Open Access Article
Open Access ArticleModification of Talc@TiO2 toward high-performance nitrile rubber application
Chao Hea,
Lin Zhanga,
Duoli Chena,
Xiaoqiang Fan *a,
Zhenbing Caib and
Minhao Zhu*ab
*a,
Zhenbing Caib and
Minhao Zhu*ab
aKey Laboratory of Advanced Technologies of Materials (Ministry of Education), School of Materials Science and Engineering, Southwest Jiaotong University, Chengdu 610031, China. E-mail: fxq@home.swjtu.edu.cn; Fax: +86 028 87600128; Tel: +86 028 87600128
bTribology Research Institute, State Key Laboratory of Traction Power, Southwest Jiaotong University, Chengdu 610031, China. E-mail: zhuminhao@swjtu.cn
First published on 11th May 2018
Abstract
To improve the dispersion of talcum powder (Talc) for polymer applications, modified nano-titania powders (TiO2) using a silane coupling agent (KH550), a titanate coupling agent (NDZ201) and sodium polyacrylate (PAAS) were well adhered to the surface of Talc with a ball milling method, thereby preparing a series of mixed Talc@TiO2 particles to realize good dispersion in carboxylated acrylonitrile–butadiene rubber (XNBR). Note that Talc@TiO2 particles modified by PAAS and NDZ201 show better colloidal dispersion in anhydrous ethanol due to organification and repulsion of charge, with original Talc and NDZ201 modified Talc@TiO2 powders as a comparison. Modified Talc@TiO2 hybrid XNBR shows good performance characteristics, including damping capacity and impact resistance, depending mainly on the excellent mechanical property of Talc, good dispersion and the high adhesive force between modified Talc@TiO2 and XNBR.
1. Introduction
The blending of organic/inorganic fillers with matrix materials is a common approach to tailoring their performance characteristics, because the introduction of fillers by simple and low-cost techniques can obtain the expected physicochemical and mechanical properties of matrix materials due to nature of the fillers, their size and shape, distribution and adhesion in the composite.1–4 Primordial Talc composed primarily of hydrated magnesium silicate with a molecular formula of 3MgO·4SiO2·H2O has aroused wide interest in academe and industry because of its bargain price, surface paintability and high mechanical strength. Talc is often encountered as an important filler to enhance the mechanical behaviors of polymer materials,5–8 but as a passive filler it has always found it difficult to achieve widespread application. And Talc particles are prone to aggregation because of their high surface energy. To realize Talc applications in polymers, scholars have taken action to obtain satisfactory compatibility with organic compounds, such as surface chemical modification, surface physical coating, plasma surface treatment, and mechano-chemistry to modify the surface of the inorganic particles.9,10Talc hybrid polypropylene (PP) composites have been extensively prepared by reactive mixing of raw materials (including PP, Talc and improvers), the addition of inorganic and organic acid modified Talc powers as fillers, and so forth, and their physicochemical and mechanical properties have been studied in the past.11–15 Talc powders which have been acid activated and modified by organic surfactant have also been prepared and used as fillers in rubber to improve its mechanical and damping properties.10,16–19 Unfortunately, up to now, there has been no simple and sufficient approach to Talc surface modification and one-step modification technology has not been able to have an impact on ameliorating aggregation, improving dispersion and enhancing the interfacial adhesion between the filler and matrix materials. Recently, an organic and inorganic coordinated modification approach has attracted attention, and hybrid composites using this method have shown excellent mechanical performance via the synergy of organic and inorganic phases at the nano-scale or even at the molecular level.20–23 Titania nanoparticles could perform with outstanding function, dispersing better in matrix materials than other nanoparticles.24 The chemical formula of Talc is 3MgO·4SiO2·H2O, so it contains the massive SiO2. As in previous studies on silica-titania nanoparticles, TiO2 performed with high chemical stability, high activity, non-toxicity and high photoelectric conversion efficiency when used in mixed particles.25,26 Surfactant molecules are essential for solving the poor dispersion and compatibility of nanoparticles through repulsive interaction.27–29 A great deal of research on diverse surfactant and modification mechanisms has been conducted,30–33 and it has been found that coupling agents can improve the surface hydrophobicity of inorganic particles by chemical bonding, including a silane coupling agent and a titanate coupling agent. And water-soluble polymers like sodium polyacrylate (PAAS) can also sufficiently improve the dispersion of inorganic particles.34,35
XNBR is the product of NBR after the introduction of carboxyl groups and it is always used as a sealing material. As a class of special high-performance rubber with the carboxyl group along the chain, it has good wear resistance and excellent mechanical and damping properties. The carboxyl groups along the chain have the possibility of reacting with the OH of the fillers.36 These reactive and polar functional groups make it a suitable candidate for exploring the interaction between the rubber matrix and the various fillers, and developing some novel composite materials.37,38
In this paper, a series of Talc@TiO2 particles with good dispersion were prepared using a collaborative modification method with reactive mixing of organic modified titania nanoparticles and Talc by ball milling. A silane coupling agent (KH550), titanate coupling agent (NDZ201) and PAAS (ACUME9300) were chosen to modify the titania nanoparticles. Then Talc@TiO2 hybrid carboxylated acrylonitrile–butadiene rubbers (XNBR) were prepared, and their mechanical behaviors were evaluated in detail.
2. Experimental section
2.1 Materials
Original Talc was obtained from the Guilin Xinhua Hui Factory of Ultrafine Powder (Guangxi, China). KH550 and NDZ201 were purchased from Dongguan City Ding Hai Plastic Chemical Co., Ltd. ACUMER9300 was commercially obtained from the DOW Chemical Company. Solid XNBR was obtained from the NANTEX Industry Co. Ltd. The performance parameters of XNBR are shown in Table 1. All of the reagents were analytical-grade.| Parameters | Typical value |
|---|---|
| Acrylonitrile content (%) | 27 |
| Carboxyl content (%) | 7 |
| Mooney viscosity ML1+4 at 100 °C | 34 |
2.2 Preparation of Talc@TiO2 fillers and hybrid XNBR
Before modification, Talc and TiO2 particles were dried in a vacuum oven at 60 °C for 12 h to remove the absorbed moisture on the surface. 2 g of TiO2 nanoparticles were dispersed in a mixed solution of 90 ml of anhydrous ethanol and 30 ml of deionized water with ultrasonic dispersion for 20 min to ensure that TiO2 was completely dispersed. 0.2 g of NDZ201, ACUMER9300 and hydrolysis treated KH550 were added, respectively. The pH value of the solutions was regulated successively at 5 and 10 at 50 °C for an hour under ultrasonic treatment. Then the products were washed by centrifugation with anhydrous ethanol and deionized water several times. The modified TiO2 particles were obtained after drying in a vacuum oven for 12 h at 60 °C.10 g of Talc particles were pre-ground by a ball mill for 1 h to stimulate their surface activity. 2 g of modified TiO2 particles were added and mixed with the Talc particles in moderate anhydrous ethanol at a constant velocity of 300 rpm for 2 h. After the mixtures were dried for 12 h at 80 °C, modified Talc@TiO2 particles were obtained and were abbreviated to KH550-Talc@TiO2, NDZ201-Talc@TiO2 and PAAS-Talc@TiO2 according to the different surfactants, with surfactant-free Talc@TiO2 particles as a comparison. The modification of TiO2 nanoparticles by these organic agents is schematically illustrated in Fig. 1.
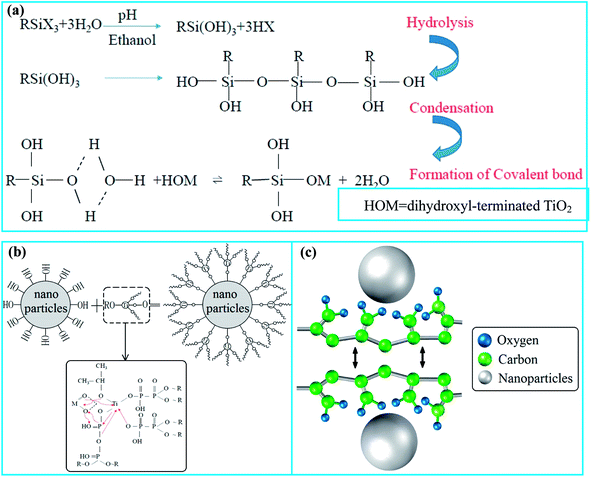 | ||
| Fig. 1 Schematic illustration of organic modification of TiO2 nanoparticles: (a) KH550-modified TiO2, (b) NDZ201-modified TiO2, (c) PAAS-modified TiO2. | ||
XNBR was plasticated by a two-roller mill at 15 rpm for 3 min, and then the fillers were mixed with XNBR using the two-roller mill for 5 min. The mixing ratios of fillers were increased according to 5 wt%, 10 wt% and 15 wt%. Samples were mould-compressed by a press moulding machine under the following conditions: 150 °C, 15 MPa and 20 min.
These modified Talc@TiO2 particles with a weight content of 10 wt% fillers were added into the XNBR and mixed for 10 min. Modified Talc@TiO2 hybrid XNBR composites were obtained by compression moulding in a twin-screw extruder at 160 °C.
2.3 Characterization of modified Talc@TiO2 fillers and hybrid XNBR
The morphology of the original and modified TiO2 particles was investigated by a JSM-7800F field-emission scanning electron microscope (SEM) (JEOL, Japan), and the functional groups on these modified TiO2 particles were investigated by Fourier transform infrared (FTIR) (PerkinElmer16PC, USA) in the wave number range 4000 cm−1 to 500 cm−1.The surface hydrophilicity of the modified Talc@TiO2 blocks was measured by a DSA100 instrument (KRUSS, Germany) at room temperature. 1 μl of water was dropped onto the surface of the modified Talc@TiO2 blocks and the contact angle data were recorded. The morphology of the modified Talc@TiO2 particles was investigated by SEM (JSM-6610LV, JEOL, Japan). FTIR spectra of the modified Talc@TiO2 particles were also obtained by a PerkinElmer 16PCFTIR spectrometer. An X-ray photoelectron spectroscope (XPS) (ESCALAB 250Xi, USA) was used to investigate the chemical states of the typical elements from the modifiers to further confirm the modification mechanism of the modified Talc@TiO2 particles.
Sedimentation of Talc and modified Talc@TiO2 was tested with a sedimentation pipe. An appropriate amount of Talc and modified Talc@TiO2 were added individually to sedimentation pipes until they were evenly dispersed using ultrasonic treatment. After a certain interval of time, the mixtures were photographed to evaluate the sedimentation of the particles.
The morphology of the modified Talc@TiO2 hybrid XNBR was investigated by JSM-6610LV SEM, and their mechanical properties (including a tensile test, damping and anti-impact behaviors) were evaluated by a tensile machine (AGS-J, SHIMADZU, China), a dynamic mechanical analyzer (DMA) (Q800, USA) and a home-made impact-sliding test rig.
3. Results and discussion
3.1 Analysis of modified TiO2 particles
To observe the morphology of three types of modified TiO2 nanoparticles and compare them with their aggregations, Fig. 2 shows the SEM micrographs of raw and modified TiO2 for contrast. Raw TiO2 particles have a relatively high degree of agglomeration due to the nano-size effects with high surface energy. After being modified by coupling agents (KH550 and NDZ201), these organic molecules stick onto the surface of TiO2 via physical adsorption and chemical bonding interaction to form an organic molecular layer which prevents their aggregation. The function of NDZ201 is superior to that of KH550, one possible reason being that KH550 does not completely react with TiO2 during polycondensation in solution. PAAS with a negative charge has the function of charge mutual repulsion in a mixed solution with TiO2 nanoparticles. PAAS is prone to transform into a gel in solution, so PAAS favors the formation of a molecular layer on the surface of the particles, suggesting that PAAS modified TiO2 particles will show good dispersibility with low agglomeration.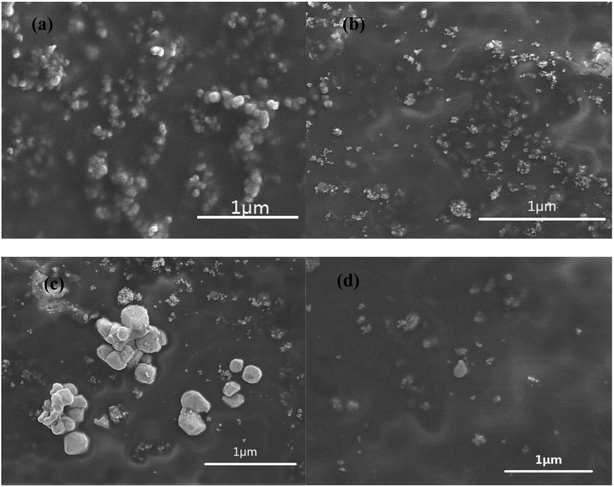 | ||
| Fig. 2 SEM images of raw and modified TiO2 particles: (a) raw TiO2, (b) NDZ201-TiO2, (c) KH550-TiO2, (d) PAAS-TiO2. | ||
Fig. 3 shows FTIR spectra of modified TiO2 to confirm the presence of functional groups, with raw TiO2 as a comparison. As shown in Fig. 3, organic modification reconciled some characteristic peaks in the region 1200–1500 cm−1, and some other characteristic peaks disappeared after modification, including bands of NDZ201-TiO2 at 1316 cm−1 and 1024 cm−1, and a band of KH550-TiO2 at 1260 cm−1. After organic modification, some new characteristic peaks appeared on the spectra, such as KH550-TiO2 with Si–O vibration absorption at 1120 cm−1, and PAAS-TiO2 with carboxyl absorption bands at 1044 cm−1 and 894 cm−1.39 The characteristic peak of TiO2 at 1127 cm−1 moved to 1120 cm−1 after modification by KH550. FTIR results indicate that TiO2 particles have been effectively modified by coupling agents and surfactant.
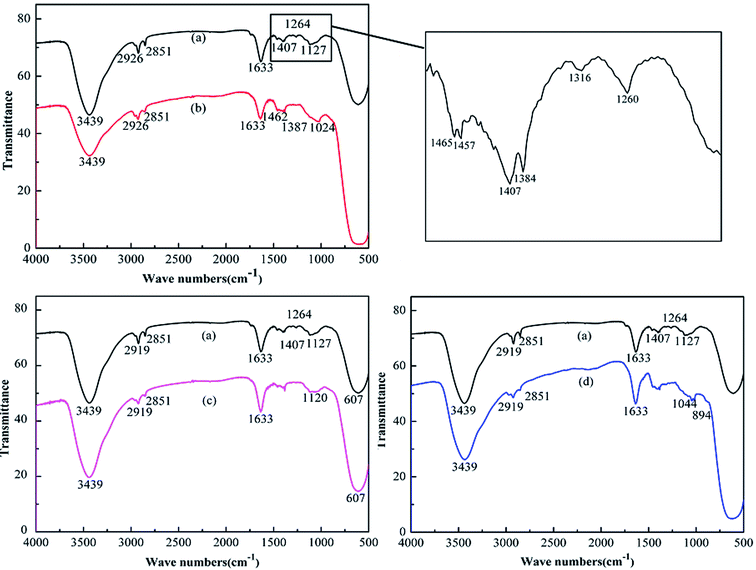 | ||
| Fig. 3 FTIR spectra of modified TiO2 particles, with raw TiO2 as a comparison: (a) TiO2, (b) NDZ201-TiO2, (c) KH550-TiO2, (d) PAAS-TiO2. | ||
3.2 Analysis of modified Talc@TiO2
The surface hydrophilicity of as-prepared Talc@TiO2 particles was evaluated to reflect the affinity between the filler and matrix materials. The contact angle data are shown in Fig. 4. Their contact angles increase in the following sequence: Talc@TiO2 (67.5°) < PAAS-Talc@TiO2 (68.3°) < KH550-Talc@TiO2 (71.5°) < NDZ201-Talc@TiO2 (90.4°). The results illustrate that the hydrophilicity of the as-prepared particles decreases with the use of an organic modifier, especially NDZ201-Talc@TiO2, which has become hydrophobic. This phenomenon could be attributed to the interaction between the carboxyl group of NDZ201 and the hydroxyl group absorbed on the surface of nano TiO2, thereby achieving the purpose of chemical coupling.40 PAAS (ACUMER9300) is a kind of water-soluble macro-molecule without a hydrophobic group and KH550 has low solubility in aqueous media which affects the condensation reaction and effective modification, so there is no substantial increase in contact angle on PAAS-Talc@TiO2 or KH550-Talc@TiO2. These indicate that the modified TiO2 nano-particles were deposited or adhered on the surface of Talc particles to form modified Talc@TiO2 composite particles.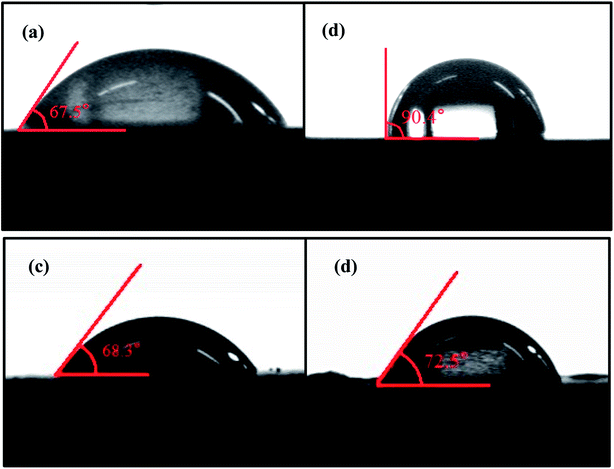 | ||
| Fig. 4 Contact angle of (a) Talc@TiO2, (b) NDZ201-Talc@TiO2, (c) KH550-Talc@TiO2, (d) PAAS-Talc@TiO2. | ||
To obtain visual information about the effect of an organic modifier on the dispersion of Talc@TiO2, Fig. 5 shows photographs of modified Talc@TiO2 in anhydrous ethanol for 10 min, 1 hour and 24 hours. All the surface modifiers have improved the dispersion of Talc@TiO2 in organic solution. As time goes on, the NDZ201-Talc@TiO2 solution has remained nearly unchanged and hardly formed any sediment throughout the entire process, suggesting that NDZ201 modified Talc@TiO2 has the best dispersion and stability in organic solution. Their dispersion and stability increase in the following sequence: KH550-Talc@TiO2 < PAAS-Talc@TiO2 < NDZ201-Talc@TiO2. Of great significance is that organic modification of Talc@TiO2 greatly improves its compatibility with polymers to achieve the desired performance.
 | ||
| Fig. 5 Sedimentation of Talc@TiO2 and modified Talc@TiO2 (including PAAS-Talc@TiO2, NDZ201-Talc@TiO2, KH550-Talc@TiO2) in anhydrous ethanol for 10 min, 1 hour and 24 hours. | ||
Fig. 6 shows the SEM images of organic modified Talc@TiO2, with Talc@TiO2 as a comparison, and their topography and structure under high magnifications are remarkably different. Talc@TiO2 shows a low modification density of TiO2 nano-particles and poor dispersion with some aggregates. A micrograph of KH550-Talc@TiO2 illustrates that KH550 modified TiO2 nanoparticles with good dispersion have adhered to the surface of Talc and formed mixed particles with local aggregation. An SEM image of PAAS-Talc@TiO2 shows that ACUMER9300 modified TiO2 particles are unevenly dispersed in Talc and there are relatively large aggregates. NDZ201-Talc@TiO2 displays a relatively regular shape, uniform size, and good dispersion morphology. The results illustrate that organic modified TiO2 particles are more inclined to form a mixed structure via effectively adhering to the surface of Talc, especially coupling agents. The agglomeration of raw TiO2 nanoparticles is easily caused in the process of preparation, but organic modified ones have good dispersion and hold a better prospect of application.
To verify the functional groups of organic modified Talc@TiO2, FTIR was used to obtain the characteristic absorption peaks of the samples. Fig. 7 shows the FTIR spectra of all the specimens. The peaks at 788 cm−1 and 872 cm−1 are assigned to the Si–O–Si stretching band and Si–C stretching band, respectively.41 The peak at around 1637 cm−1 is the OH bending vibration of the adsorbed and interlayer of TiO2. Compared with Talc@TiO2, modified Talc@TiO2 particles have peaks nearby at 2850 cm−1 and 2920 cm−1, which are assigned to the CH3 symmetric stretching vibration of saturated alkyl and CH2 antisymmetric stretching vibration of long chain alkyl.42 The characteristic peak at 1442 cm−1 is due to the bending vibration mode of the carboxyl groups. For KH550-Talc@TiO2, a sharper Si–O vibration peak at 1017 cm−1 reveals the exposure of the organic functional groups on Talc@TiO2.41 Peaks at 1317 cm−1 and 1382 cm−1 are attributed to the appearance of COO–, indicating that PAAS has been absorbed on the surface of Talc@TiO2.
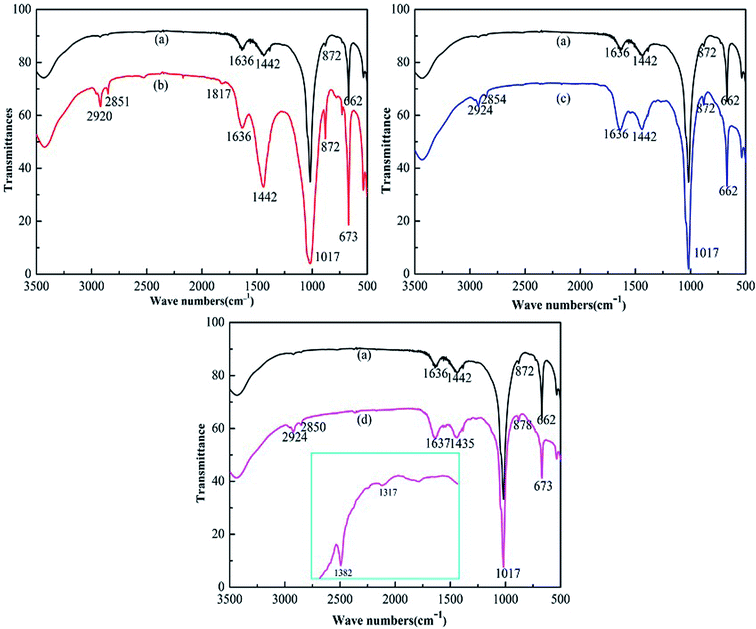 | ||
| Fig. 7 FTIR spectra of (a) Talc@TiO2, (b) NDZ201-Talc@TiO2, (c) KH550-Talc@TiO2, (d) PAAS-Talc@TiO2. | ||
To further explore the modification mechanism, XPS was used to confirm the chemical states of the typical elements. XPS spectra of the samples are shown in Fig. 8. Talc@TiO2 gives the Ti2p1/2 peak at 464.7 eV and the Ti2p3/2 peak at 459.4 eV, which are attributed to TiO2. NDZ201-Talc@TiO2 gives the Ti2p1/2 peak at 464.7 eV and the Ti2p3/2 peak at 459.1 eV, Ti2p3/2 peak has undergone chemical shift due to the change in valence state from Ti4+ to Ti2+ and their alteration.43 Compared with Talc@TiO2, the Si2p peaks of KH550-Talc@TiO2 have moved to a higher binding energy and a new peak is located at 103.4 eV, which is due to triethoxy silane,44 illustrating that the silane coupling agent has adhered to the surface/interface of Talc@TiO2 to achieve good dispersion and compatibility in the matrix materials. As can be seen in Fig. 8e, the XPS spectrum of original Talc@TiO2 offers low binding energy C1s peaks at 284.6 eV, 285.0 eV and 285.6 eV, which are mainly attributed to carbon and its compounds from the surrounding environment. The XPS spectrum of PAAS-Talc@TiO2 in Fig. 8f shows C1s peaks with higher binding energies, located at the binding energies of 288.9 eV, 286.7 eV and 285.4 eV, which are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and the alkyl chain from PAAS, respectively,45 thereby indicating the existence of PAAS on the Talc@TiO2 particles via physical adsorption and the chemical interaction between functional groups and the hydroxyl or carbonyl groups of nano-TiO2.
O, C–O and the alkyl chain from PAAS, respectively,45 thereby indicating the existence of PAAS on the Talc@TiO2 particles via physical adsorption and the chemical interaction between functional groups and the hydroxyl or carbonyl groups of nano-TiO2.
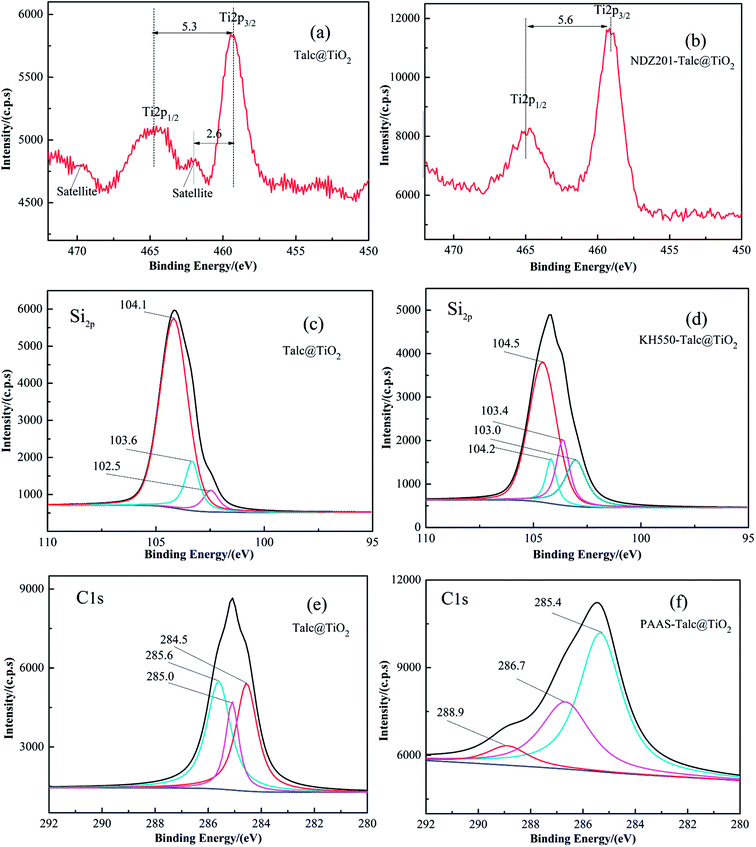 | ||
| Fig. 8 XPS spectra, the fitting of Si2p and C1s peaks of original and modified Talc@TiO2 by approximating the contribution of the background by the Shirley method. | ||
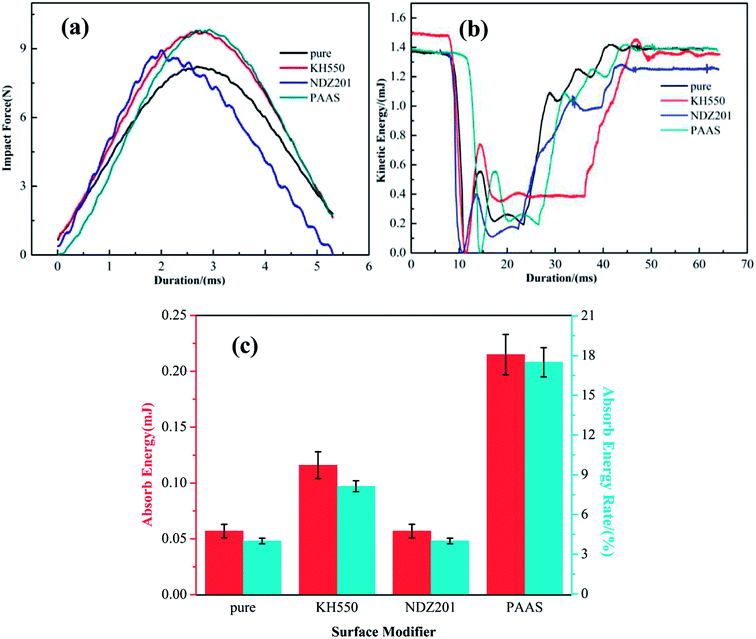
3.3 Performance characteristics of modified Talc@TiO2 hybrid XNBR
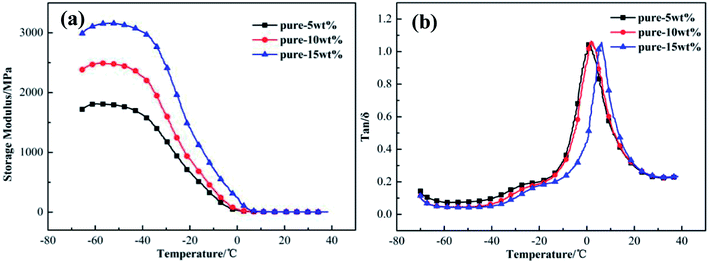
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of XNBR. The storage modulus increases with an increase in amount of filler added, but the change in tan
δ of XNBR. The storage modulus increases with an increase in amount of filler added, but the change in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is inconsistent with the storage modulus. The tan
δ is inconsistent with the storage modulus. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value is close to 1.1, and 10 wt% Talc@TiO2 as filler in XNBR gives the highest value. Therefore, XNBR with 10 wt% modified Talc@TiO2 filler will be investigated in detail in the following.
δ value is close to 1.1, and 10 wt% Talc@TiO2 as filler in XNBR gives the highest value. Therefore, XNBR with 10 wt% modified Talc@TiO2 filler will be investigated in detail in the following.
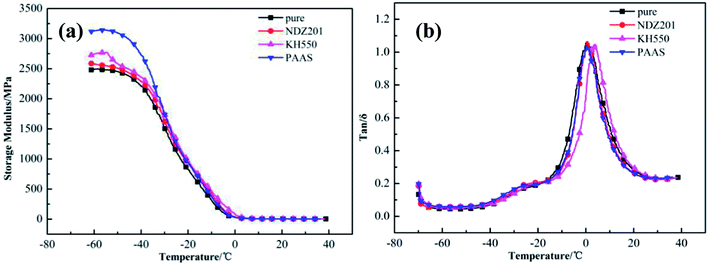
Fig. 11 shows the dynamic mechanical analysis data of modified Talc@TiO2 hybrid XNBR, including the storage modulus and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. The storage modulus of modified Talc@TiO2 hybrid XNBR reduces gradually with an increase in temperature, but they display a higher storage modulus at low temperature. Their tan
δ. The storage modulus of modified Talc@TiO2 hybrid XNBR reduces gradually with an increase in temperature, but they display a higher storage modulus at low temperature. Their tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values are generally consistent with that of original Talc@TiO2 hybrid XNBR, and all of them are close to about 1.1, because the Talc@TiO2 filler has the same basic composition, namely the same matrix material. The increase in storage modulus at lower temperature indicates that organic modification improves the compatibility and adhesion between the filler and XNBR, and modified Talc@TiO2 as a filler can strengthen the elasticity of XNBR.48
δ values are generally consistent with that of original Talc@TiO2 hybrid XNBR, and all of them are close to about 1.1, because the Talc@TiO2 filler has the same basic composition, namely the same matrix material. The increase in storage modulus at lower temperature indicates that organic modification improves the compatibility and adhesion between the filler and XNBR, and modified Talc@TiO2 as a filler can strengthen the elasticity of XNBR.48
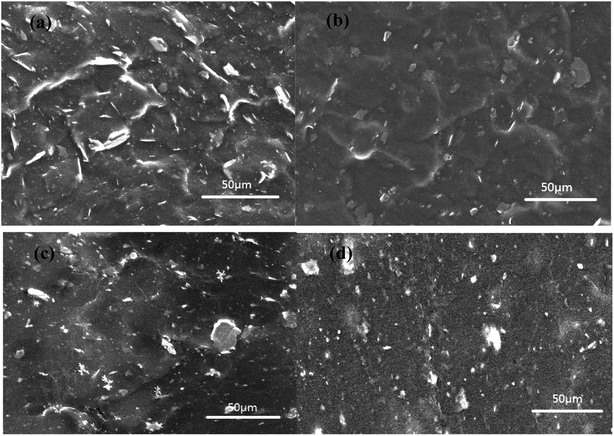 | ||
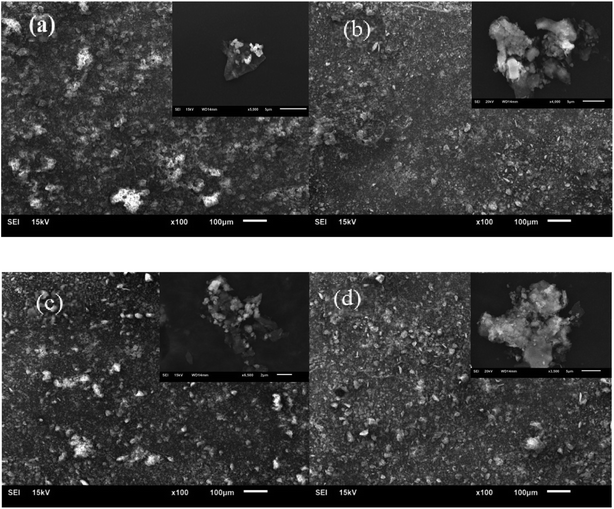
| Fig. 13 Cross-sectional SEM images of XNBR with original Talc@TiO2 (a) and modified Talc@TiO2 by (b) PAAS, (c) KH550, (d) NDZ201. | ||
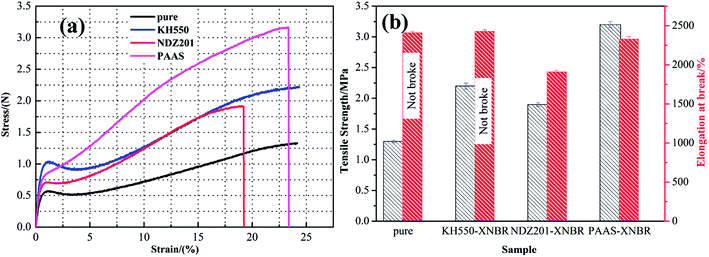
Combined with the analysis of modified Talc@TiO2 and hybrid XNBR, PAAS is the best choice as modifier according to the modification effect and performance characteristics of hybrid XNBR. By contrast, PAAS-Talc@TiO2 hybrid XNBR possesses excellent performances including good adhesion, a high storage modulus and absorbance of energy, depending mainly on the good compatibility and thickening effect of the modifier. For KH550-Talc@TiO2 hybrid XNBR, as KH550 modified Talc@TiO2 was introduced into XNBR, XNBR and KH550 modifier interacted to form KH550-coupled XNBR with modified Talc@TiO2, so this also illustrates that KH550 can offer relatively favorable mechanical properties.
4. Conclusions
Three modified Talc@TiO2 particles were successfully prepared and were introduced into XNBR to investigate their compatibility and mechanical performance, such as damping capacity and impact resistance. The following conclusions can be reached:(a) Talc@TiO2 mixed particles were prepared via the ball-milling treatment of coupling agents and surfactant modified TiO2 nanoparticles and Talc. NDZ201-Talc@TiO2 particles show hydrophobicity, and PAAS-Talc@TiO2 has good dispersion and compatibility in nitrile rubber.
(b) PAAS modified Talc@TiO2 particles hybrid XNBR shows better adhesion and good damping capacity and impact resistance, which is attributed to the excellent mechanical property of Talc, good adhesion between modified Talc@TiO2 and XNBR, and the nature of PAAS.
(c) The enhancement effect of modified Talc in XNBR was investigated in detail, and organic modification of Talc offers a great deal of flexibility. It is hoped that high-density modified Talc@TiO2 or organic clay as fillers can offer a significant step toward real-world application for polymers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No. 51705435 and No. 51627806), the Changjiang Scholarships and Innovation Team Development Plan (No. IRT1178).References
- H. Kargarzadeh, M. Mariano, J. Huang, N. Lin, I. Ahmad, A. Dufresne and S. Thomas, Polymer, 2017, 132, 368–393 CrossRef CAS.
- B. Likozar and Z. Major, Appl. Surf. Sci., 2010, 257, 565–573 CrossRef CAS.
- B. Chen, J. R. G. Evans, H. C. Greenwell, P. Boulet, P. V. Coveney, A. A. Bowden and A. Whiting, Chem. Soc. Rev., 2008, 37, 568–594 RSC.
- N. D. Bansod, B. P. Kapgate, C. Das, D. Basu, S. C. Debnath, K. Roy and S. Wiessner, RSC Adv., 2015, 5, 53559–53568 RSC.
- H. Kursun and U. Ulusoy, Int. J. Miner. Process., 2006, 78, 262–268 CrossRef CAS.
- S. DíEz-Gutiérrez, M. A. RodríGuez-Pérez, J. A. D. Saja and J. I. Velasco, Polymer, 1999, 40, 5345–5353 CrossRef.
- J. C. Dai and J. T. Huang, Appl. Clay Sci., 1999, 15, 51–65 CrossRef CAS.
- Y. P. Wu, Q. X. Jia, D. S. Yu and L. M. Zhang, J. Appl. Polym. Sci., 2010, 89, 3855–3858 CrossRef.
- A. Krysztafkiewicz and L. Domka, J. Mater. Chem., 1997, 7, 1655–1659 RSC.
- W. J. Choi and S. C. Kim, Polymer, 2004, 45, 2393–2401 CrossRef CAS.
- B. Fiorentino, R. Fulchiron, J. Duchet-Rumeau, V. Bounor-Legaré and J. C. Majesté, Polymer, 2013, 54, 2764–2775 CrossRef CAS.
- K. P. Sau, T. K. Chaki and D. Khastgir, J. Mater. Sci., 1997, 32, 5717–5724 CrossRef CAS.
- Y. Shangguan, J. Yang and Q. Zheng, RSC Adv., 2017, 7, 15978–15985 RSC.
- E. G. Bajsic, F. Veljko and V. O. Bulatovc, Adv. Mater. Res., 2014, 849, 121–126 CrossRef.
- Y. Jahani and M. Ehsani, Polym. Bull., 2009, 63, 743–754 CrossRef CAS.
- N. Petchwattana, S. Covavisaruch and S. Petthai, Polym. Bull., 2014, 71, 1947–1959 CrossRef CAS.
- A. Garnier, F. Da Cruz-Boisson, S. Rigolet, J. Brendlé and V. Bounor-Legaré, RSC Adv., 2016, 6, 75715–75723 RSC.
- L. A. Castillo, S. E. Barbosa, P. Maiza and N. J. Capiati, J. Mater. Sci., 2011, 46, 2578–2586 CrossRef CAS.
- A. Krysztafkiewicz, L. Domka and W. Wieczorek, Colloid Polym. Sci., 1985, 263, 804–811 CAS.
- J. Zhang, H. Zhang, J. Pang, L. Li, S. Wang and M. Liu, RSC Adv., 2016, 6, 104416–104424 RSC.
- L. Zang, D. Chen, Z. Cai, J. Peng and M. Zhu, Composites, Part B, 2018, 137, 217–224 CrossRef CAS.
- Q. Y. Fan and H. B. Zhan, Prog. Chem., 2012, 24, 54–60 CAS.
- Z. Zhang, X. He, J. Zhang, X. Lu, C. Yang, T. Liu, X. Wang and R. Zhang, RSC Adv., 2016, 6, 91798–91805 RSC.
- S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumara, Prog. Polym. Sci., 2013, 38, 1232–1261 CrossRef CAS.
- H. H. Huang, A. H. Sun, J. F. Zhang, B. Wang, C. Y. Chu, Y. Li and G. J. Xu, Mater. Lett., 2013, 110, 260–263 CrossRef CAS.
- S. Ullah, E. P. Ferreira-Neto, A. A. Pasa, C. C. J. Alcantara, J. J. S. Acuña, S. A. Bilmes, M. L. M. Ricci, R. Landers, T. Z. Fermino and U. P. Rodrigues-Filho, Appl. Catal., B, 2015, 179, 333–343 CrossRef CAS.
- C. Ocando, A. Tercjak and I. Mondragon, Eur. Polym. J., 2011, 47, 1240–1249 CrossRef CAS.
- S. C. Tjong, Mater. Sci. Eng., R, 2006, 53, 73–197 CrossRef.
- D. F. Schmidt and E. P. Giannelis, Chem. Mater., 2010, 22, 167–174 CrossRef CAS.
- G. Cheng, B. Tong, Z. F. Tang, X. H. Yu, H. L. Wang and G. X. Ding, Appl. Surf. Sci., 2014, 313, 954–960 CrossRef CAS.
- Z. Demjen, B. Pukánszky, E. Földes and J. Nagy, J. Colloid Interface Sci., 1997, 190, 427–436 CrossRef CAS.
- J. Z. Lu, Q. L. Wu and H. S. J. Mcnabb, Wood Fiber Sci., 2000, 32, 88–104 CAS.
- P. C. Ma, J. Kim and B. Z. Tang, Carbon, 2006, 44, 3232–3238 CrossRef CAS.
- P. Yang and M. G. Moloney, RSC Adv., 2016, 6, 111276–111290 RSC.
- A. Vermogen, K. Masenelli-Varlot and R. Séguéla, Macromolecules, 2005, 38, 9661–9669 CrossRef CAS.
- S. Pradhan, F. R. Costa, U. Wagenknecht, D. Jehnichen, A. K. Bhowmick and G. Heinrich, Eur. Polym. J., 2008, 44, 3122–3132 CrossRef CAS.
- H. L. Kang, K. H. Zuo, Z. Wang, L. Q. Zhang, L. Liu and B. C. Guo, Compos. Sci. Technol., 2014, 92, 1–8 CrossRef CAS.
- K. Sasikumar, N. R. Manoj, T. Mukundan and D. Khastgir, Composites, Part B, 2016, 92, 74–83 CrossRef CAS.
- Spectral Database for Organic Compounds. SDBS, http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi.
- D. M. Zhang, H. L. Yang, S. W. Yu and X. M. Sang, Key Eng. Mater., 2008, 368, 1477–1479 CrossRef.
- J. R. Sohn and H. J. Jang, J. Catal., 1991, 132, 563–565 CrossRef CAS.
- S. Yoon, R. J. Holiday, E. L. Sibert and F. F. Crim, J. Chem. Phys., 2003, 119, 9568–9575 CrossRef CAS.
- NIST X-ray Photoelectron Spectroscopy Database, National Institute of Standards and Technology: Gaithersburg. http://srdata.nist.gov/xps/.
- E. T. Vandenberg, L. Bertilsson, L. Bo, K. Uvdal, R. Erlandsson, H. Elwing and I. Lundström, J. Colloid Interface Sci., 1991, 147, 103–118 CrossRef CAS.
- X. Wallart, C. H. D. Villeneuve and P. Allongue, J. Am. Chem. Soc., 2005, 127, 7871–7878 CrossRef CAS PubMed.
- J. Y. Wang, H. B. Jia, Y. Y. Tang, D. D. Ji, Y. Sun, X. D. Gong and L. F. Ding, J. Mater. Sci., 2013, 48, 1571–1577 CrossRef CAS.
- I. Smaoui, A. Domatti, M. Kharrat, M. Dammak and G. Monteil, Fullerenes, Nanotubes, Carbon Nanostruct., 2017, 24, 769–778 CrossRef.
- A. K. Mehrjerdi, B. Adl-Zarrabi, S. W. Cho and M. Skrifvars, J. Appl. Polym. Sci., 2013, 129, 2128–2138 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |