Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoluminescence properties of a ScBO3:Cr3+ phosphor and its applications for broadband near-infrared LEDs†
Qiyue Shao *a,
Hao Dinga,
Leqi Yaoa,
Junfeng Xub,
Chao Liangb and
Jianqing Jiangc
*a,
Hao Dinga,
Leqi Yaoa,
Junfeng Xub,
Chao Liangb and
Jianqing Jiangc
aSchool of Materials Science and Engineering, Jiangsu Key Laboratory for Advanced Metallic Materials, Southeast University, Nanjing 211189, PR China. E-mail: qiyueshao@seu.edu.cn
bJiangsu Bree Optronics Co., Ltd., Nanjing 211103, PR China
cSchool of Materials Science and Engineering, Nanjing Forestry University, Nanjing 210037, PR China
First published on 28th March 2018
Abstract
The rapid extension of solid state lighting technologies offers the possibility to develop broadband near-infrared (NIR) phosphor-converted LEDs (pc-LEDs) as novel NIR light sources. In this paper, a new NIR-emitting phosphor ScBO3:Cr3+ was synthesized by a high temperature solid state reaction method. Phase structure, spectroscopic properties, luminescent lifetime, quantum yield, emitter concentration influences and thermal quenching behavior of ScBO3:Cr3+, as well as its applications for NIR pc-LEDs, were systematically investigated. ScBO3:Cr3+ phosphors exhibit a broad absorption band ranging from 400 to 530 nm, which matches well with the characteristic emission of the blue LED chip. Moreover, Cr3+ ions occupy the Sc3+ sites with relatively low crystal field strength in the ScBO3 host, and therefore ScBO3:Cr3+ phosphors show intense broadband emission peaking at ∼800 nm upon excitation at 460 nm, originating from spin-allowed 4T2 → 4A2 transition of Cr3+ ions. The optimum Cr3+ concentration was determined to be ∼2 mol% with a quantum yield of ∼65%. A broadband NIR pc-LED prototype device was fabricated by the combination of ScBO3:Cr3+ phosphors and a blue LED chip, which showed a maximum NIR light output power of ∼26 mW and a corresponding energy conversion efficiency of ∼7%. The results indicate the great potential of ScBO3:Cr3+ phosphors for applications in broadband NIR pc-LEDs.
Introduction
Since the first demonstration of high-brightness blue LEDs and subsequently white pc-LEDs,1,2 solid state lighting technologies have developed greatly and brought about a revolution in lighting and display industries. White LEDs are emerging as novel lighting sources to replace traditional incandescent and fluorescent lamps in numerous fields, due to their superior advantages including high luminous efficiency, environment friendliness, long lifetime, compactness and good reliability. The widely used method for producing white LED devices is the combination of a blue LED chip and certain phosphor materials with appropriate emission wavelengths. These white pc-LEDs possess simple device structure and low manufacturing cost. Besides white LEDs, the rapid developments of solid state lighting technologies also will provide a promising solution to construct broadband NIR light sources, taking advantage of the phosphor-converted LED technique.3–7NIR light sources can be widely applied in a variety of fields, such as optical communication, biomedical imaging, spectroscopy, food detection, security surveillance and face (or iris) recognition.8–12 Incandescent bulbs, halogen lamps and AlGaAs-based LEDs are normally used as NIR light sources, however, to some extent suffering from various weaknesses. Incandescent bulbs and halogen lamps can provide continuous light emissions covering from visible to infrared ranges. However, their practical applications are limited by some drawbacks, such as large sizes, short lifetimes, low luminescent efficiency and high working temperature.6 In contrast, the NIR semiconductor LEDs possess small sizes, long lifetimes and high energy-conversion efficiency. Unfortunately, the LEDs normally show narrow emission bands with the largest full width at half maximum (FWHM) of ∼40 nm,5,7 which are inappropriate for most spectroscopic applications. A possible solution to overcome the limitations of current NIR light sources is to design the NIR-emitting pc-LEDs, which are composed of well-established InGaN blue LED chips and NIR-emitting phosphors. The NIR pc-LEDs can benefit from the outstanding merits of the blue-emitting InGaAl LED chips, such as relatively higher thermal stability, continuously increased luminous efficiency and decreased manufacturing cost. Since the invention of high-brightness blue LEDs in the mid 1990s,1,2 steady improvements have been achieved for InGaN-based blue LEDs. According to Haitz's law, the light output per LED package increases by a factor of twenty every decade, and the cost per lumen falls by a factor of ten.13 On the other hand, broadband and wavelength-tunable emissions can be expected for NIR pc-LEDs through the rational selection of emitting ions and host materials of NIR-emitting phosphors. Moreover, the continuous emission spectra will be achieved by the appropriate combination between various types of phosphor materials that possess various emission wavelengths upon excitation by the blue light.
To design NIR-emitting phosphors excited by the blue light, the emitting ions should be first of all chosen reasonably. The utilization of 4f–4f transitions of trivalent lanthanide ions is hampered by their spectrally narrow and low intensity absorption.3,4 For divalent or trivalent lanthanide ions (e.g., Eu2+ and Ce3+) featuring 4f–5d optical transitions, it is difficult to achieve the light emissions in the NIR spectral range due to the large energy separation between 4f and 5d levels. Recently, Bi-doped crystals and glasses have attracted intense attention due to their super broadband emissions from visible to NIR.14–16 However, the luminescent behaviour of Bi-active centre has not been well understood, and it is still a big challenge to improve its NIR emission efficiency.6 Transition metal ions with incomplete 3d shells (3dn, n < 10) have a number of low-lying energy states, between which radiative transitions in the NIR spectral region may occur. Moreover, because they are strongly coupled to the coordination ligands in hosts, optical transitions of transition metal ions are significantly affected by the crystal field symmetry and strength. In general, transition metal ions show relatively strong and broad excitation bands, as well as wavelength-tunable broadband emissions. As typical transition metal ions, Cr3+ ions in the material ruby (Al2O3:Cr3+) have been thoroughly investigated by spectroscopists for over a hundred years.17 Laser-related spectroscopy of Cr3+ ions in a variety of crystals has also been extensively studied for the developments of tunable solid state lasers.18 Recently, NIR long-persistent luminescence of Cr3+-activated gallate phosphors has gained considerable attention due to their potential applications in optical information storage, night-vision surveillance and bio-imaging.19–23 Cr3+ ions exhibit strong absorption band in the visible spectral range and can be effectively excited by the blue light. In addition, in contrast to sharp emission lines arising from the 2E → 4A2 transition of Cr3+ ions in strong-field sites (e.g., Al2O3:Cr3+), a tunable broad emission band arising from the 4T2 → 4A2 transition can be observed when Cr3+ ions occupy lattice sites with relatively weak crystal field.17,24 Theses optical characteristics imply that Cr3+ ions are an ideal choice of luminescence centres for the development of novel NIR-emitting phosphors.
In this paper, NIR-emitting ScBO3:Cr3+ phosphors were synthesized via solid state reaction, and their crystal structure, photoluminescence (PL) and thermal quenching properties were systemically investigated. Cr3+-doped ScBO3 single crystals have been considered as room temperature near-infrared tunable laser materials by Lai et al. in the mid 1980s.25 However, structural and spectroscopic characteristics of ScBO3:Cr3+ phosphors are far from systematic studies and especially its applicability for NIR pc-LEDs deserve further investigations. In this work, a prototype of the NIR pc-LED was also fabricated on the basis of the ScBO3:Cr3+ phosphors and a blue LED chip, and its electroluminescence properties were studied. The results demonstrate the great potential of ScBO3:Cr3+ phosphors for applications in broadband NIR-emitting pc-LEDs.
Experimental section
Phosphor synthesis
Sc1−xCrxBO3 (x = 0.005–0.1) phosphors were synthesized via a solid state reaction method. Sc2O3 (99.99%), H3BO3 (99.95%) and Cr2O3 (99.95%) were used as starting materials. Stoichiometric amounts of raw materials were weighed and thoroughly mixed, except that 25% excess of H3BO3 was added to compensate its evaporation loss in course of high temperature sintering. Subsequently, the powder mixture was transferred into an alumina crucible and calcined in a muffle furnace at 1300 °C for 10 h. Finally, the as-prepared products were naturally cooled down to room temperature and ground to fine powders for subsequent characterization.Phosphor characterization
The phase structure of phosphors was identified by powder X-ray diffraction (XRD) measurements on a D8 Discover diffractometer (Bruker) with Cu Kα radiation (λ = 1.5406 Å). Rietveld structure refinements were conducted using the general structure analysis system (GSAS) program.26 The particle morphology was observed on a Sirion field-emission scanning electron microscope (FE-SEM, FEI). Diffuse reflection spectrum measurements were performed on a Cary 5000 UV-vis-NIR spectrophotometer (Varian) equipped with an internal diffuse reflectance accessory. The PL excitation spectra were measured on an F-7000 fluorescence spectrophotometer (Hitachi). The PL emission spectra were obtained on a Maya2000 portable spectrometer (Ocean Optics) using a blue LED (λem = 450 nm) as the excitation source. Temperature-dependent (30–250 °C) emission spectra were measured with the assistance of a self-designed heating system. The luminescent decay curves were measured by a FluoroMax-4 fluorescence spectrometer (Horiba Jobin Yvon). The quantum yield (QY) was measured by an integrating sphere equipped with a Maya2000 spectrometer and a blue LED, and the spectrometer was carefully calibrated by a standard tungsten lamp. The quantum yield is defined as the percentage of the number of emitted photons to that of absorbed photons, which can be calculated using the following equation:27
 | (1) |
NIR pc-LED fabrication and performance measurement
NIR pc-LEDs were fabricated by applying a mixture of ScBO3:Cr3+ phosphors and transparent silicon resin on an InGaN blue LED chip (λem = 455 nm). Photographs of the lighted pc-LED were taken by a cellphone's camera (Samsung S7), which used a Si-based photodetector covering the spectral range up to ∼1100 nm. When taking the picture, an 800 nm long-pass filter was used to remove all the visible light. For electroluminescence (EL) measurements, the packaged LED device was operated at a DC forward bias of ∼3.0 V with various injection currents, and the EL spectral distribution was recorded using an integrating sphere and a corrected Maya2000 spectrometer. The NIR light output power of the pc-LED was measured by a FieldMate light power meter (Coherent) equipped a PM10 thermopile sensor, where a 600 nm long-pass filter was used to remove the residual blue light from the LED chip. The energy conversion efficiency was defined as the ratio of the NIR light output power to the input electrical power.Results and discussion
Structural analysis
Rietveld structure refinements of as-prepared ScBO3 and ScBO3:0.02Cr3+ samples were performed to identify their phase structure. The crystallographic data (ICSD# 65010) reported by Keszler et al. was adopted as the initial model.28 Fig. 1 shows the refined XRD patterns of ScBO3 and ScBO3:0.02Cr3+, and the final refinement parameters are listed in Table 1. The refined profiles confirm the phase purity without unidentified diffraction peaks from impurity, regardless of Cr3+ doping. Both ScBO3 and ScBO3:Cr3+ are found to crystallize as a rhombohedral structure with a space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (no. 167). Fig. 2 displays the crystal cell of ScBO3 and the coordination environment of [ScO6] group. In ScBO3, Sc3+ ions are coordinated by six oxygen atoms and B3+ ions are surrounded by three oxygen atoms. ScBO3 exhibits a layered calcite-like structure, which is composed of [ScO6] octahedron and trigonal planar [BO3] group. Structural connectivity between the [ScO6] and [BO3] group occurs only via corner-sharing.
c (no. 167). Fig. 2 displays the crystal cell of ScBO3 and the coordination environment of [ScO6] group. In ScBO3, Sc3+ ions are coordinated by six oxygen atoms and B3+ ions are surrounded by three oxygen atoms. ScBO3 exhibits a layered calcite-like structure, which is composed of [ScO6] octahedron and trigonal planar [BO3] group. Structural connectivity between the [ScO6] and [BO3] group occurs only via corner-sharing.
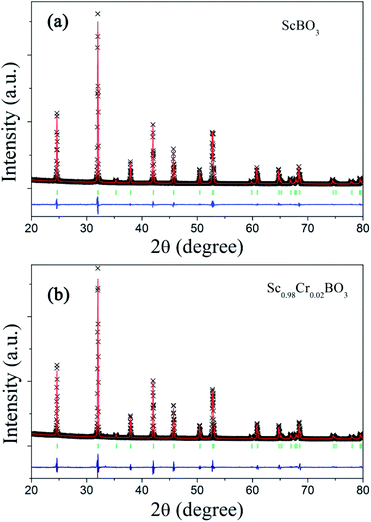 | ||
| Fig. 1 Experimental (cross), calculated (solid line), Bragg positions (tick mark) and differences (bottom) results of powder XRD refinements of (a) ScBO3 and (b) ScBO3:0.02Cr3+. | ||
| ScBO3 | ScBO3:0.02Cr3+ | |
|---|---|---|
| Space group | R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
|
| Symmetry | Rhombohedral | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Cell parameters | ||
| a = b (Å) | 4.7516(5) | 4.7481(9) |
| c (Å) | 15.2835(7) | 15.2641(1) |
| α = β, γ (°) | 90°, 120° | 90°,120° |
| Z, volume (Å3) | 6, 298.84(1) | 6, 298.03(1) |
| Rp (%), Rwp (%) | 6.43%, 8.57% | 8.45%, 11.51% |
| χ2 | 3.163 | 6.672 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Bond distance and angle | ||
| B–O (Å) | 1.37624(1) | 1.3916(18) |
| Sc–O (Å) | 2.12198(2) | 2.1124(9) |
| ∠OBO (°) | 120 | 120 |
| ∠OScO (°) | 87.686(1), 180.000(0), 92.314(1) | 87.483(25), 180.000(0), 92.517(25) |
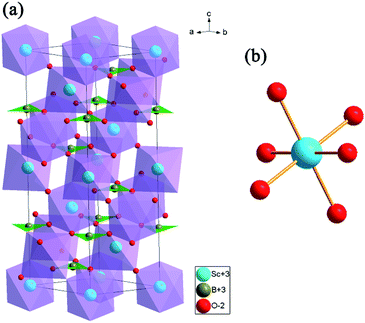 | ||
| Fig. 2 (a) The crystal structure representation of ScBO3 and (b) the coordination environment of [ScO6] octahedron. | ||
For the ScBO3 host, the lattice parameters are calculated to be a = b = 4.7516(5) Å, c = 15.2835(7) Å, V = 298.84(1) Å3, and Z = 6. For ScBO3:0.02Cr3+, the smaller lattice parameters are determined: a = b = 4.7481(9) Å, c = 15.2641(1) Å, V = 298.03(1) Å3. The effective ionic radius of Cr3+ is 0.615 Å when the coordination number (CN) is equal to 6, which is close to that of Sc3+ (0.745 Å, CN = 6) and far larger than that of B3+ (0.27 Å, CN = 6).29 Therefore, Cr3+ ions prefer to occupy the Sc3+ sites with octahedral coordination in ScBO3:Cr3+ phosphors. Due to the smaller ionic radius of Cr3+, the calculated cell parameters and Sc–O bond length of ScBO3:0.02Cr3+ are slightly smaller than that of pure ScBO3 (Table 1).
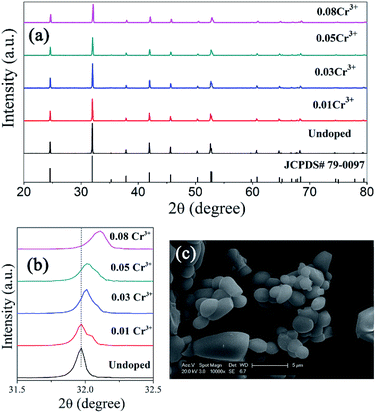
XRD patterns of ScBO3:Cr3+ phosphors with various Cr3+ concentrations are shown in Fig. 3a. All the diffraction peaks of ScBO3:xCr3+ (x = 0.01–0.08) phosphors agree well with those of a rhombohedral ScBO3 structure (JCPDS Card no. 79-0097). No impurity phase can be recognized with the increase of Cr3+ contents.
Noting that the XRD peaks of ScBO3:xCr3+ phosphors shift to higher diffraction angle with increasing the Cr3+ concentration (Fig. 3b), indicative of the decrease of lattice interplanar spacing due to the smaller ionic radius of Cr3+. The SEM image (Fig. 3c) shows that the obtained ScBO3:Cr3+ phosphor is mainly composed of a large number of spherical particles with an average size of 1–3 μm, except that a few large-sized particles (>5 μm) are also observed.
Spectroscopic properties
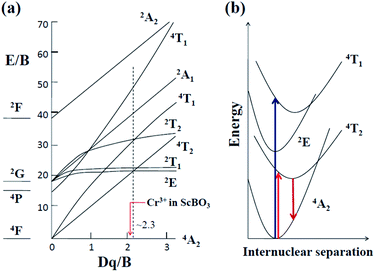
When Cr3+ (3d3) ions occupy lattice sites with octahedral coordination, their energy level distribution can be illustrated by Tanabe–Sugano diagram (Fig. 4a).30 Except for 2E and 2T1 levels, most of Cr3+ crystal field levels (e.g., 4T2, 4T1, 2A1) show strong dependences on Dq/B values, where Dq and B are the crystal field strength and Racah parameters, respectively.17,31 Optical transitions of Cr3+ ions can be understood in more detail with the aid of their configuration coordinate diagrams (Fig. 4b). Noting that 4A2 and 2E states are derived from the t3 2 crystal field orbital, whereas 4T2 and 4T1 states are originated from the t2 2e orbital.17,32 Accordingly, there is a small shift in equilibrium distance between the parabolas of 4A2 and 2E states in configuration coordinate diagram. In contrast, the parabolas of 4T2 and 4T1 states exhibit a larger offset compared to that of the ground state 4A2. The absorption spectra of Cr3+-doped compounds are normally characterized by two broad absorption bands in the visible spectral range, arising from the spin-allowed 4A2 → 4T2 and 4A2 → 4T1 transitions, respectively. The emission band shapes of Cr3+ ions are determined by the host crystal field strength, depending on the fact whether the 2E or 4T2 level is lowest. As shown in Fig. 4a, 2E and 4T2 levels cross at Dq/B ≈ 2.3.17 When Cr3+ ions occupy high-field sites (Dq/B > 2.3), 2E will be the emitting state and the Cr3+ emission spectra is characterized by sharp emission lines (2E → 4A2). When Cr3+ ions occupy low-field sites (Dq/B < 2.3), the 4T2 → 4A2 transition will dominate the Cr3+ luminescence featuring a very broad emission band (Fig. 4b).Fig. 5a shows the diffuse reflection spectra of ScBO3 and ScBO3:xCr3+ samples. Two broad absorption bands centred at ∼460 and 645 nm were detected after the incorporation of Cr3+ ions into the ScBO3 host, which are originated from the 4A2 → 4T1 and 4A2 → 4T2 transitions of Cr3+ ions (Fig. 4b), respectively. With the increase of Cr3+ contents, a continuous enhancement in absorptivity can be found. Fig. 5b represents the excitation and emission spectra of ScBO3:0.02Cr3+ phosphors. Upon excitation at 450 nm, the PL spectrum displays a broad emission band extending from 700 to 950 nm, with a maximum at ∼800 nm and a FWHM value of ∼120 nm. The broad emission band should be attributed to the spin-allowed 4T2 → 4A2 transition of Cr3+ ions (Fig. 4b). The excitation spectrum monitored at ∼800 nm is composed of two excitation bands at ∼460 and 645 nm, which is consistent with the diffuse reflection spectra of ScBO3:Cr3+ phosphors.
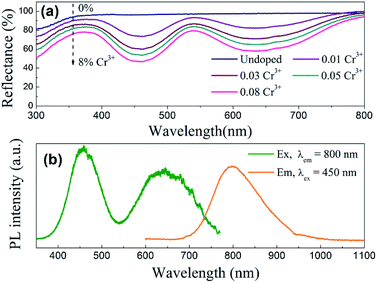 | ||
| Fig. 5 (a) Diffuse reflection spectra of ScBO3 and ScBO3:xCr3+; (b) excitation and emission spectra of ScBO3:0.02Cr3+ phosphors. | ||
For pc-LED applications, the quantum yield (QY) of the phosphors is an important factor that should be considered. The quantitative excitation profiles and emission spectra of the ScBO3:0.02Cr3+ phosphor and the reference sample were recorded upon excitation at ∼450 nm using an integrating sphere (Fig. S1†). According to eqn (1), the quantum yield of the ScBO3:0.02Cr3+ phosphor is calculated to be ∼65% under 450 nm excitation. The ∼460 nm excitation band of ScBO3:Cr3+ phosphors matches well the emissions of the blue LED chips. In combination with their broadband emission at ∼800 nm, ScBO3:Cr3+ phosphors show great potential for applications in NIR pc-LED devices based on the blue LED chip.
The Stokes shift with respect to the transition between 4A2 and 4T2 levels is determined to be ∼3004 cm−1 by the comparison between the corresponding spectral positions of room-temperature excitation (∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 504
504![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1) and emission (∼12
cm−1) and emission (∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) bands. From the spectral positions of 4A2 → 4T2 and 4A2 → 4T1 excitation bands shown in Fig. 5b, the values of the crystal field Dq and Racah B parameters can be calculated using the following equations:17,33
500 cm−1) bands. From the spectral positions of 4A2 → 4T2 and 4A2 → 4T1 excitation bands shown in Fig. 5b, the values of the crystal field Dq and Racah B parameters can be calculated using the following equations:17,33
| E(4T2) = 10Dq | (2) |
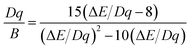 | (3) |
Cr3+ concentration effects
Fig. 6a shows the PL emission spectra of ScBO3:xCr3+ phosphors with various Cr3+ contents. The spectral profile changes little with the increase of Cr3+ concentrations. The peak wavelengths of broad emission bands of ScBO3:xCr3+ phosphors exhibit a slight red-shift with increasing x values (Fig. S2†), despite the fact that the Sc–O distance decreases at higher Cr3+ contents. We believe the re-absorption between activator ions play an important role in ScBO3:xCr3+ phosphor with larger x values. As shown in Fig. 5b, the long-wavelength excitation band of ScBO3:Cr3+ slightly overlaps with the emission band, and the re-absorption between Cr3+ ions will reduce the emission at the blue wing and result in the red-shift of emission band.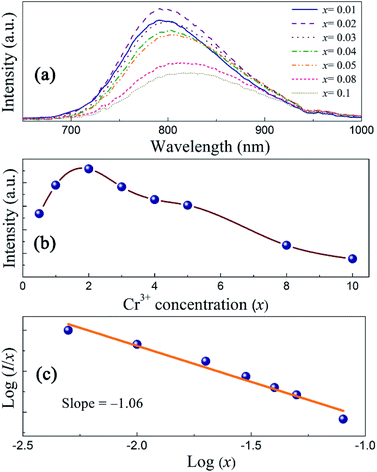 | ||
| Fig. 6 (a) Emission spectra, (b) plots of I vs. x and (c) log(I/x) vs. log(x) of ScBO3:xCr3+ phosphors (λex = 450 nm) with various Cr3+ contents. | ||
The emission intensities of ScBO3:xCr3+ phosphors increase with increasing x values and reach a maximum at a critical concentration of x = 0.02. A further increase in the Cr3+ concentration results in the decrease of the emission intensity due to the concentration quenching effect (Fig. 6b). Nonradiative energy migration among luminescence centres can occur via exchange interaction or multipole–multipole interaction. In this case, the critical energy transfer distance (Rc) can be approximately estimated by the following equation proposed by Blasse:31
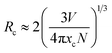 | (4) |
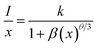 | (5) |
Fig. 7 shows the luminescent decay curves of ScBO3:xCr3+ phosphors with various x values. All the decay curves can be well fitted by a single exponential function (Fig. S3†). The luminescent lifetime (τ) are determined to approximately 105, 94, 83, 67 and 41 μs for ScBO3:xCr3+ where x = 0.01, 0.02, 0.03, 0.05, 0.08. The decrease of the Cr3+ lifetime is attributed to the increased luminescent quenching effect at higher Cr3+ concentrations. The well fitting results of luminescent decay curves by a single exponential function also indicate that the Cr3+ ions occupy only single lattice site in the ScBO3 host.
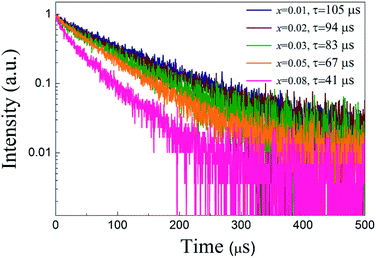 | ||
| Fig. 7 Luminescent decay curves of ScBO3:xCr3+ phosphors with various Cr3+ concentrations (λex = 450 nm). | ||
Temperature-dependent PL properties
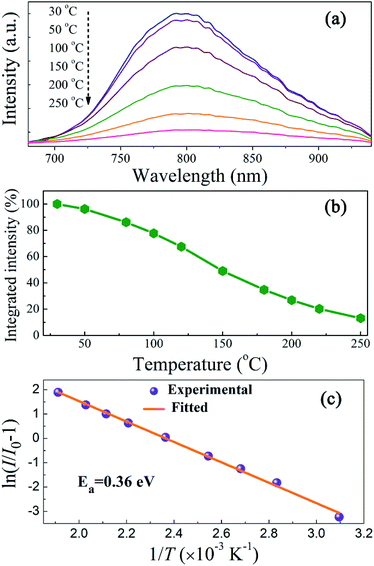
Luminescent thermal stability is another important factor of the phosphors for pc-LED applications. It is well known that the emission intensity of luminescent materials usually decreases in different degrees at elevated temperatures due to increased nonradiative transitions. The working temperature of LED devices can be higher than 100 °C,37 and therefore the phosphor materials should maintain their luminescent intensity at higher temperatures for pc-LED applications. Fig. 8a represents the PL spectra of ScBO3:0.02Cr3+ at various temperatures (30–250 °C), where a thermal quenching behaviour can be observed. With the temperature increasing from 30 to 150 °C, the emission intensity of ScBO3:0.02Cr3+ decreases by approximately 51% from the initial intensity (Fig. 8b). NIR-emitting ScBO3:Cr3+ phosphors show lower thermal stability than the commercial visible-emitting phosphors, such as Y3Al5O12:Ce3+ and CaAlSiN3:Eu2+, for which larger than 80% of the emission intensity at room temperature can be retained at 150 °C.38,39 The thermal quenching of ScBO3:Cr3+ can be caused via two possible routes:31 (1) multi-phonon emission directly across the energy gap between the excited state 4T2 and the ground state 4A2; (2) nonradiative transition through the crossing of 4T2 and 4A2 parabolas (Fig. 4b). The increased thermal quenching of ScBO3:Cr3+ is probably related to the narrow energy gap between 4T2 and 4A2 levels, as well as the larger Stokes shift of the phosphor.The temperature-dependence of the emission intensity can be described by a modified Arrhenius equation:40
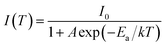 | (6) |
Fabrication and performance of NIR pc-LED
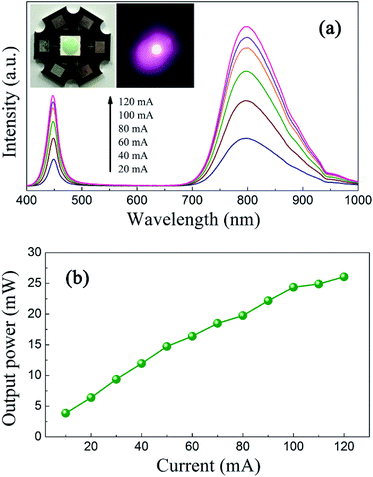
In order to demonstrate the applicability of ScBO3:Cr3+ phosphors, a NIR pc-LED prototype was fabricated by the combination of a 455 nm InGaN LED chip and the ScBO3:0.02Cr3+ phosphor. Photographs of the as-fabricated pc-LED device and the lighted one are shown in the insets of Fig. 9a. Bight NIR emission could be photographed by a cellphone's camera after the visible light was removed by an 800 nm long-pass filter. Fig. 9a shows a downshift on the emission that called here electroluminescence (EL) spectrum, even if the is no direct electron-excitation of the as-fabricated pc-LED at various drive currents. The blue emission band at ∼455 nm comes from the LED chip and the broad emission band between 700–950 nm is attributed to ScBO3:Cr3+ phosphors. With the increase in the drive current at a forward bias of ∼3 V, no obvious changes in the EL profile can be found, except for the continuous increase of the luminescence intensity.Fig. 9b represents the dependences of the NIR light output on the injection current of the pc-LED device. A continuous increase in the NIR light output power is found with increasing the injection current. A maximum output power of ∼26 mW is achieved at a drive current of 120 mA. The energy conversion efficiency is calculated to be approximately 7%, much larger than the reported values of NIR pc-LEDs based on trivalent lanthanide doped glass phosphors.3,4 Noting that the energy conversion efficiency of the LED chip used here is about 20% and the blue light from the LED chip still remains for the NIR pc-LED device. The energy conversion efficiency can be further improved by engineering the package structure and utilizing more efficient blue LED chips. These results demonstrate the great potential of ScBO3:Cr3+ phosphors as an alternative NIR component for applications in broadband NIR pc-LEDs.
Conclusions
ScBO3:Cr3+ phosphors were synthesized via a solid state reaction method and investigated for their structural and luminescent characteristics. The phase structure of as-prepared phosphors was confirmed by the Rietveld analysis. In the ScBO3 host, Cr3+ ions preferentially occupied Sc3+ sites with low-field octahedral coordination. Therefore, a broadband emission (λmax = ∼800 nm) in the NIR spectral range between 700–950 nm was observed for ScBO3:Cr3+ upon excitation by the blue light, which was attributed to 4T2 → 4A2 transitions of Cr3+ ions. The luminescent quenching was proportional to the Cr3+ concentration in Sc1−xCrxBO3 phosphors, and the maximum emission intensity was found at x = 0.02 with a quantum yield of ∼65%. The thermal quenching properties of ScBO3:Cr3+ phosphors were also inspected and the activation energy was determined to be ∼0.36 eV. By integrating ScBO3:Cr3+ phosphors on the blue LED chip, a broadband NIR pc-LED was obtained with a maximum NIR light output of ∼26 mW and the corresponding energy conversion efficiency of ∼7%. The results suggest that ScBO3:Cr3+ phosphors can potentially serve as conversion phosphors for broadband NIR pc-LED devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province of China (BK20160073) and the Jiangsu Key R&D Program (BE2015102).Notes and references
- S. Nakamura, T. Mukai and M. Senoh, Appl. Phys. Lett., 1994, 64, 1687–1689 CrossRef CAS.
- S. Nakamura and G. Fasol, The blue laser diode, GaN based light emitters and lasers, Springer, Berlin, 1997 Search PubMed.
- K. Oshima, K. Terasawa, S. Fuchi and Y. Takeda, Phys. Status Solidi C, 2012, 9, 2340–2343 CrossRef CAS.
- S. Fuchi, Y. Shimizu, K. Watanabe, H. Uemura and Y. Takeda, Appl. Phys. Express, 2014, 7, 072601 CrossRef.
- S. Möller, A. Katelnikovas, M. Haase and T. Jüstel, J. Lumin., 2016, 172, 185–190 CrossRef.
- D. Hayashi, A. M. Van Dongen, J. Boerekamp, S. Spoor, G. Lucassen and J. Schleipen, Appl. Phys. Lett., 2017, 110, 233701 CrossRef.
- B. Malysaa, A. Meijerink, W. W. Wu and T. Jüstel, J. Lumin., 2017, 190, 234–241 CrossRef.
- H. Y. Zhu, S. O. Isikman, O. Mudanyali, A. Greenbaum and A. Ozcan, Lab Chip, 2013, 13, 51–67 RSC.
- P. A. Martina, Chem. Soc. Rev., 2002, 31, 201–210 RSC.
- L. S. Magwaza, U. L. Opara, H. Nieuwoudt, P. J. R. Cronje, W. Saeys and B. Nicolaï, Food Bioprocess Technol., 2012, 5, 425–444 CrossRef CAS.
- N. Salamati, Z. Sadeghipoor and S. Süsstrunk, Proc. SPIE, 2011, 7865, 786508 CrossRef.
- J. Dowdall, I. Pavlidis and G. Bebis, Image Vis. Comput., 2003, 21, 565–578 CrossRef.
- R. Haitz and J. Y. Tsao, Phys. Status Solidi A, 2011, 208, 17–29 CrossRef CAS.
- J. Y. Zheng, L. L. Tan, L. P. Wang, M. Y. Peng and S. H. Xu, Opt. Express, 2016, 24, 2830–2835 CrossRef CAS PubMed.
- Z. Y. Zhang, J. K. Cao, Y. F. Xue, L. L. Tan, S. H. Xu, Z. M. Yang and M. Y. Peng, J. Am. Ceram. Soc., 2018, 101, 1159–1168 CrossRef.
- Y. F. Xue, J. K. Cao, Z. Y. Zhang, L. P. Wang, S. H. Xu and M. Y. Peng, J. Am. Ceram. Soc., 2018, 101, 624–633 CrossRef CAS.
- B. Henderson and G. F. Imbusch, Optical spectroscopy of inorganic solids, Clarendon, Oxford, 1989 Search PubMed.
- S. Kück, Appl. Phys. B, 2001, 72, 515–562 CrossRef.
- Z. W. Pan, Y. Y. Lu and F. Liu, Nat. Mater., 2012, 11, 58–63 CrossRef CAS PubMed.
- Z. J. Li, Y. W. Zhang, X. Wu, X. Q. Wu, R. Maudgal, H. W. Zhang and G. Han, Adv. Sci., 2015, 2, 1500001 CrossRef PubMed.
- M. Allix, S. Chenu, E. Véron, T. Poumeyrol, E. A. Kouadri-Boudjelthia, S. Alahraché, F. Porcher, D. Massiot and F. Fayon, Chem. Mater., 2013, 25, 1600–1606 CrossRef CAS.
- Y. X. Zhuang, J. P. Ueda, S. Tanabe and P. Dorenbos, J. Mater. Chem. C, 2014, 2, 5502–5509 RSC.
- Y. Li, S. F. Zhou, Y. Y. Li, K. Sharafudeen, Z. J. Ma, G. P. Dong, M. Y. Peng and J. R. Qiu, J. Mater. Chem. C, 2014, 2, 2657–2663 RSC.
- U. Hommerich and K. L. Bray, Phys. Rev. B, 1995, 51, 12133–13141 CrossRef CAS.
- S. T. Lai, B. H. T. Chai, M. Long and R. C. Morris, IEEE J. Quantum Electron., 1986, 22, 1931–1933 CrossRef.
- A. C. Larson and R. B. Von Dreele, Los Alamos National laboratory Report LAUR, 1994, pp. 86–748 Search PubMed.
- J. S. Zhong, D. Q. Chen, W. G. Zhao, Y. Zhou, H. Yu, L. F. Chen and Z. G. Jia, J. Mater. Chem. C, 2015, 3, 4500–4510 RSC.
- D. A. Keszler and H. Sun, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 1505–1507 CrossRef.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- Y. Tanabe and S. Sugano, J. Phys. Soc. Jpn., 1954, 9, 766–779 CrossRef.
- G. Blasse and B. C. Grabmaier, Luminescent materials, Springer, Berlin, 1994 Search PubMed.
- H. M. Zhu, C. C. Lin, W. Q. Luo, S. T. Shu, Z. G. Liu, Y. S. Liu, J. T. Kong, E. Ma, Y. G. Cao, R. S. Liu and X. Y. Chen, Nat. Commun., 2014, 5, 5312 CrossRef PubMed.
- S. S. Pedro, L. P. Sosman, R. B. Barthem, J. C. G. Tedesco and H. N. Bordallo, J. Lumin., 2013, 134, 100–106 CrossRef CAS.
- L. G. Van Uitert, J. Electrochem. Soc., 1967, 114, 1048–1053 CrossRef CAS.
- C. H. Huang, Y. C. Chiu, Y. T. Yeh and T. M. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 6661–6668 CAS.
- Z. G. Xia, J. Liu, Q. Li and J. Y. Sun, Electrochem. Solid-State Lett., 2007, 10, J4–J8 CrossRef CAS.
- J. McKittrick and L. E. Shea-Rohwer, J. Am. Ceram. Soc., 2014, 97, 1327–1352 CrossRef CAS.
- V. Bachmann, C. Ronda and A. Meijerink, Chem. Mater., 2009, 21, 2077–2084 CrossRef CAS.
- X. Q. Piao, K. Machida, T. Horikawa, H. Hanzawa, Y. Shimomura and N. Kijima, Chem. Mater., 2007, 19, 4592–4599 CrossRef CAS.
- W. Z. Lv, Y. C. Jia, Q. Zhao, M. M. Jiao, B. Q. Shao, W. Lü and H. P. You, Adv. Opt. Mater., 2014, 2, 183–188 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01084f |
| This journal is © The Royal Society of Chemistry 2018 |