Open Access Article
Open Access ArticleRetracted Article: Salvianolic acid B inhibits inflammatory response and cell apoptosis via the PI3K/Akt signaling pathway in IL-1β-induced osteoarthritis chondrocytes
Bin Zhua,
Xuejian Wanga and
Jiawen Teng *b
*b
aDepartment of Orthopedics, Baodi Clinical College of Tianjin Medical University, Tianjin, 301800, China
bDepartment of Orthopedics, Affiliated Hospital of Shandong Traditional Chinese Medicine University, No. 16369 Jingshi Road, Shizhong District, Jinan, Shandong 250014, PR China. E-mail: tengjiawen2018@sina.com; Tel: +86-531-68616666
First published on 29th October 2018
Abstract
Osteoarthritis (OA) is the most common joint disease among late middle-aged or elderly people. The pathological process of OA mainly involves the degeneration of cartilage tissue and deficiency of joint function. Salvianolic acid B (Sal B) is the main active ingredient of Salvia miltiorrhiza Bge, which possesses anti-inflammatory, anti apoptotic and other pharmacological activities. In this study, primary chondrocytes were cultured to investigate the effects of Sal B on the inflammatory response and apoptosis of OA induced by IL-1β, and to explore the possible mechanism. First, we determined the cytotoxicity of Sal B; the results showed that the cell activity of chondrocytes was not influenced by Sal B when the concentration was below 150 μM. Moreover, Sal B (40 and 80 μM) suppressed the expression of iNOS in OA chondrocytes induced by IL-1β, and restrained the secretion of NO, IL-6, IL-17 and TNF-α in chondrocytes obviously. Sal B (40, 80 μM) significantly alleviated the inhibitory effect of cell activity stimulated by IL-1β and up-regulated the expression of Col II and reduced the expression of Col X. Besides, Sal B down-regulated the expression level of Bax and promoted the expression of Bcl-2, showed a significant effect on promoting proliferation and inhibiting cell apoptosis. In addition, we found that IL-1β significantly reduced the ratio of p-PI3K/PI3K, p-Akt/Akt induced the nuclear translocation of AKT and inhibited the activation of the PI3K/Akt signaling pathway. Finally, the PI3K inhibitor, LY-294002, was added in IL-1β-induced chondrocytes. The results suggest that Sal B ameliorates IL-1β induced inflammation and suppresses apoptosis in OA by activating the PI3K/Akt signaling pathway. Our study reveals the mechanism of Sal B acts on OA and may provide a basis for the treatment of OA with Sal B.
Introduction
Osteoarthritis (OA) is the most common joint disease, and mostly occurs in the late middle-aged or elderly population.1 The pathological process of OA mainly involves the degeneration of cartilage tissue and the loss of joint function; joint pain and swelling are the main clinical symptoms, and it will develop into movement difficulties caused by limb stiffness, which seriously affects the life quality of patients.2,3 At present, the pathogenesis underlying OA is still unclear, the clinical treatment of OA is limited to the remission of OA symptoms, and no independent formulations can affect the pathogenesis of OA.4 Therefore, it is critical to explore the possible pathogenesis of OA and find effective therapeutic agents for the prevention and treatment of OA.In the pathological process of OA, pro-inflammatory cytokine IL-1β is the direct factor leading to cartilage destruction, which can directly inhibit the synthesis of cartilage matrix, induce chondrocyte apoptosis, simultaneously induce the production of inflammatory mediator NO through inducible nitric oxide synthase (iNOS).5–7 Recent studies have found that iNOS-mediated inflammatory pathways is of great significant importance to the development of OA.8 Overexpression of NO can inhibit chondrocyte proliferation, inhibit the synthesis of collagen, activate matrix metalloproteinase (MMPs), and promote synovial cells release TNF-α, thereby further aggravating inflammation, promoting cartilage matrix degradation and inducing cartilage apoptosis.9–11
Salvianolic acid B (Sal B) is the main active ingredient of Salvia miltiorrhiza Bge, and one of the most studied salvianolic acids at present. In traditional applications, Salvia is often used to treat cardiovascular and cerebrovascular diseases, chronic renal failure and skin diseases.12,13 Recent studies have shown that Sal B has multiple pharmacological activities, such as antioxidant, anti-inflammatory, anti apoptotic and anticancer activities.14,15 In ischemia-reperfusion injury, Sal B can protect the heart and liver through anti-oxidation, anti-inflammatory and anti apoptotic effects.12,16,17 At the same time, Sal B can inhibit the inflammatory response of OA induced by IL-1β, and exhibit protective effects on OA mice.18 Studies have also reported that PI3K/Akt signaling pathway is one of the pathways affected the degradation of cartilage matrix OA and apoptosis of cartilage cells by inhibiting the activation of its downstream protein NF-KB and thereby inhibiting the expression of MMPs.3,19,20 However, whether Sal B inhibits the inflammatory response and apoptosis of OA induced by IL-1β via PI3K/Akt pathway has not been reported.
Therefore, the purpose of this study is to investigate the effects of Sal B on inflammatory response and apoptosis of OA and its potential mechanism. We found that Sal B could inhibit the increase of iNOS expression induced by IL-1β, thereby inhibiting the secretion of NO in chondrocytes cells and reducing the mRNA level of inflammatory factors. Besides, Sal B reduced the inhibitory effect of IL-1β on chondrocyte proliferation, inhibiting the apoptosis of chondrocytes, through activation of the PI3K/Akt signaling pathway, suggesting that Sal B can inhibit inflammatory response and apoptosis induced by IL-1β by activating the PI3K/Akt signaling pathway.
Materials and methods
Primary articular chondrocyte culture
Primary articular chondrocyte was isolated from patients (male, 67 years, OA grade I) undergoing joint replacement surgery in the hospital. All experiments were performed in accordance with the Guidelines of Baodi Clinical College of Tianjin Medical University, and Experiments were approved by the ethics committee at Baodi Clinical College of Tianjin Medical University. Informed consents were obtained from human participants of this study. The soft tissue around joints in the sterile environment was peeled, and the isolated articular cartilage tissue was cleaned by D-Hank's buffer which contains penicillin and streptomycin. The cartilage tissue was digested with DMEM solution with 0.2% collagenase and 5% fetal bovine serum overnight after cut into chunks and digested with trypsin at the temperature of 37 °C for 10 min. The cells were centrifuged at 800 r/min twice for 20 min and cultured with DMEM (containing 20% FBS) in 5% CO2 incubator. When the cell fusion at 80%, IL-1β (5 ng mL−1) was used to stimulate chondrocyte cells for 6 h in vitro, and the cells were divided into four groups: the control group, control cells + IL-1β induced group, IL-1β + Sal B 20 μM group and IL-1β + Sal B 40 μM group and IL-1β + Sal B 80 μM group.Cell viability by the MTT assay
Cell viability was assessed via MTT assay. Cells were seeded in a 96-well plate with seven different concentrations (0, 20, 40, 60, 80, 100, 200 μM) of Sal B for 24 h, after incubation for 4 h with MTT, the media was removed and DMSO was added to each well. The relative number of surviving cells was assessed by measuring the optical density (OD) of cell lysates at 570 nm. All assays were performed in triplicate.Western blot analysis
Western blotting was performed to measure the protein expression. Simply put, the experimental extracted proteins from cells by protein lysis solution, measured the protein concentration by BCA method. Cell lysis samples were separated by SDS-PAGE. After transfer onto polyvinylidene fluoride membranes, the membranes were blocked in 3% bovine serum albumin (BSA) at room temperature for 1 h, then the membrane was incubated for 24 h with antibodies (inducible nitric oxide synthase (iNOS), 1:500; type II collagen (Col II), 1:1000; type X collagen (Col X), 1:1000; Bax, 1:500; Bcl-2, 1:1000; PI3K, 1:600; Akt, 1:500; p-PI3K, 1:500; p-Akt (phospho T308), 1:500). The secondary antibody was added and incubated at the temperature of 37 °C for 2 h after washing the membrane by TBST. The β-actin was used as the internal reference to normalize the data in the study and the results were analyzed by using Image plus 5.0 software.Cytokine ELISA assay
Chondrocytes were homogenized in nine volumes of ice-cold 0.9% NaCl solution and centrifuged at 3000 × g for 15 min at 4 °C. Supernatant was collected for measurement of cytokine concentrations of nitric oxide (NO, Invitrogen Inc, USA), TNF-α (Pepro tech Inc, USA), IL-6 and IL-17 (eBioscience Inc) by ELISA kits following the manufacturer's instructions at 450 nm. Determinations were performed in duplicate in 3 independent experiments. The results were expressed as pg mL−1.The CCK-8 assay
The cells were treated with Sal B (20, 40, 80 μM) and IL-1β (5 ng mL−1), and were collected and inoculated 96-well plates. Adding 100 μL fresh medium and 10 μL CCK-8 solution to each well after cultivating 24 h. And incubated in the incubator at 37 °C, 5% CO2 for 4 h. The absorbance value of each well were detected by microplate reader at the wavelength of 450 nm. Calculation the proliferation rate with the formula that proliferation rate = (D450 experimental group − background)/(D450 control group − background) × 100%.Immunofluorescence assay
The culture medium was discarded after the slides of cartilage cells were collected. Paraformaldehyde was used for fixing the cartilage cells for 30 min, then washed using 1× PBS solution and incubated for 1 h with 5% goat serum. After permeated by 0.5% TritonX-100, cells were sealed with 5% goat serum for 1 h and sealed with first antibody (Akt, 1:200) at 4 °C overnight. Cells were washed by 1× PBST and performed following incubation with second antibody for 1 h at room temperature, and 10 mg mL−1 4′,6-diamidino-2-phenylindole (DAPI) was used for staining nuclei. An confocal scanning microscope was used for observing and photographing the immunofluorescence signal.Statistical analysis
All statistical analysis was carried out using SPSS 17.0. Data were presented as the means ± SD. Analysis was performed using one-way ANOVA followed by Bonferroni post hoc testing. Statistical significance was accepted at P < 0.05.The results
Toxicity test of Sal B
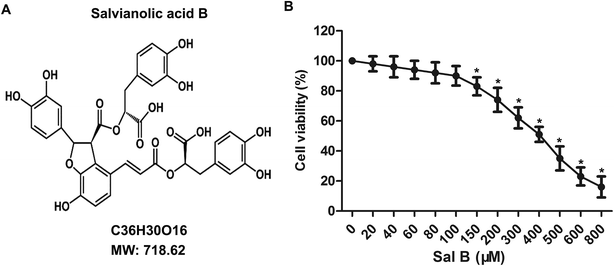
To determine the toxicity of Sal B to chondrocytes, we examined the cellular viability of chondrocytes treated with Sal B for 24 h by using MTT assay. The results showed that Sal B significantly inhibited the activity of chondrocytes over the concentration of 150 μM, (Fig. 1B, *P < 0.05), The results suggest the IC50 of Sal B was 400 μM and Sal B exhibited a certain cytotoxicity when reaching 150 μM concentration. Therefore, we selected the 20, 40 and 80 μM concentrations to continue the next study.Sal B inhibits the inflammatory response in OA by inhibiting the production of iNOS
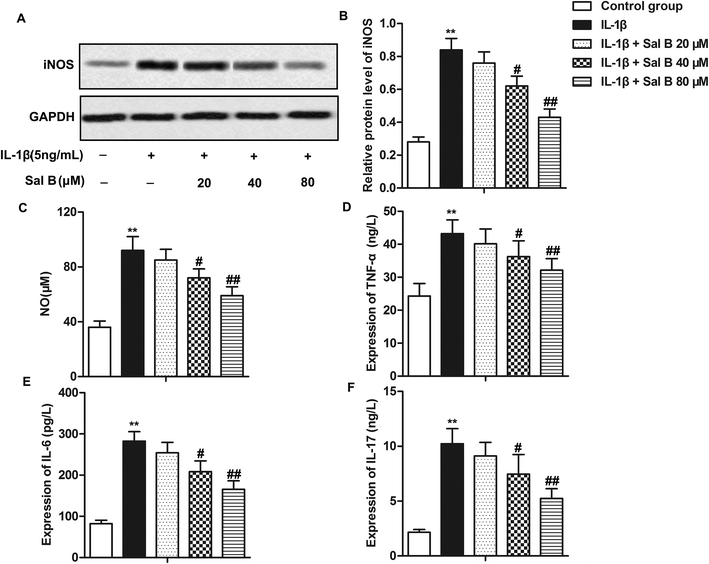
Numerous studies have reported that Sal B has a strong anti-inflammatory activity, and can inhibit the release of IL-1, IL-6, NO and TNF-β and other inflammatory factors.16,21,22 In order to investigate the effect of Sal B on the inflammatory response of OA, we detected the expression of iNOS in cells and the levels of NO, IL-6, IL-17 and TNF-α in IL-1β in supernatant. The results showed that compared with the control group, the expression level of iNOS in the IL-1β group was significantly higher (Fig. 2A and B, **P < 0.01); compared with the IL-1β group, Sal B (40 and 80 μM) significantly inhibited the expression of iNOS induced by IL-1β (Fig. 2A and B, #P < 0.05, ##P < 0.01). At the same time, Sal B (40 and 80 μM) also inhibited the induction of NO by IL-1β in the supernatant of cells, and the inhibitory effect of Sal B was dose-dependent (Fig. 2C, **P < 0.01, #P < 0.05, ##P < 0.01). In addition, compared with the normal group, the expression of IL-6, IL-17 and TNF-α was increased significantly in IL-1β group (Fig. 2D–F, **P < 0.01). Besides, IL-1β could cause the inflammatory response in chondrocytes. Sal B (40 and 80 μM) inhibited the secretion of IL-6, IL-17 and TNF-α. The inhibitory effect was gradually increased with the increasing concentration (Fig. 2D–F, #P < 0.05, ##P < 0.01). The results above showed that Sal B inhibited the inflammatory response of OA induced by IL-1β dose-dependently.Sal B promotes chondrocyte proliferation and inhibits apoptosis
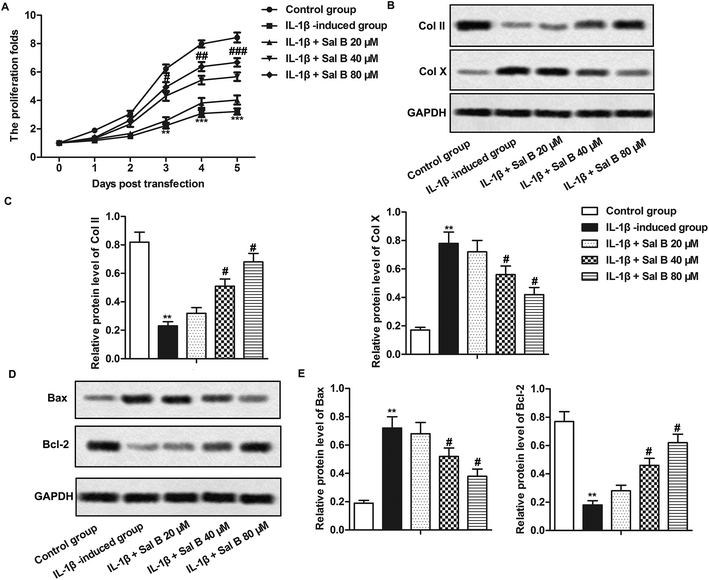
Studies have shown that cell inflammation is closely related to apoptosis,23 we have demonstrated that Sal B could inhibit the inflammatory response of OA in our experiments, so we carried out further studies to clarify the effects of Sal B on proliferation and apoptosis of OA chondrocytes, as shown by Fig. 3A, IL-1β significantly inhibited the proliferation of chondrocytes (**P < 0.01, ***P < 0.001), Sal B (80 μM) reversed the inhibitory effect of IL-1β on the proliferation of chondrocytes, and the effect was enhanced gradually with the prolongation of time (#P < 0.05, ##P < 0.01, ###P < 0.001); in addition, IL-1β significantly inhibited the expression of type II collagen (Col II) and promoted the expression of type X collagen (Col X) (Fig. 3B and C, **P < 0.01), Sal B (40 and 80 μM) reversed the inhibitory effect of IL-1β on the expression of Col II and inhibited the expression of Col X (Fig. 3B and C, #P < 0.05), these results suggest that Sal B can promote the proliferation of chondrocytes in OA. At the same time, we detected the expression of proteins related to apoptosis by western blot. The results showed that compared with the control group, the expression of Bax in the IL-1β group increased markedly, and the expression of Bcl-2 decreased significantly (Fig. 3D and E, **P < 0.01); The Sal B significantly decreased Bax and increased Bcl-2 gene expression when compared to the control. (Fig. 3D and E, #P < 0.05), it suggests that Sal B could inhibit the apoptosis of chondrocytes induced by IL-1β, and the effect was increased with the increase concentration.Sal B promotes activation of the PI3K/Akt pathway
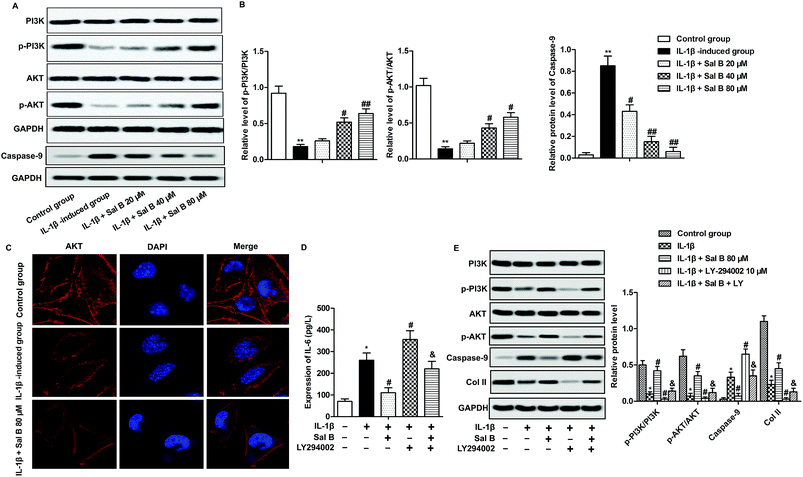
PI3K/Akt signaling pathway is one of the important signaling pathways to regulate cell activity and apoptosis.24,25 To investigate whether Sal B affect chondrocyte proliferation and apoptosis through PI3K/Akt signaling pathway, we detected the expression of proteins p-PI3K, PI3K, p-Akt and Akt on PI3K/Akt signaling pathway. The experimental results show that IL-1β greatly reduced chondrocyte p-PI3K and phosphorylation of p-Akt, reduced the ratio of p-PI3K/PI3K, p-Akt/Akt, suggesting that IL-1β could inhibit the activation of PI3K/Akt signaling pathway (Fig. 4A and B, **P < 0.01); Sal B significantly reversed the inhibitory effect of IL-1β on PI3K/Akt signaling pathway, increased the ratio of p-PI3K/PI3K, p-Akt/Akt (Fig. 4A and B, #P < 0.05, ##P < 0.01). At the same time, we selected the strongest concentration (80 μM) to activate the PI3K/Akt signaling pathway to investigate the effect of Sal B on the activation of Akt. The results showed that compared with the normal group, Akt was localized in the cell membrane, and the expression of Akt in IL-1β group mainly appeared in the cytoplasm; Sal B (80 μM) significantly promoted the expression of Akt on the cell membrane (Fig. 4C). To further convince that Sal B ameliorate inflammation and apoptosis by activating the PI3K/Akt signal signaling pathway, LY-294002, the inhibitor of PI3K was added in IL-1β-induced chondrocytes. The results displayed that Sal B (80 μM) restrained the LY-294002-induced elevating effect on the level of IL-6 and the expression level of caspase-9. Besides, Sal B (80 μM) activated LY-294002-induced inactivation of PI3K/AKT and the expression level of Col II (Fig. 4D and E). These results suggest that Sal B ameliorates inflammation and suppresses apoptosis by activating the PI3K/Akt signal signaling pathway in OA.Discussion
Chondrocytes are the only cells present in cartilage tissue and are mainly involved in the release of proinflammatory cytokines during the development of OA. Chondrocytes play a vital role in the synthesis and degradation of cartilage matrix.26 Therefore, this study has developed primary chondrocytes for the subsequent study of osteoarthritis. Studies have shown that the inflammatory factor IL-1β secreted by chondrocytes is the most important alienation factor in the development of OA.27 IL-1β can degradate chondrocyte extracellular matrix by inducing the expression of NO and MMPs, and inhibit the formation of extracellular matrix by inhibiting the synthesis of Col II, which is often used to construct OA models.28–30 IL-1β mediated overexpression of iNOS is an important factor leading to inflammatory reaction, cartilage degeneration and pain.7,31 NO is regulated by iNOS, NO is one of the important inflammatory mediators that can induce the release of inflammatory factors such as IL-6 and TNF-α.32 NO can also promote cartilage matrix degradation by activating MMPs, cyclooxygenases, inhibiting Col II synthesis, and promote the apoptosis of chondrocytes by inhibiting the regulation of cell respiration by cytochrome c.8,28,33 In addition, studies have reported that inhibition of iNOS expression can inhibit chondrocyte apoptosis, thereby slowing the process of cartilage injury.34 The iNOS knockout mice with OA recovered the ability to synthesize Col II.35 All of the above results demonstrate that overexpression of iNOS is an important factor leading to OA cartilage degeneration and chondrocyte apoptosis.Sal B has many pharmacological activities and is one of the effective constituents of Salvia miltiorrhiza, a traditional Chinese medicine. Recent studies have shown that Sal B could alleviate the inflammatory reaction of many diseases and has a strong anti-inflammatory activity.12,21 Sal B inhibits the release of inflammatory factors by inhibiting the activation of NF- kappa B and plays a protective role in the lung. Sal B also inhibits the inflammatory response caused by a high-fat diet through modulating the Nrf2 pathway.21,36 Ma et al. have proved that Sal B inhibited the inflammatory response and affected the prognosis of renal ischemia-reperfusion through PI3K/Akt signaling pathway.37 In this study, we first examined the toxicity of Sal B on human primary chondrocytes and showed that the cytotoxicity of chondrocytes when Sal B reached dose of 150 M. After the study, we found that Sal B reduced the up-regulation of iNOS induced by IL-1β at safe range, reducing the secretion of NO in cartilage cells, reducing the mRNA levels of IL-6, IL-17 and TNF-α, thus inhibiting the inflammation caused by IL-1β. This results are consistent with the Li et al. and others published researches.38 These results indicate that Sal B has anti-inflammatory activity of OA.
Chondrocytes apoptosis is one of the major pathological processes of OA, and the release of various inflammatory factors contributes to apoptosis of chondrocytes.39 Chondrocytes are the only cells of articular cartilage, and the repairing ability of chondrocytes is very poor in adults, it is difficult to achieve the requirement of repairing cartilage cells after OA. Therefore, inhibition of cell apoptosis after OA is the key to promote chondrocyte repair.40 It has been reported that Chinese herbal medicine and its extract can suppress this apoptosis after OA, but the effect of Sal B on chondrocyte apoptosis has not been reported.41–43 In this study, we examined chondrocyte activity, chondrocyte proliferation marker molecules Col II, Col X and the expression of apoptosis-related proteins Bax and Bcl-2. We also explored the effect of Sal B on chondrocyte proliferation and apoptosis. The results showed that IL-1β reduced the activity of chondrocytes, inhibited the expression of COL1A1 and Bcl-2, promoted the expression of Bax and Col X, and Sal B could reverse the effect of IL-1β on cell viability and Bcl-2 inhibition, inhibited the expression of Bax. Bcl-2 has inhibitory activity and Bax has pro-apoptotic activity.44 The results showed that Salvia Sal B had the effect of promoting chondrocytes proliferation and inhibiting apoptosis after OA.
PI3K/Akt signaling pathway is a vital pathway to regulate cell cycle, proliferation and apoptosis.45 After the activation of PI3K, inositol can be phosphorylated, and the inositol promotes the activation of Akt from the cytoplasm to the inner membrane after phosphorylation.46,47 Phosphorylated-Akt is the main form of the activation of Akt. It can inhibition of apoptosis by suppressing the activity of FKHR, NF-kappa B and GSK-3 and inhibiting phosphorylation of Bad, caspase-9 and inhibiting the release of apoptosis factors by mitochondrial to maintain cell survival.48 Studies have also reported that PI3K/Akt regulates the transcription of a variety of inflammatory response proteins and affects migration of inflammatory cells by activating the downstream factor NF-kappa B.49 Therefore, it will be beneficial to the prognosis of OA if the PI3K/Akt pathway can be activated by drugs to inhibit chondrocyte apoptosis and inflammatory response. The results showed that Sal B increased the ratio of p-PI3K/PI3K and p-Akt/Akt, which promoted the activation of PI3K and Akt, thus activating the PI3K/Akt pathway. At the same time, we found that IL-1β inhibited the activation of Akt and promoted the apoptosis of chondrocytes. Sal B reversed the inhibitory effect of IL-1β on Akt activation and transferred Akt from the cytoplasm to the cell membrane, contributing to the survival of chondrocytes after stimulation by IL-1β.
In conclusion, Sal B inhibited inflammatory response and cell apoptosis in cartilage cells induced by IL-1β and promoted chondrocyte proliferation, playing an anti-inflammatory and anti-apoptotic role in cartilage. This study clarified the role and mechanism of Sal B on OA, and provided a theoretical basis for the development of new OA drugs.
Conflicts of interest
None.Abbreviations
| OA | Osteoarthritis |
| Sal B | Salvianolic acid B |
| iNOS | Inducible nitric oxide synthase |
| MMPs | Matrix metalloproteinase |
| COL1A1 | Type II collagen |
References
- C. Wu, B. Tian, X. Qu, F. Liu, T. Tang and A. Qin, et al., MicroRNAs play a role in chondrogenesis and osteoarthritis (review), Int. J. Mol. Med., 2014, 34, 13–23 CrossRef CAS PubMed.
- H. Lu, G. Hou, Y. Zhang, Y. Dai and H. Zhao, c-Jun transactivates Puma gene expression to promote osteoarthritis, Mol. Med. Rep., 2014, 9, 1606–1612 CrossRef CAS PubMed.
- D. Fu, X. Shang, Z. Ni and G. Shi, Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis, Exp. Ther. Med., 2016, 12, 2735–2740 CrossRef CAS PubMed.
- X.-H. Zhang, X.-X. Xu and T. Xu, Ginsenoside Ro suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-κB, Chin. J. Nat. Med., 2015, 13, 283–289 CAS.
- X. Wang, Y. Guo, C. Wang, H. Yu, X. Yu and H. Yu, MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1, Inflammation, 2016, 39, 1718–1728 CrossRef CAS PubMed.
- J. Tang and Q. Dong, Knockdown of TREM-1 suppresses IL-1beta-induced chondrocyte injury via inhibiting the NF-kappaB pathway, Biochem. Biophys. Res. Commun., 2017, 482, 1240–1245 CrossRef CAS PubMed.
- P. G. Winyard, B. Ryan, P. Eggleton, A. Nissim, E. Taylor and M. L. Lo Faro, et al., Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease, Biochem. Soc. Trans., 2011, 39, 1226–1232 CrossRef CAS PubMed.
- K. Vuolteenaho, T. Moilanen, R. G. Knowles and E. Moilanen, The role of nitric oxide in osteoarthritis, Scand. J. Rheumatol., 2007, 36, 247–258 CrossRef CAS PubMed.
- S. R. Goldring and M. B. Goldring, The role of cytokines in cartilage matrix degeneration in osteoarthritis, Clin. Orthop. Relat. Res., 2004, S27–S36 CrossRef.
- J. P. Pelletier, D. Jovanovic, J. C. Fernandes, P. Manning, J. R. Connor and M. G. Currie, et al., Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase, Arthritis Rheum., 1998, 41, 1275–1286 CrossRef CAS PubMed.
- R. Maier, G. Bilbe, J. Rediske and M. Lotz, Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression, Biochim. Biophys. Acta, 1994, 1208, 145–150 CrossRef CAS.
- H. Lv, L. Wang, J. Shen, S. Hao, A. Ming and X. Wang, et al., Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats, Brain Res. Bull., 2015, 115, 30–36 CrossRef CAS PubMed.
- D. H. Zhao, Y. J. Wu, S. T. Liu and R. Y. Liu, Salvianolic acid B attenuates lipopolysaccharide-induced acute lung injury in rats through inhibition of apoptosis, oxidative stress and inflammation, Exp. Ther. Med., 2017, 14, 759–764 CrossRef CAS PubMed.
- J. Y. Han, J. Y. Fan, Y. Horie, S. Miura, D. H. Cui and H. Ishii, et al., Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion, Pharmacol. Ther., 2008, 117, 280–295 CrossRef CAS PubMed.
- A. Watzke, S. J. O'Malley, R. G. Bergman and J. A. Ellman, Reassignment of the configuration of salvianolic acid B and establishment of its identity with lithospermic acid B, J. Nat. Prod., 2006, 69, 1231–1233 CrossRef CAS PubMed.
- L. Xue, Z. Wu, X. P. Ji, X. Q. Gao and Y. H. Guo, Effect and mechanism of salvianolic acid B on the myocardial ischemia-reperfusion injury in rats, Asian Pac. J. Trop. Med., 2014, 7, 280–284 CrossRef CAS PubMed.
- R. Kong, Y. Gao, B. Sun, H. Chen, G. Wang and X. Wang, et al., The strategy of combined ischemia preconditioning and salvianolic acid-B pretreatment to prevent hepatic ischemia-reperfusion injury in rats, Dig. Dis. Sci., 2009, 54, 2568–2576 CrossRef CAS PubMed.
- Y. Lou, C. Wang, W. Zheng, Q. Tang, Y. Chen and X. Zhang, et al., Salvianolic acid B inhibits IL-1beta-induced inflammatory cytokine production in human osteoarthritis chondrocytes and has a protective effect in a mouse osteoarthritis model, Int. Immunopharmacol., 2017, 46, 31–37 CrossRef CAS PubMed.
- J. Chen, R. Crawford and Y. Xiao, Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis, J. Cell. Biochem., 2013, 114, 245–249 CrossRef CAS PubMed.
- V. Ulici, K. D. Hoenselaar, J. R. Gillespie and F. Beier, The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation, BMC Dev. Biol., 2008, 8, 40 CrossRef PubMed.
- D. F. Zhang, J. Zhang and R. Li, Salvianolic acid B attenuates lung inflammation induced by cigarette smoke in mice, Eur. J. Pharmacol., 2015, 761, 174–179 CrossRef CAS PubMed.
- S. Xu, A. Zhong, X. Bu, H. Ma, W. Li and X. Xu, et al., Salvianolic acid B inhibits platelets-mediated inflammatory response in vascular endothelial cells, Thromb. Res., 2015, 135, 137–145 CrossRef CAS PubMed.
- W. Manucha and P. G. Valles, Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy, Inflamm. Allergy Drug Targets, 2012, 11, 303–312 CrossRef CAS PubMed.
- Y. Wang, Z. Z. Zhang, Y. Wu, J. J. Ke, X. H. He and Y. L. Wang, Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway, Braz. J. Med. Biol. Res., 2013, 46, 861–867 CrossRef CAS PubMed.
- L. C. Cantley, The phosphoinositide 3-kinase pathway, Science, 2002, 296, 1655–1657 CrossRef CAS PubMed.
- E. A. Makris, A. H. Gomoll, K. N. Malizos, J. C. Hu and K. A. Athanasiou, Repair and tissue engineering techniques for articular cartilage, Nat. Rev. Rheumatol., 2015, 11, 21–34 CrossRef CAS PubMed.
- F. Wang, L. Wu, L. Li and S. Chen, Monotropein exerts protective effects against IL-1beta-induced apoptosis and catabolic responses on osteoarthritis chondrocytes, Int. Immunopharmacol., 2014, 23, 575–580 CrossRef CAS PubMed.
- A. S. Lee, M. B. Ellman, D. Yan, J. S. Kroin, B. J. Cole and A. J. van Wijnen, et al., A current review of molecular mechanisms regarding osteoarthritis and pain, Gene, 2013, 527, 440–447 CrossRef CAS PubMed.
- G. Zhang, Y. Sun, Y. Wang, R. Liu, Y. Bao and Q. Li, MiR-502-5p inhibits IL-1beta-induced chondrocyte injury by targeting TRAF2, Cell. Immunol., 2016, 302, 50–57 CrossRef CAS PubMed.
- C. Csaki, N. Keshishzadeh, K. Fischer and M. Shakibaei, Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro, Biochem. Pharmacol., 2008, 75, 677–687 CrossRef CAS PubMed.
- J. P. Pelletier, J. C. Fernandes, D. V. Jovanovic, P. Reboul and J. Martel-Pelletier, Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase, J. Rheumatol., 2001, 28, 2509–2519 CAS.
- W. Cheng, D. Wu, Q. Zuo, Z. Wang and W. Fan, Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes, Int. Orthop., 2013, 37, 2065–2070 CrossRef PubMed.
- S. B. Abramson, M. Attur, A. R. Amin and R. Clancy, Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis, Curr. Rheumatol. Rep., 2001, 3, 535–541 CrossRef CAS PubMed.
- J. P. Pelletier, D. V. Jovanovic, V. Lascau-Coman, J. C. Fernandes, P. T. Manning and J. R. Connor, et al., Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level, Arthritis Rheum., 2000, 43, 1290–1299 CrossRef CAS PubMed.
- W. B. van den Berg, F. van de Loo, L. A. Joosten and O. J. Arntz, Animal models of arthritis in NOS2-deficient mice, Osteoarthr. Cartil., 1999, 7, 413–415 CrossRef CAS PubMed.
- B. Wang, J. Sun, Y. Shi and G. Le, Salvianolic Acid B Inhibits High-Fat Diet-Induced Inflammation by Activating the Nrf2 Pathway, J. Food Sci., 2017, 82, 1953–1960 CrossRef CAS PubMed.
- Z. G. Ma, H. Q. Xia, S. L. Cui and J. Yu, Attenuation of renal ischemic reperfusion injury by salvianolic acid B via suppressing oxidative stress and inflammation through PI3K/Akt signaling pathway, Braz. J. Med. Biol. Res., 2017, 50, e5954 CAS.
- S. Li, J. W. Chen, X. Xie, J. Tian, C. Deng and J. Wang, et al., Autophagy inhibitor regulates apoptosis and proliferation of synovial fibroblasts through the inhibition of PI3K/AKT pathway in collagen-induced arthritis rat model, Am. J. Transl. Res., 2017, 9, 2065–2076 CAS.
- X. Zhang, X. Xu, T. Xu and S. Qin, Beta-ecdysterone suppresses interleukin-1beta-induced apoptosis and inflammation in rat chondrocytes via inhibition of NF-kappaB signaling pathway, Drug Dev. Res., 2014, 75, 195–201 CAS.
- H. S. Hwang and H. A. Kim, Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis, Int. J. Mol. Sci., 2015, 16, 26035–26054 CrossRef CAS PubMed.
- F. Liu, G. Liu, W. Liang, H. Ye, X. Weng and P. Lin, et al., Duhuo Jisheng decoction treatment inhibits the sodium nitroprussiateinduced apoptosis of chondrocytes through the mitochondrialdependent signaling pathway, Int. J. Mol. Med., 2014, 34, 1573–1580 CrossRef PubMed.
- P. Lin, X. Weng, F. Liu, Y. Ma, H. Chen and X. Shao, et al., Bushen Zhuangjin decoction inhibits TM-induced chondrocyte apoptosis mediated by endoplasmic reticulum stress, Int. J. Mol. Med., 2015, 36, 1519–1528 CrossRef CAS PubMed.
- Y. Zhou, S. Q. Liu, L. Yu, B. He, S. H. Wu and Q. Zhao, et al., Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling, Apoptosis, 2015, 20, 1187–1199 CrossRef CAS PubMed.
- L. L. Pan, A. Y. Wang, Y. Q. Huang, Y. Luo and M. Ling, Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line, Asian Pac. J. Cancer Prev., 2014, 15, 7065–7068 CrossRef PubMed.
- D. Wang, J. Chen, H. Chen, Z. Duan, Q. Xu and M. Wei, et al., Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway, J. Biosci., 2012, 37, 91–101 CrossRef CAS PubMed.
- X. T. Wang, D. S. Pei, J. Xu, Q. H. Guan, Y. F. Sun and X. M. Liu, et al., Opposing effects of Bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury, Cell. Signalling, 2007, 19, 1844–1856 CrossRef CAS PubMed.
- M. J. Fry, Structure, regulation and function of phosphoinositide 3-kinases, Biochim. Biophys. Acta, 1994, 1226, 237–268 CrossRef CAS.
- C. J. Malemud, The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis?, Future Med. Chem., 2015, 7, 1137–1147 CrossRef CAS PubMed.
- K. Ren, Y. J. Lu, Z. C. Mo, X. Liu, Z. L. Tang and Y. Jiang, et al., ApoA-I/SR-BI modulates S1P/S1PR2-mediated inflammation through the PI3K/Akt signaling pathway in HUVECs, J. Physiol. Biochem., 2017, 73, 287–296 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |