Open Access Article
Open Access ArticleEffect of alkali and alkaline earth metal species on the combustion characteristics of cattle manures
Baojun Yi *ab,
Qiaoxia Yuanab,
Hongliang Caoab,
Wenjuan Niuab,
Ming Wangab,
Yao Zhua and
Shuiping Yanab
*ab,
Qiaoxia Yuanab,
Hongliang Caoab,
Wenjuan Niuab,
Ming Wangab,
Yao Zhua and
Shuiping Yanab
aCollege of Engineering, Huazhong Agricultural University, No. 1, Shizishan Street, Hongshan District, Wuhan, 430070, P. R. China. E-mail: bjyi@mail.hzau.edu.cn; Fax: +86 27 87282120; Tel: +86 27 87282120
bKey Laboratory of Agricultural Equipment in the Mid-lower Yangtze River, Ministry of Agriculture, Wuhan, 430070, P. R. China
First published on 27th March 2018
Abstract
This study investigates the effects of alkali and alkaline earth metal (AAEM) species on the combustion characteristics of cattle manures (CM). Different AAEM species (K, Na, Ca, and Mg) were mixed with CM and deashing CM (D-CM) samples. The combustion characteristics of raw and char samples were compared. The effects of AAEM species on CM char were analyzed based on the structural characteristics of the char sample. Results show that K and Na exert a positive effect, and this effect varies depending on the addition amount. Ca and Mg also exhibit a positive effect, but this effect does not change with the addition amount. The positive effect of K, Na, and Ca is related to the decrease in graphitization degree and increase in specific surface area. However, the positive effect of Mg is negligible. In conclusion, CM can be mixed with fuels containing K or Na in an appropriate ratio. The amount of Ca to be mixed with fuels has no specific requirement, whereas that of Mg to be mixed with fuels should be controlled.
1. Introduction
In recent years, the problem of fecal matter in large-scale farms has attracted wide attention. Improper handling not only affects the surrounding environment but also restricts the healthy development of farms. Livestock and poultry manure can cause water, soil, and atmospheric pollution; in addition, fecal pathogens can easily lead to disease spread.1 A number of treatment methods can be used for livestock and poultry manures.2–5 These wastes can be applied as organic fertilizers directly to farmlands.6 However, the waste production of large-scale farms outnumbers the needs of local farmlands. In addition, direct fertilizer utilization generates biogas through the anaerobic fermentation of livestock and poultry manures. The smell and pollution of livestock and poultry manures can be reduced, but the processing cycle is too long. Heat treatment of livestock and poultry manures is a practical technology that ensures rapid and complete eradication of harmful pathogens in large farms.Livestock manures are heat treated through pyrolysis, gasification, and combustion.3,7,8 Biomass char can be obtained through fecal pyrolysis9–11 or by mixing pyrolysis with other biomass.12 However, utilization problems remain for subsequent char and biomass oil. The energy produced from biogas could be maximized through gasification of livestock and poultry manures.8,13 However, the tar formed during gasification and the high content of potassium in the ash limit the application of livestock and poultry manure gasification.2 By contrast, burning of poultry manures has attracted widespread attention as a direct and complete process to generate energy.14 At present, combustion of livestock and poultry manures is performed through co-firing or direct combustion. Co-firing is easier to achieve than direct combustion,15,16 but it needs a suitable coal-fired power plant surrounding. Compared with co-firing, direct combustion has less requirements and impact on the surrounding environment. Thus, direct combustion is the better choice to treat livestock and poultry manures for large-scale farms.
Most livestock and poultry manures in China are abundant in K, Ca, and Mg.17 Previous studies confirmed that K, Na, Ca, and Mg in low-rank fuels catalyze gasification18–22 and combustion.23,24 Hu et al. studied the catalytic effects of inherent alkali and alkaline earth metals (AAEMs) on pyrolysis and gasification of biomass.25,26 Cheng et al. ranked the catalytic effect of AAEMs on the combustion of pulverized coal to be Na > Fe > Ca > Al.23 However, excessive AAEMs can lead to severe fouling and slagging.27,28 A suitable proportion of CaO can significantly reduce the alkali metal content of chars and slagging.29,30 As a disadvantage of AAEM on the combustion of agriculture, forestry, and straw biomass, washed biomass can induce deashing to promote combustion.31 For livestock and poultry manures, washing needs huge amounts of water and cause secondary pollution due to the large number of organic matter and pathogens. Meanwhile, pickling is of high cost. Therefore, understanding the effects of AAEM species on the burning of livestock and poultry manures is important.
Most previous studies focused on the effects of inherent AAEM species on the pyrolysis and combustion of biomass.25,26 Few studies investigated the impact of external AAEM content on livestock and poultry manures after deashing. In the actual transfer process, external ash is easily to be mixed to livestock and poultry manure. Furthermore, whether the addition of external ash would facilitate combustion reaction need further research. In the present study, typical cattle manure (CM) was selected as the object of research, and the effect of AAEM species (K, Na, Ca, and Mg) on the combustion of CM was discussed.
2. Experimental
2.1. Materials and pretreatment
All CM samples used in the experiment were freshly collected from a dairy farm of Dongzheng Livestock and Poultry Co., Ltd. in Jiangxia District, Wuhan, Hubei Province. CM was dehydrated in air at the College of Engineering Laboratory of Huazhong Agricultural University. CM was dried in a drying oven at 105 °C for 48 h before the experiment. Then, dried CM was crushed into powder and passed through a 60-mesh sieve. Proximate analysis, ultimate analysis, low heating value, chemical composition, and inorganic substances are shown in Table 1. The proportion of volatile matter was 45.80% on the dry basis of CM, and the proportions of ash and fixed carbon were 32.19% and 12.52%, respectively. Volatile matter is the main body of combustible CM. Ultimate analysis revealed O content of 49.92% and C content of 41.13%. High O and low H caused the small lower heating values, which is 13.426 MJ Kg−1. The main chemical composition of CM was 24.05% cellulose, 26.24% hemicellulose, and 5.16% lignin. The chemical composition in biomass is responsible for the different thermochemical properties. Lignin is the most difficult one to decompose among the three components.32 Hence, low content lignin in CM is expected to result in good combustion reactivity. The main inorganic substances of raw CM are Si, Ca, K, Al, and Mg. Some AAEM species in CM is in favor of comparing the impact of internal and external AAEM species. To clarify the effect of external AAEM (K, Na, Ca, and Mg) species on the combustion of CM, K2CO3, Na2CO3, CaCO3, and MgCO3 were blended with the deashing samples. The alkali metal carbonate has a good catalytic effect.33,34 In addition, the product result from the decomposition reaction of carbonate may not affect the experiment. Deashing method was introduced everywhere.35,36 This process was carried out by mixing a 5 g CM sample with 100 mL of 50% HCl and stirring for 2 h in a water bath at 75 °C. The sample was cooled, filtered, and then washed in distilled water for three times. The sample was then mixed with 100 mL of 50% HF, then the previous step was repeated. After three times of HCl–HF washing, the sample was dried and identified as D-CM. The experimental material was pretreated several times by the above deashing process to obtain enough D-CM samples, which were placed in a sealed bag and stored in the freezer. Different contents of AAEM species were compared. The blend ratios were 2.5%, 5%, and 7.5% in weight. The blending of CM/D-CM and AAEMs were immersed in water solution (1 g of solid sample per 10 mL agent), stirred for 2 h at ambient temperature (25 ± 3 °C), and then it dried at 55 °C in an oven for 24 h. The dried samples were shaked for 10 min.| Inorganic species (g kg−1) | LHV (MJ kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Ca | K | Al | Mg | Na | Fe | Mn | Zn | Cu | ||
| CM | 203.59 | 70.08 | 16.53 | 13.33 | 9.64 | 3.51 | 2.81 | 0.78 | 0.14 | 0.04 | 13.426 |
Char was prepared in accordance with a previously described method to distinguish the effect of AAEM species on volatile matter and fixed carbon in CM.37 In the present study, we conducted 800 °C storage for 30 min under N2 atmosphere. The sample amount was 5 g each time, the blend ratio was 5% AAEM species, and the gas flow rate was set to 1 L min−1. The char samples prepared from CM and deashing CM were named as CM char and D-CM char, respectively. The method of dissolving and re-drying was chosen to blend K2CO3, Na2CO3, CaCO3, and MgCO3 in the sample.38,39
Raw CM mixed with K2CO3, Na2CO3, CaCO3, and MgCO3 were denoted by CM K2CO3, CM Na2CO3, CM CaCO3, and CM MgCO3, respectively. D-CM char mixed with K2CO3, Na2CO3, CaCO3, and MgCO3 were named as D-CM K2CO3 char, D-CM Na2CO3 char, D-CM CaCO3 char, and D-CM MgCO3 char, respectively.
2.2. Experimental methods
Inductively coupled plasma optical emission spectrometry (Perkin Elmer Optima 8000DV) was performed to analyze inorganic species. Field emission-scanning electron microscopy (JEOL JSM-6390LV) was employed to observe the surface topography of the sample. BRUKER D8 ADVANCE was used to analyze carbon crystal structure and mineral crystal type at 40 KV working voltage and 40 mA tube current with Cu-Kα radiation, respectively. The diffraction peak was collected by a step-by-step scanning method, and the order was 0.01°. 2θ in the range of 10–90° was recorded in the diffraction data. Specific surface area and pore structure were analyzed using the Accelerated Surface Area and Porosimetry System (ASAP 2020) in accordance with a previously described method.40Thermogravimetric analysis (TGA) has been widely used in the study of thermochemical conversion processes.41–44 Combustion reactivity experiments were conducted in the simultaneous DSC-TGA Q600. First, 10 ± 0.2 mg of sample was placed in an alumina crucible. The thermal analysis program was heated to 800 °C from room temperature at 20 °C min−1. The furnace atmosphere was simulated air by mixing 21% O2 and 79% N2. Total gas flow rate was 100 mL min−1.
2.3. Combustion parameters
Several main combustion characteristic temperatures were used for comparison among different atmospheres, including start weight loss temperature (Ts), ignition temperature (Tig), temperature of the maximum combustion rate (Tmax), and burnout temperature (Tb), which were obtained through thermal gravimetric curves in accordance with previously described methods.37,443. Results and discussion
3.1. Combustion characteristics curve
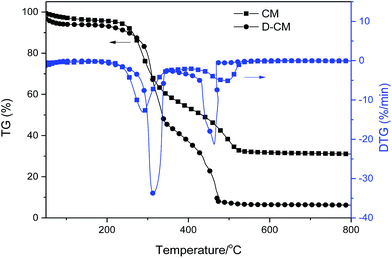
Livestock and poultry manure combustion presents a bimodal curve. One is the devolatilization stage (200–390 °C), which represents the release of volatiles and ignition, whereas the other one (400–600 °C) is related to char oxidation.45,46 Raw CM in our experiment was also divided into two stages, as depicted in Fig. 1. The first stage at 250–400 °C is for volatile matter release and combustion. The second stage at 420–550 °C is for char combustion. Meanwhile, the weight loss rates of the first and second peaks were about 41.06% and 20.56%, respectively, which are consistent with the contents of volatile matter and fixed carbon. This result indicates that CM at low-heating-rate combustion conditions features clear-phased combustion characteristics.The ash ratio of D-CM was 6%. As shown from the DTG diagram, the volatile mater of D-CM was released later than that of raw CM, indicating that some volatile matter were easily released or catalytic mineral was lost during deashing, which is in accordance with the literature.22 The peak of volatile matter and fixed carbon in D-CM is 49.09% and 36.84%, respectively, which are increased with decreasing ash content and increasing combustible content. D-CM appeared at a low burnout temperature. A similar phenomenon with other materials was reported.47,48 The phenomenon may be caused by the intense burning of D-CM in air atmosphere. As a result, the sample temperature was provisionally above the heating program's temperature. After a short time, the sample temperature returned to the program temperature, and the heating process continued.
As illustrated in Fig. 2a, the combustion curves moved to lower temperature as K2CO3 content was further increased. The volatile matter released and char combusted ahead of time, whereas Tb showed a decreasing trend. However, the rate of weight loss gradually decreased. As displayed in Fig. 2b, the combustion curves moved to a lower temperature with increasing Na2CO3. K and Na had lower reaction temperatures than the other AAEM species, indicating an improvement in reactivity. A decrease in weight loss corresponded to decreased combustible concentration with the addition of AAEM. As shown in Fig. 2c and d, only 7.5% of CaCO3 mixing was conducive to the release of volatile matter when the added contents of CaCO3 and MgCO3 were increased. Other contents played no significant role in promoting the release of volatile matter, but all the char burnout processes were improved. In conclusion, K and Na exerted a positive impact on the starting reaction temperature. The addition of K, Na, Ca, and Mg improved burnout, but combustible concentration decreased with the addition of AAEM.
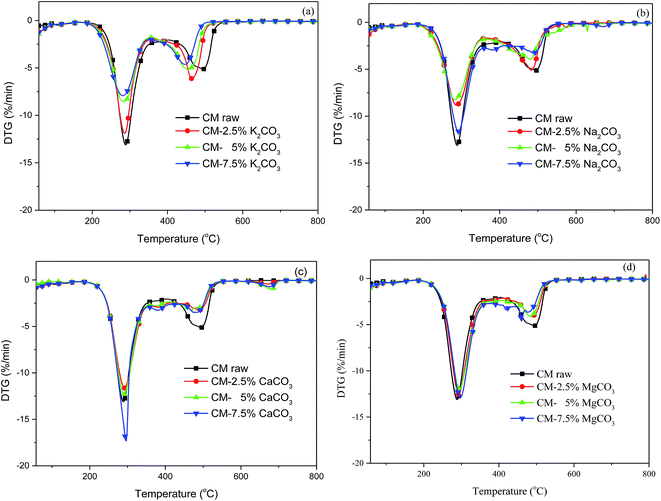 | ||
| Fig. 2 Effect of mixed content with various AAEM species on the combustion curves of CM. (a) K2CO3, (b) Na2CO3, (c) CaCO3, (d) MgCO3. | ||
To study the effect of internal and external AAEM species on CM combustion, the combustion of K, Na, Ca, and Mg blends in D-CM is shown in Fig. 3. K and Na showed similar trends. Compared with the curves of D-CM, the peaks of volatile matter release and char combustion of D-CM K2CO3 and D-CM Na2CO3 moved to a lower temperature. The blend with 2.5% content had a larger volatile matter release peak than the other blends. With the increase in amount, volatile matter release peak gradually decreased, and the decrease rate of D-CM Na2CO3 was greater than that of D-CM K2CO3. For D-CM K2CO3, the starting point of char combustion peak with 2.5% mixing content coincided with the volatile matter release peak, and the end time of its weight loss peak significantly decreased. With the increase in K2CO3 blend amount in D-CM, the char combustion peak gradually moved to a higher temperature. However, 5% mixing content for the lowest temperature of char combustion peak was observed for D-CM Na2CO3. As the amount of Na2CO3 blend increased, temperature of char combustion peak moved to a higher temperature. The temperature of char combustion peak of 7.5% mixing content was also lower than that of D-CM. Fig. 3c and d show that Ca and Mg have similar trends. The peak height of the two weight losses increased, and the volatile matter release peak increased by nearly 1 time. However, Ts increased. The char combustion peak moved to a lower temperature. This effect was not influenced by the increase in mixing amount. In summary, external K, Na, Ca, and Mg blends favored the combustion of volatile matter and char in D-CM. Promotion of K and Na decreased as the blend amount was increased within 2.5–7.5%, but the promotion of Ca and Mg did not change with the increase in mixing amount. Combined with the conclusion in Fig. 2, internal and external K, Na, Ca, and Mg in CM exert a catalytic effect on combustion.
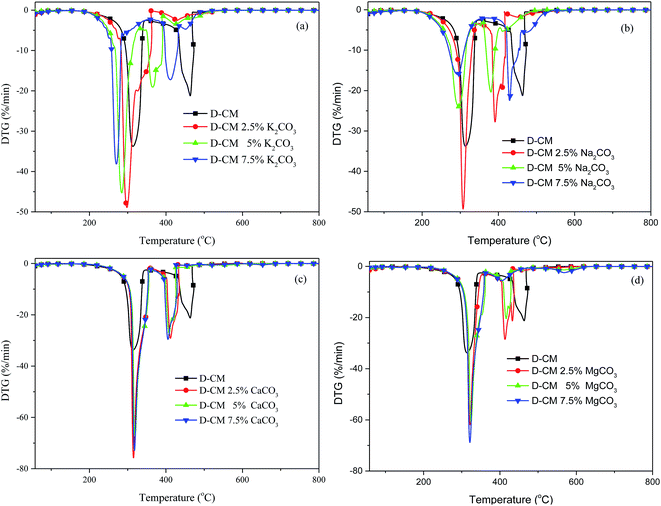 | ||
| Fig. 3 Effect of mixed content with various AAEM species on the combustion curves of D-CM. (a) K2CO3, (b) Na2CO3, (c) CaCO3, (d) MgCO3. | ||
To study the different effects of AAEM species on volatile matter and char reaction in CM, the combustion characteristics of 5% K2CO3, Na2CO3, CaCO3, and MgCO3 blend CM/D-CM char were analyzed (Fig. 4). After blending the AAEM species in CM, (dw/dt)max increased and Ts decreased, but the change in the MgCO3 blend was smaller than those in the other substances. Ts was in the order of K2CO3 > Na2CO3 > CaCO3 > MgCO3, and (dw/dt)max was CaCO3 > Na2CO3 > K2CO3 > MgCO3. The trend of Tb was the same as that of Ts. In conclusion, only Ca, Na, and K significantly influenced the reactivity of CM char. Combining the result from the Fig. 2, the AAEM species catalyzed CM combustion. The improved reactivity of K2CO3, Na2CO3, and CaCO3 on CM combustion not only existed in the volatile matter release stage but also in the combustion stage of char.
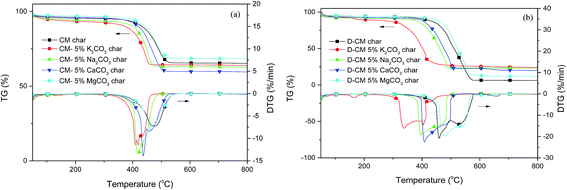 | ||
| Fig. 4 Effect of AAEM species blended in CM or D-CM on the combustion characteristics of the char sample. (a) CM, (b) D-CM. | ||
As shown in Fig. 4b, the Ts and Tb of D-CM char significantly decreased after mixing with Na2CO3, CaCO3, and, especially, K2CO3. The characteristic temperature of the K2CO3 blend in D-CM decreased up to 150 °C. The Ts and Tb for mixing with Na2CO3 and CaCO3 in D-CM char decreased by more than 50 °C. After the MgCO3 blend, the Ts of D-CM increased by 20 °C, but its Tb did not have any change. Thus, promoting the effect of K, Na, and Ca on D-CM combustion is in the order K > Na > Ca, and it is similar to AAEM species for CM char combustion.
3.2. Structural characterization
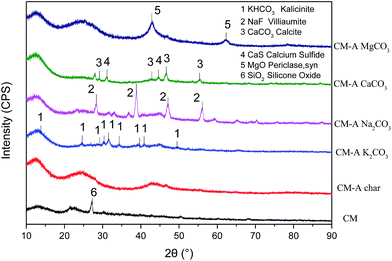
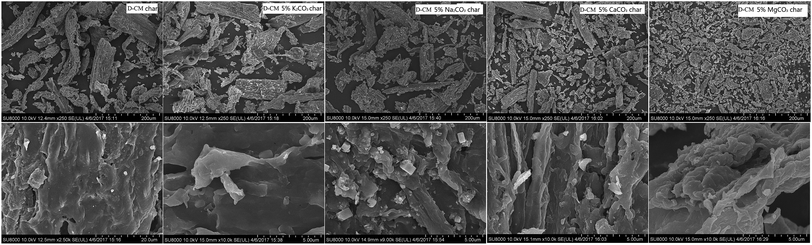
The study above revealed that the blending of K2CO3, Na2CO3, CaCO3, and MgCO3 with D-CM significantly influenced the combustion characteristics, but whether this effect is related to the carbon crystal structure and mineral crystal structure has yet to be determined. To this end, the pyrolysis char were prepared by the blends of 5% AAEM species and D-CM. XRD analysis is shown in Fig. 5 d002 and d10 peaks are related to the carbon crystal structure.49 Compared with raw CM, D-CM char had more obvious d002 and d10 peaks. D-CM MgCO3 char showed significant d002 and d10 peaks too. Both D-CM char and D-CM MgCO3 char demonstrated a high degree of graphitization, which affected the reactivity of the char. The D-CM char mixed with K2CO3, Na2CO3, and CaCO3 displayed no obvious d002 and d10 peaks. The degree of graphitization was low, and combustion reactivity was high. In summary, K2CO3, Na2CO3, and CaCO3 can improve the reactivity of CM char during pyrolysis and prevent the formation of a graphitization structure. However, Mg promoted the formation of graphitization by reducing the reactivity of char. From the blends of D-CM and AAEM species, the main minerals were KHCO3, NaF, CaCO3, and CaS, MgO in the D-CM K2CO3 char, D-CM Na2CO3, D-CM CaCO3 char, and D-CM MgCO3 char, respectively. This finding indicates that the AAEM species were converted to other substances during pyrolysis. Therefore, the promotion of K, Na, and Ca on the combustion of D-CM char are related to reducing graphitized structure while the enhanced graphitized structure of D-CM MgCO3 char leads to the low reactivity of CM with the addition of MgCO3. As is verified in the literature,50 through facilitating ion-exchangeable cations, AAEMs play an important role in the formation of light hydrocarbons, oxygen-containing species, char and tar, thus affecting release of volatile matter and formation of char. In addition to promoting breakage and restructuring of hetero atoms of tar, AAEMs also enhanced the thermal decomposition of heavier aromatics.26K2CO3, Na2CO3, and CaCO3 promoted whereas MgCO3 inhibited the combustion reaction of AAEM species blended in D-CM char. To this end, SEM images of char were analyzed as shown in Fig. 6. The D-CM char displayed a large particle size and rich porosity. In the graph magnified 250×, the char particle size after blending with K2CO3 and Na2CO3 remained large, but the average particle size of the blend CaCO3 and MgCO3 decreased. Magnification 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× revealed many small particles attached to the surface of mixed K2CO3, Na2CO3, and CaCO3 particles, and these particles formed micro-pores. The surface of sample blended with MgCO3 was sintered into blocks and showed no obvious fine particle adhesion. Less reaction surface to contact with oxygen was available, which is one of the reasons leading to poor combustion characteristics.
000× revealed many small particles attached to the surface of mixed K2CO3, Na2CO3, and CaCO3 particles, and these particles formed micro-pores. The surface of sample blended with MgCO3 was sintered into blocks and showed no obvious fine particle adhesion. Less reaction surface to contact with oxygen was available, which is one of the reasons leading to poor combustion characteristics.
Previous SEM images showed that D-CM chars mixed with K2CO3, Na2CO3, and CaCO3 have more fine particles, which form larger pore structures. The BET test was carried out to determine the effect of AAEM species on the pore structure of D-CM char. Results are shown in Fig. 7 and Table 2. As shown in Fig. 7, the specific surface area of the micropores in D-CM mixed with K2CO3 was greater than that in the other blend conditions. The specific surface areas of the mesopores in D-CM CaCO3 char and mesopores and macropores in D-CM Na2CO3 char were also greater than those in the other conditions. Statistical results of the pore structure test are shown in Table 2. Compared with raw CM, D-CM char had a larger specific surface area, pore volume, and smaller pore size. Compared with CM char, D-CM char mixed with K2CO3, Na2CO3, and CaCO3 showed larger specific surface area, pore volume, and pore size in different degrees. The specific surface area was in the order of CaCO3 > K2CO3 > Na2CO3 > MgCO3. The D-CM MgCO3 char had a smaller specific surface area and a larger pore volume and size than the other samples. Combined with the curve of characteristic combustion in Fig. 4b, the addition of K2CO3, Na2CO3, and CaCO3 in D-CM increased the specific surface area of char, which consequently improved the combustion characteristics. Moreover, the low pore structure of D-CM MgCO3 char was further explained as a serious sinter and a decreasing porosity, which consequently declined reactivity.
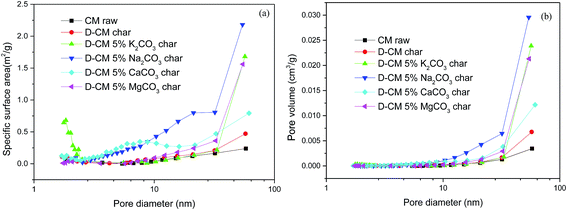 | ||
| Fig. 7 Distribution characteristics of pore structure with pore size of the char prepared under various AAEM species blended in D-CM. (a) Specific surface area, (b) pore volume. | ||
| Sample | Specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| CM raw | 0.3276 | 0.0061 | 75.0179 |
| D-CM char | 3.6274 | 0.0107 | 12.9783 |
| D-CM K2CO3 char | 7.3350 | 0.0288 | 15.9371 |
| D-CM Na2CO3 char | 6.6835 | 0.0489 | 29.2414 |
| D-CM CaCO3 char | 8.0680 | 0.0246 | 13.0130 |
| D-CM MgCO3 char | 3.0662 | 0.0272 | 36.0255 |
3.3. Characteristic temperature
The effect of blend AAEM species on the combustion of CM was qualitatively analyzed. The combustion characteristic temperatures of the K2CO3, Na2CO3, CaCO3, and MgCO3 blends with different proportions in CM and D-CM are shown in Fig. 8. The various proportions of CaCO3 and MgCO3 blends increased Tig and Tmax, and the Tig and Tmax increase levels of the MgCO3 blend in CM/D-CM were higher than those of the CaCO3 blend. Various blend proportions of K2CO3 and Na2CO3 reduced Tig and Tmax, and the decrease range of Tig and Tmax was lower in the K2CO3 blend than in the Na2CO3 blend. This result indicates that the effect of the AAEM species on CM/D-CM was the same before reaching the reaction peak. The Tig and Tmax of D-CM were higher than those of CM, indicating that the internal AAEM species played a positive role in the initial reaction period.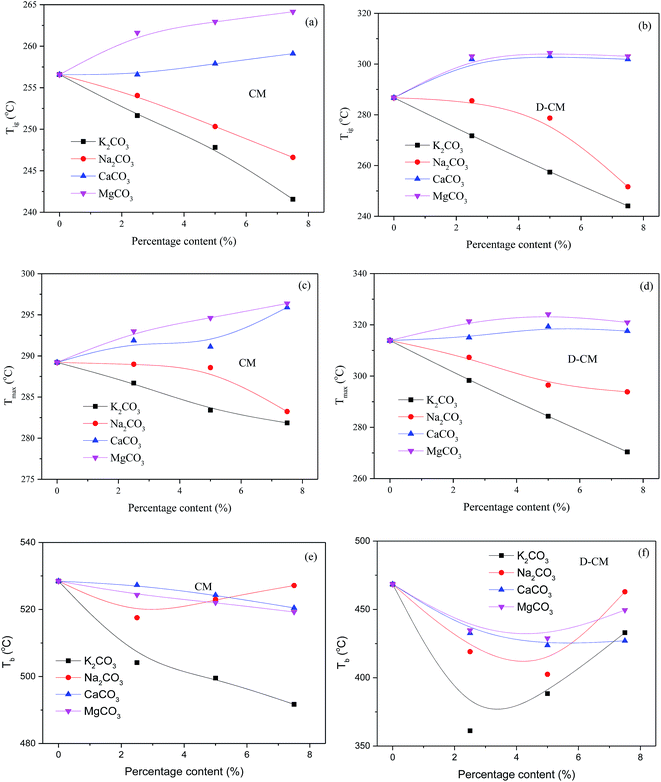 | ||
| Fig. 8 Effect of AAEM amounts on the characteristic temperature of CM/D-CM combustion. (a) Tig, CM; (b) Tig, D-CM; (c) Tmax, CM; (d) Tmax, D-CM; (e) Tb, CM; (f) Tb, D-CM. | ||
Tb decreased as the addition amount of AAEM species was increased, but a different trend was observed between CM and D-CM. Except for blending Na2CO3 in CM, the positive effect of mixing AAEM on Tb was enhanced as the amount of AAEM was increased. However, the Tb of D-CM initially decreased and then further decreased as the amount of AAEM species was increased from 2.5% to 7.5%. Meanwhile, the Tb of D-CM was lower than that of CM. Various amounts of AAEM species mixing with CM and D-CM indicates a competitive relationship, which plays a positive role in the reactivity and an inhibitory role in the contact between C and O2 at the core of CM particles during the burnout period. The amount of external AAEM species to be added needs to be controlled within 5%. In conclusion, the effect of mixing AAEM in CM or D-CM follows K, Na, Ca, and Mg, gradually.
Volatile matter and char combustion occur during solid fuel combustion. The content of volatile matter in CM reached 45.80%, whereas that of fixed carbon was only 12.52% (Table 1). In this case, the impact of AAEM species on volatile matter and char combustion was unclear. As shown in Fig. 9, the Tig and Tmax of char were much higher than those of the raw sample, and the change rule of char after mixing K2CO3, Na2CO3, CaCO3, and MgCO3 was much higher than that of raw char. Tig, Tmax, and Tb increased after blending these substances in sequence, but a difference in the range was observed. The Tig and Tmax of CM partly reflect the reaction of volatile matter, especially for a high volatile matter content of CM. During the combustion of CM, the catalytic effect of AAEM species is unclear. However, the catalytic effect during the combustion of CM char without volatile matter content was obvious. For D-CM char, the catalytic effect of AAEM species was further reflected. In summary, the influence of K2CO3, Na2CO3, CaCO3, and MgCO3 on combustion was mainly reflected in char combustion, and the existence of volatile matter weakened the effect.
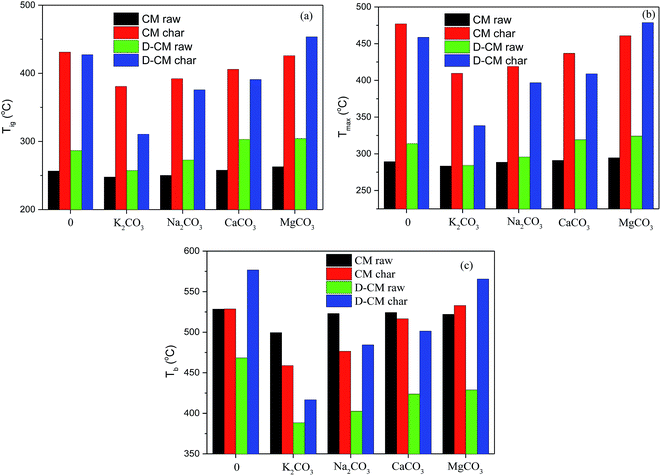 | ||
| Fig. 9 Effect of AAEM species on the characteristics temperature of raw and char samples. (a) Tig, (b) Tmax, (c) Tb. | ||
4. Conclusion
This study investigated the effects of AAEM species on the combustion characteristics of CM. K and Na in raw CM catalyzed the volatile release phase, and all of the AAEM species (K, Na, Ca, and Mg) in raw CM catalyzed the burnout phase. With the external addition of AAEM to D-CM, K and Na exerted an obvious positive effect on combustion, and this effect changed with their addition amount. Meanwhile, the positive effect of Ca and Mg did not change with the addition amount. K, Na, and Ca demonstrated a positive effect on both CM char and D-CM char, but the effect on the latter was more significant than that on the former. Mg exhibited no obvious promotion effect on CM char and D-CM char. Analysis of D-CM char mixed with 5% K, Na, Ca, and Mg revealed that the positive effect of K, Na, and Ca is related to the decrease in graphitization degree and the increase in specific surface area. Meanwhile, the negligible positive effect of Mg is related to the increase in graphitization degree and decrease in specific surface area.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the Natural Science Foundation of Hubei Provincial (2017CFB231), the Special Fund for Agro-scientific Research in the Public Interest of China (201303091) and the Fundamental Research Funds for the Central Universities (2662015QD048 and 2015PY077).References
- D. Gao, T. Chen and B. Liu, Geographical Research, 2006, 25, 311–319 Search PubMed.
- C. Font-Palma, Energy Convers. Manage., 2012, 53, 92–98 CrossRef CAS.
- K. B. Cantrell, T. Ducey, K. S. Ro and P. G. Hunt, Bioresour. Technol., 2008, 99, 7941–7953 CrossRef CAS PubMed.
- M. Fatih Demirbas, M. Balat and H. Balat, Energy Convers. Manage., 2011, 52, 1815–1828 CrossRef CAS.
- L. Zhang, C. Xu and P. Champagne, Energy Convers. Manage., 2010, 51, 969–982 CrossRef CAS.
- X. Flotats, A. Bonmatí, B. Fernández and A. Magrí, Bioresour. Technol., 2009, 100, 5519–5526 CrossRef CAS PubMed.
- N. H. Florin, A. R. Maddocks, S. Wood and A. T. Harris, Waste Management, 2009, 29, 1399–1408 CrossRef CAS PubMed.
- Y. Xin, H. Cao, Q. Yuan and D. Wang, Waste Management, 2017, 68, 618–625 CrossRef CAS PubMed.
- X. Liu, Z. Li, Y. Zhang, R. Feng and I. B. Mahmood, Waste Management, 2014, 34, 1619–1626 CrossRef CAS PubMed.
- W.-T. Tsai, C.-N. Huang, H.-R. Chen and H.-Y. Cheng, Waste Biomass Valorization, 2015, 6, 975–981 CrossRef CAS.
- S.-Y. Zhang, R.-Y. Hong, J.-P. Cao and T. Takarada, Bioresour. Technol., 2009, 100, 4278–4283 CrossRef CAS PubMed.
- S. Xiu, H. K. Rojanala, A. Shahbazi, E. H. Fini and L. Wang, J. Therm. Anal. Calorim., 2012, 107, 823–829 CrossRef CAS.
- D. S. Pandey, M. Kwapinska, A. Gómez-Barea, A. Horvat, L. E. Fryda, L. P. L. M. Rabou, J. J. Leahy and W. Kwapinski, Energy Fuels, 2016, 30, 3085–3096 CrossRef CAS.
- W.-T. Tsai and S.-C. Liu, Biomass Convers. Biorefin., 2016, 6, 71–77 CrossRef CAS.
- K. Annamalai, B. Thien and J. Sweeten, Fuel, 2003, 82, 1183–1193 CrossRef CAS.
- S. Yurdakul, Renewable Energy, 2016, 89, 215–223 CrossRef CAS.
- X. Shen, G. Huang, Z. Yang and L. Han, Appl. Energy, 2015, 160, 108–119 CrossRef CAS.
- C.-Z. Li, Fuel, 2013, 112, 609–623 CrossRef CAS.
- D. Lv, M. Xu, X. Liu, Z. Zhan, Z. Li and H. Yao, Fuel Process. Technol., 2010, 91, 903–909 CrossRef CAS.
- T. P. Vispute, H. Zhang, A. Sanna, R. Xiao and G. W. Huber, Science, 2010, 330, 1222–1227 CrossRef CAS PubMed.
- H. Zhang, S. Shao, X. Rui, D. Shen and J. Zeng, Energy Fuels, 2014, 28, 52–57 CrossRef CAS.
- L. Jiang, S. Hu, L.-s. Sun, S. Su, K. Xu, L.-m. He and J. Xiang, Bioresour. Technol., 2013, 146, 254–260 CrossRef CAS PubMed.
- J. Cheng, F. Zhou, X. Xuan, J. Liu, J. Zhou and K. Cen, Fuel, 2017, 187, 398–402 CrossRef CAS.
- R. Jiménez, X. García, T. López and A. L. Gordon, Fuel Process. Technol., 2008, 89, 1160–1168 CrossRef.
- L. Jiang, S. Hu, Y. Wang, S. Su, L. Sun, B. Xu, L. He and J. Xiang, Int. J. Hydrogen Energy, 2015, 40, 15460–15469 CrossRef CAS.
- S. Hu, L. Jiang, Y. Wang, S. Su, L. Sun, B. Xu, L. He and J. Xiang, Bioresour. Technol., 2015, 192, 23–30 CrossRef CAS PubMed.
- L. J. R. Nunes, J. C. O. Matias and J. P. S. Catalão, Renewable Sustainable Energy Rev., 2016, 53, 235–242 CrossRef CAS.
- D. Lynch, A. M. Henihan, W. Kwapinski, L. Zhang and J. J. Leahy, Energy Fuels, 2013, 27, 4684–4694 CrossRef CAS.
- P. Billen, J. Costa, L. van der Aa, L. Westdorp, J. Van Caneghem and C. Vandecasteele, Energy Fuels, 2014, 28, 5455–5462 CrossRef CAS.
- K. Wang, Z. Yin, P. Zhao, D. Han, X. Hu and G. Zhang, Energy Fuels, 2015, 29, 4428–4435 CrossRef CAS.
- V. Stanković, M. Gorgievski and D. Božić, Biomass Bioenergy, 2016, 88, 17–23 CrossRef.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS.
- F. Zhang, M. Fan, X. Huang, M. D. Argyle, B. Zhang, B. Towler and Y. Zhang, Fuel Process. Technol., 2017, 161, 145–154 CrossRef CAS.
- A. Karimi and M. R. Gray, Fuel, 2011, 90, 120–125 CrossRef CAS.
- B. Feng, S. K. Bhatia and J. C. Barry, Carbon, 2002, 40, 481–496 CrossRef CAS.
- X. Wu, J. Tang and J. Wang, Fuel, 2016, 165, 59–67 CrossRef CAS.
- B. Yi, L. Zhang, Z. Mao, F. Huang and C. Zheng, Fuel Process. Technol., 2014, 128, 17–27 CrossRef CAS.
- X. Gong, Z. Guo and Z. Wang, Combust. Flame, 2010, 157, 351–356 CrossRef CAS.
- E. Abbasi-Atibeh and A. Yozgatligil, Fuel, 2014, 115, 841–849 CrossRef CAS.
- B. Yi, L. Zhang, Q. Yuan, S. Yan and C. Zheng, Fuel Process. Technol., 2016, 152, 294–302 CrossRef CAS.
- M. V. Kok and E. Özgür, Fuel Process. Technol., 2013, 106, 739–743 CrossRef CAS.
- M. Otero, M. E. Sanchez, X. Gómez and A. Morán, Waste Management, 2010, 30, 1183–1187 CrossRef CAS PubMed.
- B. Yi, L. Zhang, F. Huang, Z. Xia, Z. Mao, J. Ding and C. Zheng, Energy Convers. Manage., 2015, 103, 439–447 CrossRef CAS.
- B. Yi, L. Zhang, F. Huang, Z. Mao and C. Zheng, Appl. Energy, 2014, 132, 349–357 CrossRef CAS.
- M. Baniasadi, A. Tugnoli, R. Conti, C. Torri, D. Fabbri and V. Cozzani, Renewable Energy, 2016, 90, 458–468 CrossRef CAS.
- A. Gani and I. Naruse, Renewable Energy, 2007, 32, 649–661 CrossRef CAS.
- Z. Zhou, X. Hu, Z. You, Z. Wang, J. Zhou and K. Cen, Thermochim. Acta, 2013, 553, 54–59 CrossRef CAS.
- L. Jiang, X. Yuan, H. Li, Z. Xiao, J. Liang, H. Wang, Z. Wu, X. Chen and G. Zeng, Energy Convers. Manage., 2015, 106, 282–289 CrossRef CAS.
- B. Feng, S. K. Bhatia and J. C. Barry, Energy Fuels, 2003, 17, 744–754 CrossRef CAS.
- C.-Z. Li, Fuel, 2007, 86, 1664–1683 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |