for nearly 50% of the printing production worldwide. The main factors for its widespread use are the quality of the prints, applicability to almost every type of fiber or mixture, ability to withstand any washing processes after fixation, its simplicity, low cost, and minimum requirements for wet-processing.1,2 However, the synthetic dyes or pigments and their auxiliaries used in the printing process may be toxic and produce harmful effects on humans and the environment. In addition, several synthetic and natural dyes have low durability to washing and UV light, leading to color fading.3On the other hand, textile functionalization with noble metal (gold and silver) nanoparticles (NPs) has increased in recent years. Many attempts have been made to investigate different methods for their preparation4–10 and to enhance the functions of the textiles such as flame retarding ability, hydrophobicity, self-cleaning, and resistance to wrinkles as well as antistatic, antimicrobial and UV-protective properties.11–13 In addition, these NPs are endowed with unique localized surface plasmon resonance properties (SPR) and thus exhibit brilliant and different colors. With such excellent properties, novel shades of elegant hues and excellent multifunctional activities on different textile materials have been achieved.14,15
Nevertheless, many dyes are not stable under irradiation of sunlight and have low resistance to washing and rubbing, causing color fading. Metal NPs are different from traditional dyes: it is not the chromophore of traditional dyes but the shape and size of NPs that define the colors. This property makes all the colors stable towards UV light, and they do not change provided that there is no change in the particle size through the growth or reduction of discrete nanoparticles or agglomeration.16,17 According to the previous literatures, most printing methods are based on the utilization of pigments or dyes as colorants incorporated with metal NPs as finishing agents to produce multifunctional printed textiles.18–21 However, these methods consume a lot of chemicals, time, and energy.
Recently, several attempts have been carried out to use gold and silver NPs to dye fabrics with colors and simultaneously implement functionality to the dyed fabric, e.g., antibacterial activity22,23 and/or UV-protection.24 Based on the literature, the incorporation of gold and silver NPs into fabric or fiber materials is applied using three different procedures: impregnation of fabrics in the prepared metal NP colloidal solution,25–27 preparing metal NPs in situ in fabrics,14,28–32 and synthesizing polymer-NP composites followed by a spinning process to form colored fibers.33
To the best of our knowledge, textile printing with metal nanoparticles as a colorant has not been reported to date. Therefore, the main task of this study is to investigate a novel, simple, one-step, green approach for printing natural and synthetic fabrics, namely, cotton and polyester fabrics using gold and silver NPs as stable and fast colorants. This is an environmentally friendly solution for the current harmful printing, coloration and finishing processes.
2. Experimental
2.1. Materials
Scoured and bleached plain weave polyester (100%, 109 g m−2) and mill-scoured, bleached knitted cotton (200 g m−2) fabrics were used.Sera® Binder M-CPB liquid (acrylate-based copolymer, anionic, Clariant) and Sera® print M-CPK thickener 160 EG liquid (synthetic thickening agent based on ammonium polyacrylate, Clariant) were used.
Chloroauric acid (HAuCl4·3H2O, Sigma-Aldrich) was purchased from Skyspring Nanomaterials, Inc. USA.
All other chemicals used during this study such as AgNO3 (Sigma) and tri-sodium citrate Na3C6H5O7·2H2O (99.5%) were of commercial grade.
2.2. Methods
2.2.2.1. Preparation of extract. Pluchea dioscoridis leaves were collected from Damietta, Egypt. The leaves were cleaned well and washed using tap water followed by distilled water. The clean leaves were dried for a week at room temperature and then was ground to powder form. Five g of the leaf powder was introduced into boiling distilled water (200 ml) in an appropriate flask for 5 min. Then, the mixture was filtered, and the filtrate was stored at 40 °C for further use.
2.2.2.2. Synthesis of silver nanoparticles (Ag-NPs). Various volumes (1 and 12 ml) of Pluchea dioscoridis extracts (Pd) were placed in 100 mL Erlenmeyer flasks. Then, 45 ml AgNO3 with different concentrations (1–5 mM) was titrated drop by drop in each flask for 10 min. The formation of Ag-NPs was confirmed by the change in colour from colorless to reddish-brown.35
| Printing paste components | g kg−1 paste |
|---|---|
| Thickener | 20 |
| Binder | 100 |
| Nano-material | 20 |
| Water | 840 |
| Total | 1000 |
Printed fabric samples were then simultaneously dried and fixed in a high temperature steamer at 150 °C for 4 min followed by washing using 2 g l−1 non-ionic detergent at a liquor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 at 60 °C for 10 min.
50 at 60 °C for 10 min.
2.3. Characterization
2.4. Testing
2.5. Functional properties of metal NP-printed fabrics
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1996, and protection was rated as good, very good or excellent if the UPF values ranged from 15 to 24, 25 to 39, or above 40, respectively.
1996, and protection was rated as good, very good or excellent if the UPF values ranged from 15 to 24, 25 to 39, or above 40, respectively.3. Results and discussion
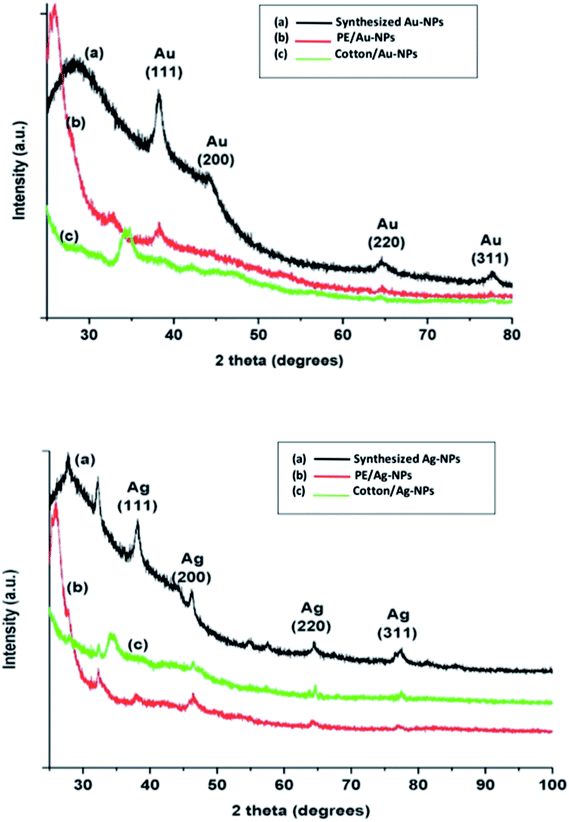
The main task of the current study is to obtain a facile novel one step green procedure for printing cotton and polyester fabrics using colloidal gold (Au-NPs) and silver (Ag-NPs) as stable and fast colorants to attain multifunctional prints based on nanomaterials. The results obtained and the appropriate discussions are presented below.3.1. Characterization of the synthesized nanoparticles
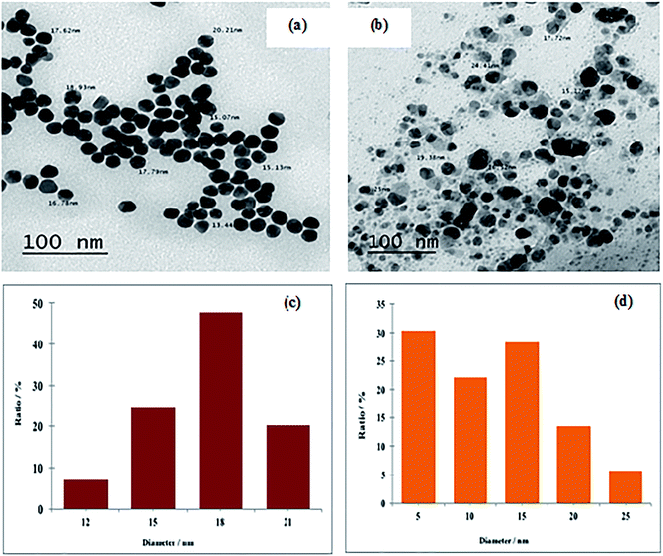 | ||
| Fig. 1 TEM images and size distribution histograms of the synthesized metal NPs: (a and c) Au NPs and (b and d) Ag-NPs. | ||
The size of numerous particles were measured using the TEM images, and histograms providing different size distributions of the well-dispersed suspensions of Au and Ag NPs were given in Fig. 1c and d, respectively. The width of the Au-NP bins was 3 nm, as illustrated in Fig. 1c, and they were centered at 12, 15, 18 and 21 nm. Au-NPs around 18 nm in size exhibited the highest percentage value (47.79%) in comparison with other Au-NPs present in the colloid. From Fig. 1d, for Ag-NPs, the bins of the histogram were 5 nm wide, and they were centered at 5, 10, 15, 20 and 25 nm. The majority of particles (30–33%) were located at around 5 nm, which may be the main reason for the high antimicrobial activity of Ag-NPs.
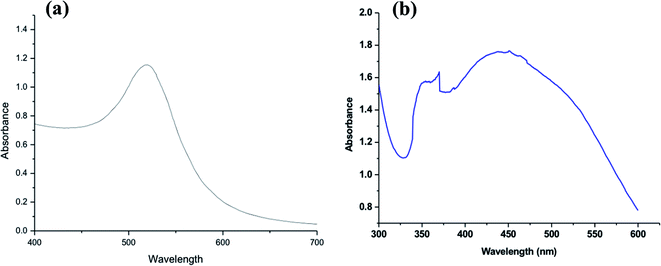
Fig. 2a shows the UV-vis spectrum of the synthesized Au-NPs, and a maximum absorbance peak of the colloidal Au-NPs occurred at about 520 nm. Generally, Au-NPs displayed a single absorption peak in the visible range between 510 and 550 nm due to its SPR and exhibited heavy absorption of visible light at 520 nm. This was resulted in a brilliant wine-red color for the gold NPs, which varied according to its size and shape. Alaqad et al.40 also observed the absorbance spectrum of Au-NPs at around 520 nm, whereas HAuCl4 did not exhibit any absorbance at the same wavelength.
Fig. 2b shows the UV-vis spectrum of the synthesized Ag-NPs; it can be clarified that due to the yellowish color of the colloidal Ag-NPs, the maximum absorption peak was at about 450 nm, which was specific to SPR of spherical Ag-NPs.37 This result confirmed the proposed formation according to transmission electron microscopy (TEM). Both the UV-vis absorption (Fig. 2b) and TEM (Fig. 1b) results verified that the yellow color of the colloidal Ag-NPs was a result of the presence of spherical Ag-NPs.
The observed broad peak illustrates that the synthesized Ag-NPs were different in size and shape, which was in agreement with the TEM analysis (Fig. 1b). Krishnaraj et al.41 reported that a single SPR band indicates spherical nanoparticles, whereas two or more SPR bands indicate anisotropic particles. Furthermore, it was observed that another strong UV absorption peak appeared at about 350 nm, which may be due to the residual plant extract used in the synthesis of Ag-NPs or trace iron impurities, which appeared in the ppm level.42
3.2. Color measurements
The color of printed fabrics is of key significance since it may impact the product demand and consumer inclination. To investigate the color properties of nano-printed fabric surfaces, their colorimetric data were measured for Au-NP- and Ag-NP-printed fabrics using the CIELAB system in terms of L*, a*, and b* to study the effect of gold and silver NPs as new colorants for cotton and polyester fabrics. The color coordinates are listed in Tables 2 and 3. After printing fabrics using colloidal solutions of Au-NPs and Ag-NPs, red-wine and yellow printed polyester and cotton fabrics were obtained with different shades and very good homogeneity, as shown in Fig. 5.| Sample | Cotton | Polyester | ||||||
|---|---|---|---|---|---|---|---|---|
| Au-NP Conc. | L* | a* | b* | K/S | L* | a* | b* | K/S |
| a Values in parentheses indicate K/S after 10 washing cycles. | ||||||||
| 0.3 mμ | 69.58 | 20.17 | 0.71 | 1.4 (1.2)a | 65.39 | 27.71 | 4.36 | 1.3 (1.2) |
| 0.5 mμ | 60.09 | 29.05 | 1.14 | 3.6 (3.2) | 57.24 | 28.54 | 9.1 | 2.6 (2.5) |
| 0.8 mμ | 50.16 | 36.74 | 5.15 | 5.7 (5.3) | 48.95 | 34.62 | 10.7 | 4.6 (4.5) |
| 2.5 mμ | 47.69 | 36.45 | 9.6 | 6.8 (6.1) | 43.92 | 34.82 | 11.53 | 6.7 (6.6) |
| 4 mμ | 43.89 | 37.79 | 6.07 | 8.2 (8.01) | 38.63 | 38.11 | 12.83 | 9.3 (9.1) |
| Sample | Cotton | Polyester | ||||||
|---|---|---|---|---|---|---|---|---|
| Ag-NP Conc. | L* | a* | b* | K/S | L* | a* | b* | K/S |
| a Values in parentheses indicate K/S after 10 washing cycles. | ||||||||
| 5 mμ | 85.72 | 6.52 | 6.69 | 2.7 (2.5)a | 81.87 | 4.68 | 20.76 | 1.2 (1.2) |
| 6 mμ | 82.91 | 4.54 | 21.54 | 4.6 (4.6) | 69.55 | 13.93 | 33.6 | 2.5 (2.4) |
| 7 mμ | 78.94 | 5.95 | 30.67 | 5.7 (5.6) | 61.97 | 18.8 | 37.38 | 3.7 (3.5) |
| 8 mμ | 75.7 | 11.1 | 39.74 | 7.4 (7.2) | 60.35 | 23.43 | 46.59 | 6.1 (6) |
| 9 mμ | 58.91 | 21.57 | 43.44 | 9.4 (9.3) | 46.59 | 24.59 | 52.01 | 8.9 (8.7) |
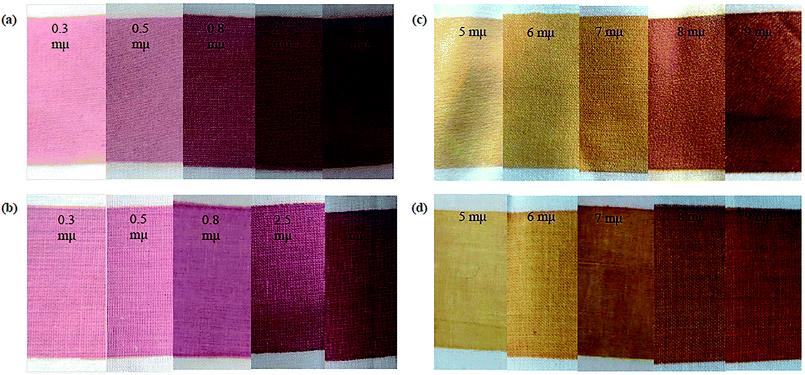 | ||
| Fig. 5 Photographs of the metal NP-printed fabrics: (a) polyester/Au-NPs, (b) cotton fabrics with Au-NPs, (c) polyester fabrics with Ag-NPs, and (d) cotton fabrics with Ag-NPs. | ||
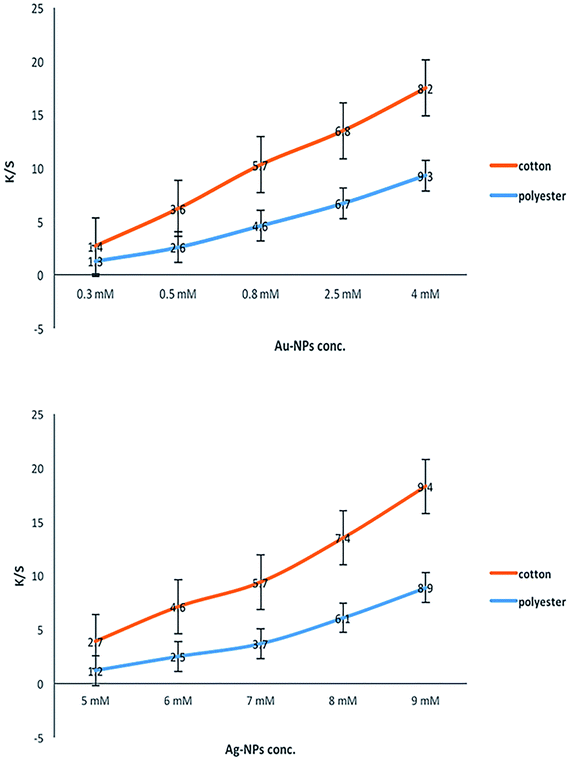
Table 2 shows the colorimetric data for the printed fabrics using Au-NPs and Ag-NPs. At low concentrations of NPs, the colors of the printed cotton and polyester fabrics were light red-wine for Au-NPs and light-yellow for Ag-NPs, as shown by the low positive a* and b* values. With an increase in the concentration of Au-NPs, the positive a* value progressively increased giving a reddish tone to the printed fabrics. The color of the Ag-NP-printed fabrics gradually increased, giving a dark yellowish tone with an increase in the concentration of Ag-NPs, as shown by the positive high b* values. Furthermore, the lightness (L*) values gradually decreased for all the printed fabrics. This decrease in L* was a result of the coloration of the fabrics, which became deeper by increasing the concentrations of Au and Ag metal NPs.
The color strength (K/S) data were measured at the wavelength of 425 nm, which is related to the yellow color of Ag-NPs, whereas the wine-red color of Au-NPs was measured at the wavelength of 520 nm. Fig. 6 shows that increasing the concentration of the synthesized Au-NPs and Ag-NPs resulted in a significant improvement in the color depth of the obtained metal Au and Ag NP prints expressed as the K/S values. The observed data indicated that the darker yellow and red-wine colors of the printed fabrics were achieved by using higher concentrations of Au-NPs and Ag-NPs. This can be explained by the fact that a high number of spherical NPs were formed in the fabrics under investigation with the use of more concentrated Au-NP and Ag-NP colloidal solutions.
Moreover, the fabrics printed with Au-NPs had a red-wine color, and the fabrics printed with Ag-NPs had a yellow color with different shades [from faint (red-wine/yellow) to dark (red-wine/yellow)], but the nature of the color did not change regardless of the concentration of NPs. This may be regarded to the fact that the change in SPR of Au-NPs and Ag-NPs with different shapes and sizes on the polyester and cotton samples led to bright colors of the printed fabrics.
These results support the suggestion that cotton and polyester fabrics can be printed by synthesized silver and gold colloidal NPs as stable and fast colorants, and the color strength (K/S) of these fabrics can be controlled by changing the concentration of synthesized Au-NPs and Ag-NPs.
3.3. Fastness properties
The coloration requirements are not limited to imparting color to the fabrics, but the acquired color should withstand the action of certain tests. Thus, different color fastness properties (washing, rubbing and light) of all the printed cotton and polyester fabrics were measured according to standard AATCC Test Methods (61-1972) and (8-1972), and (16A-1972), respectively, and the results are reported in Table 4. The results showed that all the printed fabrics exhibited excellent fastness levels to washing and rubbing and a very good fastness level to light. The obtained results reflected the ability of the suggested technique to yield good printed fabrics with excellent fastness properties without the use of any other chemicals (e.g., cross-linkers or coating materials).3.4. Antimicrobial activity
The antimicrobial properties of the Au-NP- and Ag-NP-printed fabrics are shown in Table 5. The results revealed that the unprinted samples were not affected, and there were no inhibition areas. On the other hand, the cotton samples printed using Ag and Au-NPs as colorants exhibited excellent antimicrobial activities against S. aureus, Bacillus cereus, E. coli and Candida utilis. It is known that nano-materials have strong inhibiting effects towards a broad spectrum of bacteria and yeasts, especially those Ag-NPs displaying good activity against all the indicator pathogens, which shows the potential broad spectrum antimicrobial activity.45 The remarkable improvement in the antimicrobial functionality is ascribed to the interaction of the negatively charged cell walls of the pathogens with the positively charged cationic sites of the antimicrobial agent, which changes its chemical and physical properties. This action interrupts cell membrane functions and protein activity as well as the ability to multiply. In addition, the silver and gold nanoparticles released silver and gold ions, which produced higher biocidal effect on the microorganisms.11,453.5. UV-protection activity
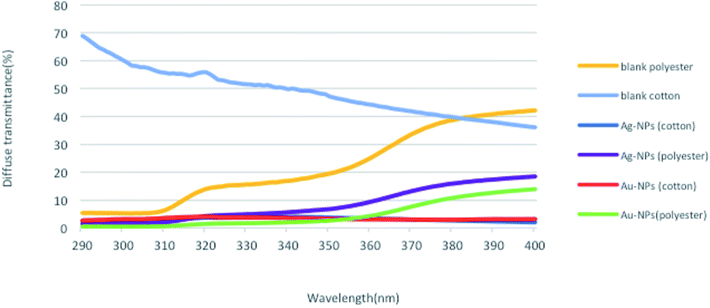
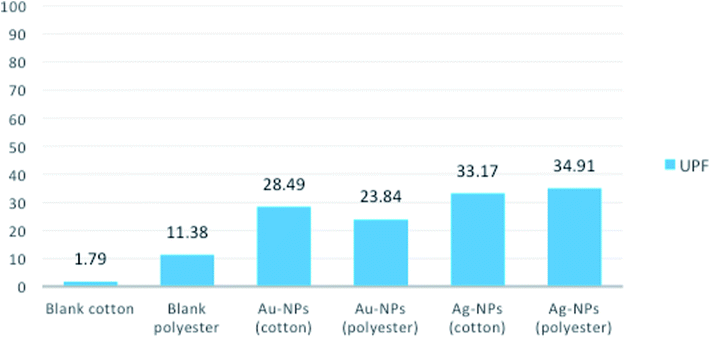
To investigate the UV-protection ability of the blank and polyester and cotton fabrics printed with Au-NPs and Ag-NPs, their UV light transmittance and UPF values were measured, and the results were illustrated in Fig. 7 and 8.As expected, the blank samples are not good UV filters and a high percentage of UV light can penetrate into the fabric. However, the average transmittance values in the UVA (315–400 nm) and UVB (280–315 nm) regions for the fabrics decreased clearly after printing the fabrics with Au-NPs and Ag-NPs, which indicated that the gold and silver NPs noticeably improved the UV-blocking abilities of the polyester and cotton samples.
The UPF values of Au-NP- and Ag-NP-printed fabrics exhibit very good UV-protection according to the Australian classification scheme compared with those of blank fabrics, as shown in Fig. 8. Therefore, these results confirmed the UV reflection ability of Au-NPs and Ag-NPs, which can effectively decrease aging and reduce human skin damage caused by harmful UV-radiation.
3.6. Durability to washing
The results illustrated in Table 6 reveal that the retention of the imparted functional properties, i.e., antimicrobial and UV-protection together with the fastness properties as well as the color strength (K/S) of the obtained metal NPs prints was still high even after 10 washing cycles. This means that the metal NPs (Au-NPs and Ag-NPs) were still tightly loaded and fixed onto the simultaneously printed and finished fabric surfaces.| Type of NPs | Type of fabric | Fastness properties | Functional properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WFa | RFb | LFc | ZId (mm) | UPF | |||||||
| St. | Alt. | Wet | Dry | G+ve | G−ve | Yeast | |||||
| S. aureus | Bacillus Cereus | E. coli | Candida utilis | ||||||||
| a Wash fastness.b Rubbing fastness.c Light fastness.d Zone of inhibition. | |||||||||||
| Au-NPs | Polyester | 5 | 4/5 | 4/5 | 5 | 4/5 | 14 | 16 | 18 | 14 | 22.34 |
| Cotton | 5 | 4/5 | 4/5 | 5 | 4 | 16 | 20 | 14 | 12 | 26.87 | |
| g-NPs | Polyester | 5 | 4/5 | 4/5 | 5 | 4 | 23 | 21 | 25 | 14 | 32.88 |
| Cotton | 5 | 4/5 | 4/5 | 5 | 4/5 | 21 | 25 | 18 | 16 | 30.52 | |
4. Conclusion
A facile and feasible strategy was devised to produce colorful and multifunctional printed polyester and cotton fabrics using synthesized NPs. This method featured the novelty of employing gold and silver NPs as eco-friendly stable colorants and functional components in a one-step process for printing and functional finishing of polyester and cotton fabrics. The results of the UV-visible, TEM, SEM and XRD measurements confirmed the successful synthesis and uniform distribution of spherical Au-NPs and Ag-NPs on polyester and cotton fabrics. The brilliant colored appearance, excellent color fastness properties, highly durable antibacterial activity and very good UV-protective properties of the nano-printed fabrics indicated their ability to produce good printed/finished fabrics in a one-step process without using traditional dyes or auxiliary chemicals.Conflicts of interest
There are no conflicts to declare.References
- W. H. Solangi, Z. A. Noonari, A. A. Channa, M. Q. Khan and A. B. Siyal, Influence of Binders and Thickeners of Pigment Printing paste on Light Fastness and Crocking Fastness of the Fabric, Int. J. Sci. Res. Dev., 2014, 3, 1024–1032 Search PubMed.
- T. Feczko, K. Samu, K. Wenzel, B. Nerald and B. Voncina, Textiles screen-printed with photochromic ethyl cellulose–spirooxazine composite nanoparticles, Color. Technol., 2013, 129, 18–23 Search PubMed.
- A. B. Rezaie, M. Montazer and M. M. Rad, A cleaner route for nano-colouration of wool fabric via green assembling of cupric oxide nanoparticles along with antibacterial and UV protection properties, J. Cleaner Prod., 2017, 221–231 CrossRef.
- H. E. Emam and H. B. Ahmed, Carboxymethyl cellulose macromolecules as generator of anisotropic nanogold for catalytic performance, Int. J. Biol. Macromol., 2018, 111, 999–1009 CrossRef PubMed.
- H. E. Emam, M. K. Zahran and H. B. Ahmed, Generation of biocompatible nanogold using H2O2-starch and their catalytic/antimicrobial activities, Eur. Polym. J., 2017, 90, 354–367 CrossRef; H. B. Ahmed, M. K. Zahran and H. E. Emam, Heatless synthesis of well dispersible Au nanoparticles using pectin biopolymer, Int. J. Biol. Macromol., 2016, 91, 208–219 CrossRef PubMed.
- H. B. Ahmed, A. M. Abdel-Mohsen and H. E. Emam, Green-assisted tool for nanogold synthesis based on alginate as a biological macromolecule, RSC Adv., 2016, 6, 73974 RSC.
- A. A. Hebeish, M. H El-Rafie, F. A. Abdel-Mohdy, E. S. Abdel-Halim and H. E. Emam, Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles, Carbohydr. Polym., 2010, 82, 933–941 CrossRef.
- M. K. Zahran, H. B. Ahmed and M. H. El-Rafie, Facile size-regulated synthesis of silver nanoparticles using pectin, Carbohydr. Polym., 2014, 111, 971–978 CrossRef PubMed.
- M. K. Zahran, H. B. Ahmed and M. H. El-Rafie, Alginate mediate for synthesis controllable sized AgNPs, Carbohydr. Polym., 2014, 111, 10–17 CrossRef PubMed.
- N. A. Ibrahim, B. M. Eid, E. Abd El-Aziz, T. M. Abou Elmaaty and S. M. Ramadan, Loading of Chitosan-Nano metal oxide hybrids onto cotton/polyester fabrics to impart permanent and effective multifunctions, Int. J. Biol. Macromol., 2017, 105, 769–776 CrossRef PubMed.
- N. A. Ibrahim, B. M. Eid, E. Abd El-Aziz, T. M. Abou Elmaaty and S. M. Ramadan, Multifunctional cellulose-containing fabrics using modified finishing formulations, RSC Adv., 2017, 7, 3219–3230 Search PubMed.
- B. Tang, Y. Yao, J. Li, S. Qin, H. Zhu, J. Kaur, W. Chen, L. Sun and X. Wang, Functional Application of Noble Metal Nanoparticles in situ Synthesized on Ramie Fibers, Nanoscale Res. Lett., 2015, 10, 2–9 CrossRef PubMed.
- X. Lin, F. Zou, X. Chen and B. Tang, Functional modification of Nylon fabrics based on noble metal nanoparticles, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 231, 1–7 Search PubMed.
- S. Islam, B. S. Butola and F. Mohammad, Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials, RSC Adv., 2016, 6, 44232–44247 RSC.
- H. Oda, Improving Light Fastness of Natural Dye: Photostabilisation of Gardenia Blue, Color. Technol., 2012, 128, 68–73 Search PubMed.
- J. H. Johnston and K. A. Lucas, Nanogold synthesis in wool fibres: novel colourants, Gold Bull., 2011, 44, 85–89 CrossRef.
- N. A. Ibahima, B. M. Eid, E. Abd El-Aziz and T. M. Abou Elmaaty, Functionalization of linen/cotton pigment prints using inorganic nano structure materials, Carbohydr. Polym., 2013, 97, 537–545 CrossRef PubMed.
- N. A. Ibrahim, B. M. Eid, T. M. Abou Elmaaty and E. Abd El-Aziz, A smart approach to add antibacterial functionality to cellulosic pigment prints, Carbohydr. Polym., 2013, 94, 612–618 CrossRef PubMed.
- N. A. Ibrahim, T. M. Abou Elmaaty, B. M. Eid and E. Abd El-Aziz, Combined antimicrobial finishing and pigment printing of cotton/polyester blends, Carbohydr. Polym., 2013, 95, 379–388 CrossRef PubMed.
- N. A. Ibrahim, E. Abd El-Aziz, B. M. Eid and T. M. Abou Elmaaty, Single-stage process for bifunctionalization and eco-friendly pigment coloration of cellulosic fabrics, J. Text. Inst., 2015, 107, 1022–1029 Search PubMed.
- N. A. Ibrahim, B. M. Eid and M. S. Abdel-Aziz, Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics, Appl. Surf. Sci., 2017, 392, 1126–1133 CrossRef.
- N. A. Ibrahim, B. M. Eid and M. S. Abdel-Aziz, Green Synthesis of AuNPs for Eco-friendly Functionalization of Cellulosic Substrates, Appl. Surf. Sci., 2016, 389, 118–125 CrossRef.
- H. E. Emam and R. M. Abdelhameed, Anti-UV Radiation Textiles Designed by Embracing with Nano-MIL (Ti,In)-Metal Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9(33), 28034–28045 CrossRef PubMed.
- H. B. Ahmed, H. E. Emam, H. M. Mashalyc and M. Rehan, Nanosilver leverage on reactive dyeing of cellulose fibers: color shading, color fastness and biocidal potentials, Carbohydr. Polym., 2018, 186, 310–320 CrossRef PubMed.
- J. H. Johnston and K. A. Lucas, Nanogold synthesis in wool fibres: novel colourants, Gold Bull., 2011, 44, 85–89 CrossRef.
- A. J. Kiyan, L. Karimi and A. Davodiroknabadi, Producing colored cotton fabrics with functional properties by combining silver nanoparticles with nano titanium dioxide, Cellulose, 2017, 24, 3083–3094 CrossRef.
- S. Mowafi, M. Rehan, H. M. Mashaly, A. Abou El-Kheir and H. E. Emam, Influence of silver nanoparticles on the fabrics functions prepared by in- situ technique, J. Text. Inst., 2017, 108, 1828–1839 CrossRef.
- H. E. Emam, M. Rehan, H. M. Mashaly and H. B. Ahmed, Large scaled strategy for natural/synthetic fabrics functionalization via immediate assembly of AgNPs, Dyes Pigm., 2016, 133, 173–183 CrossRef.
- M. Rehan, H. M. Mashaly, S. Mowafi, A. Abou El-Kheir and H. E. Emam, Multi-functional textile design using in situ Ag NPs incorporation into natural fabric matrix, Dyes Pigm., 2015, 118, 9–17 CrossRef.
- H. E. Emam, N. S. El-Hawary and H. B. Ahmed, Green technology for durable finishing of viscose fibers via self-formation of AuNPs, Int. J. Biol. Macromol., 2017, 96, 697–705 CrossRef PubMed.
- H. E. Emam, N. H. Saleh, K. S. Nagy and M. K. Zahran, Functionalization of medical cotton by direct incorporation of silver nanoparticles, Int. J. Biol. Macromol., 2015, 78, 249–256 CrossRef PubMed.
- H. B. Ahmed, N. S. El-Hawary and H. E. Emam, Self-assembled AuNPs for ingrain pigmentation of silk fabrics with antibacterial potency, Int. J. Biol. Macromol., 2017, 105, 720–729 CrossRef PubMed.
- T. V. Sreekumar, D. Arunashish, C. Lal, S. Anurage and R. K. Bhasker, Inherently colored antimicrobial fibers employing silver nanoparticles, J. Biomed. Nanotechnol., 2009, 5, 115–120 CrossRef PubMed.
- S. Honary, P. Ebrahim and M. Ghsemitabar, Preparation of gold nanoparticles for biomedical applications using chemometric technique, Trop. J. Pharm. Res., 2013, 12, 295–298 Search PubMed.
- N. Azzaz, S. El-Kadi, K. S. Ahmed and M. Mahmoud, Antimicrobial Activities for Green Synthesis of Silver Nanoparticles using Stevia rebaudiana and Pluchea dioscoridis Leaves, International Journal of Agricultural and Biosystems Engineering, 2017, 2, 54–66 Search PubMed.
- H. N. Verma, P. Singh and R. M. Chavan, Gold nanoparticle: synthesis and characterization, Vet. World, 2014, 7, 72–77 CrossRef.
- H. E. Emam, S. Mowafi, H. M. Mashaly and M. Rehan, Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles, Carbohydr. Polym., 2014, 110, 148–155 CrossRef PubMed.
- S. Al-Azad, M. N. Morshed, H. Deb, M. A. Alam, K. M. F. Hasan and X. Shen, Localized Surface Plasmon Resonance Property of Ag-Nanoparticles and Prospects as Imminent Multi-Functional Colorant, American Journal of Nanoscience and Nanotechnology Research, 2017, 5, 2–20 Search PubMed.
- A. R. Prado, J. P. Oliveira, W. J. Keijok, B. A. Milaneze, B. V. Nogueira, M. C. Guimarães, M. J. Pontes and M. R. Ribeiro, Comparison between the synthesis of gold nanoparticles with sodium citrate and sodium tetraborate, BMC Proc., 2014, 8, 252 CrossRef.
- K. Alaqad and T. Saleh, Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs, Journal of Environmental and Analytical Toxicology, 2016, 6, 1–10 Search PubMed.
- C. Krishnaraj, E. G. Jagan, S. Rajasekar, P. Selvakumar, P. Kalaichelvan and N. Mohan, Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens, Colloids Surf., B, 2010, 76, 50–56 CrossRef PubMed.
- A. M. Abdelghany, A. H. Oraby, A. A. Hindi, D. M. El-Nagar and F. S. Alhakami, Green synthesis of mixed metallic nanoparticles using room temperature self-assembly, J. Adv. Phys., 2017, 13, 4671–4676 Search PubMed.
- K. Anandalakshmi and J. Venugobal, Green Synthesis and Characterization of Silver Nanoparticles Using Vitex negundo (Karu Nochchi) Leaf Extract and its Antibacterial Activity, Med. Chem., 2017, 7, 218–225 Search PubMed.
- M. Chelladurai, R. Shanmugam, V. Mahendran, P. Kanniah, G. Gnanadhas and A. Gurusamy, Eco-friendly synthesis and characterization of gold nanoparticles using Klebsiella pneumonia, J. Nanostruct. Chem., 2013, 3, 1–7 Search PubMed.
- N. Gokarneshan and K. Velumani, Application of Nano Silver Particles on Textile Materials for Improvement of Antibacterial Finishes, Glob. J. Nanomed., 2017, 2, 1–4 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |