Open Access Article
Open Access ArticleTransition metal triflate catalyzed conversion of alcohols, ethers and esters to olefins†
J. Keskiväli ,
A. Parviainen
,
A. Parviainen ,
K. Lagerblom
,
K. Lagerblom and
T. Repo
and
T. Repo *
*
Department of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, P.O. Box 55, 00014, Finland. E-mail: timo.repo@helsinki.fi
First published on 23rd April 2018
Abstract
Herein, we report an efficient transition metal triflate catalyzed approach to convert biomass-based compounds, such as monoterpene alcohols, sugar alcohols, octyl acetate and tea tree oil, to their corresponding olefins in high yields. The reaction proceeds through C–O bond cleavage under solvent-free conditions, where the catalytic activity is determined by the oxophilicity and the Lewis acidity of the metal catalyst. In addition, we demonstrate how the oxygen containing functionality affects the formation of the olefins. Furthermore, the robustness of the used metal triflate catalysts, Fe(OTf)3 and Hf(OTf)4, is highlighted by their ability to convert an over 2400-fold excess of 2-octanol to octenes in high isolated yields.
Introduction
There is a great aspiration to replace fossil feedstocks with sustainable biomass-based resources. Biomass provides a large supply of different organic compounds, mostly in the form of cellulose, hemicellulose and lignin.1 As a result, the selective defunctionalization and upgrading of these oxygen rich biomass-based substrates has been intensely researched recently, and various approaches have been devised to obtain value-added biochemicals, such as alkanes, isosorbide and furfurals.2–6 In addition to lignocellulose, terpenes are a versatile class of organic compound widely available from biomass, containing different functionalities, such as alcohols, esters and ethers.7,8 In this respect, the defunctionalization of terpenes offers an interesting opportunity to produce high value biomass-based olefins for biopolymer applications as well as for other purposes.9 However, more attention should be focused on finding abundant and sustainable catalysts to produce valuable olefins from renewable natural resources.The dehydration of alcohols to alkenes is usually conducted using Brønsted acid catalysts, including H2SO4, HCl or H-ZSM5.10–12 However, these catalysts are associated with various problems, such as low product selectivities, corrosive nature as well as environmental hazards.3,5,10 Accordingly, the development of more benign and selective catalysts is preferred. The use of Lewis acidic metal dehydration catalysts has been sparsely reported in the synthesis of olefins from alcohols, ethers and esters.10 On the other hand, metal triflates, such as those of Eu, Hf and La, have previously been reported to catalyze C–O bond cleavage at elevated temperatures in the presence of Pd/C and at high hydrogen pressures, producing alkanes as products.13–18 In this respect, the use of metal triflates in the synthesis of olefins is plausible, and ideally the more expensive metals should be replaced with more abundant and inexpensive transition metal-based Lewis acid catalysts in the conversion of biomass-based oxygen containing compounds to olefins.
Herein, we present the correlation between the oxophilicity and the Lewis-acidity of metal triflates in the dehydration of alcohols, and the aptitude of the selected transition metal triflates, Fe(OTf)3 and Hf(OTf)4, to produce olefins from various oxygen containing biomass-based compounds under solvent-free conditions. The durability and robustness of Fe(OTf)3 and Hf(OTf)4 are demonstrated with a large scale synthesis of octenes from 2-octanol. As a proof of concept for the upgrading of essential oils to alkenes, tea tree oil is converted to olefins using the aforementioned metal triflate catalysts.
Results and discussion
We initiated the study by investigating different catalysts in the catalytic cleavage of the alcohol C–O bond of 2-octanol (Table 1). The catalyst scope covered different lanthanide and transition metal salts, which were mainly their triflates. Triflic acid (HOTf) was used as a reference Brønsted acid. The experiments were conducted under solvent-free reaction conditions to exclude possible solvent-effects, as, for example, coordinating solvents have been shown to reduce the dehydration activity of Hf(OTf)4 under hydrodeoxygenation conditions.13,15 To rationalize the results, the octene yield and conversion were plotted as a function of the oxophilicity and Lewis acidity of the used metal cations, respectively (Fig. 1). The oxophilicity was assessed in terms of the dissociation energy of the M–O bond, and the Lewis acidity of the metal cations was calculated using the formula: Lewis acidity = Z/r3 (Z = charge of the metal and r = ionic radius of the cation in pm).19–21| Entry | Catalyst | Oxophilicity (kJ mol−1)21 | Lewis acidityc (×10−6 pm−3) | Conv. (%) | Octene yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 3 h, 150 °C, 0.5 mol% of catalyst using a closed batch type reaction vessel.b Mixture of 1-octene and cis/trans isomers of 2-, 3- and 4-octene, yields obtained with GC-FID using calibration curves.c Lewis acidity = Z/r3, unit 1/pm3. The effect of anions has not been taken into account in the calculated Lewis acidity. | |||||
| 1 | Fe(OTf)3 | 409 | 11.18 | 48 | 30 |
| 2 | Hf(OTf)4 | 791 | 11.18 | >99 | 93 |
| 3 | Eu(OTf)3 | 557 | 3.53 | 9 | 2 |
| 4 | Sc(OTf)3 | 674 | 7.26 | 20 | 6 |
| 5 | Yb(OTf)3 | 398 | 4.59 | 8 | 1 |
| 6 | Y(OTf)3 | 715 | 4.11 | 5 | 1 |
| 7 | La(OTf)3 | 799 | 2.73 | 11 | 5 |
| 8 | Nd(OTf)3 | 703 | 3.16 | 10 | 2 |
| 9 | Al(OTf)3 | 512 | 19.59 | 75 | 34 |
| 10 | Mg(OTf)2 | 394 | 5.36 | 10 | 4 |
| 11 | Pr(OTf)3 | 753 | 3.09 | 10 | 1 |
| 12 | Ti(OTf)4 | 662 | 18.06 | >99 | 71 |
| 13 | Cr(OTf)3 | 427 | 12.90 | 73 | 35 |
| 14 | FeCl3 | 409 | 11.18 | 11 | 0 |
| 15 | Fe(NO3)3 | 409 | 11.18 | 11 | 0 |
| 16 | Fe(CO2CH3)3 | 409 | 11.18 | 13 | 0 |
| 17 | Fe2(SO4)3 | 409 | 11.18 | 14 | 0 |
| 18 | HOTf | — | — | >99% | 84 |
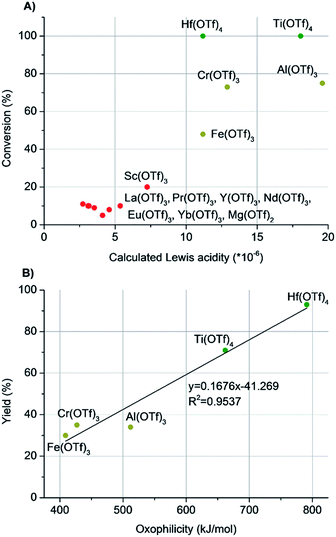 | ||
| Fig. 1 (A) The conversion of 2-octanol as a function of the Lewis acidity of the metal triflate. The Lewis acidity is calculated for the metal cations, and the effect of the triflate anion is not included. (B) The yield of octenes as a function of the oxophilicity of the metal cation in the dehydration of 2-octanol. The oxophilicity is the measured M–O bond dissociation energy.21 | ||
Interestingly, the plots revealed a clear dependence of the reaction outcome on the oxophilicity and Lewis acidity of the metal cations (Table 1 & Fig. 1). In general, the metals with relatively high Lewis acidity (>10 × 10−6) gave significantly better conversions than those catalysts with lower Lewis acidity (<10 × 10−6, Fig. 1A). Further comparison of the octene yields as a function of oxophilicity with the five most Lewis acidic metal cations (Fe(OTf)3, Cr(OTf)3, Al(OTf)3, Ti(OTf)4 and Hf(OTf)4) revealed a linear correlation (Fig. 1B). While Fe(OTf)3, Cr(OTf)3 and Al(OTf)3 gave octenes in moderate yields of 30–40%, the most oxophilic Hf(OTf)4 and Ti(OTf)4 generated quantitative conversion of 2-octanol, and octenes were formed in high 93% and 71% yields, respectively. The reference Brønsted acid catalyst, HOTf, generated quantitative conversion of 2-octanol with 84% yield of octenes (Table 1, entry 18). In addition to olefin products, unidentified by-products were formed in all of the experiments.
Additional experiments were conducted with Fe(OTf)3, Cr(OTf)3 and Al(OTf)3 at an elevated temperature of 165 °C using 2-octanol as a substrate. This resulted in quantitative conversions and enhanced yields of 80–85%, matching those of HOTf, Hf(OTf)4 and Ti(OTf)4 at 150 °C (see Table S1 in ESI†). The replacement of the triflate anion with chloride resulted in a significantly reduced yield and conversion (Table 1, entries 14–17). The better reactivity with triflates can be explained by the weak electron donor and strong electron acceptor nature of this anion, which increases the Lewis acidity of the central metal atom.22 In contrast, chloride, acetate, nitrate and sulphate are π-donating middle and low field ligands, according to the spectrochemical series, and because of this they are unlikely to impart greater Lewis acidity.23 Additionally, some of the metal chlorides form HCl when they come into contact with water (e.g. AlCl3, HfCl4 and TiCl4), thus possessing similar unwanted properties to Brønsted acids, such as HOTf.
Next, we measured the conversion rates of 2-octanol (6.3 mmol) with 0.5 mol% loadings of Fe(OTf)3, Cr(OTf)3, Al(OTf)3, Ti(OTf)4 and Hf(OTf)4 at 150 °C. The reactions were monitored using GC-FID with sampling intervals of 5 min, 15 min, 30 min, 1 h, 1.5 h, 2 h and 3 h. The conversion had a linear correlation with time, indicating that the reaction follows zero-order kinetics (Fig. 2). This is common in catalytic reactions where a catalyst is in large excess of substrates. The order of the reaction arises through saturated catalyst activity, forcing the catalyst to work at the maximum capacity until the substrate is nearly consumed. The observed rates of the reactions with different metal triflates were in a similar order as the initial catalyst screening. Hf(OTf)4 generated the highest conversion rate of 2.76 mmol h−1, which was three times higher than that with Fe(OTf)3. Overall, the order of the conversion rates is Hf(OTf)4 > Ti(OTf)4 > Al(OTf)3 > Cr(OTf)3 > Fe(OTf)3. For further studies we selected Fe(OTf)3, due to its benign and non-toxic nature, and the most efficient Hf(OTF)4 as catalyst. The catalytic activity and efficiency of Ti(OTf)4, Al(OTf)3 and Cr(OTf)3 in the following experiments could be expected to be in between that of Hf(OTf)4 and Fe(OTf)3.
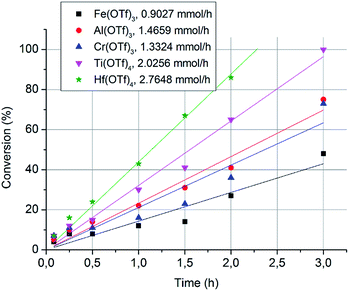 | ||
| Fig. 2 The conversion of 2-octanol with respect to time-on-stream. The conversion rates were calculated based on the slopes of the fitted curves. | ||
The effect of the position of the hydroxyl group on the Hf(OTf)4 and Fe(OTf)3 catalyzed dehydration reactions was studied using primary, secondary and tertiary alcohols (Table 2). The reactions were conducted in a solvent-free manner in a flask connected to micro distillation apparatus, allowing the distillation and isolation of the alkenes upon formation. To ensure efficient conversion, the reactions catalyzed by Fe(OTf)3 were conducted at higher temperatures than those with Hf(OTf)4. The tertiary alcohol, 3-ethylpentan-3-ol as a substrate, was converted to 3-ethylpent-3-ene in isolated yields of 79% and 84% using Fe(OTf)3 and Hf(OTf)4, respectively (Table 2, entry 3). With the secondary, alcohols 2-octanol and cyclohexanol, higher reaction temperatures were required than with the tertiary alcohol. With Fe(OTf)3, octenes from 2-octanol were obtained in a slightly higher 91% yield than the 85% yield with Hf(OTf)4 (Table 2, entry 1). Various isomers of octene were observed, and the product ratio of 2-, 3- and 4-octene to 1-octene was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with both the catalysts (see Fig. S5 in ESI†). Cyclohexanol was converted to cyclohexene in 80% and 78% yield with Hf(OTf)4 and Fe(OTf)3, respectively (Table 2, entry 4). Full conversion of the primary alcohol, 1-octanol, was achieved after 6 h at 180 °C using Fe(OTf)3 as a catalyst (Table 2, entry 2). Unexpectedly, dioctyl ether was the main product with 80% yield, which was accompanied by octene in only 2% yield, and the rest being unidentified side products. Due to this, no further Fe(OTf)3 catalyzed experiments with primary alcohols were conducted. With Hf(OTf)4, 1-octanol was converted to octenes, generating 46% yield in 6 hours and 65% yield in 12 hours at 180 °C (Table 2, entry 2). The ratio of 2-, 3- and 4-octene to 1-octene was 30
1 with both the catalysts (see Fig. S5 in ESI†). Cyclohexanol was converted to cyclohexene in 80% and 78% yield with Hf(OTf)4 and Fe(OTf)3, respectively (Table 2, entry 4). Full conversion of the primary alcohol, 1-octanol, was achieved after 6 h at 180 °C using Fe(OTf)3 as a catalyst (Table 2, entry 2). Unexpectedly, dioctyl ether was the main product with 80% yield, which was accompanied by octene in only 2% yield, and the rest being unidentified side products. Due to this, no further Fe(OTf)3 catalyzed experiments with primary alcohols were conducted. With Hf(OTf)4, 1-octanol was converted to octenes, generating 46% yield in 6 hours and 65% yield in 12 hours at 180 °C (Table 2, entry 2). The ratio of 2-, 3- and 4-octene to 1-octene was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reactivity of the alcohols was found to decrease in the order of tert- > sec- > prim-alcohols, which is analogous to the order of the stability of the carbocations, and is in line with previous publications.10 Furthermore, the dehydration of diethylene glycol produced a good 84% yield of 1,4-dioxane with Hf(OTf)4 (Table 2, entry 7). The dehydration of 1-phenylpropan-1-ol and 3-phenylpropan-1-ol resulted in formation of non-volatile compounds, and little of the desired dehydrated products were obtained (Table 2, entries 5 & 6).
1. The reactivity of the alcohols was found to decrease in the order of tert- > sec- > prim-alcohols, which is analogous to the order of the stability of the carbocations, and is in line with previous publications.10 Furthermore, the dehydration of diethylene glycol produced a good 84% yield of 1,4-dioxane with Hf(OTf)4 (Table 2, entry 7). The dehydration of 1-phenylpropan-1-ol and 3-phenylpropan-1-ol resulted in formation of non-volatile compounds, and little of the desired dehydrated products were obtained (Table 2, entries 5 & 6).
| Entry | Substrate (mmol) | Fe(OTf)3 catalyst | Hf(OTf)4 catalyst | ||||
|---|---|---|---|---|---|---|---|
| t (h) | T (°C) | Yieldb (%) | t (h) | T (°C) | Yieldb (%) | ||
| a N.d. = not detected.b Isolated olefin yields obtained by distillation of the products during the reaction. Analyzed using GC-MS, 1H NMR and 13C NMR (see ESI).c 1,4-Dioxane was the reaction product. | |||||||
| 1 | 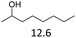 |
3 | 165 | 91 | 1.5 | 150 | 85 |
| 2 | 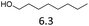 |
6 | 180 | 2 | 12 | 180 | 65 |
| 3 | 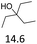 |
0.5 | 130 | 79 | 0.5 | 110 | 84 |
| 4 | 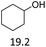 |
2.5 | 165 | 80 | 3.5 | 150 | 78 |
| 5 | 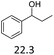 |
0.5 | 130 | 3 | 0.5 | 110 | 3 |
| 6 | 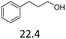 |
— | — | — | 6 | 180 | N.d. |
| 7 | 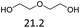 |
— | — | — | 4.5 | 180 | 85c |
The efficiency and robustness of Fe(OTf)3 and Hf(OTf)4 in the dehydration reaction was studied by conducting experiments using 0.06 mmol of the metal triflates and 144.9 mmol of 2-octanol, which is equal to 0.04 mol% catalyst loading. During the reaction, 2-octanol was added dropwise using a dropping funnel to keep the amount of substrate constant, while the octenes formed were distilled. The catalysts converted an over 2400-fold excess of 2-octanol during the reaction, and with the use of Hf(OTf)4 octenes were generated in 84% isolated yield in 10 hours, while Fe(OTf)3 produced octenes in 80% yield in 24 hours at 165 °C. These experiments underscore the robust performance of the metal triflate catalysts in the dehydration.
We moved on to study the formation of the isomers during the dehydration reactions. Based on the literature, two possible pathways were recognized: (i) migration of the charge in the carbocation intermediate and (ii) alkene hydration (Scheme 1).24,25 The isomerization mechanisms were studied by heating 1-octene in the presence of Hf(OTf)4 with and without water at 150 °C. In the absence of water, 1-octene seemingly underwent oligomerization, forming a viscous dark brown oil. The measured 1H NMR spectrum shows that the signals corresponding to the alkene protons were suppressed and shifted, and also that the saturated carbon chain C–H signals were clearly broadened (see Fig. S23 in ESI†). This oligomerization might explain the formation of the non-volatile compounds from the aromatic alcohols.26 Based on the 1H NMR analysis, octene isomers or oligomers do not form in the presence of water, which indicates that the isomers are formed through the migration of the carbocation in the transition state. This proposition is in agreement with the previous report on the isomerization of alkenes.24 The addition of water decreases the oligomerization through the formation of a biphasic system and the solvation of Hf(OTf)4, thus limiting the interaction of the catalyst and the alkenes.
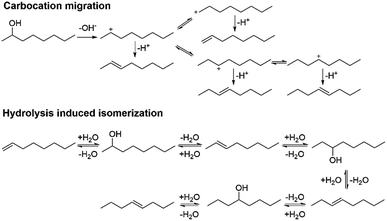 | ||
| Scheme 1 Two putative pathways for octene isomerization: carbocation migration and hydrolysis induced isomerization. | ||
Due to the promising results obtained using the model alcohols, we conducted Fe(OTf)3 and Hf(OTf)4 catalyzed dehydration of the biomass-based monoterpene alcohols. The reactions were conducted in a solvent-free manner using micro distillation apparatus to isolate the products as they formed (Table 3). Some reactions were conducted under reduced pressure to ensure the distillation of the products. The main emphasis was on the secondary and tertiary monoterpene alcohols, due to their more reactive nature. Using menthol and dihydromyrcenol, 71–84% isolated yields of the corresponding terpenes and dihydromyrcenes were obtained with both metal triflates (Table 3, entries 1 & 2). With Fe(OTf)3, α-terpineol was successfully converted to limonene, α-terpinen, γ-terpinen and α-terpinolene in 76% yield. Meanwhile, Hf(OTf)4 gave only 16% yield of the olefins and mainly formed a viscous non-volatile oily substance similar to with the aromatic alcohols (Table 3, entry 3). The formation of non-volatile oils was also observed with linalool and citronellol (Table 3, entries 4 & 5). In fact, all of the secondary and tertiary alcohols forming these non-volatile oils had electron donating groups, such as methyl, connected to the sp2-hybridized carbon. This is known to facilitate cationic polymerization.9 It is also known that metal halides are capable of catalyzing cationic polymerization, and thus it is likely that the non-volatile oils herein are a result of metal triflate catalyzed cationic oligomerization.27,28 It is therefore rational that the more Lewis acidic and oxophilic, and thus stronger dehydration catalyst, Hf(OTf)4 also catalyzes the oligomerization more efficiently than Fe(OTf)3 (Table 3, entry 3). The dehydration of sugar alcohols was studied using sorbitol, which is readily available from cellulose- and hemicellulose-based glucose.29 As a result, isosorbide was generated in yields of 77% and 78% with Fe(OTf)3 and Hf(OTf)4 as the catalysts, respectively (Table 3, entry 6). In comparison to the previously published metal triflate-catalyzed conversion of sorbitol to isosorbide, the use of Fe(OTf)3 and Hf(OTf)4 afforded isosorbide in similar yields but in shorter reaction times and with lower catalyst loadings.30,31
| Entry | Substrate (mmol) | Fe(OTf)3 catalyst | Hf(OTf)4 catalyst | Formed productsd | ||||
|---|---|---|---|---|---|---|---|---|
| t (h) | T (°C) | Yielda (%) | t (h) | T (°C) | Yielda (%) | |||
| a Isolated yields obtained by distillation of the olefins during the reaction. Analyzed using GC-MS, 1H NMR and 13C NMR (see ESI).b Batch type reaction was conducted in vacuo to increase the efficiency of the dehydration, and product yields were measured using HPLC-FID and calibration curves, with isosorbide as the reaction product.c Product yields were determined using HPLC-FID and calibration curves, with isosorbide as the reaction product.d Product distributions are presented in the ESI. | ||||||||
| 1 | 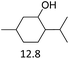 |
4 | 165 | 71 | 3 | 150 | 82 |  |
| 2 |  |
0.5 | 130 | 82 | 0.5 | 110 | 84 |  |
| 3 |  |
0.5 | 130 | 76 | 0.5 | 110 | 16 | 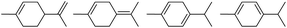 |
| 4 | 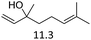 |
0.5 | 110 | 3 | 0.5 | 110 | 3 | 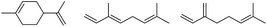 |
| 5 | 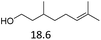 |
— | — | — | 24 | 180 | 7 | 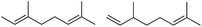 |
| 6b | 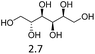 |
3 | 150 | 77c | 3 | 130 | 78c | 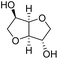 |
As metal triflates could potentially catalyze the conversion of essential oils to olefins, we composed an experiment as a proof of concept using tea tree oil as a substrate in Fe(OTf)3 and Hf(OTf)4 catalyzed dehydration reactions. Tea tree oil consists mainly of terpinen-4-ol, thus providing an ample source of biomass-based alcohol for the synthesis of olefins.32,33 With the use of Fe(OTf)3, a mixture of products, mainly α- and γ-terpinenes, was generated in 53 wt% yield from tea tree oil in 1 hour under a reduced pressure of 57 mmHg at 130 °C. However, the Hf(OTf)4-catalyzed reaction produced non-volatile products at 110 °C under reduced pressure, and as a result no olefins were obtained. The reaction results resemble those of the α-terpineol dehydration, where Fe(OTf)3 gave a good olefin yield while Hf(OTf)4 generated mostly non-volatile products (Table 3, entry 3).
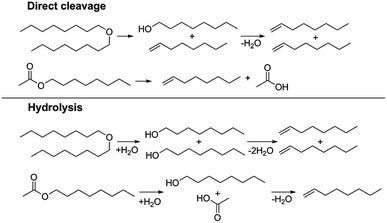
We also investigated two metal triflate-catalyzed non-hydrogenolytic approaches to the scission of ester and ether C–O bonds: (i) direct cleavage and (ii) hydrolysis, followed by the dehydration of the hydroxyl groups to alkenes (Scheme 2). Previously, ester and ether C–O bond cleavage has been achieved through hydrogenolysis catalyzed by Hf(OTf)4 and Pd/C.18,34 Accordingly, on dioctyl ether and octyl acetate we carried out several reactions with and without water to see the effect of hydrolysis (Table 4). It turned out that the use of Hf(OTf)4 and water (1–2 mol eq.) resulted in the hydrolysis of dioctylether, forming 1-octanol. Subsequently, the as-formed 1-octanol was dehydrated to octenes, although in relatively low yields. The highest yield of 29% was obtained after 24 hours at 180 °C (Table 4, entries 8 & 9). No alkenes were detected using Hf(OTf)4 without additional water (Table 4, entry 10). Evidently, water is an essential additive to convert the ethers to alcohols through hydrolysis, followed by the yield-reducing cumbersome dehydration of the primary alcohols to alkenes. However, Fe(OTf)3 could not convert dioctyl ether to alkenes or alcohols in significant amounts with either approach (see Table S2 in ESI†). The attempted conversion of dimethyl tetrahydrofuran to olefins through hydrolysis and subsequent dehydration failed to produce any olefin products with either of the used catalysts, Fe(OTf)3 or Hf(OTf)4.
| Entry | Catalyst | Substrate (mmol) | Time (h) | Temp. (°C) | Octene yieldb (%) |
|---|---|---|---|---|---|
| a N.d. = not detected.b Yield determined using GC-FID and calibration curves.c Isolated yields obtained by distillation of the olefins during the reaction. Analyzed using GC-MS, 1H NMR and 13C NMR.d 1 equivalent of H2O added to induce hydrolysis of the ether/ester bond. | |||||
| 1 | Fe(OTf)3 | 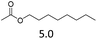 |
3 | 170 | 4 |
| 2 | Fe(OTf)3d | 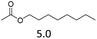 |
3 | 170 | 4 |
| 3 | Fe(OTf)3 | 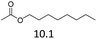 |
36 | 180 | 68c |
| 4 | Hf(OTf)4 | 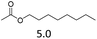 |
3 | 150 | 13 |
| 5 | Hf(OTf)4d | 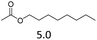 |
3 | 170 | 5 |
| 6 | Hf(OTf)4 | 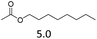 |
3 | 170 | 20 |
| 7 | Hf(OTf)4 | 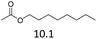 |
22 | 180 | 80c |
| 8 | Hf(OTf)4d | 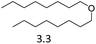 |
8 | 180 | 7 |
| 9 | Hf(OTf)4d | 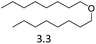 |
24 | 180 | 29 |
| 10 | Hf(OTf)4 |  |
8 | 180 | N.d. |
The hydrolysis experiments with octyl acetate generated octenes with both approaches and catalysts (Table 4, entries 2 & 5). In general, the addition of water resulted in the formation of 1-octanol and acetic acid as the hydrolysis products. However, this approach leads to low alkene yields, as dehydration of the primary hydroxyl group is difficult even with Hf(OTf)4 (see above). In this respect, the addition of water should be avoided. Without water, the direct ester C–O cleavage approach resulted in the formation of acetic acid and octene from octyl acetate through the scission of the alkoxy C–O bond (Table 4, entries 1, 4 & 6). This direct approach generated high isolated yields of 68% and 80% of octenes with Fe(OTf)3 and Hf(OTf)4, respectively (Table 4, entries 3 & 7). Compared to the previous Lewis acid catalyzed synthesis of olefins, our setup allows the use of benign and abundant transition metal triflate catalysts as well as various alcohols, esters and ethers as substrates.10,35 Furthermore, the solvent-free approach enables the isolation of the products in good yields as they form.
The compatibility of aldehydes and carboxylic acids as substrates was also investigated. Under the applied conditions, aldehydes underwent an immediate oligomerization to non-volatile oils, while carboxylic acids did not undergo any conversion using Fe(OTf)3 and Hf(OTf)4, although the deoxygenation of carboxylic acids has been reported to occur under similar conditions with Pd or Pt as catalysts.36
Conclusions
In summary, highly oxophilic and Lewis acidic metal triflates can be used to catalyze the cleavage of C–O bonds of biomass-based alcohols, esters and ethers to give the corresponding olefins. The catalytic activity of the transition metal triflates improved with increasing oxophilicity and Lewis-acidity. Understanding of this correlation aids in the development of new transition metal based defunctionalization catalysts. The reactivity order of the different oxygen containing species to alkenes is tert-alcohols > sec-alcohols > esters > prim-alcohols > ethers. Alcohols and esters can be converted efficiently to olefins under solvent-free conditions using metal triflate catalysts. However, the conversion of ethers to alkenes requires initial hydrolysis of the ether bond to an alcohol, which is then followed by the dehydration of the formed alcohol. The proof of concept experiments demonstrated that the studied Fe(OTf)3 and Hf(OTf)4 are very robust catalysts, as in the solvent-free continuous distillation process they converted an over 2400-fold excess of 2-octanol into octenes in high yields of 84% and 80%, respectively. Also, the usability of essential oils as substrates was established by the dehydration of tea tree oil, producing alkenes in up to 53 wt% yield.Experimental
General procedure for the synthesis of olefins
The desired amount of catalyst (0.5 mol%, unless otherwise stated) was placed into a reaction vessel, to which an appropriate amount of substrate and any additives, such as water, were added. The vessel was heated with an oil bath at a suitable temperature. Batch type reactions were conducted in a closed vial, and the distillation reactions were conducted using micro distillation apparatus at atmospheric or reduced pressure, depending on the substrate. The forming products and side products, water or acetic acid, were distilled and collected in a product flask, which was cooled with an ice bath to minimize evaporation of the products. The distillation reactions were continued until completion, and no more products could be recovered. After the reaction, when water was the side product the product flask was placed in a freezer for ease of separation of the olefin products from the frozen water. In the case of acetic acid as the side product, the acetic acid–olefin mixture was washed with 1 M NaOH(aq) to neutralize the acetic acid. Pure olefin products were obtained after drying with Na2SO4 and filtration. The reaction products were then analysed using GC-FID, GC-MS, 1H NMR and 13C NMR. For a detailed description of the experimental setups see the ESI.†GC-analysis
The reaction products were analysed using an Agilent Technologies 7890B GC System equipped with a flame ionization detector (FID) and an Agilent Technologies 5977C MSD mass detector (MS). HP-5MS UI (30 m, 0.25 mm, 0.25 μm) was used as the column in the GC-analysis.NMR-analysis
Nuclear magnetic resonance (NMR) experiments (1H and 13C) were conducted using a Varian Mercury Plus 300 MHz (1H frequency 300 MHz and 13C frequency 75 MHz) and deuterated solvents.HPLC-analysis
High-performance liquid chromatography (HPLC) analysis was conducted using an Agilent Technologies 1200 Series HPLC equipped with Phenomenex Rezex ROA – organic acid H+ (8%) (300 × 7.80 mm) column and refractive index detector (RID). A 5 mM H2SO4 aqueous solution was used as the eluent with the flow rate of 0.35 ml min−1.Conflicts of interest
There are no conflicts to declare.Notes and references
- S. V. Vassilev, D. Baxter, L. K. Andersen, C. G. Vassileva and T. J. Morgan, Fuel, 2012, 94, 1–33 CrossRef CAS.
- G. W. Huber, J. Chheda, C. B. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS PubMed.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- R. Karinen, K. Vilonen and M. Niemelä, ChemSusChem, 2011, 4, 1002–1016 CrossRef CAS PubMed.
- M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS PubMed.
- A. Corma Canos, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef PubMed.
- O. Lasekan and K. A. Abbas, Crit. Rev. Food Sci. Nutr., 2012, 52, 726–735 CrossRef CAS PubMed.
- P. A. Wilbon, F. Chu and C. Tang, Macromol. Rapid Commun., 2013, 34, 8–37 CrossRef CAS PubMed.
- T. J. Korstanje, E. F. de Waard, J. T. B. H. Jastrzebski and R. J. M. Klein Gebbink, ACS Catal., 2012, 2, 2173–2181 CrossRef CAS.
- M. Eisenacher, S. Beschnitt and W. Hölderich, Catal. Commun., 2012, 26, 214–217 CrossRef CAS.
- M.-H. Lee, S.-W. Lee, Y.-M. Jeong, D.-Y. Park and J.-Y. Ryu, WO 2005035468, 2005.
- Z. Li, R. S. Assary, A. C. Atesin, L. A. Curtiss and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 104–107 CrossRef CAS PubMed.
- J. Keskiväli, P. Wrigstedt, K. Lagerblom and T. Repo, Appl. Catal., A, 2017, 534, 40–45 CrossRef.
- H. J. Song, J. Deng, M. S. Cui, X. L. Li, X. X. Liu, R. Zhu, W. P. Wu and Y. Fu, ChemSusChem, 2015, 8, 4250–4255 CrossRef CAS PubMed.
- A. D. Sutton, F. D. Waldie, R. Wu, M. Schlaf, A. Louis III and J. C. Gordon, Nat. Chem., 2013, 5, 428–432 CrossRef CAS PubMed.
- A. E. King, T. J. Brooks, Y.-H. Tian, E. R. Batista and A. D. Sutton, ACS Catal., 2015, 5, 1223–1226 CrossRef CAS.
- T. L. Lohr, Z. Li and T. J. Marks, ACS Catal., 2015, 5, 7004–7007 CrossRef CAS.
- K. Mikami, M. Terada and H. Matsuzawa, Angew. Chem., Int. Ed., 2002, 41, 3554–3572 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- J. A. Dean, Lange’s Handbook of chemistry, McGraw-Hill, Inc, New York, 1985 Search PubMed.
- S. Voigt, H. Glaum, S. Schlecht, J. Magull, D. Fenske and K. Dehnicke, Communications, 1997, 3, 1994–1995 Search PubMed.
- Y. Shimura, Bull. Chem. Soc. Jpn., 1988, 61, 693–698 CrossRef CAS.
- V. Macho, M. Králik, E. Jurecekova, J. Hudec and L. Jurecek, Appl. Catal., A, 2001, 214, 251–257 CrossRef CAS.
- M. B. Smith and J. March, March’s advanced organic chemistry, John Wiley & Sons, Inc., New Jersey, 6th edn, 2007 Search PubMed.
- C. H. Bartholomew, Appl. Catal., A, 2001, 212, 17–60 CrossRef CAS.
- W. J. Roberts and A. R. Day, J. Am. Chem. Soc., 1950, 72, 1226–1230 CrossRef CAS.
- J. Lu, M. Kamigaito, M. Sawamoto, T. Higashimura and Y.-X. Deng, J. Appl. Polym. Sci., 1996, 61, 1011–1016 CrossRef CAS.
- W. Deng, X. Tan, W. Fang, Q. Zhang and Y. Wang, Catal. Lett., 2009, 133, 167–174 CrossRef CAS.
- F. Liu, K. De Oliveira Vigier, M. Pera-Titus, Y. Pouilloux, J.-M. Clacens, F. Decampo and F. Jérôme, Green Chem., 2013, 15, 901–909 RSC.
- K. Stensrud, E. Hagberg, S. Howard and E. M. Rockafellow, WO 2014137619 A1, 2014.
- J. J. Brophy, N. W. Davies, I. A. Southwell, I. A. Stiff and L. R. Williams, J. Agric. Food Chem., 1989, 37, 1330–1335 CrossRef CAS.
- G. Swords and G. L. K. Hunter, J. Agric. Food Chem., 1978, 26(3), 734–737 CrossRef CAS.
- T. L. Lohr, Z. Li, R. S. Assary, L. A. Curtiss and T. J. Marks, Energy Environ. Sci., 2016, 9, 550–564 CAS.
- Z. Zhu and J. H. Espenson, J. Org. Chem., 1996, 61, 324–328 CrossRef CAS.
- M. Snåre, I. Kubičková, P. Mäki-Arvela, K. Eränen and D. Y. Murzin, Ind. Eng. Chem. Res., 2006, 45, 5708–5715 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, experimental and analysis details. See DOI: 10.1039/c8ra02437e |
| This journal is © The Royal Society of Chemistry 2018 |