Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanolayer-like-shaped MgFe2O4 synthesised via a simple hydrothermal method and its catalytic effect on the hydrogen storage properties of MgH2
N. A. Alia,
Nurul Hayati Idrisa,
M. F. Md Dinb,
N. S. Mustafaa,
N. A. Sazeleea,
F. A. Halim Yapa,
N. N. Sulaimana,
M. S. Yahyaa and
M. Ismail *a
*a
aSchool of Ocean Engineering, Universiti Malaysia Terengganu, 21030 Kuala Terengganu, Malaysia. E-mail: mohammadismail@umt.edu.my; Fax: +609-6683991; Tel: +609-6683487
bDepartment of Electrical and Electronic Engineering, Faculty of Engineering, National Defence University of Malaysia, Kem Sungai Besi, Kuala Lumpur, Malaysia
First published on 25th April 2018
Abstract
In this study, the effect of nanolayer-like-shaped MgFe2O4 that is synthesised via a simple hydrothermal method on the performance of MgH2 for hydrogen storage is studied. MgH2 + 10 wt% MgFe2O4 is prepared by using the ball milling method. The MgFe2O4-doped MgH2 sample started to release H2 at approximately 250 °C, 90 °C and 170 °C lower than the milled and pure MgH2 respectively. At 320 °C, the isothermal desorption kinetic study has shown that the doped sample has desorbed approximately 4.8 wt% H2 in 10 min while the milled MgH2 desorbed less than 1.0 wt% H2. For isothermal absorption kinetics, the doped sample can absorb approximately 5.5 wt% H2 in 10 min at 200 °C. Meanwhile, the undoped sample absorbs only 4.0 wt% H2 in the same condition. The activation energy of 10 wt% MgFe2O4-doped MgH2 composite is 99.9 kJ mol−1, which shows a reduction of 33.1 kJ mol−1 compared to the milled MgH2 (133.0 kJ mol−1). X-ray diffraction spectra display the formation of new species which are Fe and MgO after dehydrogenation, and these new species are believed to act as the real catalyst that plays a crucial role in improving the sorption performance of the MgFe2O4-doped MgH2 system by providing a synergetic catalytic effect.
1. Introduction
To prepare for the future and ensure global environmental viability, energy systems have to be reliable, clean, low cost, environmentally friendly and flexible. Humanity is expected to use 40 TW of power (40 billion of kW) in the future. To satisfy this demand, different sources of renewable energy, such as hydrogen, are needed. Sustainable hydrogen is an ideal clean energy carrier because there is no carbon dioxide or other greenhouse gas emission at the end-user level. Commonly, there are 3 forms of storing hydrogen which is high-pressure gas, cryogenic liquid hydrogen in tanks (stored at 21.2 K) and as solid state hydrogen storage by either reacting with chemical compounds or absorbing. Among these approaches, solid-state hydrogen storage has higher potential for higher hydrogen density and may yield greater utility towards the practical implementation of hydrogen storage. Among the various materials for solid-state hydrogen storage, MgH2 considered as one of the most potential material due to its high hydrogen storage capacity (7.6 wt%), excellent reversibility and low cost.1 However, MgH2 is restricted by the decomposition temperature, which is high with slow sorption kinetics and is excessively stable thermodynamically.2 Many research have been conducted to overcome these disadvantages by altering the thermodynamics and improve the kinetic properties by producing nanostructures3,4 and utilizing catalysts such as carbon-based materials,5,6 metals,7–10 metal hydrides,11,12 metal oxides,13–18 metal halides,19–21 and nanosized alloys.22–24Previous research has proved that catalyst based on ternary metal oxides greatly improved the hydrogen storage performance of MgH2.15,25–35 Zhang et al.25 demonstrate that ferrite nanoparticles (MnFe2O4, ZnFe2O4, Mn0.5Zn0.5Fe2O4 and CoFe2O4) can greatly lower the decomposition temperature of MgH2. CoFe2O4 provides the best catalytic effect compared with other ferrites. New by-products are found after the dehydrogenation process, and its phase shows a great catalytic effect on the properties of hydrogen storage of MgH2. Furthermore, Li et al.26 shows a significant improvement in the desorption performance of MgH2 when catalysed with MnFe2O4. X-ray photoelectron spectroscopy and X-ray diffraction (XRD) tests show that Fe0.872O and Mg2MnO4 phases take part as significant role in enhancing the dehydriding performance of MgH2. Meanwhile, we showed in our previous study that MnFe2O4 synthesised via a simple hydrothermal method provides a remarkable effect in improving the hydrogen storage performance of MgH2.27 Interestingly, our result showed that Fe metal formed after dehydrogenation instead of Fe0.872O species, as claimed by Li et al.26 This variation paved the way for the debate on how ternary metal oxides, particularly ferrites, work as catalysts in improving the hydrogen sorption performance of MgH2. Moreover, the difference in the synthesis method of the catalysts may also provide a different effect in the catalytic role.
Inspired by the role of active species that formed during the heating process in the MgH2-ternary metal oxides catalyst system, it is quite interesting to investigate the use of other ferrites (e.g. MgFe2O4) as catalysts to improve the hydrogen sorption performance of MgH2. Therefore, in this work, MgFe2O4 was synthesised by using a simple hydrothermal method, and its catalytic effects on the hydrogen sorption performance of MgH2 were systematically studied. To the best of authors' knowledge, this paper is the first to study the hydrogen sorption performance of MgH2 catalysed with MgFe2O4. The possible catalysis mechanisms of MgFe2O4 in the sorption performances of MgH2 are also discussed in this paper.
2. Experimental details
The nanolayer-like-shaped MgFe2O4 was synthesised via a hydrothermal method. In a typical synthesis, a stoichiometric amount of Mg(NO3)2·6H2O (Sigma-Aldrich) and Fe(NO3)3·9H2O (Sigma-Aldrich) were dissolved in 50 ml distilled water. A total of 10 ml of H4N2·H2O (Sigma-Aldrich) was added dropwise to the above solution to attain the resultant pH of >9. The mixture was then transferred into a sealed Teflon lined stainless-steel autoclave (125 ml capacity) and heated for 12 h at 180 °C. The final product was washed several times with deionised water and dried overnight at 60 °C under vacuum. A total of 10 wt% of as-prepared MgFe2O4 was mixed with 300 mg of MgH2 (95% pure; Sigma-Aldrich) and undergo intensive ball milling for 1 h in a planetary ball mill at the rate of 400 rpm. For comparison, pure MgH2, and MgH2 added with 10 wt% Fe (Alfa Aesar) and 10 wt% MgO (R&M Chemicals), respectively were also prepared under the same conditions. All preparations, including loading and weighing, were conducted in an argon atmosphere glove box (MBraun Unilab).The onset decomposition temperature and sorption kinetic measurement for doped and undoped samples were characterised by using Sievert-type pressure–composition–temperature apparatus (Advanced Materials Corporation). For onset decomposition temperature measurement, the samples were heated from room temperature to 450 °C at a heating rate of 5 °C min−1 in vacuum chamber. Meanwhile, the sorption kinetics was conducted under 1.0 atm at 320 °C for desorption kinetic measurement and under 33.0 atm at 200 °C for absorption kinetic measurement. The thermal properties of the doped and undoped samples were performed using differential scanning calorimeter (DSC)/thermogravimetric analysis from Metler Toledo. With a flow of 50 ml min−1 argon, the samples were heated with 15, 20, 25 and 30 °C min−1 heating rate from 25 °C to 500 °C.
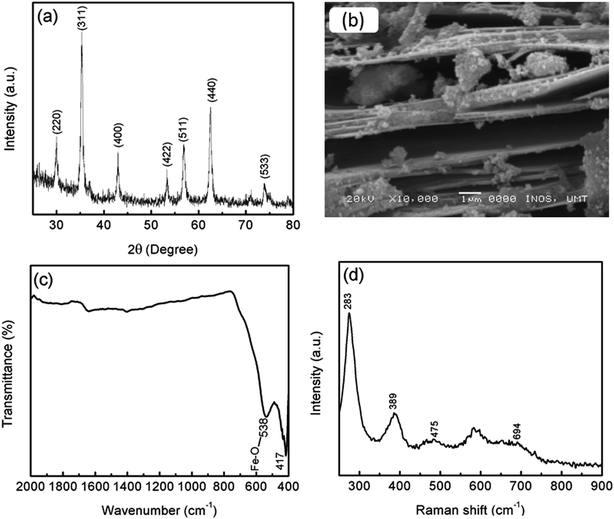
The phase composition of the samples was analysed by XRD via a Rigaku MiniFlex X-ray diffraction apparatus equipped with Cu Kα radiation. Data were collected in the 2θ range 20° to 80° at 2° min−1. The morphologies of the samples were observed by scanning electron microscopy (SEM) (JEOL JSM-6350LA). Fourier transform infrared (FTIR) spectrometry was recorded on an IR Shimadzu Tracer-100 between 400 and 2000 cm−1. Raman spectra were recorded on Renishaw Raman spectroscopy (532 nm radiation) extended with 0.1% power laser measurement at room temperature.
3. Results and discussion
Before milling with MgH2, the phase structure of MgFe2O4 was confirmed by XRD, as shown in Fig. 1(a). The crystallographic planes of (220), (311), (400), (422), (511), (440) and (533) correspond to the diffraction peaks at 2θ of 30.1°, 35.4°, 43.1°, 53.5°, 57.0°, 62.6° and 74.1°, respectively. This result is in good agreement with the standard cubic spinel structure (JCPDS 71-1232). No other peaks were detected in the sample. The average crystallite size of MgFe2O4 was approximately 19 nm, as determined by using Scherrer's formula:
L = kλ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, θ,
| (1) |
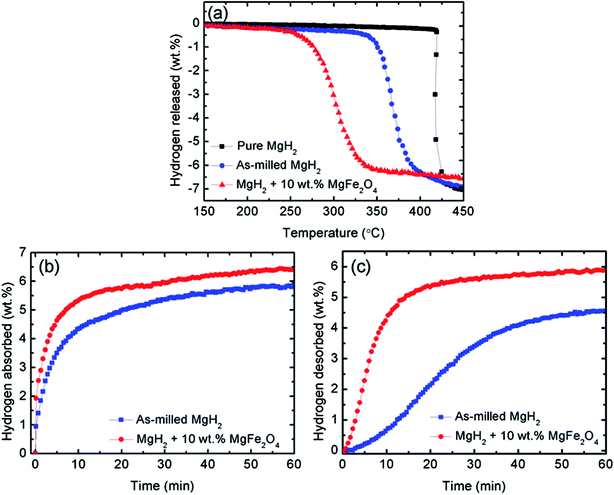
Fig. 2(a) shows the onset decomposition temperature results for the pure MgH2, milled MgH2 and MgH2 with 10 wt% MgFe2O4. Before milling, pure MgH2 started to desorb hydrogen at approximately 420 °C. The total amount of hydrogen desorbed is approximately 7.0 wt%. After milling for 1 h, the onset decomposition temperature of MgH2 was decreased to approximately 340 °C. This phenomenon demonstrate that the sorption performance of MgH2 also influenced by the milling process. From the curve, it can be seen that after milling, the amount of hydrogen desorb of MgH2 slightly decreases. This might be ascribed to the hydrogen released from MgH2 during the milling process. After doping with 10 wt% of MgFe2O4, it is clear that the onset decomposition temperature of the MgH2 was dramatically reduced to 250 °C, 90 °C and 170 °C lower than that for the milled and pure MgH2, respectively. However, the hydrogen desorption capacity decrease slightly to approximately 6.5 wt% because the dopant used in this study, namely, MgFe2O4, does not contain hydrogen.40 From Fig. 2(a), it can be concluded that MgFe2O4 additive plays a positive role in decreasing the decomposition temperature of MgH2.
To further examine the sorption properties of the MgFe2O4-doped MgH2 sample, the isothermal absorption kinetic was studied. The amount of hydrogen absorbed from the milled MgH2 and the MgFe2O4-doped MgH2 sample was measured under 33.0 atm H2 and at constant temperature of 200 °C, as shown in Fig. 2(b). The MgFe2O4-doped MgH2 sample shows better absorption kinetics than undoped MgH2. For the MgFe2O4-doped MgH2 sample, the amount of 5.5 wt% H2 was absorbed in 10 min, whereas the milled MgH2 only absorbed approximately 4.0 wt% H2 within the same time. From the result, it clearly shows that the addition of MgFe2O4 enhanced the rehydrogenation kinetics of MgH2.
For further studies on the catalytic effect of MgFe2O4 on the sorption kinetic of MgH2, isothermal desorption kinetic was performed under 1.0 atm at 320 °C. As shown in Fig. 2(c), the MgFe2O4-doped MgH2 sample shows significant enhancement compared with the milled MgH2. The results shows that the undoped MgH2 released less than 1.0 wt% H2 after 10 min, whereas the doped sample can released approximately 4.8 wt% H2 under the same condition. In contrast, it can be seen that MgFe2O4 also plays a significant role in enhancing the dehydrogenation kinetic of MgH2.
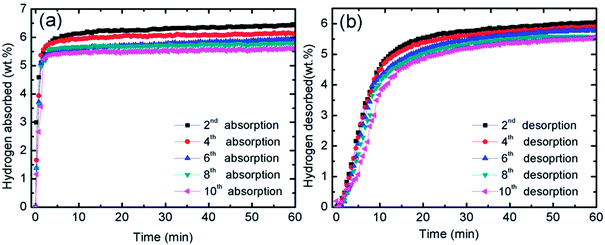
The catalytic effect of MgFe2O4 was further studied with the cycling performances of MgFe2O4-doped MgH2 system. Fig. 3(a) presents the isothermal absorption kinetics of the 10 wt% MgFe2O4 doped with MgH2 at 320 °C under a hydrogen pressure of 33.0 atm over 10 cycles. From the result, it can be seen that after the ten cycles, the absorption kinetics show a small reduction in the hydrogen capacity. After completing the 10th cycle, the system is able to absorb 5.6 wt% of hydrogen in 60 minutes. The result shows that the doped system displays good absorption properties even after 10 cycles. As for the desorption kinetics, Fig. 3(b) shows the isothermal desorption kinetics for 10 cycles that was carried out at 320 °C and under 1.0 atm of pressure. Like the absorption kinetics, a small hydrogen capacity degradation is shown after completing the 10th cycle. The doped system possesses a good performance after completing the 10th cycle as it is able to desorb about 5.5 wt% of hydrogen within 60 minutes. These results demonstrated that MgFe2O4 plays a vital catalytic role for the cycle life of MgH2.
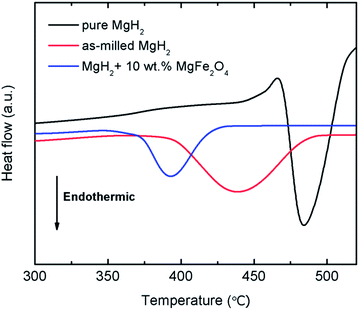
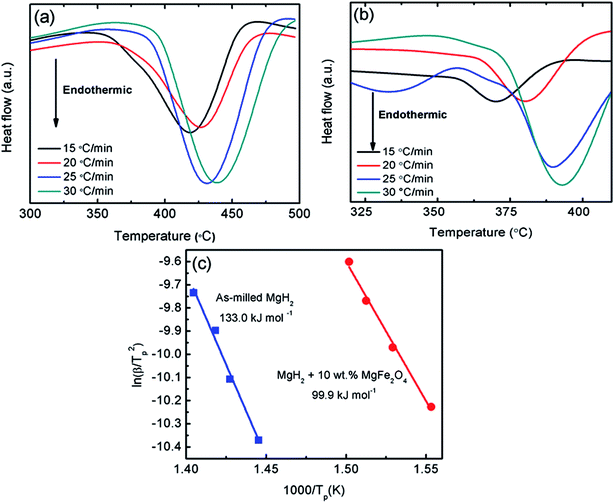
The thermal properties of the 10 wt% MgFe2O4-doped MgH2 and undoped MgH2 sample were further studied by DSC at heating rate of 30 °C min−1 and under a flow of 50 ml min−1 argon (Fig. 4). Obviously, the DSC trace for the pure MgH2 showed one endothermic peak at approximately 482.9 °C. This strong endothermic peak related to the released of hydrogen from the MgH2. Similar to the pure MgH2, DSC traces of the milled MgH2 and MgFe2O4-doped MgH2 showed only one strong endothermic peak at 438.8 °C and 393.3 °C respectively. The peaks correlated to the decomposition of MgH2 but at lower temperatures.
The improvement in desorption behaviour is correlated with the kinetic barrier of the hydrogen desorbed from the MgH2. By doping MgH2 with MgFe2O4, low value of activation energy for released hydrogen is obtained. Kissinger analysis41 (eqn (2)) was conducted to determine the activation energy of doped and undoped MgH2 samples.
| ln[β/Tp2] = −EA/RTp + A, | (2) |
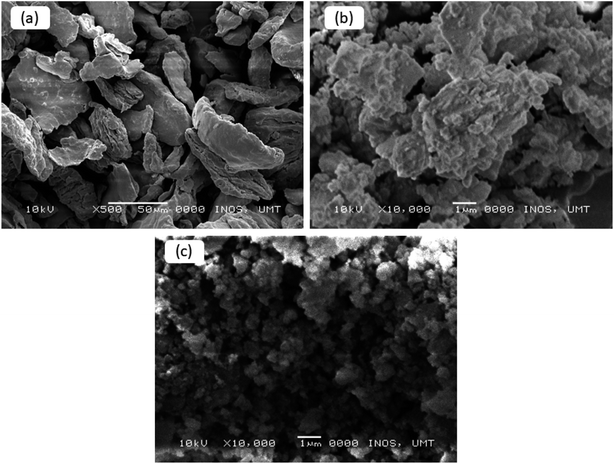
Fig. 6 presents the microstructures of the pure and milled MgH2, and MgFe2O4-doped MgH2. From the images, it can be seen clearly that the particle size of the pure MgH2 is around 50–100 μm (Fig. 6(a)). Fig. 6(b) shows the image of the MgH2 after 1 h ball milling. The size of the milled MgH2 was decreased dramatically compared to the pure MgH2. However, the image shows agglomeration and inconsistent particle sizes. Fig. 6(c) shows that the particle size of 10 wt% MgFe2O4-doped MgH2 was the smallest and had less agglomeration than the pure and milled MgH2. Smallest particle size gives a larger region of contact to the MgH2, thus resulting in the higher rate of reaction of MgH2.
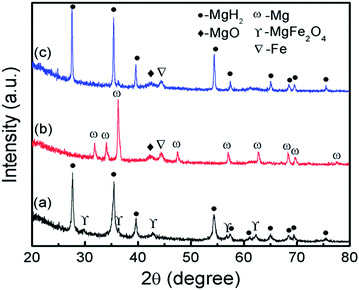
To investigate the phase structure, XRD measurement was performed on the 10 wt% MgFe2O4-doped MgH2 sample, as shown in Fig. 7. From Fig. 7(a), it can be observed that the MgH2 and MgFe2O4 phases are present in the as-milled MgFe2O4-doped MgH2 sample. No additional peaks were found from the spectra. After dehydrogenation at 450 °C (Fig. 7(b)), the XRD pattern showed that the MgH2 was completely dehydrogenated to Mg. This result demonstrates that the decomposition of MgH2 was completed after heating for up to 450 °C. Furthermore, a small peak of MgO and Fe formed after the desorption process, thus demonstrate that the partial reaction of MgH2 with MgFe2O4 may occur during the heating process as follows:
| 4MgH2 + MgFe2O4 → Mg + 4MgO + 2Fe + 4H2 | (3) |
 | ||
| Fig. 7 XRD spectra of the MgH2 + 10 wt% MgFe2O4 (a) after ball milling for 1 h, (b) after desorption under 1.0 atm at 450 °C and (c) after absorption under 33.0 atm at 320 °C. | ||
The standard Gibbs Free energy,  , of MgH2, MgFe2O4 and MgO are −35.9824, −1317.1232 and −569.024 kJ mol−1, respectively.42 The total change ΔG correlated with the reaction in eqn (3) is −815.0168 kJ mol−1. These values can confirm the possibility of the reaction in eqn (3) from thermodynamic potentials. Fig. 7(c) shows the XRD patterns for the rehydrogenated sample under 33.0 atm H2 at 320 °C. The result illustrates that the phase of Mg was fully converted into MgH2. Furthermore, the peak of Fe and MgO still remained after undergo rehydrogenation.
, of MgH2, MgFe2O4 and MgO are −35.9824, −1317.1232 and −569.024 kJ mol−1, respectively.42 The total change ΔG correlated with the reaction in eqn (3) is −815.0168 kJ mol−1. These values can confirm the possibility of the reaction in eqn (3) from thermodynamic potentials. Fig. 7(c) shows the XRD patterns for the rehydrogenated sample under 33.0 atm H2 at 320 °C. The result illustrates that the phase of Mg was fully converted into MgH2. Furthermore, the peak of Fe and MgO still remained after undergo rehydrogenation.
From the above analyses, the improvements in the sorption properties of MgH2 doped with 10 wt% MgFe2O4 may be resulted from the formations of Fe and MgO. Fe and MgO may act as the real catalysts that play a vital role in the improvements of MgH2 sorptions. Therefore, to verify the effect of Fe and MgO on MgH2, samples of 10 wt% MgO-doped MgH2 and 10 wt% Fe-doped MgH2 were prepared and the TPD profiles for the dehydrogenations were shown as in Fig. 8. It is clearly seen that the decomposition temperature of MgH2 are reduced with the addition of MgO or Fe as compared to the pure and milled MgH2. However, the performance of these MgO and Fe are not significant as the sample of 10 wt% of MgFe2O4 doped with MgH2. This demonstrated that the in situ generated MgO and Fe from the reaction of MgH2 + 10 wt% of MgFe2O4 may play a significant role that introduce a synergetic catalytic effect that cause a significant improvement on the dehydrogenation performances of MgH2 doped with 10 wt% of MgFe2O4. In addition, the in situ formed Fe and MgO in the MgH2 + 10 wt% of MgFe2O4 sample are speculated to have a higher degree of dispersion and more compact phase segregation as compared to the milled MgH2 + 10 wt% Fe and milled MgH2 + 10 wt% MgO. This would be likely to lead the improvement of the sorption kinetics.
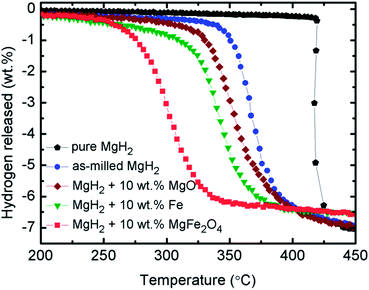 | ||
| Fig. 8 Decomposition temperature profile of pure and milled MgH2, 10 wt% MgO-doped MgH2, 10 wt% Fe-doped MgH2 and 10 wt% MgFe2O4-doped MgH2 sample. | ||
From the result obtained, we postulate that formation of fresh and fine MgO and Fe species which resulted from the reaction between MgH2 and MgFe2O4 during the dehydrogenation process may play significant role in improving the sorption performances of MgH2. Numerous studies have claimed that the newly active species formed during the de/absorption process may play as a real catalyst in the enhancement of MgH2 sorption.43,44 Many works have proven that Fe is an excellent catalyst for sorption performance in MgH2.7–9 It is believed that the presence of fresh and fine Fe could interact with H2 molecules, thus possibly leading to the dissociation of H2 molecules and the improvement of the de/rehydrogenation kinetic. Furthermore, previous studies have shown that the sorption performance in MgH2 can be enhanced with the addition of MgO. Ares-Fernández and Aguey-Zinsou45 claimed that the addition of MgO to MgH2 during the milling process led to an enhancement of sorption kinetics because of the high electronegativity MgO. In another study, the same group also claimed that during the milling process, MgO may act as a process control agent that can lead to the reduction of the particle agglomeration of MgH2 by an optimal breakage rate, thus aiding the high stability of these particles and evading the use of cold welding.46 Shan et al.15 also revealed that MgO has a great catalytic effect on the MgH2 sorption performance. Their study showed that during the heating process in CoFe2O4-doped MgH2 composite system, MgO is formed. The catalytic effect of MgO could work together with the catalytic role of the Fe metal to create a synergetic effect. Therefore, the in situ active species of Fe and MgO may actually act as real catalysts and further enhance the hydrogen sorption performance of MgH2. However, further studies are needed to elucidate more details on the exact role of MgFe2O4 addition in MgH2.
4. Conclusion
In this study, nanolayer-like-shaped MgFe2O4 was successfully synthesised through a rapid, simple hydrothermal method. The addition of 10 wt% as-synthesised MgFe2O4 to MgH2 reduces the onset decomposition temperature and enhances sorption kinetics. The MgFe2O4-doped MgH2 sample has started to release H2 at approximately 250 °C, 90 °C and 170 °C lower than milled and pure MgH2 respectively. In a duration of 10 min, the isothermal desorption kinetic study showed that the doped sample can release approximately 4.8 wt% H2 at 320 °C while the milled MgH2 only desorbed less than 1.0 wt% H2 under the same condition. For isothermal absorption kinetics, the doped sample can absorb approximately 5.5 wt% H2 in 10 min at 200 °C. By contrast, the milled MgH2 sample absorbed only 4.0 wt% H2 in the same condition. From the Kissinger analysis, the apparent activation energies, EA, for the MgFe2O4-doped MgH2 sample were calculated to be 99.9 kJ mol−1, which is decreased by 33.1 kJ mol−1 compared with the milled MgH2 (133.0 kJ mol−1). The XRD exploration displays the formation of new species of Fe and MgO after the dehydrogenation process, and these species remained unchanged after rehydrogenation. It is believed that the new species of Fe and MgO play a synergistic role in enhancing the hydrogen storage performances of MgH2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Ministry of Higher Education of Malaysia for financial support through the Fundamental Research Grant Scheme (FRGS 59362). The authors also would like to give appreciation to Universiti Malaysia Terengganu for the good facilities to accomplish this project.References
- I. P. Jain, C. Lal and A. Jain, Int. J. Hydrogen Energy, 2010, 35, 5133–5144 CrossRef CAS.
- L. Ouyang, J. Tang, Y. Zhao, H. Wang, X. Yao, J. Liu, J. Zou and M. Zhu, Sci. Rep., 2015, 5, 10776 CrossRef CAS PubMed.
- M. Polanski, J. Bystrzycki and T. Plocinski, Int. J. Hydrogen Energy, 2008, 33, 1859–1867 CrossRef CAS.
- J. Huot, G. Liang, S. Boily, A. Van Neste and R. Schulz, J. Alloys Compd., 1999, 293–295, 495–500 CrossRef CAS.
- N. N. Sulaiman and M. Ismail, Dalton Trans., 2016, 45, 19380–19388 RSC.
- M. A. Lillo-Ródenas, Z. X. Guo, K. F. Aguey-Zinsou, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2008, 46, 126–137 CrossRef.
- N. Hanada, T. Ichikawa and H. Fujii, J. Phys. Chem. B, 2005, 109, 7188–7194 CrossRef CAS PubMed.
- H. Chen, H. Yu, Q. Zhang, B. Liu, P. Liu, X. Zhou, Z. Han and S. Zhou, J. Power Sources, 2016, 322, 179–186 CrossRef CAS.
- G. Liang, J. Huot, S. Boily, A. Van Neste and R. Schulz, J. Alloys Compd., 1999, 292, 247–252 CrossRef CAS.
- S. A. Shevlin and Z. X. Guo, J. Phys. Chem. C, 2013, 117, 10883–10891 CAS.
- J. Lu, Y. J. Choi, Z. Z. Fang, H. Y. Sohn and E. Ronnebro, J. Am. Chem. Soc., 2009, 131, 15843–15852 CrossRef CAS PubMed.
- N. Patelli, M. Calizzi, A. Migliori, V. Morandi and L. Pasquini, J. Phys. Chem. C, 2017, 121, 11166–11177 CAS.
- N. S. Mustafa and M. Ismail, J. Alloys Compd., 2017, 695, 2532–2538 CrossRef CAS.
- D. Pukazhselvan, N. Nasani, P. Correia, E. Carbó-Argibay, G. Otero-Irurueta, D. G. Stroppa and D. P. Fagg, J. Power Sources, 2017, 362, 174–183 CrossRef CAS.
- J. Shan, P. Li, Q. Wan, F. Zhai, J. Zhang, Z. Li, Z. Liu, A. A. Volinsky and X. Qu, J. Power Sources, 2014, 268, 778–786 CrossRef CAS.
- Z. Jalil, A. Rahwanto, F. Mulana and M. Mustanir, Int. J. Technol., 2016, 7, 1301–1306 CrossRef.
- T. Ma, S. Isobe, Y. Wang, N. Hashimoto and S. Ohnuki, J. Phys. Chem. C, 2013, 117, 10302–10307 CAS.
- M. S. Yahya and M. Ismail, Int. J. Hydrogen Energy, 2018, 43, 6244–6255 CrossRef CAS.
- N. N. Sulaiman, N. S. Mustafa and M. Ismail, Dalton Trans., 2016, 45, 7085–7093 RSC.
- L. P. Ma, X. D. Kang, H. B. Dai, Y. Liang, Z. Z. Fang, P. J. Wang, P. Wang and H. M. Cheng, Acta Mater., 2009, 57, 2250–2258 CrossRef CAS.
- N. N. Sulaiman, N. Juahir, N. S. Mustafa, F. A. Halim Yap and M. Ismail, J. Energy Chem., 2016, 25, 832–839 CrossRef.
- D. Wu, L. Ouyang, C. Wu, H. Wang, J. Liu, L. Sun and M. Zhu, J. Alloys Compd., 2015, 642, 180–184 CrossRef CAS.
- X. B. Yu, Y. H. Guo, H. Yang, Z. Wu, D. M. Grant and G. S. Walker, J. Phys. Chem. C, 2009, 113, 5324–5328 CAS.
- X. B. Yu, Z. X. Yang, H. K. Liu, D. M. Grant and G. S. Walker, Int. J. Hydrogen Energy, 2010, 35, 6338–6344 CrossRef CAS.
- J. Zhang, J. Shan, P. Li, F. Zhai, Q. Wan, Z. Liu and X. Qu, J. Alloys Compd., 2015, 643, 174–180 CrossRef CAS.
- P. Li, Q. Wan, Z. Li, F. Zhai, Y. Li, L. Cui, X. Qu and A. A. Volinsky, J. Power Sources, 2013, 239, 201–206 CrossRef CAS.
- N. H. Idris, N. S. Mustafa and M. Ismail, Int. J. Hydrogen Energy, 2017, 42, 21114–21120 CrossRef CAS.
- N. Juahir, N. S. Mustafa, A. Sinin and M. Ismail, RSC Adv., 2015, 5, 60983–60989 RSC.
- Q. Wan, P. Li, J. Shan, F. Zhai, Z. Li and X. Qu, J. Phys. Chem. C, 2015, 119, 2925–2934 CAS.
- T. Zhang, S. Isobe, A. Jain, Y. Wang, S. Yamaguchi, H. Miyaoka, T. Ichikawa, Y. Kojima and N. Hashimoto, J. Alloys Compd., 2017, 711, 400–405 CrossRef CAS.
- T. Zhang, S. Isobe, Y. Wang, H. Oka, N. Hashimoto and S. Ohnuki, J. Mater. Chem. A, 2014, 2, 4361–4365 CAS.
- G. Xu, N. Shen, L. Chen, Y. Chen and W. Zhang, Mater. Res. Bull., 2017, 89, 197–203 CrossRef CAS.
- N. S. Mustafa, N. N. Sulaiman and M. Ismail, RSC Adv., 2016, 6, 110004–110010 RSC.
- M. Baricco, M. W. Rahman, S. Livraghi, A. Castellero, S. Enzo and E. Giamello, J. Alloys Compd., 2012, 536(supplement 1), S216–S221 CrossRef CAS.
- M. S. Yahya and M. Ismail, J. Energy Chem., 2018 DOI:org/10.1016/j.jechem.2017.10.020.
- A. Pradeep, P. Priyadharsini and G. Chandrasekaran, J. Magn. Magn. Mater., 2008, 320, 2774–2779 CrossRef CAS.
- K. B. Modi, M. C. Chhantbar and H. H. Joshi, Ceram. Int., 2006, 32, 111–114 CrossRef CAS.
- Y. Yin, W. Liu, N. Huo and S. Yang, ACS Sustainable Chem. Eng., 2017, 5, 563–570 CrossRef CAS.
- Z. Yan, J. Gao, Y. Li, M. Zhang and M. Guo, RSC Adv., 2015, 5, 92778–92787 RSC.
- Y. Liu, C. Liang, H. Zhou, M. Gao, H. Pan and Q. Wang, Chem. Commun., 2011, 47, 1740–1742 RSC.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- http://www.chemistry-reference.com/StandardThermodynamicValues.pdf.
- Y. Cheng, W. Zhang, J. Liu, K. Cheng and Z. Zhao, Int. J. Hydrogen Energy, 2017, 42, 356–365 CrossRef CAS.
- M. Ismail, N. S. Mustafa, N. Juahir and F. A. H. Yap, Mater. Chem. Phys., 2016, 170, 77–82 CrossRef CAS.
- J.-R. Ares-Fernández and K.-F. Aguey-Zinsou, Catalysts, 2012, 2, 330 CrossRef.
- K. F. Aguey-Zinsou, J. R. Ares Fernandez, T. Klassen and R. Bormann, Mater. Res. Bull., 2006, 41, 1118–1126 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |