Open Access Article
Open Access ArticleRetracted Article: MiR-320d suppresses the progression of breast cancer via lncRNA HNF1A-AS1 regulation and SOX4 inhibition
Shuai Shia,
Xiaoling Hub,
Jianpo Xuc,
Hong Liua and
Libo Zou†
 *a
*a
aReproductive Medicine Center of Jinhua People's Hospital, Biomedical Research Center of Zhejiang Normal University, Jinhua, 321000, China. E-mail: Shuai5189188@qq.com; Tel: (+86) 18258950539
bDepartment of Reproductive Endocrinology, Zhejiang University School of Medicine Affiliated Obstetrics and Gynecology Hospital, Hangzhou, 310006, China
cLife Sciences Institute of Zhejiang University, Hangzhou, 310058, China
First published on 24th May 2018
Abstract
MicroRNA-320d (miR-320d) is a novel cancer-related miRNA and functions as a tumor suppressor in human cancers. However, the expression pattern and function of miR-320d in breast cancer remain largely unknown. In the present study, we found that the expression level of miR-320d in breast cancer tissues and cells was significantly lower than in non-tumor tissues and MCF-10A cells. Decreased miR-320d was associated with poor overall survival in patients with breast cancer. Overexpression of miR-320d inhibited proliferation, migration, and invasion and promoted apoptosis of breast cancer cells. In addition, the long non-coding RNA, HNF1A antisense RNA 1 (HNF1A-AS1) was up-regulated in both breast cancer tissues and cell lines. HNF1A-AS1 suppressed the expression and function of miR-320d. Moreover, SRY-related HMG-box 4 (SOX4) was speculated and confirmed as a target of miR-320d. We also demonstrated that HNF1A-AS1 may function as a sponge competitive endogenous RNA for miR-320d, and thus regulate the expression of SOX4. Taken together, our study has identified a novel signaling pathway through which miR-320d exerts its anti-carcinogenic roles and suggested that the HNF1A-AS1/miR-320d/SOX4 may be a potential target for the therapy of breast cancer.
Introduction
Breast cancer is one of the most common cancers among women and is the fifth leading cause of cancer death in the world.1 It was reported that more than 1676 million new cases of breast cancer and 521![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 breast cancer deaths have occurred worldwide.2 Breast cancer is a heterogeneous disease, which has diverse biological characteristics contributing to cancer initiation, development and metastasis and leading to different responses to treatments.3 Although great advances have made in the mechanism and treatment of breast cancer in recent years, further understanding is needed to provide novel molecules for early diagnosis and prognostic predications.
900 breast cancer deaths have occurred worldwide.2 Breast cancer is a heterogeneous disease, which has diverse biological characteristics contributing to cancer initiation, development and metastasis and leading to different responses to treatments.3 Although great advances have made in the mechanism and treatment of breast cancer in recent years, further understanding is needed to provide novel molecules for early diagnosis and prognostic predications.
MicroRNA (miRNA) is a conserved family of small non-coding RNA molecules. It regulates gene expression at the post-transcriptional level through binding to the 3′-untranslated region (3′UTR) of mRNAs.4–6 MiRNA is reported to be involved in diverse biological processes, such as cell proliferation, apoptosis and death.7 Abnormal miRNA expression has been frequently observed in various human cancers, either as an oncogene or a tumor-suppressor.8–10 MiR-320d is a member of miR-320 family. I was reported that miR-320 family is down-regulated in colorectal adenoma and affects tumor proliferation.11 Down-regulation of miRNA-320d predicts poor overall survival and promotes the growth and invasive abilities in glioma.12 However, the expression pattern and biological function of miR-320d in breast cancer are largely unknown.
Long non-coding RNAs (lncRNAs) are defined as transcripts containing more than 200 nucleotides. LncRNAs play important roles in various types of cancer and exert multiple functions in cancer cell proliferation, apoptosis, migration, and invasion.13–15 LncRNA HNF1A-AS1 is a novel identified single-exon gene with 2455 nucleotides and localized at the chromosome 12q24.31.16 HNF1A-AS1 was identified as a potential biomarker and was associated with the progression of multiple cancers.17–20 However, the expression and function of HNF1-AS1 remains largely unknown in the development of breast cancer.
In the present study, we investigated the role of miR-320d and HNF1A-AS1 in breast cancer. We found that miR-320d is down-regulated, whereas HNF1A-AS1 is up-regulated in breast cancer. Mechanically, miR-320d suppresses the progression of breast cancer via HNF1A-AS1 regulation and SOX4 inhibition.
Materials and methods
Tissue samples
Breast cancer tissues and adjacent normal tissues were obtained from 34 patients undergoing surgery at Jinhua People's Hospital (Jinhua, China). The tumor tissues were evaluated independently by three pathologists. After surgery, patients were further followed up per 2 months. Informed consents were obtained from all patients, and the study was approved by the Research Ethics Committee of Jinhua People's Hospital.Cell culture
Human breast cancer cells (MDA-MB-468, SKBR3, MCF-7, MDA-MB-453, T47D, MDA-MB-231) and MCF-10A cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cell lines have been tested and authenticated. Cells were grown in DMEM complemented with 10% FBS (Life Technologies, Grand Island, USA), and incubated at 37 °C in a 5% CO2 incubator.Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells or tissues using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's protocol. Equal amounts of RNA were reversely-transcribed to cDNA with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Then the mRNA level of miR-320d, HNF1A-AS1 and SOX4 was analyzed by SYBR Green PCR Master Mix (Vazyme, Nanjing, China) in ViiA™ 7 Real-time PCR System (Applied Biosystems, USA). Results were normalized to the expression of GAPDH (for HNF1A-AS1 and SOX4) or U6 (for miR-320d).Cell viability analysis
MCF-7 and MDA-MB-231 cells were cultured on a 96-well plate. After various treatments, the cell viability was then measured by the CCK-8 kit (Beyotime Biotechnology, Shanghai, China) under 540 nm according to the manufacturer's instructions.Cell apoptosis assay
MCF-7 and MDA-MB-231 cells were transfected with miR-320d mimics for 24 h. After wash with ice-cold PBS, cells were resuspended in annexin V binding buffer and incubated with FITC-conjugated annexin V/PI (ThermoFisher, USA) for 15 min at room temperature. The cells were then analyzed with CytoFLEX system (Beckman, USA).Western blot
Cells were lysed with RIPA Lysis Buffer (Solarbio Life Sciences, Beijing, China). Equivalent amounts of proteins from each sample were separated by 10% SDS-PAGE and then transferred to PVDF membranes (Millipore). After blocking with 5% non-fat milk at room temperature for 1 h, the membranes were incubated with respectively primary antibodies overnight at 4 °C and then were incubated with HRP-conjugated secondary antibodies (BOSTER Biological Technology, Wuhan, China) at room temperature. ECL system (Bio-Rad Laboratories, USA) was used for detection of antibody-bound proteins according to manufacturer's instructions. β-Actin was used as endogenous reference.Detection of caspase 3 activity
MCF-7 and MDA-MB-231 cells were transfected with miR-320d mimics for 24 h. The activity of caspase 3 was detected by Caspase 3 Assay Kit (Abcam, Cambridge, MA) according to the manufacturer's instruction.Plasmid constructs and transfection assays
For HNF1A-AS1 and SOX4 overexpression, HNF1A-AS1 and SOX4 cDNA sequences were synthesized (Genepharma, Shanghai, China) and subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, USA), and the empty pcDNA3.1 vector was served as a negative control. siRNA against HNF1A-AS1 and si-control were obtained from RIBOBIO (Guangzhou, China). MiR-320d mimic and control mimic were purchased from Genepharma (Shanghai, China). Plasmid, siRNA or mimic were transfected to MCF-7 or MDA-MB-231 cells by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions.Cell migration assay
MCF-7 or MDA-MB-231 cells were transfected with miR-320d mimics were cultured in a 24-well chamber. The confluent cell monolayer was stroked with a pipette tip. Cells were washed to remove detached and damaged cells. After 24 h incubation, the cell migration was monitored microscopically and the migration distance was measured from 5 points per 1 wound area by the ImageJ software.Transwell invasion assay
The invasion capacity of MCF-7 or MDA-MB-231 cells was performed using transwell chamber (0.4 μm pore size, Corning) inserts precoated with matrigel. Briefly, cells transfected with miR-320d mimics or mimic control were seeded in the upper chamber with serum-free medium, and the lower chamber was filled with complete medium (containing 10% FBS). After 24 h incubation, the bottom cells were fixed in 95% ethanol, stained with crystal violet, and the number of invaded cells was counted microscopically at 200× magnification on 10 random fields.Statement
The animal experiments in this study were approved by the Animal Care and Research Committee of Zhejiang Normal University. All experiments were performed in compliance with relevant laws and guidelines. Besides, all experiments were conducted following institutional guidelines of Zhejiang Normal University.Animal work and experimental protocols
Female athymic BALB/c mice (nu/nu, 20–25 g body weight) were obtained from the Experimental Animal Center of Zhejiang Normal University (Jinhua, China). Mice were housed under controlled conditions (25 ± 2 °C, 70% humidity and 12 h light–dark periods) and fed on regular sterile chow diet and water ad libitum. A total of 10 mice were equally divided into 2 groups. One group was subcutaneously inoculated in the back with 5 × 106 MCF-7 cells which have been transfected with miR-320d mimics, and another group was subcutaneously inoculated in the back with equal amount of MCF-7 cells which have been transfected with mimic control. Mice were monitored for signs of tumor growth. Tumor volumes were measured per 5 days according to the formula: [length (mm) × width (mm) × width (mm) × 0.52]. Upon termination, tumors were harvested for further experiments.Immunohistochemistry
Tumor sections were prepared essentially by standard protocol. After the sequential processes of rehydration and antigen retrieval, the sections were incubated with antibody against PCNA overnight, and subsequently incubated with secondary antibody for 30 minutes. Diaminobenzidine (DAB) was used for performing color reactions. Sections were visualized with a fluorescent microscope.Apoptotic cells were identified using the One Step TUNEL Apoptosis Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's instructions.
Luciferase reporter assay
The 3′UTR of HNF1A-AS1 and SOX4 mRNA containing predicted miR-320d binding sites was PCR-amplified and inserted into pGL3 luciferase reporter plasmid (Promega, USA). Mutant plasmid was generated using the MutanBEST Kit (Takara, Japan), according to the bioinformatics analysis. For luciferase reporter assays, MCF-7 cells were co-transfected with wild-type/mutated reporter plasmids and miR-320d mimics/mimic control by using Lipofectamine 2000 reagent. The luciferase activities were measured with a dual luciferase reporter assay system (Promega, Madison, USA) according to the manufacturer's instructions.Bioinformatics data set
Prediction of the interaction between miR-320d and HNF1A-AS1 or SOX4 was performed using online software miRanda (http://www.microrna.org) and TargetScan (http://www.targetscan.org).Statistical analysis
Data were analyzed by GraphPad Prism software and the results were expressed as mean ± standard deviation (SD). The statistical significance of the studies was analyzed using one way ANOVA. The difference was considered significant at P < 0.05.Results
MiR-320d is down-regulated in breast cancer and is associated with poor survival
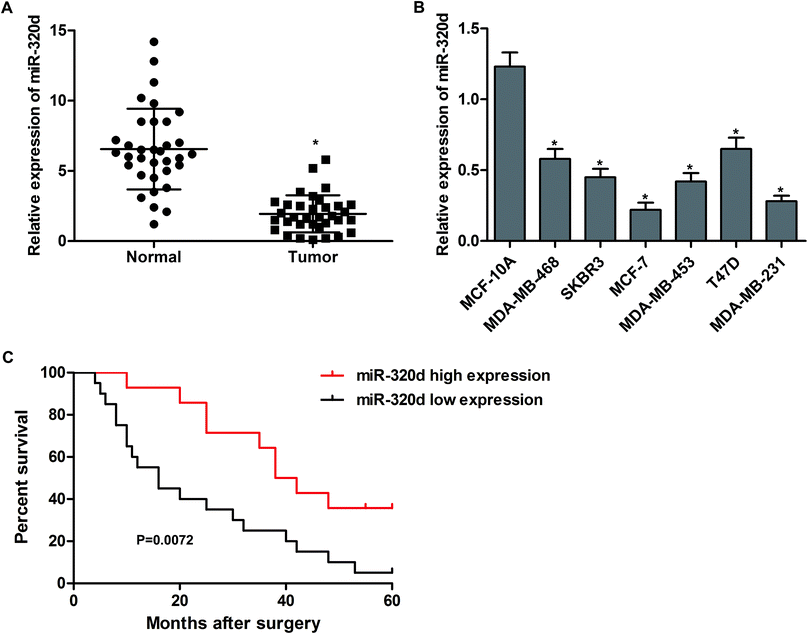
To understand the biological significance of miR-320d in breast cancer, the mRNA levels of miR-32d in tumor tissues and adjacent noncancerous tissues from 34 patients were detected by qPCR. The results showed that the expressions of miR-320d in breast cancer tissues were significantly higher than in normal tissues (Fig. 1A). In addition, the expression levels of miR-320d were significantly decreased in human breast cancer cells (MDA-MB-468, SKBR3, MCF-7, MDA-MB-453, T47D and MDA-MB-231) when compared to that in human mammary epithelial cell MCF-10A (Fig. 1B). We also followed the survival status of these patients, and the Kaplan–Meier survival analysis showed that sufferers with low miR-320d expression (n = 20) had a shorter survival than those with high miR-320d expression (n = 14, Fig. 1C). These results indicate that low miR-320d expression may be associated with the progression of breast cancer.MiR-320d inhibits proliferation and promotes apoptosis of breast cancer cells
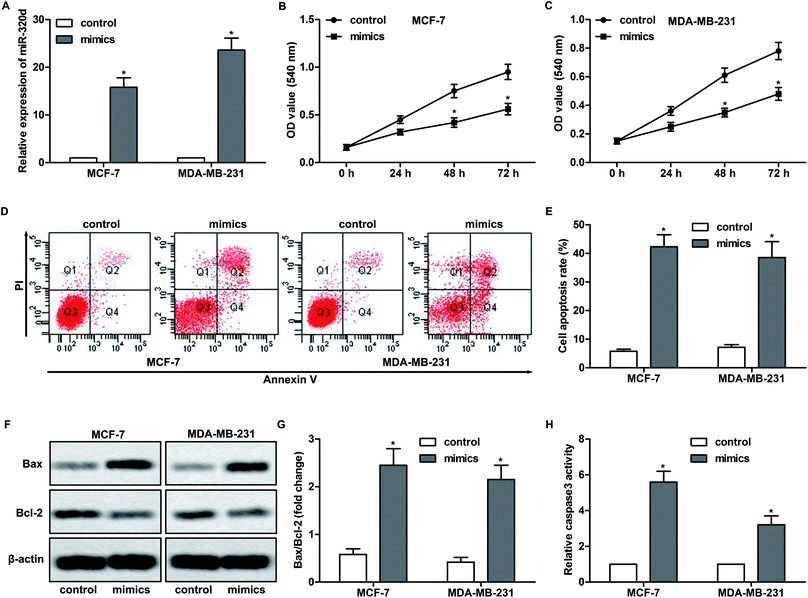
Based on the observation above, we performed gain-of-function studies. MiR-320d mimics were transfected into MCF-7 and MDA-MB-231 cells to overexpress miR-320d. As shown in Fig. 2A, miR-320d mimic significantly increased the expression of miR-320d in these cells. In addition, miR-320d mimic dramatically inhibited the proliferation of MCF-7 and MDA-MB-231 cells (Fig. 2B and C). Furthermore, flow cytometry analysis showed that miR-320d overexpression promoted the apoptosis of MCF-7 and MDA-MB-231 cells (Fig. 2D and E). Since the balance between Bcl-2 and Bax play a crucial role in cell apoptosis,21 the expressions of these proteins were detected by western blot. The results showed that miR-320d overexpression significantly increased the ratio of Bax/Bcl-2 (Fig. 2F and G). MiR-320d overexpression also enhanced the activity of caspase 3, which is an executioner of apoptosis (Fig. 2H). Taken together, these results indicate that miR-320d inhibits proliferation and promotes apoptosis of breast cancer cells.MiR-320d suppresses migration and invasion of breast cancer cells
We next examined the effect of miR-320d on cell migration and invasion. MCF-7 and MDA-MB-231 cells were transfected with miR-320d mimics or mimic control. And then, the wound healing assay and matrigel transwell assay were carried out. The results showed that the miR-320d mimic-mediated overexpression effectively inhibited the migration (Fig. 3A and B) and invasion (Fig. 3C and D) of MCF-7 and MDA-MB-231 cells. Since VEGF and MMP-9 play an important role in tumor progression by promoting epithelial–mesenchymal transition (EMT),22,23 the effect of miR-320d overexpression on the expression of these proteins was determined by western blot. As anticipated, the expressions of VEGF and MMP-9 were significantly decreased after transfection of miR-320d mimic (Fig. 3E–G). These findings indicate that miR-320d could inhibit the migration and invasion of breast cancer cells.MiR-320d suppresses tumor growth
To investigate the function of miR-320d in vivo, mice were injected with MCF-7 cells which have been transfected with miR-320d mimic or mimic control to build tumor xenograft model. Tumor volume was measured per 5 days. The results showed that miR-320d significantly inhibited the tumor growth (Fig. 4A and B). In addition, immunohistochemistry showed that the expression proliferation-related protein PCNA was dramatically decreased in miR-320d-overexpressed tumor tissues (Fig. 4C and D). TUNEL staining demonstrated that miR-320d also promoted cell apoptosis in tumor tissues (Fig. 4C and D).HNF1A-AS1 suppresses miR-320d expression and accelerates proliferation of breast cancer cells
Previous studies have demonstrated that lncRNA play an important role in the regulation of miRNA. According to the bioinformatic analysis, we found a target site of miR-320d in lncRNA HNF1A-AS 3′UTR (Fig. 5A). To investigate the significance of HNF1A-AS in breast cancer, the expression of HNF1A-AS in breast cancer tissues and cells was detected by qPCR. As shown in Fig. 5B and C, The RNA level of HNF1A-AS in breast cancer tissues and cells was significantly higher than in normal tissues and cells. In addition, a negative correlation was found between the expression of HNF1A-AS and miR-320d in breast cancer tissues (Fig. 5D, r = −0.699, P < 0.0001). To confirm the binding domain between HNF1A-AS and miR-320d, the 3′UTR of HNF1A-AS which contain the predicted site or mutant site (Fig. 5A) of miR-320d was inserted into pGL3 reporter plasmid. The results showed that miR-320d notably decreased the luciferase activity of wide type plasmid but not mutant plasmid (Fig. 5E). In addition, si-HNF1A-AS-mediated knockdown of HNF1A-AS increased the expression of miR-320d (Fig. 5F), and pcDNA3.1-HNF1A-AS-mediated overexpression of HNF1A-AS decreased the expression of miR-320d (Fig. 5G). Furthermore, si-HNF1A-AS significantly inhibited the proliferation of MCF-7 and MDA-MB-231 cells (Fig. 5H and I). These results indicate that HNF1A-AS1 promotes the proliferation of breast cancer cells by down-regulating miR-320d.SOX4 is a downstream target of miR-320d in breast cancer cells
The miRNA target algorithms TargetScan was used to explore the downstream target of miR-320d. We found that SOX4 may be a potential target of miR-320d (Fig. 6A). In addition, the expression of SOX4 in tumor tissues was much higher than in adjacent normal tissues (Fig. 6B). We also found a negative correlation between the mRNA level of SOX4 and miR-320d in breast cancer tissues (Fig. 6C, r = −0.831, P < 0.0001). To verify the binding effect between SOX4 and miR-320d, the luciferase reporter plasmid containing WT or mutant SOX4 binding site was constructed and transfected into MCF-7 cells. As shown in Fig. 6D, miR-320d significantly decreased the luciferase activity of WT plasmid rather than mutant plasmid. MiR-320d mimic also up-regulated the protein level of SOX4 and its downstream proteins including β-catenin, cyclin D1 and c-Myc (Fig. 6E and F). Furthermore, overexpression of SOX4 attenuated the inhibitory effect of miR-320d on cell proliferation (Fig. 6G and H). These results suggest that SOX4 is a functional target of miR-320d.HNF1A-AS1 up-regulates SOX4 expression through sponging miR-320d
Based on the observations above, we found a positive correlation between the mRNA level of SOX4 and HNF1A-AS1 in breast cancer tissues (Fig. 7A, r = 0.826, P < 0.0001). The expression of SOX4 was significantly down-regulated by HNF1A-AS1 knockdown, whereas was increased by HNF1A-AS1 overexpression. HNF1A-AS1 also restored miR-320d mimic-induced decrease of SOX4 in breast cancer cells (Fig. 7B–F). These results demonstrate that HNF1A-AS1 up-regulates the expression of SOX4 through sponging miR-320d.Discussion
In recent years, increasing evidence has demonstrated that the aberrant expression of miRNAs, which may function as oncogene or tumor-suppressor, play important roles in the development and progression of human cancers.24–26 Numerous studies have reported the abnormal expression of miRNAs in breast cancer. MiR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer.27 MiR-133a acts as a tumor suppressor in breast cancer through targeting LASP1.27 In addition, overexpression of suppressive miRNAs, such as miR-30a, miR-200c and miR-500a-5p are associated with improved survival in breast cancer patients.28,29 MiR-320d is an important member in miR-320 family. Previous studies have reported the function of miR-320d in glioma, colorectal adenoma and B-cell lymphoma.11,12,30 In the present study, we first investigated the clinical significance of miR-320d and explored its role in cell growth, apoptosis, migration and invasion of breast cancer. The results demonstrated that the expression of miR-320d in breast cancer tissues was significantly lower than that in normal tissues. Low expression of miR-320d was positively correlated with poor overall survival in patients with breast cancer. Overexpression of miR-320d inhibited proliferation, migration, invasion and promoted apoptosis of breast cancer cells. Thus, miR-320d potentially functions as a tumor suppressor in breast cancer.Numerous studies have suggested that lncRNAs were implicated in a variety of cellular processes, and were thought of as diagnostic and prognostic markers for breast cancer. For example, lncRNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR-320a.31 LncRNA PTENP1 inhibits proliferation and migration of breast cancer cells via AKT and MAPK signaling pathways.32 HNF1A-AS1 is transcribed from the opposite strand of HNF1A gene transcription. It contains two Alu-like sequences. Abnormal HNF1A-AS1 was first found in human primary esophageal adenocarcinoma.33 HNF1A-AS1 was also up-regulated in lung adenocarcinoma, hepatocellular carcinoma and osteosarcoma, acting as an oncogene.34–36 In the present study, we first found a target site of miR-320d in HNF1A-AS1 3′UTR by using bioinformatics analysis. In addition, HNF1A-AS1 was up-regulated in breast cancer tissues and cells compared with the non-cancer tissues and normal cells. Overexpression of HNF1A-AS1 significantly increased the expression of miR-320d, conversely, HNF1A-AS1 knockdown decreased the expression of miR-320d and inhibited the proliferation of breast cancer cells. These data suggest that HNF1A-AS1 acts as an oncogene by down-regulating the expression of miR-320d in the progression of breast cancer.
SOX4, a member of the SOX family, play instrumental roles in the regulation of in tumorigenicity and cancer stemness.37 SOX4 is up-regulated in various cancers, such as esophagus cancer, lung cancer and breast cancer.38–40 Interestingly, it was reported that the expression of SOX4 was post-transcriptionally regulated by miR-187, miR-204 and miR-363.41–43 In this study, SOX4 was predicted to be a downstream target of miR-320d. The expression of SOX4 was up-regulated in breast cancer tissues and was negatively correlated with the expression of miR-320d. Moreover, SOX4 overexpression partly reversed the inhibitory effect of miR-320d mimic on cell proliferation. Saegusa et al. demonstrated that SOX4 functions as a positive regulator of β-catenin signaling through upregulation of TCF4 during morular differentiation of endometrial carcinomas.44 Inoue et al. suggested that SOX4 promoted the development of sarcomatous components in UCSs through transcriptional regulation of the Slug gene by cooperating with the β-catenin/p300 signal pathway,45 indicating that β-catenin is a function target of SOX4. Our study demonstrated that miR-320d increased the expression of β-catenin and its downstream proteins including cyclin D1 and c-Myc, suggesting that the SOX4/β-catenin signaling pathway was also regulated by miR-320d. Furthermore, our results demonstrated that the level of SOX4 was positively correlated with HNF1A-AS1 in breast cancer tissues. Mechanism research showed that HNF1A-AS1 up-regulated the expression of SOX4 through sponging miR-320d.
Conclusions
Our study showed that miR-320d acted as a tumor suppressor in breast cancer. The data suggested that HNF1A-AS1 up-regulated the expression of SOX4 via acting as a competing endogenous RNA for miR-320d binding. Since breast cancer shares the pathogenesis with many other types of cancer, our experimental data also suggested that the HNF1A-AS1-miR-320d-SOX4 axis may be a promising therapeutic target for breast cancer or other cancers.Conflicts of interest
There are no conflicts to declare.References
- F. Schettini, G. Buono, C. Cardalesi, I. Desideri, S. De Placido and L. Del Mastro, Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going, Cancer Treat. Rev., 2016, 46, 20–26 CrossRef PubMed.
- Y. Liang, Y. Li, X. Song, N. Zhang, Y. Sang, H. Zhang, Y. Liu, B. Chen, W. Zhao, L. Wang and others, Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance in breast cancer, Cancer Biol. Ther., 2018, 1–12 Search PubMed.
- J. Kravchenko, I. Akushevich, V. L. Seewaldt, A. P. Abernethy and H. K. Lyerly, Breast cancer as heterogeneous disease: contributing factors and carcinogenesis mechanisms, Breast Cancer Res. Treat., 2011, 128(2), 483–493 CrossRef PubMed.
- D. P. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 2004, 116(2), 281–297 CrossRef PubMed.
- S. Bagga, J. Bracht, S. Hunter, K. Massirer, J. Holtz, R. Eachus and A. E. Pasquinelli, Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation, Cell, 2005, 122(4), 553–563 CrossRef PubMed.
- N. Nohata, Y. Sone, T. Hanazawa, M. Fuse, N. Kikkawa, H. Yoshino, T. Chiyomaru, K. Kawakami, H. Enokida, M. Nakagawa and others, miR-1 as a tumor suppressive microRNA targeting TAGLN2 in head and neck squamous cell carcinoma, Oncotarget, 2011, 2(1–2), 29–42 Search PubMed.
- R. Hamam, D. Hamam, K. A. Alsaleh, M. Kassem, W. Zaher, M. Alfayez, A. Aldahmash and N. M. Alajez, Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers, Cell Death Dis., 2017, 8(9), e3045 CrossRef PubMed.
- C. M. Wong, F. H. Tsang and I. O. Ng, Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications, Nat. Rev. Gastroenterol. Hepatol., 2018, 15(3), 137–151 CrossRef PubMed.
- Y. Guo, H. Yu, J. Wang, Q. Sheng, S. Zhao and Y. Y. Zhao, The Landscape of Small Non-Coding RNAs in Triple-Negative Breast Cancer, Genes, 2018, 9(1), 29 CrossRef PubMed.
- K. Konicke, A. Lopez, J. L. Munoz, L. S. Servin, A. Flores, E. Olasz and Z. Lazarova, The microRNA landscape of cutaneous squamous cell carcinoma, Drug Discovery Today, 2018, 864–870 CrossRef PubMed.
- T. Tadano, Y. Kakuta, S. Hamada, Y. Shimodaira, M. Kuroha, Y. Kawakami, T. Kimura, H. Shiga, K. Endo, A. Masamune and others, MicroRNA-320 family is downregulated in colorectal adenoma and affects tumor proliferation by targeting CDK6, World Journal of Gastrointestinal Oncology, 2016, 8(7), 532–542 CrossRef PubMed.
- C. Z. Qin, Q. L. Lv, Y. T. Yang, J. M. Zhang, X. J. Zhang and H. H. Zhou, Downregulation of microRNA-320d predicts poor overall survival and promotes the growth and invasive abilities in glioma, Chem. Biol. Drug Des., 2017, 89(5), 806–814 Search PubMed.
- Y. Cheng, I. Jutooru, G. Chadalapaka, J. C. Corton and S. Safe, The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration, Oncotarget, 2015, 6(13), 10840–10852 CrossRef PubMed.
- H. Niu, Z. Hu, H. Liu, G. Hu, B. Yang, S. Wu and F. Li, Long non-coding RNA AK027294 involves in the process of proliferation, migration, and apoptosis of colorectal cancer cells, Tumor Biol., 2016, 37(8), 10097–10105 CrossRef PubMed.
- J. Wen, J. Xu, Q. Sun, C. Xing and W. Yin, Upregulation of long non coding RNA PCAT-1 contributes to cell proliferation, migration and apoptosis in hepatocellular carcinoma, Mol. Med. Rep., 2016, 13(5), 4481–4486 CrossRef PubMed.
- G. Zhang, X. An, H. Zhao, Q. Zhang and H. Zhao, Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer, Biomed. Pharmacother., 2017, 98, 594–599 CrossRef PubMed.
- X. Zhang, Y. Xiong, F. Tang, Y. Bian, Y. Chen and F. Zhang, Long noncoding RNA HNF1A-AS1 indicates a poor prognosis of colorectal cancer and promotes carcinogenesis via activation of the Wnt/beta-catenin signaling pathway, Biomed. Pharmacother., 2017, 96, 877–883 CrossRef PubMed.
- C. Fang, S. Qiu, F. Sun, W. Li, Z. Wang, B. Yue, X. Wu and D. Yan, Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA, Cancer Lett., 2017, 410, 50–62 CrossRef PubMed.
- L. Cai, J. Lv, Y. Zhang, J. Li, Y. Wang and H. Yang, The lncRNA HNF1A-AS1 is a negative prognostic factor and promotes tumorigenesis in osteosarcoma, J. Cell. Mol. Med., 2017, 21(11), 2654–2662 CrossRef PubMed.
- Y. Zhan, Y. Li, B. Guan, Z. Wang, D. Peng, Z. Chen, A. He, S. He, Y. Gong, X. Li and others, Long non-coding RNA HNF1A-AS1 promotes proliferation and suppresses apoptosis of bladder cancer cells through upregulating Bcl-2, Oncotarget, 2017, 8(44), 76656–76665 CrossRef PubMed.
- M. S. Mohamed, A. O. Abdelhamid, F. M. Almutairi, A. G. Ali and M. K. Bishr, Induction of apoptosis by pyrazolo[3,4-d]pyridazine derivative in lung cancer cells via disruption of Bcl-2/Bax expression balance, Bioorg. Med. Chem., 2017, 623–629 Search PubMed.
- K. J. Kim, B. Li, J. Winer, M. Armanini, N. Gillett, H. S. Phillips and N. Ferrara, Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo, Nature, 1993, 362(6423), 841–844 CrossRef PubMed.
- S. Curran and G. I. Murray, Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis, Eur. J. Cancer, 2000, 36(13 Spec No), 1621–1630 CrossRef PubMed.
- I. Koturbash, F. J. Zemp, I. Pogribny and O. Kovalchuk, Small molecules with big effects: the role of the microRNAome in cancer and carcinogenesis, Mutat. Res., 2011, 722(2), 94–105 Search PubMed.
- N. Lynam-Lennon, S. G. Maher and J. V. Reynolds, The roles of microRNA in cancer and apoptosis, Biol. Rev. Cambridge Philos. Soc., 2009, 84(1), 55–71 CrossRef PubMed.
- R. Hummel, D. J. Hussey and J. Haier, MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types, Eur. J. Cancer, 2010, 46(2), 298–311 CrossRef PubMed.
- X. J. Li, Z. J. Ren, J. H. Tang and Q. Yu, Exosomal MicroRNA MiR-1246 Promotes Cell Proliferation, Invasion and Drug Resistance by Targeting CCNG2 in Breast Cancer, Cell. Physiol. Biochem., 2017, 44(5), 1741–1748 CrossRef PubMed.
- T. Kawaguchi, L. Yan, Q. Qi, X. Peng, E. M. Gabriel, J. Young, S. Liu and K. Takabe, Overexpression of suppressive microRNAs, miR-30a and miR-200c are associated with improved survival of breast cancer patients, Sci. Rep., 2017, 7(1), 15945 CrossRef PubMed.
- D. Degli Esposti and V. N. Aushev, MiR-500a-5p regulates oxidative stress response genes in breast cancer and predicts cancer survival, Sci. Rep., 2017, 7(1), 15966 CrossRef PubMed.
- P. Y. Wu, X. D. Zhang, J. Zhu, X. Y. Guo and J. F. Wang, Low expression of microRNA-146b-5p and microRNA-320d predicts poor outcome of large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone, Hum. Pathol., 2014, 45(8), 1664–1673 CrossRef PubMed.
- J. Liu, Z. Song, C. Feng, Y. Lu, Y. Zhou, Y. Lin and C. Dong, The long non-coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR-320a, Am. J. Transl. Res., 2017, 9(12), 5594–5602 Search PubMed.
- S. Chen, Y. Wang, J. H. Zhang, Q. J. Xia, Q. Sun, Z. K. Li, J. G. Zhang, M. S. Tang and M. S. Dong, Long non-coding RNA PTENP1 inhibits proliferation and migration of breast cancer cells via AKT and MAPK signaling pathways, Oncol. Lett., 2017, 14(4), 4659–4662 CrossRef PubMed.
- X. Yang, J. H. Song, Y. Cheng, W. Wu, T. Bhagat, Y. Yu, J. M. Abraham, S. Ibrahim and W. Ravich, Roland BC and others. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells, Gut, 2014, 63(6), 881–890 CrossRef PubMed.
- Y. Wu, H. Liu, X. Shi, Y. Yao, W. Yang and Y. Song, The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma, Oncotarget, 2015, 6(11), 9160–9172 CrossRef PubMed.
- Z. Liu, X. Wei, A. Zhang, C. Li, J. Bai and J. Dong, Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p, Biochem. Biophys. Res. Commun., 2016, 473(4), 1268–1275 CrossRef PubMed.
- H. Zhao, W. Hou, J. Tao, Y. Zhao, G. Wan, C. Ma and H. Xu, Upregulation of lncRNA HNF1A-AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/beta-catenin signaling pathway, Am. J. Transl. Res., 2016, 8(8), 3503–3512 Search PubMed.
- X. Ye and R. A. Weinberg, Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression, Trends Cell Biol., 2015, 25(11), 675–686 CrossRef PubMed.
- M. Kang, Y. Li, W. Liu, R. Wang, A. Tang, H. Hao, Z. Liu and H. Ou, MiR-129-2 suppresses proliferation and migration of esophageal carcinoma cells through downregulation of SOX4 expression, Int. J. Mol. Med., 2013, 32(1), 51–58 CrossRef PubMed.
- Y. Li, L. Zu, Y. Wang, M. Wang, P. Chen and Q. Zhou, MiR-132 inhibits lung cancer cell migration and invasion by targeting SOX4, J. Thorac. Dis., 2015, 7(9), 1563–1569 Search PubMed.
- J. Zhang, Q. Liang, Y. Lei, M. Yao, L. Li, X. Gao, J. Feng, Y. Zhang, H. Gao, D. X. Liu and others, SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression, Cancer Res., 2012, 72(17), 4597–4608 CrossRef PubMed.
- C. C. Yu, P. N. Chen, C. Y. Peng, C. H. Yu and M. Y. Chou, Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis, Oncotarget, 2016, 7(15), 20180–20192 CrossRef PubMed.
- F. Hu, J. Min, X. Cao, L. Liu, Z. Ge, J. Hu and X. Li, MiR-363-3p inhibits the epithelial-to-mesenchymal transition and suppresses metastasis in colorectal cancer by targeting SOX4, Biochem. Biophys. Res. Commun., 2016, 474(1), 35–42 CrossRef PubMed.
- F. Zhang, Y. Luo, Z. Shao, L. Xu, X. Liu, Y. Niu, J. Shi, X. Sun, Y. Liu, Y. Ding and others, MicroRNA-187, a downstream effector of TGFbeta pathway, suppresses Smad-mediated epithelial-mesenchymal transition in colorectal cancer, Cancer Lett., 2016, 373(2), 203–213 CrossRef PubMed.
- M. Saegusa, M. Hashimura and T. Kuwata, SOX4 functions as a positive regulator of beta-catenin signaling through upregulation of TCF4 during morular differentiation of endometrial carcinomas, Lab. Invest., 2012, 92(4), 511–521 CrossRef PubMed.
- H. Inoue, H. Takahashi, M. Hashimura, K. Eshima, M. Akiya, T. Matsumoto and M. Saegusa, Cooperation of SOX4 with beta-catenin/p300 complex in transcriptional regulation of the Slug gene during divergent sarcomatous differentiation in uterine carcinosarcoma, BMC Cancer, 2016, 16, 53 CrossRef PubMed.
Footnote |
| † Address: No. 228 Xinhua Road, Wucheng District, 321000, Jinhua, Zhejiang, PR China. |
| This journal is © The Royal Society of Chemistry 2018 |