Open Access Article
Open Access ArticleBioactive properties: enhancement of hepatoprotective, antioxidant and DNA damage protective effects of golden grey mullet protein hydrolysates against paracetamol toxicity
Intidhar Bkhairia *a,
Sabah Dhibi
*a,
Sabah Dhibi bc,
Rim Nasria,
Abdelfettah Elfekib,
Najla Hfaiyedhbc,
Ibtissem Ben Amarad and
Moncef Nasria
bc,
Rim Nasria,
Abdelfettah Elfekib,
Najla Hfaiyedhbc,
Ibtissem Ben Amarad and
Moncef Nasria
aLaboratory of Enzyme Engineering and Microbiology, University of Sfax, National School of Engineering of Sfax (ENIS), B. P. 1173, 3038, Sfax, Tunisia. E-mail: ibkhairia@yahoo.com; Fax: +216 74 275 595; Tel: +216 96 287 128
bLaboratory of Environmental Physiopathology, Valorization of Bioactive Molecules and Mathematical Modeling, Faculty of Sciences of Sfax, Road Soukra km 3.5, PB no. 1171-14 3000, Sfax, Tunisia
cLaboratory Animal Eco Physiology, Faculty of Sciences, Sidi Ahmed Zarrouk, 2112, Gafsa, Tunisia
dHigher Institute of Biotechnology of Sfax, University of Sfax, 3000, Sfax, Tunisia
First published on 26th June 2018
Abstract
This study was undertaken to examine the hepatoprotective, antioxidant, and DNA damage protective effects of protein hydrolysates from Liza aurata, against paracetamol overdose induced liver injury in Wistar rats. L. aurata protein hydrolysates (LAPHs) were mainly constituted by glutamic acid (Glu) and glutamine (Gln) and lysine (Lys). In addition, they contained high amounts of proline (Pro), leucine (Leu) and glycine (Gly). The molecular weight distribution of the hydrolysates was determined by size exclusion chromatography, which analyzed a representative hydrolysate type with a weight range of 3–20 kDa. The hepatoprotective effect of LAPHs against paracetamol liver toxicity was investigated by in vivo assay. Rats received LAPHs daily by gavage, for 45 days. Paracetamol was administrated to rats during the last five days of treatment by intraperitoneal injection. Paracetamol overdose induced marked liver damage in rats was noted by a significant increase in the activities of serum aspartate amino transferase (AST) and alanine amino transferase (ALT), and oxidative stress which was evident from decreased activity of the enzymatic antioxidants (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)), and level of glutathione (GSH), and increased concentration of lipid peroxidation products (MDA). Furthermore, paracetamol increased the DNA damage with liver histopathological changes. LAPH pretreatment significantly attenuated paracetamol-induced hepatotoxic effects, including oxidative damage, histopathological lesions, and apoptotic changes in the liver tissue. Interestingly, LAPHs restored the activities of antioxidant enzymes and the level of GSH, ameliorated histological and molecular aspects of liver cells. The present data suggest that paracetamol high-dose plays a crucial role in the oxidative damage and genotoxicity of the liver and therefore, some antioxidants such us LAPHs might be safe as hepatoprotectors. Altogether, our studies provide consistent evidence of the beneficial effect of LAPHs on animals treated with a toxic dose of paracetamol and might encourage clinical trials.
1. Introduction
Minimizing toxicity remains one of the major barriers to bringing a drug to market. Approximately 92% of all developed compounds fail because of adverse effects of the candidate during clinical development.1Paracetamol (acetaminophen – APAP) is used by millions of people worldwide as a safe analgesic drug at therapeutic doses. An overdose of paracetamol results in an increase in the levels of the toxic metabolite N-acetyl-p-benzoquinoneimine (NAPQI), which extensively depletes hepatocellular glutathione (GSH) along with generation of reactive oxygen species (ROS).2 There are an estimated 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of paracetamol overdose annually, with most cases being intentional suicide gestures.3 Nearly 26
000 cases of paracetamol overdose annually, with most cases being intentional suicide gestures.3 Nearly 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 overdose patients are hospitalized each year, and an estimated 1% develops severe coagulopathy or encephalopathy. The mortality attributed to acetaminophen toxicity is 500 per annum, and at least 20% of these deaths occur in patients with non-intentional paracetamol overdose.3 High dose of paracetamol is well known to be toxic to the liver.4 When acetaminophen is used in therapeutic doses, most of the drug is metabolized via glucuronidation and sulfation, a very small amount of acetaminophen is metabolized to NAPQI by the hepatic enzyme cytochrome P450 2E1 (CYP2E1).5
000 overdose patients are hospitalized each year, and an estimated 1% develops severe coagulopathy or encephalopathy. The mortality attributed to acetaminophen toxicity is 500 per annum, and at least 20% of these deaths occur in patients with non-intentional paracetamol overdose.3 High dose of paracetamol is well known to be toxic to the liver.4 When acetaminophen is used in therapeutic doses, most of the drug is metabolized via glucuronidation and sulfation, a very small amount of acetaminophen is metabolized to NAPQI by the hepatic enzyme cytochrome P450 2E1 (CYP2E1).5
The antidote n-acetylcysteine (NAC) is highly effective at preventing toxicity, provided it is administered within a few hours of the overdose. Subsequently efficacy declines as the interval between the overdose and NAC administration increases.6 However, despite its efficacy in reducing mortality due to paracetamol poisoning, intravenous NAC can cause anaphylactoid reactions.7 Due to the complexity of preparation of NAC as well as its side effects, mistakes are possible.8 However, by reason of the narrow treatment and limited timing of NAC, new therapeutic interventions are necessary to be developed for the treatment of paracetamol toxicity.
In recent years, a great deal of interest has been expressed regarding the productions, characterizations, and applications of protein hydrolysates and food-derived biopeptides due to their numerous beneficial health effects. Peptides with biological activities have shown promise as pharmaceutics with the potential to treat a wide variety of diseases. Indeed, increasing demands for functional foods for health care and disease risk reduction are prevalent throughout the world.9 The past decade has also witnessed intense interest in functional foods or dietary photochemical which can influence the pharmacological activity of drugs and their toxicities by modifying metabolism system, including drug-metabolizing enzymes and transporters.10
In the pharmaceutical industry, liver, a vital organ in the body responsible for metabolizing and detoxification of substances,11 is one of the routinely assessed organs during preclinical safety evaluations. Indeed several biological compounds with antioxidant properties proved effective in protecting the liver against deleterious effects of paracetamol overdose. In addition, protein hydrolysates from fish are considered as reservoirs of structurally diverse bioactive materials with numerous biological effects for human's body affects either directly or indirectly in maintaining good health.12 Depending on the composition and the sequence of amino acids, these protein hydrolysates, which contain a complex mixture of peptides, can exhibit diverse activities.
In fact, experimental data showed the antihypertensive,13 antioxidative14,15 and anti-diabetic16 properties from marine protein hydrolysates.
In a previous study, we demonstrated that LAPHs obtained from L. aurata proteins using a commercial, endogenous and microbial proteases, exhibited a variable extend antioxidant activities.15 In addition, the RP-HPLC analysis demonstrates that the bioactivities of LAPHs were directly related to the hydrophobic properties of peptides. However, the protective role of biopeptides from L. aurata against paracetamol induced liver injury has not been investigated. Hence, we proposed to scrutinize hepatoprotective effect of LAPHs against paracetamol-induced acute liver injury. In addition, the feasible molecular mechanisms underlying this hepatoprotective effect are discussed, involving antioxidant activities.
Golden grey mullet (L. aurata) is one of the mullet species which is widely distributed in the Mediterranean Sea and Black Sea, Atlantic coasts from the Azores and Madeira northward to the British Isles, and the southern coasts of Norway and Sweden.17 L. aurata is relatively important in the fish catches of Tunisia, and is utilized for human consumption.
The aim of the present study was to evaluate, for the first way, the hepatoprotective potential and antioxidative effects of LAPHs in subacute toxicity induced by paracetamol overdose.
2. Materials and methods
2.1. Materials
Fresh golden grey mullet was purchased from the fish market of Sfax City, Tunisia. The samples were packed in polyethylene bags, placed in ice and transported to the research laboratory within 30 min. After the fishes were washed with water, their viscera and the muscles were separated and rinsed with cold distilled water. The muscle of L. aurata was stored in sealed plastic bags at −20 °C until it was used for protein hydrolysate production. The viscera were used immediately for the extraction of digestive enzymes.2.2. Enzymes
Enzyme preparations from Bacillus subtilis A26 and Pseudomonas aeruginosa A2, crude enzyme extract from viscera of golden grey mullet, and commercial Trypsin and Esperase® were used. The protease activities were determined by the method of Kembhavi et al.18 using casein as a substrate.2.3. Production of L. aurata protein hydrolysates (LAPHs)
LAPHs were produced as previously reported by Bkhairia et al.15 The protein hydrolysates obtained following treatment with the endogenous alkaline enzyme extract from the viscera of L. aurata, commercial enzymes (Trypsin and Esperase®) and proteases from B. subtilis A26 and P. aeruginosa A2, were named as PH-LA, PH-TR, PH-ES, PH-A26, and PH-A2, respectively.2.4. Molecular weight distribution
The molecular mass distribution of LAPHs was determined by size exclusion chromatography (SEC), using a high-performance liquid chromatography (HPLC) system. SEC analysis was performed using Agilent PL aquagel-OH MIXED-H preparative-column (300 mm × 25 mm, Agilent, LC1260, USA), and elution was performed in 50% methanol at a flow rate of 1.5 ml min−1.1 20 μl of sample (50 mg ml−1) was injected onto the column which was previously calibrated with bovine serum albumin (66 kDa), egg albumin (44.5 kDa), pepsin (34 kDa), trypsin (24 kDa) and casein k (19 kDa). Detection was monitored at 214 nm.2.5. In vitro gastrointestinal simulated digestion
The digestion procedure mimicking the physiological situation in the upper tract (stomach and small intestine) was adapted from a published method.19 Briefly, the LAPHs (10 mg ml−1) were resolved in suitable volume of phosphate buffer (10 mM; pH 6.8) and incubated for 2 min at 37 °C. To produce an acidic condition of the simulated stomach solution, 1 ml of 1 M HCl was added, followed by adding 5 ml of pepsin solution (32 U ml−1) in 1 M HCl–KCl buffer (pH 1.5). The mixture was incubated for 1 hour at 37 °C with continuous shaking. At the end of the post-gastric digestion, the mixture was immediately cooled down with ice bath and then an aliquot of 1 ml was removed, frozen and taken for analysis of the five hydrolysates. Thereafter, the pH of the reaction mixture was adjusted to 6.8 with 1 M NaHCO3, and 1 ml mixture of bile and pancreatic juice that contained bile extract (13.5 mg ml−1), pancreatin (10 mg ml−1), and trypsin (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 U ml−1) in 10 mM phosphate buffer (pH 8.2), was added to the solution. The mixture was allowed to stand for 3 hours at 37 °C to create duodenal condition. The enzymes were inactivated by heating at 90 °C for 10 min. The antioxidant activities were tested using the β-carotene-linoleate bleaching model during the digestion after 30, 60, 120, 180, and 240 min. The relative activities were measured and compared to that without any treatment (at 0 min).
600 U ml−1) in 10 mM phosphate buffer (pH 8.2), was added to the solution. The mixture was allowed to stand for 3 hours at 37 °C to create duodenal condition. The enzymes were inactivated by heating at 90 °C for 10 min. The antioxidant activities were tested using the β-carotene-linoleate bleaching model during the digestion after 30, 60, 120, 180, and 240 min. The relative activities were measured and compared to that without any treatment (at 0 min).
2.6. Determination of protein oxidation
To quantify protein oxidation, after treatment, protein samples were subjected to SDS-PAGE. Bovine serum albumin (BSA) was dissolved in phosphate buffer (100 mM, pH 7.4) at a final concentration of 0.5 mg ml−1, and then incubated with or without LAPHs at 37 °C. After 30 min, Cu2+/H2O2 (0.1/0.25 mM) were added to the mixture. Reactions were performed in a water bath at 37 °C for 1.5 hour. In these experiments, the same amount of phosphate buffer was added to the control groups. BSA samples were mixed with loading buffer and heated at 100 °C for 10 min. 20 μl of the mixture solution was separated by 10% SDS-PAGE. After running for about 2 hours, gels were stained with 0.1% Coomassie brilliant blue R-250 for 30 min. Band intensities were measured using Quantity one analysis software.2.7. Animals and treatment
Male Wistar rats, weighing 180 ± 20 g were obtained from the Central Pharmacy (SIPHAT, Tunisia). Animal maintenance and experimental procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tunis University and approved by the Animal Ethics Committee of National Institute of Health (1985).20 They were housed at ambient temperature (22 ± 2 °C) in a 12 hours light/dark cycle and a minimum relative humidity of 60% ± 5%. Food (SNA, Sfax, Tunisia) (Table 1) and water were available. One week after acclimatization to laboratory conditions, the rats were randomly divided into seven groups of six each and treatments were carried out over a period of 45 days.| Nutritional properties (%) | |
| Moisture (maximal) | 14 |
| Fibers (maximal) | 5 |
| Proteins (minimal) | 18 |
| Fat (maximal) | 3 |
| Ash (maximal) | 13.5 |
| Carbohydrate | 46.5 |
| Calorific value (kcal kg−1) | 2846 |
| Amino acid (%) | |
| Methionine | 0.36 |
| Cysteine | 0.26 |
| Threonine | 0.62 |
| Tryptophan | 0.2 |
| Mineral mix (mg kg−1) | |
| Manganese | 80 |
| Fer | 48 |
| Cuivre | 18 |
| Zinc | 64 |
| Selenium | 0.28 |
| Cobalt | 0.2 |
| Iode | 2 |
| Vitamin and antioxidant (mg kg−1) | |
| Vitamine A | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| Vitamine D3 | 2800 |
| Vitamine H | 25 |
| Antioxidant (BHA–BHT) | 100 |
Group 1: normal rats (NR) fed with the standard diet and water, Group 2: rats treated with 325 mg of paracetamol/kg body weight (bw), by intraperitoneal injection, during the last five days of treatment period. This dose provoked toxicity without lethality. Groups 3, 4, 5, 6 and 7: rats received 350 mg kg−1 bw of PH-LA, PH-ES, PH-TR, PH-A2, and PH-A26, daily by gavage, during 45 days. They were treated intraperitoneally with paracetamol for the last five days of treatment period.
At the end of the experimental period, animals of the different groups were sacrificed by cervical decapitation to avoid stress.
Trunk blood samples were collected into EDTA tubes. Some of them were immediately used for the determination of hematological parameters. The sera samples were collected after centrifugation (2200 × g, 15 min, 4 °C), and the liver of each rat was carefully excised.
Some liver samples were immediately removed, rinsed in ice-cold physiological saline solution, fixed in 10% buffered formalin solution and embedded in paraffin for histological studies. The other ones were homogenized in Tris-buffer-saline (pH 7.4) and then centrifuged (3500 × g, 20 min) at 4 °C. The supernatants were frozen and stored for further use in subsequent enzymatic assays. All samples were stored at −80 °C until further use.
2.8. DNA fragmentation assay in liver
The DNA was isolated from the liver of the different groups by ZR Genomic DNATM-Tissue MidiPrep, Irvine, CA 92614, USA, according to manufacturer's instructions. The quality and quantity of DNA was evaluated spectrophotometrically at 260/280 nm. DNA samples were analyzed on a standard 1% (w/v) agarose gel containing ethidium bromide. Images of the ethidium bromide stained DNA agarose gel were acquired using UVP BIODOC-IT™ system.2.9. Biochemical estimations
2.9.3.1. Catalase (CAT). CAT activity was determined by the method of Aebi.23 The enzymatic reaction was initiated by adding 500 mM H2O2 to the supernatant (20 μl). A decrease in absorbance due to H2O2 degradation was monitored spectrophotometrically at 240 nm for 1 min and CAT activity was expressed as U mg−1 protein in liver.
2.9.3.2. Glutathione peroxidase (GPx). GPx activity was measured according to Flohé and Günzler.24 Glutathione (GSH) oxidation by GPx is coupled to the transformation of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) into 2-nitro-5-thiobenzoate (TNB) which absorbs at 412 nm. The enzyme activity was expressed as U g−1 protein in liver.
2.9.3.3. Superoxide dismutase (SOD). SOD activity in the liver of rats from different groups, was assayed by the method of Sun et al.25 Enzymatic activity is proportional to the inhibition rate of nitroblue tetrazolium (NBT) oxidation by O2− anion. The developed blue color of the reaction was measured at 560 nm. Units of SOD activity were expressed as the amount of enzyme required to inhibit the reduction of NBT by 50% and the activity was expressed as U mg−1 of protein.
2.10. Hematological parameters examination
The blood samples collected into EDTA tubes were used for the determination of hematological parameters. White blood cells (WBCs), red blood cells (RBCs), hematocrit (Ht), hemoglobin (Hb), mean corpuscular volume (MCV), and platelets count (Plt) were analyzed by an electronic automate Coulter MAXM (Beckman Coulter, Inc., Fullerton, CA).2.11. Histopathological examination
After sacrificing the rats, liver tissues from the different groups of rats were fixed in formol solution for 24 hours for histological examination, and then transferred in a 10% formalin solution. The fixed tissues were embedded in paraffin and cutted in 4 μm sections. Sections were then stained with hematoxylin–eosin for histological examination. The histological liver architecture of the control group was compared with that of the treated groups.2.12. Statistical analysis
All data were expressed as mean ± standard error mean (SEM). The significance of the differences between group means for the variables of interest were assessed by the one-way analysis of variance (ANOVA) followed by the Fisher test (Stat View). Multiple mean comparisons were performed using a student test. Comparisons were done using either control rats or rats treated with paracetamol as references. The level of statistical significance was set at p < 0.01.3. Results and discussion
3.1. Molecular weight distribution and amino acid composition of LAPHs
Bioactive peptides are mostly obtained from the original proteins through enzymatic proteolysis. Since enzymes display various spectra of substrate specificity, different proteases were applied to L. aurata muscle proteins in order to obtain several types of protein hydrolysates containing peptides with different functional properties, molecular weights and amino acids compositions.The focus of recent research has been on different protein hydrolysates which were prepared at the same enzyme/substrate ratio (E/S = 3 U mg−1) and after incubation for 6 hours, PH-LA showed the highest degree of hydrolysis (DH = 13.05%), followed by PH-TR (DH = 12.67%), PH-ES (DH = 12.5%), PH-A26 (DH = 9.25%), and PH-A2 (DH = 8.0%).
Treatment of protein with different enzymes produced a mixture of bioactive peptides with a different degree of hydrolysis (DH) which also could be responsible for the different range of antioxidant capacity.15
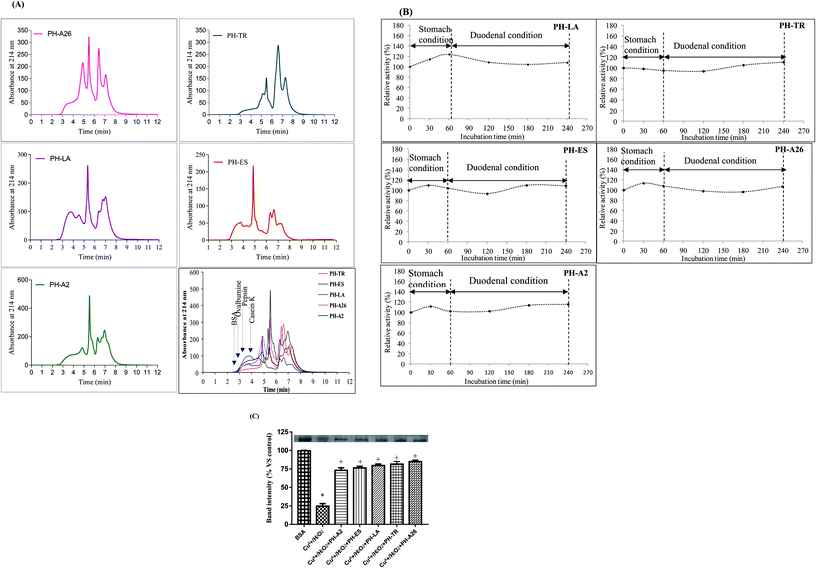
The molecular mass distribution of the LAPHs was carried out using HPLC-SEC analysis. The SEC spectra of the molecular weight (MW) peptides are shown in Fig. 1(A). The profiles of SEC spectra revealed the differences in the molecular mass distribution depending on the proteases used and consequently on the degree of hydrolysis (DH). Further, the elution profile of PH-TR and PH-LA gave the highest intensities for the last eluting peptides, characterized by their low MW. This result is in accordance with the high DH obtained for this hydrolysate. Indeed, more the DH value increased more the low MW peptides (<10 kDa) content increased. As shown in Table 2, the levels of peptides below 3 kDa obtained in PH-LA, PH-TR, PH-ES, PH-A26, and PH-A2, were 34.09%, 29.92%, 37.9%, 22.3% and 15.18%, respectively, which is in accordance with their DHs.
| SEC distribution | ||||||
|---|---|---|---|---|---|---|
| a SEC method was used to calculate the MW distribution of the LAPHs. | ||||||
| Mass (kDa) | >20 | 20–10 | 10–5 | 5–3 | <5 | <3 |
| PH-LA | 9.16 | 12.61 | 20.53 | 23.61 | 57.7 | 34.09 |
| PH-TR | 9.27 | 12.05 | 37.1 | 11.66 | 41.58 | 29.92 |
| PH-ES | 6.7 | 16.43 | 27.64 | 11.52 | 49.23 | 37.9 |
| PH-A26 | 11.13 | 18.14 | 28.67 | 19.76 | 42.06 | 22.3 |
| PH-A2 | 16.29 | 20.48 | 27.92 | 20.13 | 35.31 | 15.18 |
Protein hydrolysates obtained after proteins hydrolysis are composed of free amino acids and short chain peptides, and exhibit many advantages as nutraceuticals or functional foods because of their amino acid profile.
The amino acids composition of any food protein has a significant role in various physiological activities of the human body and affects either directly or indirectly the maintenance of good health.27 Table 3 shows the amino acid composition of the LAPHs. It is obviously shown that the LAPHs contained almost all the essential and non-essential amino acids with dominance of Glu, Gln, and Lys. Pro, Leu and Gly were also present in relatively high amounts. However, the contents of His, Met, Phe and Ser were very low, as demonstrated by our results. Eight key amino acids were observed in the hydrolysate products, namely leucine, isoleucine, valine, lysine, methionine, tyrosine and phenylalanine. These amino acids are essential daily food intakes to assure normal human growth. Furthermore, amino acid compositions may also be important to antioxidant activity. Himaya et al.28 argue that hydrophobic amino acids facilitated interactions with hydrophobic targets, such as the cell membrane, and thereby, enhanced the bioavailability. Additionally, aromatic amino acids increased the antioxidant activities of peptides and protein hydrolysates because they easily donate protons to electron-deficient radicals and maintain their stabilities via resonance structures and enhance radical scavenging activities.29 Therefore, the antioxidant activities of LAPHs could be related to the high hydrophobic and aromatic amino acid contents.
| Amino acids | PH-A2 | PH-A26 | PH-LA | PH-TR | PH-ES |
|---|---|---|---|---|---|
| a Essential amino acids. HAA: hydrophobic amino acid. Values are given as mean SD from triplicate determinations (n = 3), a,b,c,d,e in the same line indicate significant differences (p < 0.01). | |||||
| Hydrophilic amino acid | |||||
| Aspartic acid and asparagine (Asp and Asn) | 9.84 ± 0.10b | 10.02 ± 0.80a | 10.06 ± 0.06a | 9.92 ± 0.80a | 10.13 ± 1.10a |
| Glutamic acid and glutamine (Glu and Gln) | 15.75 ± 1.00d | 16.80 ± 0.10a | 16.18 ± 0.20b | 15.2 ± 0.35e | 16.00 ± 0.71c |
| Serine (Ser) | 3.93 ± 0.01d | 4.39 ± 0.10a | 4.01 ± 0.19c | 3.83 ± 0.21e | 4.19 ± 0.30b |
| Glycine (Gly) | 7.41 ± 0.02d | 8.33 ± 0.11a | 8.09 ± 0.20b | 6.63 ± 0.01e | 7.95 ± 0.42c |
| Histidinea (His) | 2.04 ± 0.02c | 2.15 ± 0.12b | 2.20 ± 0.27a | 1.84 ± 0.02d | 2.26 ± 0.61a |
| Arginine (Arg) | 5.01 ± 0.01a | 4.02 ± 0.13b | 2.18 ± 0.31e | 4.21 ± 0.10c | 3.95 ± 0.10d |
| Threoninea (Thr) | 4.48 ± 0.20d | 5.00 ± 0.14b | 5.40 ± 0.35a | 4.10 ± 0.32e | 4.89 ± 0.30c |
| Tyrosine (Tyr) | 2.48 ± 0.52b | 2.42 ± 0.15b | 2.60 ± 0.39a | 2.40 ± 0.02b | 2.63 ± 0.95a |
| Lysinea (Lys) | 11.09 ± 0.03b | 8.02 ± 0.16e | 10.45 ± 0.43c | 16.67 ± 1.23a | 8.91 ± 0.10d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Hydrophobic amino acid | |||||
| Proline (Pro) | 8.81 ± 0.00b | 8.73 ± 0.17c | 8.26 ± 0.47d | 8.27 ± 0.10d | 8.94 ± 0.14a |
| Alanine (Ala) | 7.04 ± 0.00b | 7.02 ± 0.17b | 7.08 ± 0.51b | 6.41 ± 0.10c | 7.87 ± 0.00a |
| Valinea (Val) | 4.56 ± 0.00b | 4.53 ± 0.18b | 4.86 ± 0.55a | 4.23 ± 0.21c | 4.51 ± 0.1b |
| Methioninea (Met) | 2.81 ± 0.00c | 2.97 ± 0.19b | 3.04 ± 0.59a | 2.51 ± 0.15d | 3.03 ± 0.14ab |
| Isoleucinea (Ile) | 4.18 ± 0.05c | 4.47 ± 0.20b | 4.65 ± 0.63a | 4.56 ± 0.61a | 4.42 ± 0.16b |
| Leucinea (Leu) | 7.31 ± 0.10d | 7.58 ± 0.21c | 7.87 ± 0.68b | 6.28 ± 0.13a | 7.44 ± 0.94c |
| Phenylalaninea (Phe) | 3.26 ± 0.10b | 3.50 ± 0.72a | 3.01 ± 0.72c | 2.90 ± 0.75d | 2.85 ± 0.27d |
| Total | 100 | 100 | 100 | 100 | 100 |
3.2. Evaluation of the stability of LAPHs in gastrointestinal model system
The oral bioactive agents administration route provides a valuable option for treating various deadly diseases because of its several advantages like cutbacks the likeliness of disease transmission, reduces medical cost and allows flexible intake schedule of administration.30 The resistance of bioactive peptides against gastrointestinal proteases, play a key role in their physiological effect in the human system and in their exploitation for human nutrition as functional foods.31 Therefore, in this study, the stability of LAPHs (10 mg ml−1), were subjected to two stages of hydrolysis that mimicking the gastrointestinal conditions. Interestingly, as shown in Fig. 1(B), the antioxidant activity, monitored by β-carotene-linoleate bleaching assay, increased slightly after pepsin and duodenal digestion, suggesting that peptides in these hydrolysates are resistant to digestion in the gastrointestinal tract. The LAPHs are resistant to gastric digestion, as is stable in the presence of acids and proteolytic enzymes, hence, it tends to remain intact during passage through the stomach. The stability of the peptides during gastrointestinal digestion promotes its specific bioactivities and biological functions. Further, the slight enhancement of antioxidant activity could be explained by the release of potent bioactive peptides, from initially inactive and/or active peptides, which might enhance the protection of β-carotene from discoloration. Nasri et al.13 reported an increase in antioxidant activity of protein hydrolysates after being digested in the simulated model system. Furthermore, bioactive peptides that cannot be absorbed through the gastrointestinal tract may exert a direct role upon the intestinal lumen, or through interaction with receptors in the intestinal wall itself. Some of these receptors have been implicated in such diseases as cancer, diabetes, osteoporosis, stress, obesity, and cardiovascular complications.3.3. Protective effect of LAPHs on Cu2+/H2O2 induced protein oxidation in vitro
Proteins are susceptible to damage by ROS according to previous “in vitro”15 and “in vivo”26 studies. Their oxidation may lead to structural alterations and functional inactivation.32Antioxidative activity of LAPHs, using protein oxidation with Cu2+/H2O2 assay, was objectified, in this study, by a decrease in the BSA degradation induced by Cu2+/H2O2. Densitometric analysis of the gel electrophoretic samples is presented in Fig. 1(C). The Cu2+/H2O2 system is a metal-catalyzed system and can produce ROS and altered spectroscopic properties of albumin, increased protein carbonyl content and resulted in several conformational changes. Interestingly, LAPHs reduced significantly protein damage and protein band intensity was restored to control levels. PH-A26 (3 mg ml−1), was a better protector against the metal catalyzed protein oxidative damage. The incubation of BSA with PH-A26, PH-TR, PH-LA, PH-ES, and PH-A2, increased significantly the band intensities by 86.20%, 84.0%, 81.0%, 78.0%, and 75.5%, respectively, compared to the BSA incubated only with Cu2+/H2O2 (Fig. 1(C)). The inhibitory effect of LAPHs might be explained by the scavenging of hydroxyl radical generated in Cu2+/H2O2 system. The results obtained above are in agreement with our previously findings,15 with regards to the scavenging effects of LAPHs.
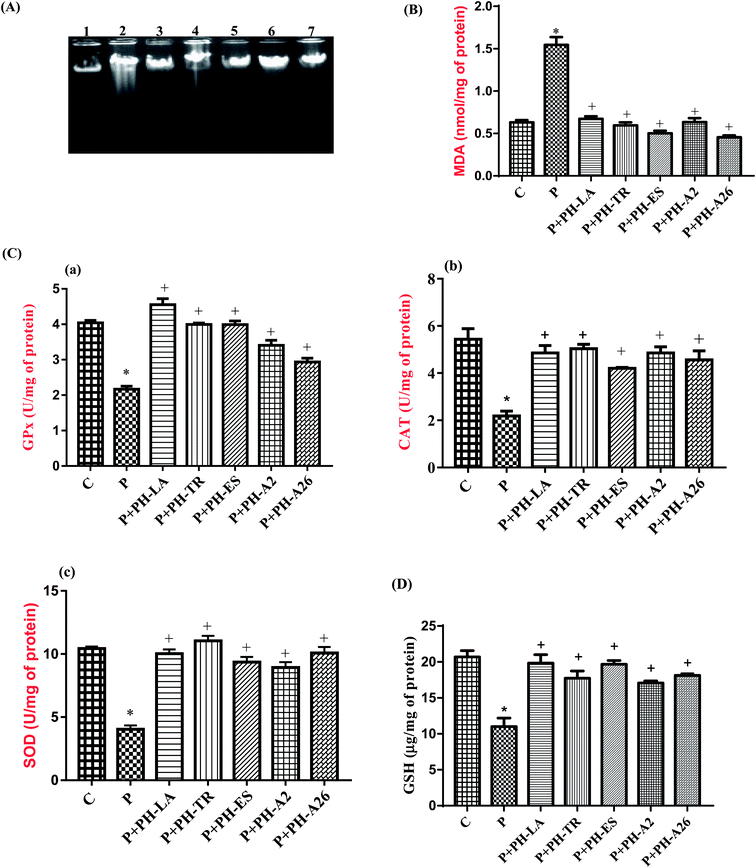
3.4. Effect of LAPHs on liver DNA damage against paracetamol toxicity
In addition to association with lipid and protein oxidation, there is clear evidence to implicate oxidative stress as a mechanism of damage in genomic DNA. The effect of paracetamol and LAPHs on the integrity of liver genomic DNA from treated rats was studied by DNA electrophoresis.Agarose gel electrophoresis showed undetectable DNA laddering in hepatic tissues of the control rats (Fig. 2, lane 1). However administration of paracetamol (325 mg kg−1 bw) caused DNA damage resulted in DNA shearing with DNA ladder pattern (a hallmark of necrosis) (Fig. 2(A), lane 2). According to Scott et al.33 free radical generation, following xenobiotic exposure, may lead to an extensive DNA damage giving rise to mutations and/or cell death. Oxidative stress induced by oxygen-derived radicals can produce numerous modifications in DNA including base and sugar lesions, strand breaks, DNA–protein cross-links and base-free sites. Flaks and Flaks34 reported that paracetamol overdose could increase the mutation rates through oxidative damage. DNA gel electrophoresis results supported the view that LAPHs protected liver cells from necrosis and/or apoptotic death induced by paracetamol overdose. Interestingly, LAPHs prevented DNA fragmentation as evidenced by the absence of DNA laddering patter (Fig. 2(A), lane 3–7).
The protection of the DNA could be caused by the DNA repair enzyme(s) such as OGG1, stimulated by LAPHs, which must be present in the nucleus to reduce the 8-oxo-deoxyguanosine (8-oxodG) incision activity.
3.5. Effect of LAPHs on hepatic toxic markers
The liver plays a major role in regulating various physio-chemical functions of the body, including synthesis, secretion and metabolism of xenobiotics.The AST, ALT and LDH activities in the serum of the paracetamol treated group increased by 37%, 71.5% and 55.5%, respectively, compared to the control (Table 4). A significant increase in the ALP (71.8%) was also noted in paracetamol treated group. Similar results were reported by Venkatachalam and Muthukrishnan.35 An elevation in transaminases ALP and LDH activities are attributed to the liver injury. When the liver cell plasma membrane is alterated, a variety of enzymes usually located in the cytosol are relegated into blood stream. The increased production of serum enzymes in blood stream was associated with central submassive necrosis of liver which causes severe hepatic injury.36
| Groups | AST (U L−1) | ALT (U L−1) | Glucose (mmol L−1) | LDH (U L−1) | ALP (U L−1) |
|---|---|---|---|---|---|
| a AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; glucose; ALP, alkaline phosphatase; PH-LA, PH-TR, PH-ES, PH-A2 and PH-A26: protein hydrolysate obtained with crude enzyme from L. aurata, trypsin, esperase, Pseudomonas aeruginosa A2 and Bacillus subtilis A26 respectively. Data expressed as mean ± SD in each group (n = 6). a,b,c,d,e,f The means with no common superscripts differ significantly (p < 0.01). | |||||
| Control | 196 ± 13.3b | 88.32 ± 3.21d | 5.41 ± 0.39de | 1006 ± 24ef | 341 ± 27ef |
| Paracetamol (P) | 269.15 ± 27.32a | 151.3 ± 6.35a | 9.45 ± 0.41a | 1563 ± 38a | 586 ± 27a |
| PH-LA/(P) | 177.96 ± 15.32c | 111.32 ± 7.32bc | 6.34 ± 0.47c | 1241 ± 17b | 407 ± 8.0bc |
| PH-TR/(P) | 145.32 ± 18.32e | 120.96 ± 6.18b | 7.21 ± 0.12b | 1205 ± 21bc | 413 ± 15b |
| PH-ES/(P) | 156.89 ± 11.66de | 117.69 ± 5.32b | 6.89 ± 0.61bc | 1102 ± 33d | 397 ± 19c |
| PH-A2/(P) | 166.32 ± 14.32d | 99.64 ± 4.62c | 5.21 ± 0.14e | 1086 ± 27de | 388 ± 10cd |
| PH-A26/(P) | 198.25 ± 15.26b | 89.34 ± 2.94d | 5.91 ± 0.36cd | 1074 ± 35ef | 361 ± 22e |
A significant increase in the serum glucose (74.67%) levels was also detected. Hinson et al.37 reported hyperglycemia (500 mg kg−1 bw) and glycosuria in an acetaminophen-overdosed patients. The data presented in Table 4 show, compared to the paracetamol treated group, a significant decrease in all parameters cited above in all protein hydrolysates treated groups, and values obtained were similar or slightly higher than those of the normal rats (p < 0.01).
The findings of the present study indicated that, the PH-TR treated rats showed the highest decrease in AST activity (46%), followed by PH-ES (41.7%), PH-A2 (38.25%), PH-LA (33.8%), and PH-A26 (26.35%), whereas the PH-S treated rats showed the highest decrease in ALT, LDH, and ALP activities (41%, 31.25%, and 38.40%, respectively) (p < 0.01).
The significant decrease in ALT and AST activities in the LAPHs pretreated rats demonstrated their hepatoprotective effects against paracetamol damage. Similar result was reported by Galal et al.38 The possible hepatoprotective mechanisms of LAPHs may be due to preventing the process of lipid peroxidation, inhibiting the cytochrome P-450 activity and stabilizing the hepatocellular membrane.
The mechanism of hepatoprotection by protein hydrolysates generally exerts multiple effects. Although they show hepatoprotection due to antioxidant effect, there are other effects like immunomodulatory39 and anti-inflammatory.40
3.6. Effect of LAPHs on levels of oxidative stress parameters in liver tissue
The results presented in Fig. 2(B) show that the level of MDA in liver tissue increased in the paracetamol-treated rats by 147%, compared to the control group, which indicate the oxidative effect of paracetamol. Several authors have also reported an increase in lipid peroxydation following administration of high doses of paracetamol in rats.41 Increased lipid peroxidation in paracetamol group, as evidenced by the elevated level of MDA in hepatic tissues, could be expected owing to the depletion in GSH stores and reduced GPx activity.
In our study LAPHs have shown to have a protective effect against damage caused by oxidative stress which is provoked by liver toxicity induced by overdose of paracetamol. We have seen that PH-LA, PH-TR, PH-ES, PH-A2, and PH-A26 were able to reduce the concentration of hepatic MDA by 57.77%, 61.50%, 70.20%, 62.75%, and 70.81%, respectively, compared to the paracetamol treated group. Hepatic MDA contents of PH-ES and PH-A26 were even lower than that of the control group.
The obtained results indicate that the administration of LAPHs effectively inhibited lipid peroxidation induced by paracetamol and demonstrate their beneficial effects.
The activities of antioxidant enzymes in liver of experimental rats are shown in Fig. 2(C). Our results revealed that hepatotoxicity induced by paracetamol overdose, significantly decreased the activities of SOD, GPx, and CAT by 59.5%, 48.8%, and 59.6%, respectively, as compared to the control group (p < 0.01). This might lead to decreased antioxidant defense and increased oxidative stress and thereby the tissue injury occurs. Similar results have been reported by Athira et al.,43 paracetamol has been reported to decrease the antioxidant enzymes activities in hepatic cells.
Pretreatment with LAPHs before paracetamol administration prevented the reduction of antioxidant enzyme activities. This might reflect the antioxidant potency of LAPHs. Our results are in agreement with reports of other workers which demonstrated that the administration of goby and zebra blenny to rats fed high fat-high fructose diet44 or alloxan induced diabetic rats27 increased SOD, CAT and GPx activities. The increase of antioxidant enzyme activities demonstrates the existence of the antioxidant peptides with potent free radical-scavenging activities in LAPHs.
These findings were attributed to small peptides that preventing the generation of free radicals. The improvement in the expression of these antioxidant enzymes in rats treated with LAPHs suggest that this hepatic antioxidant defense is reactivated by peptides with a resulting increase in the capacity of detoxification through enhanced scavenging of reactive oxygen radicals.
The measurement of non-enzymatic antioxidant content is a great biomarker for paracetamol intoxication. As shown in Fig. 2(D), the level of GSH in hepatic tissue of the paracetamol treated group, was markedly reduced by 49% as compared to the control group (p < 0.01). The decrease in the concentration of GSH in paracetamol-treated group is also an indicator of oxidative damage. The excess of NAPQI first depletes the GSH level, and then covalently binds to thiol groups of intracellular proteins, thus generating reactive oxygen species (ROS) that triggers hepatocellular necrosis.45 However, the levels of GSH, in rats pretreated with different LAPHs, were significantly restored by 90.5%, 70.05%, 84.7%, 95.26%, and 68.84%, respectively after PH-LA, PH-TR, PH-ES, PH-A2, and PH-A26 treatment, compared to the paracetamol group.
In summary, the present findings demonstrate the capability of LAPHs in promoting natural defense against ROS produced by paracetamol induced hepatotoxicity.
3.7. Effects of LAPHs on hematological parameters
It is well known that the liver plays a major role in homeostasis, as most of the coagulation factors, anticoagulant proteins and components of the fibrinolytic system are synthesized by hepatic parenchymal cells. Additionally, the reticuloendothelial system of the liver helps to regulate coagulation factors from the circulation. Finally, because the liver is a highly vascularized organ with vital systems draining through the parenchyma, liver diseases can affect blood flow and predispose patients to significant bleeding problems. The etiology of impaired haemostasis resulting from abnormal liver function is often multifactorial and may impaired coagulation factor synthesis, synthesis of dysfunctional coagulation factors, altered clearance of activated coagulation factors, and quantitative and qualitative platelet disorders.In fact, we obtained in the present study an increase in the platelet number after paracetamol administration (Table 5). The hematological profile of the control and treated groups are presented in Table 5. Treatment with paracetamol resulted in a significant decrease in the levels of RBC (red blood cell), Hb (hemoglobin) and Ht (hematocrit) suggesting an anemia installation for rats treated with paracetamol. The diminution of blood cell count is proved by the decrease of Ht level. As known, RBCs are responsible for carrying oxygen to the body' tissues thus, changes in the numbers and/or morphology of the RBCs may indicate abnormalities or some hematological conditions.
| Groups | RBC (106 μl−1) | Ht (%) | Hb (g dl−1) | CMV (fentolitre) | WBC (103 μl−1) | Plt (103 mm−3) |
|---|---|---|---|---|---|---|
| a RBC, red blood cell; WBC, white blood cell; Ht, hematocrit; Hb, hemoglobin; CMV, mean cell volume; Plt, platelets. Data are expressed as mean ± SD in each group (n = 6). a,b,c,d,e,f,g The means with no common superscripts differ significantly (p < 0.01) | ||||||
| Control | 8.65 ± 0.37a | 40.7 ± 0.86a | 13.7 ± 0.95a | 55.1 ± 0.85g | 10.32 ± 0.5g | 645 ± 22.3e |
| Paracetamol (P) | 5.23 ± 0.47e | 36.6 ± 0.8d | 9.6 ± 0.9e | 22.1 ± 0.97f | 13.56 ± 0.13a | 1234 ± 31.6a |
| PH-LA/(P) | 6.98 ± 0.44d | 38.6 ± 0.57b | 10.32 ± 0.81d | 38.9 ± 0,67e | 11.62 ± 0.48c | 820 ± 43.6g |
| PH-TR (P−1) | 7.31 ± 0.63c | 40.5 ± 0.35a | 10.97 ± 0.68c | 40.1 ± 0.69c | 11.32 ± 0.72d | 836 ± 63.1f |
| PH-ES (P−1) | 8.07 ± 0.42b | 37.6 ± 0.97c | 12.95 ± 0.67a | 47.32 ± 0,37a | 10.67 ± 0.35f | 969 ± 38.6c |
| PH-A2/(P) | 8.32 ± 0.19a | 39.6 ± 0.81ab | 10.99 ± 0.63c | 39.6 ± 0,77d | 12.36 ± 0.29b | 987 ± 55.32b |
| PH-A26/(P) | 8.04 ± 0.72ab | 38.1 ± 0.44bc | 11.27 ± 0.52b | 45.12 ± 0.15b | 10.93 ± 0.77e | 852 ± 34.33d |
Generally, paracetamol overdose induced hematotoxicity which was marked by several abnormalities such as leukopenia, granulocytosis and neutropenia, thrombocytopenia, and pancytopenia in rats. The recorded hematotoxicity could be secondary to the deleterious effect of paracetamol on organs of hematopoiesis in the body which include liver.46
Interestingly, this study shows that the LAPHs could contain candidate bioactive peptides reversing the hematotoxic effect of paracetamol, with ensuing improvement of hematopoiesis.
3.8. Histopathological observations
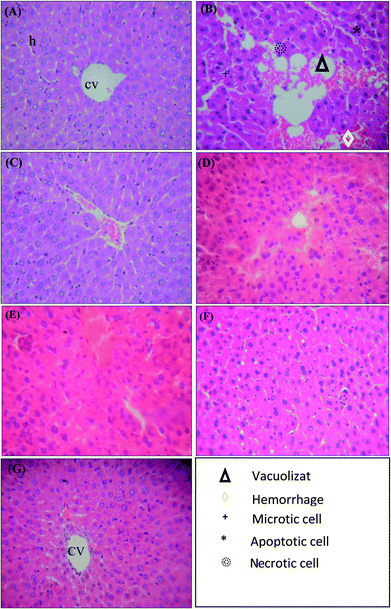
Histology of the liver sections of control animals showed normal hepatic architecture with well-preserved cytoplasm, and visible central veins (Fig. 3(A)). Liver sections of paracetamol-intoxicated rats revealed degenerative changes that involved the sinusoidal congestion, hemorrhages, confluent necrosis and massive inflammatory cell infiltration around perivenular area (Fig. 3(B)). Necrosis of hepatocyte is the important experimental feature in the hepatotoxicity studies. The extensive cell necrosis was the development of an inflammatory response with the recruitment of neutrophils and mononuclear cells into the liver.47 The damage extended to the majority of the hepatic lobule with marked loss of its normal pattern. The intracellular mechanisms of injury due to paracetamol in hepatocytes are the formation of reactive metabolites, depletion of glutathione and alkylation of proteins, especially the mitochondrial proteins.48 These initiating events trigger mitochondrial membrane permeability transition. The breakdown of the mitochondrial membrane permeability transition precedes the plasma membrane failure with cell swelling and leakage of cell content, i.e., cell death through oncotic necrosis.49However, the rat groups pre-treated with PH-LA, PH-A2 and PH-A26 exhibited significant liver protection against paracetamol-induced liver damage, as indicated by the presence of normal hepatic cells and absence of necrosis (Fig. 3(C), (F) and (G)). These results suggest the protective effect of LAPHs against chemical toxicity induced in rats. It was evidenced from the histopathological observation; the ability of LAPHs to reverse the hepatic lesions. The livers of animals treated with showed marked improvement in hepatocyte architecture in different areas around the central veins and portal tracts. This finding was consistent with the levels of the enzyme markers. Sigala et al.50 reported that the presence of mitotic cells in hepatocytes was assessed as an index of liver proliferative capacity in response to toxin-induced injury.
4. Conclusion
The present study demonstrated that pretreatment of rats with LAPHs protected the rats against paracetamol-induced hepatic damage as evidenced by a decrease in AST and ALT levels. Moreover, pretreatment with LAPHs was found to increase the antioxidant enzymes activities and GSH level as well as to decrease concentration of lipid peroxidation products. Further, liver histopathology showed that LAPHs reduced the incidence of liver lesions induced by paracetamol in rats. The obtained results suggest that protein hydrolysates from L. aurata contained some active peptides which possess hepato, hemato, and antioxidant potential against paracetamol-induced hepatotoxicity. Owing to its significant functionality, the incorporation of protein hydrolysates into food-based products is likely to be a promising practice for development of functional foods and nutraceuticals. Thus LAPHs may be used as a safe, cheap, and effective alternative chemopreventive and protective agent in the management of liver diseases.Ethical committee guidelines
All animal procedures were conducted in strict conformation with the local Institute Ethical Committee Guidelines for the Care and Use of laboratory animals of our Institution.Conflicts of interest
The authors declare that they have no competing interests to disclose.Acknowledgements
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research and the Tunisian Ministry of Public Health. The authors are grateful to Dr Khaled Dhibi, head of the surgery block of the Gafsa Hospital for his valuable encouragement and assistance in the realization of this work.References
- Frost and sullivan, Drug discovery and development, ed. H. P. Rang, Churchill Livingstone: Elsevier, 2007 Search PubMed.
- R. L. Esterline, S. D. Ray and S. Ji, Biochem. Pharmacol., 1989, 38, 2387–2390 CrossRef PubMed.
- P. Nourjah, S. R. Ahmad, C. Karwoski and M. Willy, Pharmacoepidemiol. Drug Saf., 2006, 15, 398–405 CrossRef PubMed.
- A. D. B. Vliegenthart, J. M. Shaffer, J. I. Clarke, L. E. J. Peeters, A. Caporali, D. N. Bateman, D. M. Wood, P. I. Dargan, D. G. Craig, J. K. Moore, A. I. Thompson, N. C. Henderson, D. J. Webb, J. Sharkey, D. J. Antoine, B. K. Park, M. A. Bailey, E. Lader, K. J. Simpson and J. W. Dear, Sci. Rep., 2015, 5, 15501 CrossRef PubMed.
- J. D. Ogilvie, M. J. Rieder and R. Lim, Can. Med. Assoc. J., 2012, 184, 1492–1496 CrossRef PubMed.
- S. H. L. Thomas, Toxicol. Lett., 2016, 258(suppl.), S34–S35 CrossRef.
- K. R. Atkuri, J. J. Mantovani, L. A. Herzenberg and L. A. Herzenberg, Curr. Opin. Pharmacol., 2007, 7, 355–359 CrossRef PubMed.
- G. A. Mahmoudi, P. Astaraki, A. Z. Mohtashami and M. Ahadi, Int. Med. Case Rep. J., 2015, 8, 65–69 Search PubMed.
- H.-T. Yao, M.-N. Luo and C.-C. Li, J. Funct. Foods, 2015, 12, 262–270 CrossRef.
- S. Nguyen, H. Huang, B. C. Foster, T. W. Tam, T. Xing, M. L. Smith, J. T. Arnason and H. Akhtar, J. Pharm. Pharm. Sci., 2014, 17, 254–265 Search PubMed.
- E. L. Andrade, A. F. Bento, J. Cavalli, S. K. Oliveira, R. C. Schwanke, J. M. Siqueira, C. S. Freitas, R. Marcon and J. B. Calixto, Braz. J. Med. Biol. Res., 2016, 49, e5646 Search PubMed.
- M. Nasri, in Advances in Food and Nutrition Research, Elsevier, 2017, vol. 81, pp. 109–159 Search PubMed.
- R. Nasri, I. Younes, M. Jridi, M. Trigui, A. Bougatef, N. Nedjar-Arroume, P. Dhulster, M. Nasri and M. Karra-Châabouni, Food Res. Int., 2013, 54, 552–561 CrossRef.
- R. Ben Slama-Ben Salem, I. Bkhairia, O. Abdelhedi and M. Nasri, J.Food Sci.Technol., 2017, 54, 1442–1454 CrossRef PubMed.
- I. Bkhairia, R. Ben Slama Ben Salem, R. Nasri, M. Jridi, S. Ghorbel and M. Nasri, J.Food Sci.Technol., 2016, 53, 2902–2912 CrossRef PubMed.
- R. Ben Slama-Ben Salem, N. Ktari, I. Bkhairia, R. Nasri, L. Mora, R. Kallel, S. Hamdi, K. Jamoussi, T. Boudaouara, A. El-Feki, F. Toldrá and M. Nasri, Food Res. Int., 2018, 106, 952–963 CrossRef PubMed.
- G. Şener, A. Ö. Şehirli and G. Ayanoğlu-Dülger, J. Pineal Res., 2003, 35, 61–68 CrossRef.
- A. A. Kembhavi, A. Kulkarni and A. Pant, Appl. Biochem. Biotechnol., 1992, 38, 83–92 CrossRef.
- H. Enari, Y. Takahashi, M. Kawarasaki, M. Tada and K. Tatsuta, Fish. Sci., 2008, 74, 911–920 CrossRef.
- National Research Council, Guide for the Care and the Use of Laboratory Animals, National Institute of Health, Bethesda, 1985, vol. 20, p. 85 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 Search PubMed.
- K. Yagi, Biochem. Med., 1976, 15, 212–216 CrossRef PubMed.
- H. Aebi, Methods Enzymol., 1984, 105, 121–126 Search PubMed.
- L. Flohé and W. A. Günzler, Methods Enzymol., 1984, 105, 114–121 Search PubMed.
- Y. Sun, L. W. Oberley and Y. Li, Clin. Chem., 1988, 34, 497–500 Search PubMed.
- G. L. Ellman, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef PubMed.
- N. Ktari, K. Mnafgui, R. Nasri, K. Hamden, I. Bkhairia, A. B. Hadj, T. Boudaouara, A. Elfeki and M. Nasri, Food Funct., 2013, 4, 1691–1699 RSC.
- S. W. A. Himaya, B. Ryu, D.-H. Ngo and S.-K. Kim, J. Agric. Food Chem., 2012, 60, 9112–9119 CrossRef PubMed.
- N. Rajapakse, W.-K. Jung, E. Mendis, S.-H. Moon and S.-K. Kim, Life Sci., 2005, 76, 2607–2619 CrossRef PubMed.
- Y. Ting, Y. Jiang, C.-T. Ho and Q. Huang, J. Funct. Foods, 2014, 7, 112–128 CrossRef.
- R. Nasri, G. Chataigné, A. Bougatef, M. K. Chaâbouni, P. Dhulster, M. Nasri and N. Nedjar-Arroume, J. Proteomics, 2013, 91, 444–452 CrossRef PubMed.
- M. J. Davies, S. Fu, H. Wang and R. T. Dean, Free Radicals Biol. Med., 1999, 27, 1151–1163 CrossRef PubMed.
- D. Scott, S. M. Galloway, R. R. Marshall, M. Ishidate, D. Brusick, J. Ashby and B. C. Myhr, Mutat. Res., 1991, 257, 147–205 Search PubMed.
- A. Flaks and B. Flaks, Carcinogenesis, 1983, 4, 363–368 CrossRef PubMed.
- U. Venkatachalam and S. Muthukrishnan, Biomedicine & Preventive Nutrition, 2013, 3, 273–277 Search PubMed.
- P. Madhu kiran and B. Ganga Rao, International Journal of Pharmaceutical Research and Development, 2011, 3, 2 Search PubMed.
- J. A. Hinson, J. B. Mays and A. M. Cameron, Biochem. Pharmacol., 1983, 32, 1979–1988 CrossRef PubMed.
- R. M. Galal, H. F. Zaki, M. M. Seif El-Nasr and A. M. Agha, Arch. Iran. Med., 2012, 15, 674–680 Search PubMed.
- Y.-S. Kim, C.-B. Ahn and J.-Y. Je, Food Chem., 2016, 202, 9–14 CrossRef PubMed.
- H. Hou, Y. Fan, S. Wang, L. Si and B. Li, J. Funct. Foods, 2016, 24, 37–47 CrossRef.
- A. C. B. Bandeira, T. P. da Silva, G. R. de Araujo, C. M. Araujo, R. C. da Silva, W. G. Lima, F. S. Bezerra and D. C. Costa, Chem.-Biol. Interact., 2017, 263, 7–17 CrossRef PubMed.
- B. Dimitrios, Trends Food Sci. Technol., 2006, 17, 505–512 CrossRef.
- S. Athira, B. Mann, R. Sharma and R. Kumar, J. Dairy Sci., 2013, 96, 1431–1437 CrossRef PubMed.
- R. Nasri, O. Abdelhedi, I. Jemil, I. Daoued, K. Hamden, C. Kallel, A. Elfeki, M. Lamri-Senhadji, A. Boualga, M. Nasri and M. Karra-Châabouni, Chem.-Biol. Interact., 2015, 242, 71–80 CrossRef PubMed.
- M. P. Singh, K. Y. Kim and H.-Y. Kim, Biochem. Biophys. Res. Commun., 2017, 484, 189–194 CrossRef PubMed.
- O. O. Iroanya, O. A. Adebesin and J. Okpuzor, J. Clin. Diagn. Res., 2014, 8, HC15–HC21 Search PubMed.
- H. Jaeschke, J. Pharmacol. Exp. Ther., 1990, 255, 935–941 Search PubMed.
- S. D. Nelson and S. A. Bruschi, in Drug-Induced Liver Disease, Informa Healthcare, 2007, pp. 353–388 Search PubMed.
- K. Kon, J.-S. Kim, H. Jaeschke and J. J. Lemasters, Hepatology, 2004, 40, 1170–1179 CrossRef PubMed.
- F. Sigala, S. Theocharis, K. Sigalas, S. Markantonis-Kyroudis, E. Papalabros, A. Triantafyllou, G. Kostopanagiotou and I. Andreadou, J. Pineal Res., 2006, 40, 270–279 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |