Open Access Article
Open Access ArticleRetracted Article: Ligustrazine attenuates renal damage by inhibiting endoplasmic reticulum stress in diabetic nephropathy by inactivating MAPK pathways
Hongling Yangab and
Shukun Wu *ab
*ab
aDepartment of Nephrology, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, No. 23, Section 2, 1st Ring Road (West), Chengdu, Sichuan 610000, China. E-mail: wushukuncd@163.com; Tel: 028-873939999
bChinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, Sichuan 610000, China
First published on 13th June 2018
Abstract
Background: Diabetic nephropathy (DN) is a major cause of chronic kidney disease around the world. Endoplasmic reticulum (ER) stress plays an important role in DN progression. Ligustrazine (Lig) is derived from the Chinese herb Ligusticum wallichii and is reported to exert anti-oxidant, anti-inflammation and anti-fibrosis effects. The aim of our study was to investigate the influence of Lig on the treatment of DN. Methods: Streptozotocin (STZ) was used to induce diabetes in Sprague–Dawley (SD) rats. Then, STZ-induced rats were treated with different concentrations of Lig (50 or 150 mg kg−1 day−1) for 8 weeks of treatment. Urinary albumin concentrations, blood urea nitrogen (BUN), serum creatinine (Scr) and creatinine clearance (Ccr) were determined. The levels of proinflammatory cytokines (IL-8, IL-6, IL-1β and TNF-α) were estimated by ELISA. TUNEL assay was used for apoptosis index measurement. Western blot was used for the detection of GRP78, CHOP, p-eIF2α, eIF2α, p-p38, p-38, p-ERK1/2 and ERK1/2. Results: Lig treatment significantly reduced urinary albumin excretion, BUN and Scr and increased Ccr in STZ-induced rats. Lig also suppressed the levels of IL-8, IL-6, IL-1β and TNF-α and inhibited apoptosis dose-dependently. In addition, Lig inhibited GRP78 and CHOP expression and prevented the phosphorylation of eIF2α, p-38 and ERK1/2. Conclusion: Our study indicated that Lig attenuated renal damage by inhibiting ER stress in DN by inactivating MAPK pathways.
Introduction
Diabetic nephropathy (DN) is a major cause of chronic kidney disease around the world and has a high morbidity and mortality rate due to end stage renal disease.1 The common therapies for DN include good control of hyperglycemia and blood pressure, and inhibition of the renin–angiotensin–aldosterone system.2,3 However, these treatments can only suppress the progression of DN, and can't reverse the established complications. Hence, developing novel and effective strategies for DN treatment is urgently required.The endoplasmic reticulum (ER) is an important organelle for the folding and processing of newly synthesized proteins. Accumulation of misfolded and unfolded proteins in the ER under pathophysiological stress will lead to ER stress and activate a well-conserved intracellular signaling pathway known as the unfolded protein response (UPR).4,5 UPR consists of three highly coordinated signaling pathways including the activating transcription factor-6 (ATF6), PKR-like ER protein kinase (PERK) and the inositol-requiring enzyme-1 (IRE1α).6 Moderate ER stress promotes cell survival while excessive ER stress may lead to cell injury and death. It is reported that ER stress plays vital roles in various types of diseases such as cancer, diabetes and cardiovascular diseases.7–9 Recent studies indicated that ER stress was also involved in the pathophysiology of various renal diseases including DN.10 Research by Cheang reported that ER stress was augmented and correlates with podocyte injury, proteinuria and disease in diabetic mice and DN patients.11 Therefore, reducing ER stress may attenuate renal damage during DN progression.
Ligustrazine (Lig) is an alkaloid isolated from the rhizoma of traditional Chinese herb Ligusticum wallichii and is reported to exert anti-oxidant, anti-inflammation and anti-fibrosis effects.12 Lig is often used to treat a variety of vascular diseases.13 Chen elucidated that Lig could alleviate acute pancreatitis by accelerating acinar cell apoptosis.14 Moreover, Lig can also protect kidneys from renal ischemia/reperfusion injury.15,16 However, the effects of Lig on DN and the underlying mechanism haven't been discussed before.
In our present study, we aimed to study the influence of Lig on the treatment of DN. Our study demonstrated that the renoprotective effects of Lig are associated with the attenuation of ER stress via inactivating MAPK pathways.
Materials and methods
Statement
The animal experiments in this study were approved by the Animal Care and Research Committee of Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital. All experiments were performed in compliance with relevant laws and guidelines. Besides, all experiments were conducted following institutional guidelines of Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital.Animal models
All animal experiments were carried out according to the guidelines for Institutional Animal Care and Use, with approval of Animal Research Ethics Committee of Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital. Male, 6 week-old Sprague–Dawley (SD) rats weighing 180–200 g were kept under pathogen-free conditions. Rats were injected intraperitoneally with 100 mg kg−1 body weight streptozotocin (STZ, Sigma-Aldrich, St Louis, MO, USA, dissolved in 0.01 M citrate buffer, pH 4.5) for 2 consecutive days. One week after STZ injection, rats with a blood glucose levels over 350 mg dL−1 were considered as having diabetes and were divided into 3 groups. Lig (purity > 99%) was purchased from the Chinese Medicines and Biological Products Institute (Beijing, CN). Then, rats in these 3 groups were treated with Lig 50 mg kg−1 day−1, Lig 150 mg kg−1 day−1 and equal volume of sterile saline by daily gavage for 8 weeks, respectively. Normal rats without STZ treatment were randomly divided into 2 groups and administered respectively with Lig 150 mg kg−1 day−1 and equal volume of sterile saline respectively. Rats were kept in individual metabolic cages for 24 h urine collection at the end of treatment. Urine was centrifuged at 800 × g for 10 min at 25 °C prior to testing. At the end of 8 weeks of treatment, rats were anesthetized with pentobarbital sodium and blood samples taken from the abdominal aorta, and then centrifuged for 15 min at 2000 × g to obtain plasma for measuring biochemical parameters. After sacrificed, the kidneys from rats were also collected for RNA preparation/protein extraction or histology and immunostaining.Assessment of renal dysfunction
Urinary albumin concentrations were measured using an ELISA Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. Blood urea nitrogen (BUN), serum creatinine (Scr) and urea creatinine were measured using an automatic biochemistry analyzer (Hitachi Model 7600, Hitachi High-Technologies, Tokyo, Japan). Creatinine clearance (Ccr) was computed using the following formula: creatinine clearance [Ccr] (ml min−1) = [urea creatinine/serum creatinine] × urine volume (ml).Enzyme linked immunosorbent assay (ELISA)
The levels of proinflammatory cytokines (IL-8, IL-6, IL-1β and TNF-α) in kidney homogenate were estimated by specific ELISA kits according to the manufacturer's instructions (Sigma Aldrich (St. Louis, MO, USA)). The concentration of cytokines was determined spectrophotometrically at 450 nm. Standard plots were constructed and the concentrations for unknown samples were calculated from the standard plot. The analysis was done according to the manufacturer's instructions.TUNEL assay
Colorimetric TUNEL Apoptosis Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China) was used for apoptosis index measurement. Tissues slices were incubated in the TUNEL reaction mixture followed by 3% H2O2. DAB was used for visualizing the sections. Hematoxylin was used for counter-staining. The numbers of TUNEL-positive cells of 6 random fields were counted under light microscopy. The apoptosis index was calculated as the percent of TUNEL-positive cells relative to the total cells.Western blot analysis
Proteins were extracted from tissues using RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and total protein concentration was measured using the BCA method. Equal amount of proteins were separated by 10% SDS-PAGE gel and then transferred into PVDF membranes. After blocking with nonfat milk at room temperature for 1 h, membranes were incubated with primary antibodies at 4 °C overnight. The following antibodies were used (at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution): GRP78 (ab21685, Abcam, Cambridge, UK), CHOP (sc-575, Santa Cruz, Dallas, TX, USA), phospho-eIF2α (#3398, Cell Signaling Technology) (CST), eIF2α (#5324, CST), p-p38 (#4511, CST), p-38 (#8690, CST), p-ERK1/2 (#4370, CST), ERK1/2 (#4695, CST) and GAPDH (#5174, CST). After incubating with horseradish peroxidase (HRP)-linked secondary antibody for 1 h at room temperature, immunodetection was performed by chemiluminescence.
1000 dilution): GRP78 (ab21685, Abcam, Cambridge, UK), CHOP (sc-575, Santa Cruz, Dallas, TX, USA), phospho-eIF2α (#3398, Cell Signaling Technology) (CST), eIF2α (#5324, CST), p-p38 (#4511, CST), p-38 (#8690, CST), p-ERK1/2 (#4370, CST), ERK1/2 (#4695, CST) and GAPDH (#5174, CST). After incubating with horseradish peroxidase (HRP)-linked secondary antibody for 1 h at room temperature, immunodetection was performed by chemiluminescence.
Statistical analysis
Data was expressed as the mean ± standard deviation (SD) of independent experiments. All statistical analysis was performed with SPSS 19.0. Group comparisons were performed using Student's t test or one-way ANOVA. The difference was considered statistically significant at P < 0.05.Results
Lig attenuates renal damage in DN
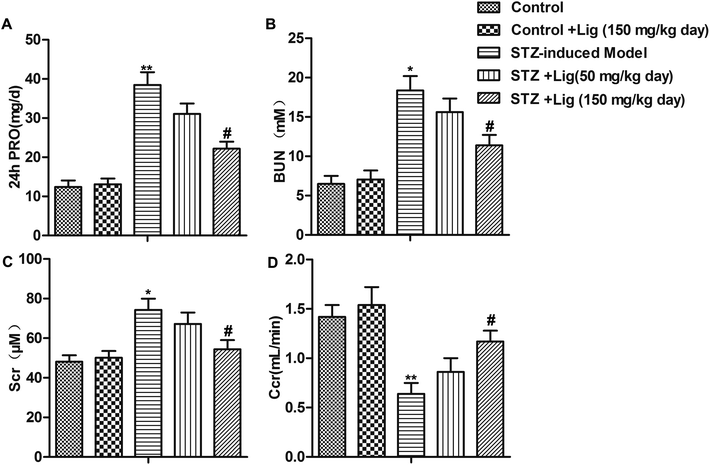
Assessment of renal dysfunction was conducted in our study and the results were shown in Fig. 1. Compared with the control group, the rats injected with STZ to induce diabetes (STZ-induced model group) showed significantly higher 24 h urinary protein (PRO) extraction, BUN, Scr levels and lower Ccr level. After STZ-induced diabetes rats received Lig treatment for 8 weeks, the 24 h PRO, BUN and Scr levels were reduced while Ccr level was increased in a dose-dependent manner (*P < 0.05, **P < 0.01, #P < 0.05). These results indicated that Lig attenuated the functional abnormalities present in diabetic nephropathy dose-dependently.Lig suppresses inflammation and apoptosis in DN
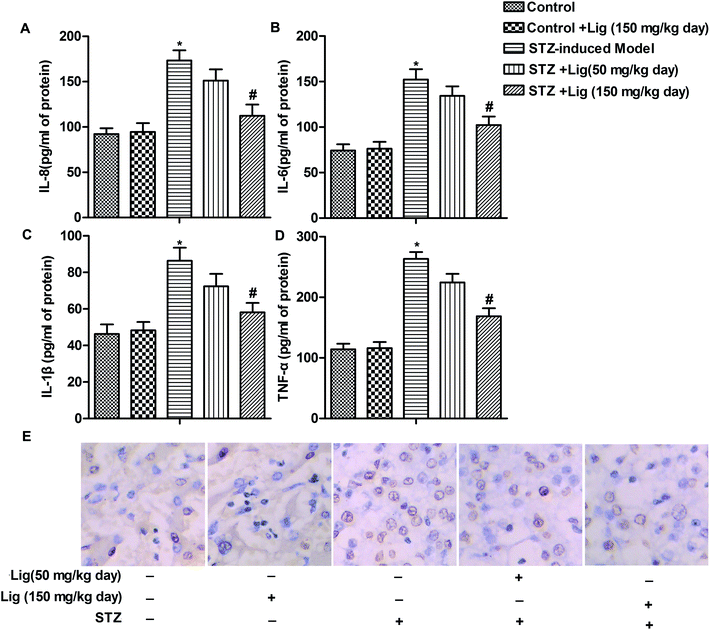
The levels of inflammatory cytokines IL-8, IL-6, IL-1β and TNF-α in kidney of rats were determined by ELISA assay, and the results were shown in Fig. 2A–D. As shown, the levels of IL-8, IL-6, IL-1β and TNF-α were remarkably up-regulated after induction of diabetes by STZ treatment in rats. However, Lig treatment decreased the high levels of IL-8, IL-6, IL-1β and TNF-α induced by STZ in a dose-dependent manner (Fig. 2A–D, *P < 0.05, #P < 0.05). Moreover, results from TUNEL assay showed that apoptosis rate was increased in STZ-induced diabetes mice and Lig suppressed this high apoptosis rate induced by STZ dose-dependently (Fig. 2E). Our data suggested that Lig suppressed inflammation and apoptosis in DN.Lig alleviates ER stress in DN
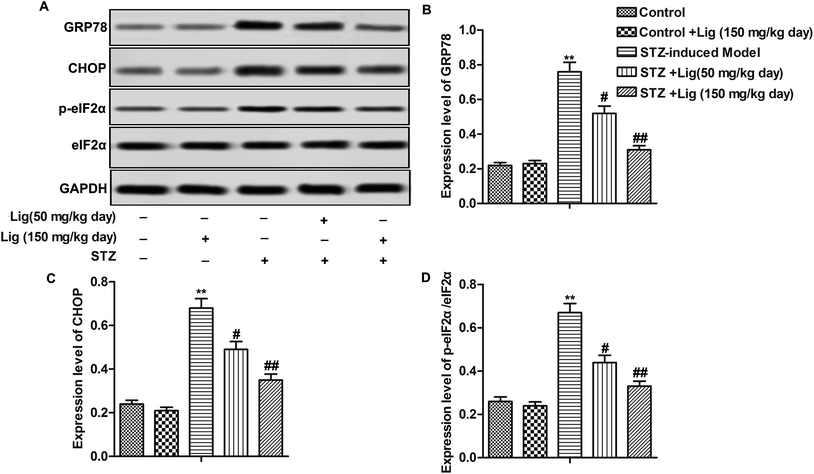
Compared with control group, relative expression of ER stress related proteins GRP78, CHOP and p-eIF2α/eIF2α ratio were significantly increased, indicating that STZ did induce ER stress in the kidney. However, the induction of ER stress by STZ was blocked by Lig treatment in a dose-dependent manner (Fig. 3A–D, **P < 0.01, #P < 0.05, ##P < 0.01). Our finding demonstrated that Lig alleviated STZ-induced ER stress in DN.Lig inactivates MAPK pathways in DN
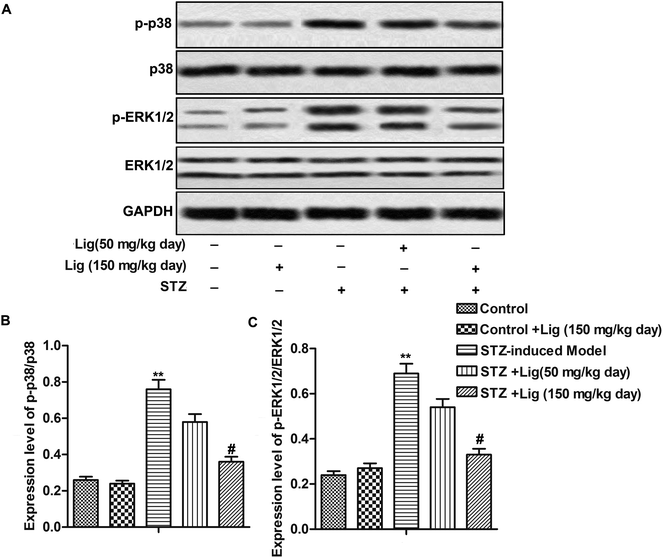
As shown in Fig. 4, the level of p-38 and ERK phosphorylation in STZ-induced mice model was much higher than the control group, indicating that p-38 and ERK MAPK pathways were activated in STZ-induced diabetes mice. After Lig treatment for 8 weeks, the ratios of p-p-38/p-38 and p-ERK1/2/ERK1/2 were significantly decreased in a dose-dependent manner (Fig. 4A–C, **P < 0.01, #P < 0.05), suggesting that Lig inactivated MAPK pathway in DN.Discussion
DN is one of the most common causes of chronic kidney disease. STZ-induced diabetes animal model is often considered as a suitable experimental model for DN study. This animal model is characterized by moderate and stable hyperglycemia, considerably altered glucose-roused insulin secretion and glucose intolerance which are similar to the features of human diabetes.17 In our present study, Lig was used for the treatment of STZ-induced diabetes rat model and our results demonstrated that Lig attenuated renal damage in STZ-induced DN by inhibiting ER stress via inactivating MAPK pathways.Development of DN is characterized by increased plasma levels of creatinine and BUN in STZ-induced diabetic rats.18 In our present study, we observed higher 24 h urine protein, BUN, Scr and lower Ccr in STZ-induced diabetes rats compared with control group, which in agreement with earlier reports.19,20 Administration of Lig significantly restored renal functions by decreasing 4 h urine protein, BUN, Scr and increasing Ccr dose-dependently in STZ-induced diabetes rats, suggesting that Lig might potentially be an effective agent that could exert beneficial effects on kidney tissues.
Accumulating evidence indicated that pro-inflammatory cytokines including IL-8, IL-6, IL-1β and TNF-α played important roles in the development and progression of DN. Theses pro-inflammatory cytokines were reported to be involved in the development of interstitial fibrosis in both diabetic patients and experimental models.21,22 TNF-α can generate reactive free radicals in mesangial cells to produce direct renal damage. IL-1β is also involved in the development of irregularities in intraglomerular hemodynamics.23 IL-1β in association with TNF-α may aggravate expression of induced nitric oxide synthase and cellular cyclic guanosine monophosphate (cGMP) concentrations in mesangial cells, which finally causes tissue injury and contributes to hyperfiltration and microalbuminuria.24,25 Recent studies have demonstrated a significant association between IL-6 and glomerular basement membrane thickening.22 Therefore, we determined the levels of IL-8, IL-6, IL-1β and TNF-α in kidney tissues with or without STZ treatment. Our results showed that the levels were significantly elevated in STZ-induced model group which kept consistent with previous studies. Administration of Lig down-regulated the levels of IL-8, IL-6, IL-1β and TNF-α remarkably. Moreover, STZ causes pancreatic β cell apoptosis and develops an experimental model for the type 1 diabetes mellitus.17 Here, we observed that STZ also increased the number of apoptosis cells in the kidney. Lig treatment decreased apoptosis cells number in a dose-dependent manner. Hence, we concluded that Lig could inhibit leakage of pro-inflammatory cytokines to suppress inflammatory reaction and also inhibit cell apoptosis in kidney.
ER stress occurs when the capacity of ER to fold proteins is overwhelmed. In response, UPR pathways are activated to increase the capacity to fold proteins. However, if the response is insufficient, then apoptosis ensues. Previous study indicated that proteinuria and hyperglycemia generated reactive oxygen species and required increasing synthesis of membrane proteins in the kidney, which could induce ER stress in renal epithelial cells.26 ER stress was also reported to contribute to glomerular diseases with proteinuria.27 Qi reported that suppressing ER stress could attenuate STZ-induced DN in rats.28 GRP78 is a crucial regulator of ER homeostasis and is involved in the activation of the ER stress response.6 CHOP is an important mediator of cell death in response to ER stress.29 During ER stress, the eIF2α-ATF4 pathway is activated and eventually leads to apoptosis.30 In our study, we found that Lig significantly alleviated ER stress induced by STZ through inhibiting GRP78 and CHOP expression and phosphorylation of eIF2α.
MAPK signaling pathways control the expression activity of a broad range of transcription factor genes. The activation mechanism of the MAPKs family is characterized by 3 levels of enzymatic reaction of phosphorylation.31 The family includes ERK, JNK, p-38 and ERK5.32 Inhibition of MAPK pathways was reported to attenuate DN in a great number of previous studies.33,34 Moreover, study from Chen demonstrated that Lig alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression on p38 and Erk MAPK pathways.14 In agreement with these previous studies, we observed that phosphorylation of p-38 and ERK1/2 was highly up-regulated in STZ-induced model group. However, Lig treatment suppressed phosphorylation of p-38 and ERK1/2 in a dose-dependent manner. Our results suggested that Lig suppressed activated p-38 and ERK MAPK pathways in DN.
Taken together, our study showed that the use of Lig seems to be effective to attenuate renal damage by inhibiting ER stress in DN via suppressing p-38 and ERK MAPK pathways. Therefore, Lig may have a therapeutic effect on patients with DN.
Conflicts of interest
NoneAbbreviations
| DN | Diabetic nephropathy |
| ER | Endoplasmic reticulum |
| Lig | Ligustrazine |
| STZ | Streptozotocin |
| BUN | Blood urea nitrogen |
| Scr | Serum creatinine |
| Ccr | Creatinine clearance |
References
- D. Pan, D. Zhang, J. Wu, C. Chen, Z. Xu, H. Yang and P. Zhou, A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice, Food Chem. Toxicol., 2014, 63, 111–118 CrossRef PubMed.
- R. G. Nelson, K. R. Tuttle, R. W. Bilous, J. M. Gonzalez-Campoy, M. Mauer, M. E. Molitch, K. Sharma, J. E. Fradkin, A. S. Narva, T. J. Wilt, A. Ishani, T. S. Rector, Y. Slinin, P. Fitzgerald, M. Carlyle, M. V. Rocco, J. S. Berns, J. V. Nally Jr, H. Kramer, M. J. Choi, K. Willis, E. Howell, M. Cheung and S. Slifer, KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update, Am. J. Kidney Dis., 2012, 60(5), 850–886 CrossRef PubMed.
- R. G. Nelson and K. R. Tuttle, Prevention of diabetic kidney disease: negative clinical trials with renin–angiotensin system inhibitors, Am. J. Kidney Dis., 2010, 55(3), 426–430 CrossRef PubMed.
- D. Ron and S. R. Hubbard, How IRE1 reacts to ER stress, Cell, 2008, 132(1), 24–26 CrossRef PubMed.
- D. Dong, M. Ni, J. Li, S. Xiong, W. Ye, J. J. Virrey, C. Mao, R. Ye, M. Wang and L. Pen, et al., Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development, Cancer Res., 2008, 68(2), 498–505 CrossRef PubMed.
- Z. S. Wang, F. Xiong, X. H. Xie, D. Chen, J. H. Pan and L. Cheng, Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress, BMC Nephrol., 2015, 16, 44 CrossRef PubMed.
- A. H. Schonthal, Targeting endoplasmic reticulum stress for cancer therapy, Front. Biosci., Scholar Ed., 2012, 4, 412–431 CrossRef.
- W. M. McKimpson, J. Weinberger, L. Czerski, M. Zheng, M. T. Crow, J. E. Pessin, S. C. Chua Jr. and R. N. Kitsis, The apoptosis inhibitor ARC alleviates the ER stress response to promote beta-cell survival, Diabetes, 2013, 62(1), 183–193 CrossRef PubMed.
- C. S. McAlpine and G. H. Werstuck, The development and progression of atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling, Cardiovasc. Hematol. Disord.: Drug Targets, 2013, 13(2), 158–164 CrossRef.
- Y. Fan, W. Xiao, Z. Li, X. Li, P. Y. Chuang, B. Jim, W. Zhang, C. Wei, N. Wang and W. Jia, et al., RTN1 mediates progression of kidney disease by inducing ER stress, Nat. Commun., 2015, 6, 7841 CrossRef PubMed.
- W. S. Cheang, W. T. Wong, L. Zhao, J. Xu, L. Wang, C. W. Lau, Z. Y. Chen, R. C. Ma, A. Xu and N. Wang, et al., PPARdelta Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice, Diabetes, 2017, 66(2), 519–528 CrossRef PubMed.
- L. Xiong, Z. Y. Fang, X. N. Tao, M. Bai and G. Feng, Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a rat asthma model, Am. J. Chin. Med., 2007, 35(6), 1011–1020 CrossRef PubMed.
- F. Jiang, J. Qian, S. Chen, W. Zhang and C. Liu, Ligustrazine improves atherosclerosis in rat via attenuation of oxidative stress, Pharmaceutical Biology, 2011, 49(8), 856–863 CrossRef PubMed.
- J. Chen, J. Chen, X. Wang, C. Wang, W. Cao, Y. Zhao, B. Zhang, M. Cui, Q. Shi and G. Zhang, Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways, Biomed. Pharmacother., 2016, 82, 1–7 CrossRef PubMed.
- L. Feng, N. Ke, F. Cheng, Y. Guo, S. Li, Q. Li and Y. Li, The protective mechanism of ligustrazine against renal ischemia/reperfusion injury, J. Surg. Res., 2011, 166(2), 298–305 CrossRef PubMed.
- L. Sun, Y. Li, J. Shi, X. Wang and X. Wang, Protective effects of ligustrazine on ischemia–reperfusion injury in rat kidneys, Microsurgery, 2002, 22(8), 343–346 CrossRef PubMed.
- S. R. Kolati, E. R. Kasala, L. N. Bodduluru, J. R. Mahareddy, S. K. Uppulapu, R. Gogoi, C. C. Barua and M. Lahkar, BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-kappaB pathway, Environ. Toxicol. Pharmacol., 2015, 39(2), 690–699 CrossRef PubMed.
- G. G. Wang, X. H. Lu, W. Li, X. Zhao and C. Zhang, Protective Effects of Luteolin on Diabetic Nephropathy in STZ-Induced Diabetic Rats, Evid.-Based Complementary Altern. Med., 2011, 2011, 323171 Search PubMed.
- M. A. Baig, V. B. Gawali, R. R. Patil and S. R. Naik, Protective effect of herbomineral formulation (Dolabi) on early diabetic nephropathy in streptozotocin-induced diabetic rats, J. Nat. Med., 2012, 66(3), 500–509 CrossRef PubMed.
- T. S. Ye, Y. W. Zhang and X. M. Zhang, Protective effects of Danggui Buxue Tang on renal function, renal glomerular mesangium and heparanase expression in rats with streptozotocin-induced diabetes mellitus, Exp. Ther. Med., 2016, 11(6), 2477–2483 CrossRef PubMed.
- M. Morcos, A. A. Sayed, A. Bierhaus, B. Yard, R. Waldherr, W. Merz, I. Kloeting, E. Schleicher, S. Mentz and R. F. Abd el Baki, et al., Activation of tubular epithelial cells in diabetic nephropathy, Diabetes, 2002, 51(12), 3532–3544 CrossRef PubMed.
- J. F. Navarro-Gonzalez and C. Mora-Fernandez, The role of inflammatory cytokines in diabetic nephropathy, J. Am. Soc. Nephrol., 2008, 19(3), 433–442 CrossRef PubMed.
- C. Mora and J. F. Navarro, Inflammation and diabetic nephropathy, Curr. Diabetes Rep., 2006, 6(6), 463–468 CrossRef.
- W. Eberhardt and J. Pfeilschifter, Nitric oxide and vascular remodeling: spotlight on the kidney, Kidney Int. Suppl., 2007, 106, S9–S16 CrossRef PubMed.
- J. Pfeilschifter and H. Schwarzenbach, Interleukin 1 and tumor necrosis factor stimulate cGMP formation in rat renal mesangial cells, FEBS Lett., 1990, 273(1–2), 185–187 CrossRef PubMed.
- M. T. Lindenmeyer, M. P. Rastaldi, M. Ikehata, M. A. Neusser, M. Kretzler, C. D. Cohen and D. Schlondorff, Proteinuria and hyperglycemia induce endoplasmic reticulum stress, J. Am. Soc. Nephrol., 2008, 19(11), 2225–2236 CrossRef PubMed.
- A. V. Cybulsky, Endoplasmic reticulum stress in proteinuric kidney disease, Kidney Int., 2010, 77(3), 187–193 CrossRef PubMed.
- W. Qi, J. Mu, Z. F. Luo, W. Zeng, Y. H. Guo, Q. Pang, Z. L. Ye, L. Liu, F. H. Yuan and B. Feng, Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response, Metab., Clin. Exp., 2011, 60(5), 594–603 CrossRef PubMed.
- L. Fang, X. Li, Y. Luo, W. He, C. Dai and J. Yang, Autophagy inhibition induces podocyte apoptosis by activating the pro-apoptotic pathway of endoplasmic reticulum stress, Exp. Cell Res., 2014, 322(2), 290–301 CrossRef PubMed.
- A. Bertolotti, Y. Zhang, L. M. Hendershot, H. P. Harding and D. Ron, Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response, Nat. Cell Biol., 2000, 2(6), 326–332 CrossRef PubMed.
- C. A. Hazzalin, E. Cano, A. Cuenda, M. J. Barratt, P. Cohen and L. C. Mahadevan, p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient, Curr. Biol., 1996, 6(8), 1028–1031 CrossRef PubMed.
- Y. Q. Wang, C. C. Fan, B. P. Chen and J. Shi, Resistin-Like Molecule Beta (RELM-beta) Regulates Proliferation of Human Diabetic Nephropathy Mesangial Cells via Mitogen-Activated Protein Kinases (MAPK) Signaling Pathway, Med. Sci. Monit., 2017, 23, 3897–3903 CrossRef PubMed.
- Y. Qiao, K. Gao, Y. Wang, X. Wang and B. Cui, Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-beta1 pathway, Exp. Ther. Med., 2017, 13(6), 3223–3230 CrossRef PubMed.
- Z. J. Xu, S. Shu, Z. J. Li, Y. M. Liu, R. Y. Zhang and Y. Zhang, Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-beta/SMADS, MAPK, and NF-kB and upregulating expression of cytoglobin in renal tissues, Medicine, 2017, 96(3), e5879 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |