Open Access Article
Open Access ArticleSynergistic effects of pH and organosolv lignin addition on the enzymatic hydrolysis of organosolv-pretreated loblolly pine
Chenhuan Lai ab,
Maobing Tu
ab,
Maobing Tu *c,
Qiang Yong
*c,
Qiang Yong a and
Shiyuan Yua
a and
Shiyuan Yua
aCo-Innovation Center for Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, 159 Longpan Road, Nanjing, Jiangsu 210037, China
bForest Products Laboratory and Center for Bioenergy and Bioproducts, Auburn University, Auburn, Alabama 36849, USA
cDepartment of Chemical and Environmental Engineering, University of Cincinnati, 2901 Woodside Drive, Cincinnati, OH 45221-0048, USA. E-mail: tumg@uc.edu
First published on 12th April 2018
Abstract
The effect of ethanol organosolv lignin (EOL) on enzymatic hydrolysis was examined at pH 4.8–6.0. The addition of EOL prepared from sweetgum enhanced the enzymatic hydrolysis of organosolv-pretreated loblolly pine (OPLP) by 38.8% and 88.0% at pH 4.8 and 5.6, respectively. The addition of EOL prepared from loblolly pine inhibited the enzymatic hydrolysis of OPLP at pH 4.8 but improved it by 43.0% at pH 5.6. This suggests that the addition of EOL and increase in pH act synergistically to improve the enzymatic hydrolysis of OPLP. The effect of EOL addition on cellulase adsorption onto residual lignins was examined. The results revealed that increasing the pH intensified the suppression of non-productive binding between enzymes and residual lignins by EOL. The potential stabilization effects of EOL on enzymes can contribute to the improvement of enzymatic hydrolysis with EOL at higher pH.
Introduction
Lignocellulosic materials, which are important sustainable resources, have been converted to fuels, chemicals and materials via thermal-chemical or biochemical methods.1,2 During a typical bioconversion process, the enzymatic saccharification of lignocellulosic materials is one of the major technical and economical bottlenecks.3,4 To improve the enzymatic hydrolysis of lignocelluloses, significant efforts have been devoted to study the catalytic behavior of cellulases. Prior to their hydrolysis action, cellulases must be adsorbed on the substrates through the cellulose binding domain (CBD) of cellulase. Enzyme adsorption increases the enzyme concentration on the substrate surface, significantly accelerating the two-phase catalytic hydrolysis.5 Nevertheless, the undesired non-productive adsorption of enzymes on substrates (especially on lignins) can occur irreversibly. This limits the enzymatic hydrolysis of lignocelluloses by reducing the amounts of available enzyme and the enzyme activity.6The non-productive binding of enzymes on lignins results from hydrophobic interactions, electrostatic interactions and hydrogen bonding,7–9 which can be affected by lignin content and physicochemical properties (e.g., hydrophobicity, negative charge, and specific functional groups or chemical structures).10–13 Hydrophobic interactions are believed to be a dominant interactive force in non-productive binding between enzymes and lignin.9 Typically, higher lignin hydrophobicity results in stronger hydrophobic interactions between enzymes and lignins.14 Electrostatic interactions are mainly controlled by the association or dissociation of functional groups in enzymes and lignins (e.g., carboxyl and hydroxyl groups in lignins and amino acid residues of enzymes).15 The cellulases from Trichoderma reesei are mostly negatively charged when the pH value is above 4.8.16 These negative charges on the lignin surfaces lead to stronger electrostatic repulsion and thus weaker adsorption affinity of cellulases on lignins.17 The presence of phenolic hydroxyl groups on the surfaces of lignins has been reported to play a role in the formation of hydrogen bonding between enzymes and lignins.18 Moreover, the condensed phenolic moieties in lignins result in increased non-productive binding or even enzyme deactivation.19,20 However, lignin alkylation likely reduces the affinity of enzymes on lignins.21
With the understanding of enzyme–lignin interactions, a variety of strategies have been developed to suppress the non-productive binding of cellulases. The genetic engineering of enzymes with weak lignin binding has been proposed as a useful strategy.22 Furthermore, non-ionic surfactants, such as poly(ethylene glycol) and Tween, along with bovine serum albumin have been frequently applied to prevent undesired enzyme absorption by occupying the hydrophobic sites on lignin.23,24 Lignin modification is also an efficient method to suppress the non-productive adsorption of enzymes by increasing acidic groups or hydrophilic groups in lignins.25–29 More interestingly, it has been reported that non-productive binding can be decreased by simply increasing the pH of enzymatic hydrolysis.30 This might be because increasing the pH increases the negative charges on the lignins and enzymes, thereby enhancing the electrostatic repulsion between lignins and enzymes.30–32
Increasing pH seems to be a facile way to relieve lignin inhibition during enzymatic hydrolysis. It has been reported that the application of lignosulfonate at higher pH can further enhance the enzymatic hydrolysis of pretreated biomass.33 However, studies on the mechanism of the synergistic effect of pH and lignosulfonate are limited. To examine the potential synergistic effects of elevated pH and lignin addition on enzymatic hydrolysis, EOLs from sweetgum and loblolly pine were applied in this study. Previously, EOLs from sweetgum and loblolly pine showed contrasting effects on enzymatic hydrolysis at the regular hydrolysis pH (4.8).14 The addition of EOL from sweetgum improved enzymatic hydrolysis; however, EOL from loblolly pine (EOL-LP) inhibited it. This work is original because it investigates if pH can change the negative lignin effect to a positive one. The effects of pH on the roles of both stimulative lignin and inhibitory lignin in enzymatic hydrolysis were evaluated. Moreover, their potential underlying mechanisms were explored, including their impacts on enzyme non-productive binding and cellulase stability. Understanding the synergistic effects of pH and EOL addition will help us better design biomass pretreatment and enzymatic hydrolysis processes.
Experimental
Enzymes
Commercial cellulase, Novozym 22C, was obtained from Novozymes (Franklinton, NC) and used in the enzymatic hydrolysis and enzyme adsorption experiments. Its filter paper enzyme activity and β-glucosidase activity were 100 FPU mL−1 and 343 IU mL−1, as measured using Whatman no. 1 filter paper and p-nitrophenyl-β-cellobiosidase substrates, respectively.34 Protease from Streptomyces griseus (P5147, ≥3.5 U mg−1) was purchased from Sigma-Aldrich and used for the enzymatic residual lignin preparation.Substrates
Avicel purchased from Sigma-Aldrich was used as a pure cellulose substrate. OPLP was prepared according to our previous study and used as the lignocellulosic substrate.14 Loblolly pine wood chips (80 g, dry weight) were cooked with 75% (v/v) ethanol solution, 1.0% (w/w) sulfuric acid, and a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in a 1.0 L Parr reactor at 170 °C for 1 h. After cooking, the reactor was cooled down in a water bath. To remove the solvent-extractable lignins and water-soluble compounds, OPLP was washed sequentially with warm ethanol and excess water. The major components in OPLP were glucan (79.64%), mannan (3.49%), xylan (1.88%) and lignin (11.48%).
7 in a 1.0 L Parr reactor at 170 °C for 1 h. After cooking, the reactor was cooled down in a water bath. To remove the solvent-extractable lignins and water-soluble compounds, OPLP was washed sequentially with warm ethanol and excess water. The major components in OPLP were glucan (79.64%), mannan (3.49%), xylan (1.88%) and lignin (11.48%).
Lignin preparation
Two EOL lignins were prepared from organosolv pretreatment according to our previous study.14 EOL-SG was precipitated from the organosolv pretreatment spent liquor of sweetgum (organosolv pretreatment conditions: 75% ethanol, 1% (w/w) sulfuric acid, 160 °C, and 1 h) by adding a three-fold volume of water. Similarly, EOL-LP was precipitated from the organosolv pretreatment spent liquor of loblolly pine (organosolv pretreatment conditions: 75% ethanol, 1% (w/w) sulfuric acid, 170 °C, and 1 h). The EOLs were then collected by filtration on Whatman no. 1 filter paper, washed with warm water to remove the water-soluble compounds, and air-dried in the fume hood. The lignin contents of EOL-SG and EOL-LP were greater than 94.0%; the glucan contents were less than 0.3%.The enzymatic residual lignin (ERL) was isolated from OPLP by hydrolyzing the cellulose and hemicellulose with enzymes.35 The ERL isolation involved two main steps: (1) the nearly complete enzymatic hydrolysis of OPLP, and (2) the removal of enzyme protein in the lignin-rich residues using protease. Briefly, to achieve nearly complete enzymatic hydrolysis, two-stage enzymatic hydrolysis was carried out on OPLP. The OPLP substrate with 2% glucan (w/v) was incubated with 20 FPU g−1 glucan of cellulase (Novozym 22C). After 72 h, the enzymatic hydrolysis residues were subjected to a second round of enzymatic hydrolysis by re-suspending the residues in fresh buffer containing another 20 FPU g−1 glucan of cellulase. Subsequently, the obtained lignin-rich residues were treated with 1 U mL−1 protease (P5147) at 37 °C and pH 7.0 for 12 h. The protease enzyme was then deactivated by incubation at 90 °C for 1 h. Finally, the residues were extensively washed, air-dried and ground. The lignin and glucan contents in ERL were 77.1% and 11.9%, respectively.
Enzymatic hydrolysis
To examine the synergistic effects of pH and lignin addition, the enzymatic hydrolysis of OPLP or Avicel with the addition of 4 g L−1 lignin (EOL-SG, EOL-LP, or ERL) was performed under pH 4.8–6.0 using sodium citrate buffer with 2% glucan (w/v) and cellulase enzyme (Novozym 22C) and incubation at 50 °C and 150 rpm for 72 h. The pH was controlled by buffer with different concentration ratios of sodium citrate to citric acid. The enzymatic hydrolysis of OPLP or Avicel without lignin addition was carried out as a control. To achieve comparable hydrolysis yields, the enzymatic hydrolysis of OPLP was conducted at a loading of 10 FPU g−1 glucan of cellulase, while the enzymatic hydrolysis of Avicel was carried out at 5 FPU g−1 glucan of cellulase. The samples were taken from the hydrolysis solution at 72 h and analyzed by HPLC using an Aminex HPX-87P column. The hydrolysis yield of the substrate was calculated from the released glucose content as a percentage of the theoretical sugars available in the substrate.Determination of lignin surface charge
The surface charges of EOL-SG, EOL-LP, and ERL lignins at pH 4.8 and 5.6 were determined by potentiometric titration.14,15 Briefly, the lignin sample (120 mg dry weight) was dissolved in 10.0 g of NaOH solution (0.1 M). The solution containing the lignin sample was then acidified with 3.0 g of 1.0 M HCl and stirred for 10 min. The obtained sample solution was neutralized by 30.0 g of 0.1 M NaOH and titrated with 0.1 M HCl using an automatic titrator (AUT-701, DKK-TOA) until the pH value decreased to approximately 2.0. Blank solution (solution without lignin) was titrated as the control. The surface charges (mmol g−1) on the lignins at different pH were calculated as follows: Q = (Vblank − Vsample) × M/W, where Q is the surface charge (mmol g−1), Vblank and Vsample are the titration volumes consumed by the blank solution and lignin sample solution, respectively, when the solution pH reached a certain value, M is the concentration of HCl (0.1 M), and W is the dry weight of the lignin sample (0.120 g).Cellulase adsorption onto lignins
To evaluate enzyme adsorption on lignins, cellulase with the same enzyme concentration as in the hydrolysis experiments was mixed with 10 g L−1 lignin sample (ERL, EOL-SG or EOL-LP) and incubated at pH 4.8 or 5.6 at 50 °C and 150 rpm for 3 h. To examine the effects of the EOL lignins (EOL-SG and EOL-LP) on enzyme adsorption on ERL, 10 g L−1 EOL and 10 g L−1 ERL were mixed with cellulase at pH 4.8 or 5.6 at 50 °C and 150 rpm for 3 h. The enzyme concentration in the supernatant was determined by Bradford assay.36 The adsorbed enzyme concentration was calculated as the difference between the enzyme concentration in the supernatant and the initial enzyme concentration. Enzyme adsorption was presented as the ratio of the adsorbed enzyme concentration to the initial protein concentration.Determination of enzyme activity in the presence of lignins
To determine the effects of lignins on cellulase activity, the filter paper activities of enzyme were measured in the presence of lignins at pH 4.8 and 5.6. EOL-SG or EOL-LP (4 g L−1) was mixed with 0.5 mL of enzyme solution (Novozym 22C) with the same enzyme concentration as in hydrolysis experiments in test tubes with stoppers. The pH was controlled at pH 4.8 or 5.6 by adding 1.0 mL of 0.05 M sodium citrate buffer. After the filter paper was added, the test tubes were incubated at 50 °C for 1.0 h. Next, 3.0 mL of dinitrosalicylic acid was added to stop the enzyme catalytic reaction, and the test tubes were boiled for 5 min. To avoid interference from the lignins in the determination of enzyme activity, samples with fresh buffer and 4 g L−1 EOLs were used as controls. Finally, all tubes were diluted with water, and the absorbance at 540 nm was determined. The enzyme activity was calculated from the absorbance.34 The enzyme activities are presented as the percentage of initial enzyme activity without lignins at pH 4.8.Results and discussion
Synergistic effects of pH and EOL addition on the enzymatic hydrolysis of organosolv-pretreated loblolly pine and Avicel
To investigate the synergistic effects of pH and EOL addition, the enzymatic hydrolysis of OPLP with the addition of EOL-SG or EOL-LP (4 g L−1) was performed at pH 4.8–6.0 (Fig. 1a). The results showed that the yields after 72 h of OPLP hydrolysis were highest at pH 5.6 with EOL-SG addition. EOL-SG and EOL-LP had contrasting effects at pH 4.8, but the effects of both EOL lignins on enzymatic hydrolysis were positive at pH 5.2–6.0.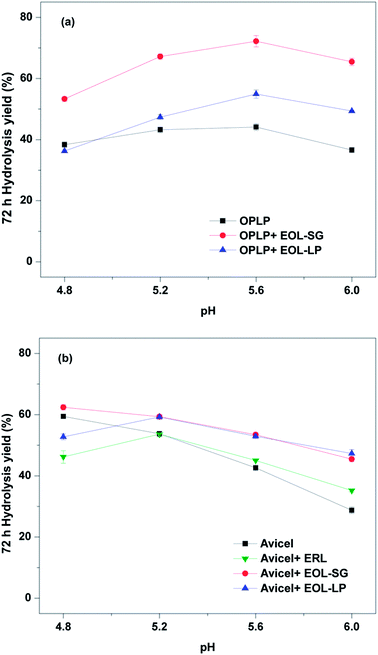 | ||
| Fig. 1 Effects of pH on the 72 h hydrolysis yields of OPLP (a), and Avicel (b) with and without EOL addition (4 g L−1). | ||
Without the addition of EOL lignins, the enzymatic hydrolysis of OPLP (44.1%) appeared to be best at pH 5.6, which was 14.8% higher than that at pH 4.8. Although pH 4.8 has long been suggested for cellulase enzymatic hydrolysis,37 different biomass substrates may have different optimal pH values for the process. Similar suggestions have been made for the enzymatic hydrolysis of sulfite-pretreated lodgepole pine and aspen.31 Higher pH has been proposed to reduce non-productive binding by increasing the electrostatic repulsion between enzymes and residual lignins in substrates.31,32 Nevertheless, as the pH increased further, the positive effects of reducing non-productive binding were offset by a decline in enzyme activity. For example, when the pH increased to 6.0, the 72 h hydrolysis yield of OPLP decreased to 36.6% (Fig. 1a).
With the addition of EOL-SG, the 72 h hydrolysis yield of OPLP reached 72.2% at pH 5.6 (Fig. 1a). Specifically, EOL-SG improved the 72 h hydrolysis yields of OPLP by 38.8%, 75.0%, 88.0%, and 70.3% compared to the control (without EOL-SG at pH 4.8) at pH 4.8, 5.2, 5.6, and 6.0, respectively. These results are explained in two ways. First, EOL-SG increased the hydrolysis yield at pH 4.8 by reducing non-productive binding. Second, increasing the pH further increased the hydrolysis yield by intensifying electrostatic repulsion and non-productive binding. The improvement in the 72 h hydrolysis yield of OPLP at pH 5.6 was greater than that at pH 4.8. This indicated that adding EOL-SG and increasing pH had a synergistic effect. A similar effect of lignosulfonate on enzymatic hydrolysis was reported.33 In contrast, the addition of EOL-LP decreased the 72 h hydrolysis yield of OPLP at pH 4.8 but increased the 72 h hydrolysis yield at pH 5.2, 5.6 and 6.0 by 23.4%, 43.0%, and 28.6%, respectively (Fig. 1a). This suggested that the synergistic effect of EOL-LP addition and increased pH also enhanced the enzymatic hydrolysis of OPLP. It should be noted that EOL-SG showed greater positive effects than EOL-LP.
The effects of pH and lignins on the enzymatic hydrolysis of Avicel were also examined (Fig. 1b). Without the addition of lignin, increasing the pH from 4.8 to 6.0 decreased the 72 h hydrolysis yield of Avicel from 59.4% to 28.8%. This indicated that pH 4.8 was best for the enzymatic hydrolysis of pure cellulose, and higher pH resulted in the partial denaturation of cellulases. In contrast, for the lignocellulosic substrate (OPLP), pH 5.6 resulted in the highest hydrolysis yield. This suggested that the residual lignin in OPLP affected the optimal pH for enzymatic hydrolysis, which was confirmed by the enzymatic hydrolysis of Avicel in the presence of ERL. The optimal hydrolysis pH for the enzymatic hydrolysis of pure cellulose in the presence of ERL was 5.2.
With the addition of EOL-SG, the 72 h hydrolysis yield of Avicel was increased by 5.0% at pH 4.8. As the pH increased, the 72 h hydrolysis yield of Avicel in the presence of EOL-SG decreased. However, the 72 h hydrolysis yields with EOL-SG were still higher than those without EOL-SG addition at higher pH (5.2–6.0). This showed that the addition of EOL-SG slowed the linear decrease in the 72 h hydrolysis yield of Avicel with increasing pH in the absence of lignin. These results suggest the potential stabilization of cellulases by EOL-SG. Similarly, an amphiphilic lignin derivative was reported to function as a cellulase stabilizer.38 The addition of EOL-LP first decreased the 72 h hydrolysis yield of Avicel from 59.4% to 52.7% at pH 4.8 and then increased it to 59.2% at pH 5.2 (Fig. 1b). This indicated that the negative effect of EOL-LP on enzymatic hydrolysis was suppressed at higher pH, likely due to an increase in electrostatic repulsion between enzymes and lignins. The 72 h hydrolysis yields with EOL-LP were higher than those without EOL addition at higher pH (5.2–6.0). This indicated that EOL-LP showed a similar stabilization effect at pH 5.6 and 6.0.
Compared to the enzymatic hydrolysis of Avicel, the improvement in hydrolysis yield was more significant for the enzymatic hydrolysis of OPLP with the addition of EOL lignins at elevated pH. This suggested that the EOL lignins might interact with the residual lignins in OPLP, potentially leading to a decline in non-productive binding. Our previous study showed that EOL lignins precipitated on the organosolv-pretreated substrates.12 To increase enzymatic hydrolysis, the residual EOLs in organosolv-pretreated substrates should be reserved by eliminating the ethanol washing process after pretreatment, and enzymatic hydrolysis should be performed at pH 5.2–6.0.
Effects of pH on cellulase adsorption onto lignins
To investigate the lignin effects on enzymatic hydrolysis, enzyme adsorption on lignins was evaluated at pH 4.8 and 5.6 (Fig. 2a). The enzyme adsorption ratios on EOL-SG, EOL-LP, and ERL were 13.0%, 56.4%, and 61.1% at pH 4.8, respectively. The molecular weights (Mw) of EOL-SG and EOL-LP were 5457 and 5210, respectively. The functional groups (phenolic hydroxyl, aliphatic hydroxyl and methoxyl) of EOL-SG and EOL-LP were previously characterized by 1H NMR.14 The hydrophobicity of EOL-LP (1.07 L g−1) was reported to be higher than that of EOL-SG (0.56 L g−1), and EOL-LP had a higher content of phenolic hydroxyl groups (2.81 mmol g−1) compared to EOL-SG (2.37 mmol g−1).14 This resulted in stronger hydrophobic interactions and hydrogen bonding between enzymes and EOL-LP. While the detailed characteristics of ERL were not determined, the condensed lignin structure in ERL led to its relatively high hydrophobicity.19 Additionally, the content of phenolic hydroxyl groups in ERL could be similar to that in EOL-LP. Therefore, both of the high hydrophobicity and the high content of phenolic hydroxyl groups in ERL contributed to the strong enzyme adsorption.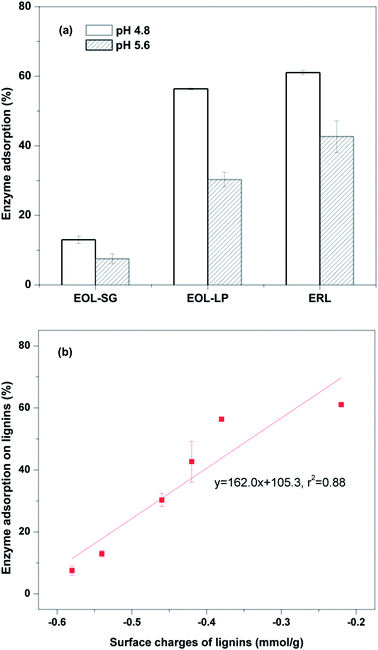 | ||
| Fig. 2 Cellulase adsorption on EOL and ERL at pH 4.8 and 5.6 (a) and the correlation between lignin surface charge and the corresponding enzyme adsorption (b). | ||
As the pH increased to 5.6, the enzyme adsorption ratios on EOL-SG, EOL-LP, and ERL respectively decreased to 7.5%, 30.3%, and 42.7%. It was reported that increasing pH could increase lignin surface charge, which enhanced the repulsive force between lignins and enzymes.32 To verify the increased lignin surface charge, the surface charges of EOL-SG, EOL-LP and ERL at pH 4.8–6.0 were determined by potentiometric titration (Table 1). The results showed that the higher pH enabled lignins to be more negatively charged. Specifically, the negative charges of EOL-SG, EOL-LP, and ERL at pH 4.8 increased from −0.54, −0.38, and −0.22 mmol g−1 to −0.58, −0.46, and −0.42 mmol g−1 at pH 5.6, respectively. A good correlation (r2 = 0.88) was observed between lignin surface charge and enzyme adsorption (Fig. 2b), indicating that more negative surface charges resulted in lower enzyme adsorption. This likely explains the improvement in the hydrolysis yield of Avicel with the addition of EOL-LP or ERL as the pH increased from 4.8 to 5.2. Similarly, more negatively charged groups (e.g., carboxylic acid groups) in isolated lignin and synthesized lignin model compounds were reported to result in lower non-productive adsorption and thus lower inhibition of enzymatic hydrolysis.14,26
| Lignin | Surface charge (mmol g−1) | |||
|---|---|---|---|---|
| pH 4.8 | pH 5.2 | pH 5.6 | pH 6.0 | |
| a ERL refers to enzymatic residual lignin isolated from organosolv-pretreated loblolly pine.b EOL-SG refers to ethanol organosolv lignin prepared from sweetgum.c EOL-LP refers to ethanol organosolv lignin prepared from loblolly pine. | ||||
| ERLa | −0.221 | −0.333 | −0.417 | −0.458 |
| EOL-SGb | −0.542 | −0.563 | −0.583 | −0.583 |
| EOL-LPc | −0.375 | −0.417 | −0.458 | −0.458 |
However, this observation was not sufficient to explain the significant improvement in the enzymatic hydrolysis of OPLP generated by EOL addition. We believe that the addition of EOL-SG and EOL-LP reduced non-productive binding between enzyme and residual lignin in OPLP. A previous study found that the addition of solvent-extractable lignin reduced enzyme adsorption on isolated residual lignins in pretreated substrates.35 In this study, the effects of EOL-SG and EOL-LP addition on enzyme adsorption on ERL (designated as residual lignins) were determined at pH 4.8 and 5.6 (Fig. 3a). At pH 4.8, EOL-SG addition reduced enzyme adsorption on ERL from 61.1% to 33.2%, while EOL-LP addition increased enzyme adsorption on ERL to 77.7%. These results agreed well with the positive effect of EOL-SG and negative effect of EOL-LP on the 72 h hydrolysis yield of OPLP at pH 4.8. However, when the pH increased to 5.6, the additions of both EOL-SG and EOL-LP decreased enzyme adsorption on ERL from 42.7% to 25.1% and 38.0%, respectively. This suggested that the higher pH could intensify the reduction of non-productive binding between enzyme and residual lignins by EOL-SG and EOL-LP. This finding corresponded well to the strong positive effects of EOL-SG and EOL-LP on the 72 h hydrolysis yield of OPLP at pH 5.6. A good correlation (r2 = 0.76) was observed between enzyme adsorption on ERL and the 72 h hydrolysis yield of OPLP (Fig. 3b). This indicated that the residual lignin inhibited the enzymatic hydrolysis of OPLP, but the additions of EOL-SG and EOL-LP enhanced enzymatic hydrolysis by reducing the non-productive binding between enzyme and residual lignins, especially at higher pH.
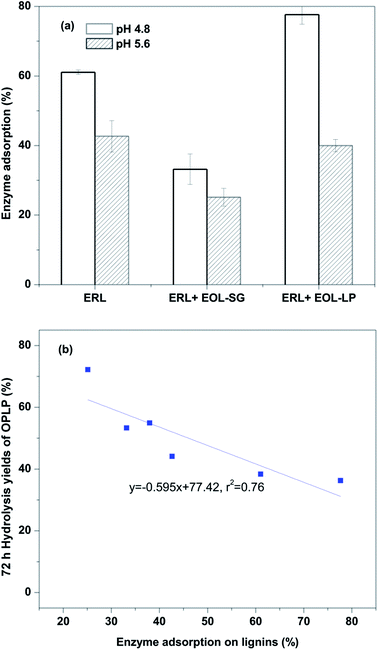 | ||
| Fig. 3 Effects of EOL addition on enzyme adsorption on ERL (a) and the correlation between enzyme adsorption and the 72 h hydrolysis yields of OPLP with EOL addition (b). | ||
Effects of pH on enzyme activity in the presence of EOL lignins
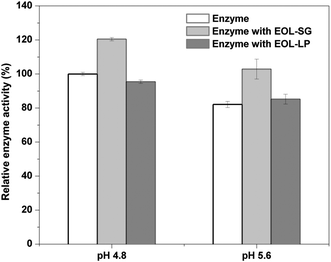
EOL-SG and EOL-LP showed potential benefits for reducing enzyme denaturation in the enzymatic hydrolysis of Avicel (Fig. 1b). The effects of pH on cellulase activity in the presence of EOL-SG and EOL-LP were examined (Fig. 4). The results showed that at pH 4.8, the addition of EOL-SG increased the relative cellulase activity to 120.5%, while the addition of EOL-LP decreased the relative cellulase activity to 95.4%. When pH changed to 5.6, the relative cellulase activity decreased to 82.1% in the control. In the presence of EOL-SG, the relative cellulase activity remained at 102.9%, and the addition of EOL-LP resulted in a relative cellulase activity of 85.2%. This indicated that the higher pH (5.6) caused the denaturation of cellulase enzymes; however, the addition of EOL-SG and EOL-LP stabilized the enzymes and reduced denaturation, especially at pH 5.6. Similar observations were reported for cellulase immobilization on lignophenols and cellulase stabilization by an amphiphilic lignin derivative.38,39 One may doubt that non-productive binding on lignins could limit the mobility of enzymes. However, the enzymes adsorbed on lignin could transfer to the cellulose chains due to the stronger affinity of cellulases for celluloses.14,40 Moreover, the higher pH reduced the binding strength between the enzyme and lignins. This moderate binding may enable the mobility and stability of cellulases.Conclusions
Synergistic effects of pH and organosolv lignins on enzymatic hydrolysis were observed. EOL-SG enhanced the enzymatic hydrolysis OPLP by reducing the non-productive binding between enzymes and residual lignins. This enhancement was intensified at higher pH (5.6). A similar effect of EOL-LP on enzymatic hydrolysis was observed. However, the higher enzyme adsorption on EOL-LP resulted in a negative effect of EOL-LP on enzymatic hydrolysis at pH 4.8. This negative effect was reversed at higher pH (5.6) due to the stronger electrostatic repulsion between enzyme and lignin. Additionally, EOL-SG and EOL-LP could also stabilize cellulases at higher pH. This study suggested that lignocellulosic substrates should be hydrolyzed at higher pH (such as 5.6), and that organosolv lignin can potentially reduce non-productive binding between enzyme and residual lignin and stabilize cellulase enzymes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was supported by grants from Alabama Agricultural Experimental Station, Sun Grant and USDA NIFA (Grant No. 2011-68005-30410), the National Natural Science Foundation for Youth (31600463), Natural Science Foundation of Jiangsu Province for Youth (BK20150874), and Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).Notes and references
- J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr, J. P. Hallett, D. J. Leak and C. L. Liotta, Science, 2006, 311, 484–489 CrossRef PubMed.
- Y. Sun and J. Y. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS PubMed.
- S. H. Mood, A. H. Golfeshan, M. Tabatabaei, G. S. Jouzani, G. H. Najafi, M. Gholami and M. Ardjmand, Renewable Sustainable Energy Rev., 2013, 27, 77–93 CrossRef.
- V. Arantes and J. N. Saddler, Biotechnol. Biofuels, 2010, 3, 4 CrossRef PubMed.
- M. Linder and T. T. Teeri, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 12251–12255 CrossRef CAS.
- X. Li and Y. Zheng, Biotechnol. Adv., 2017, 35, 466–489 CrossRef CAS PubMed.
- W. Norde, Macromol. Symp., 1996, 103, 5–18 CrossRef CAS.
- H. Palonen, F. Tjerneld, G. Zacchi and M. Tenkanen, J. Biotechnol., 2004, 107, 65–72 CrossRef CAS PubMed.
- C. Qin, K. Clarke and K. Li, Biotechnol. Biofuels, 2014, 7, 65 CrossRef PubMed.
- F. M. Mendes, G. Siqueira, W. Carvalho, A. Ferraz and A. M. F. Milagres, Biotechnol. Prog., 2011, 27, 395–401 CrossRef CAS PubMed.
- N. Sathitsuksanoh, Z. Zhu and Y. H. Zhang, Bioresour. Technol., 2012, 117, 228–233 CrossRef CAS PubMed.
- C. Lai, M. Tu, M. Li and S. Yu, Bioresour. Technol., 2014, 156, 92–99 CrossRef CAS PubMed.
- X. Ju, M. Engelhard and Z. Xiao, Bioresour. Technol., 2013, 132, 137–145 CrossRef CAS PubMed.
- C. Lai, M. Tu, Z. Shi, K. Zheng, L. G. Olmos and S. Yu, Bioresour. Technol., 2014, 163, 320–327 CrossRef CAS PubMed.
- J. L. Rahikainen, J. D. Evans, S. Mikander, A. Kalliola, T. Puranen, T. Tamminen, K. Marjamaa and K. Kruus, Enzyme Microb. Technol., 2013, 53, 315–321 CrossRef CAS PubMed.
- J. P. Hui, P. Lanthier, T. C. White, S. G. McHugh, M. Yaguchi, R. Roy and P. Thibault, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 752, 349–368 CrossRef CAS.
- S. Heiss-Blanquet, D. Zheng, F. N. Lopes, C. Lapierre and S. Baumberger, Bioresour. Technol., 2011, 102, 5938–5946 CrossRef CAS PubMed.
- X. Pan, J. Biobased Mater. Bioenergy, 2008, 2, 25–32 CrossRef.
- S. Sun, Y. Huang, R. Sun and M. Tu, Green Chem., 2016, 18, 4276–4286 RSC.
- T. Pielhop, G. O. Larrazábal, M. H. Studer, S. Brethauer, C. M. Seidel and P. R. V. Rohr, Green Chem., 2015, 17, 3521–3532 RSC.
- C. Lai, M. Tu, C. Xia, Z. Shi, S. Sun, Q. Yong and S. Yu, Energy Fuels, 2017, 31, 12317–12326 CrossRef CAS.
- A. Berlin, N. Gilkes, A. Kurabi, R. Bura, M. Tu, D. Kilburn and J. Saddler, Appl. Biochem. Biotechnol., 2015, 124, 163–170 Search PubMed.
- T. Eriksson, J. Börjesson and F. Tjerneld, Enzyme Microb. Technol., 2002, 31, 353–364 CrossRef CAS.
- B. Yang and C. E. Wyman, Biotechnol. Bioeng., 2006, 94, 611–617 CrossRef CAS PubMed.
- H. Zhou, H. Lou, D. Yang, J. Zhu and X. Qiu, Ind. Eng. Chem. Res., 2013, 52, 8464–8470 CrossRef CAS.
- S. Nakagame, R. P. Chandra, J. F. Kadla and J. N. Saddler, Biotechnol. Bioeng., 2011, 108, 538–548 CrossRef CAS PubMed.
- X. Lin, X. Qiu, L. Yuan, Z. Li, H. Lou, M. Zhou and D. Yang, Bioresour. Technol., 2015, 185, 165–170 CrossRef CAS PubMed.
- J. Wang, T. Q. Lan and J. Y. Zhu, Biotechnol. Biofuels, 2013, 6, 9 CrossRef PubMed.
- C. Lai, S. Tang, B. Yang, Z. Gao, X. Li and Q. Yong, Bioresour. Technol., 2017, 244, 92–99 CrossRef CAS PubMed.
- T. Q. Lan, H. Lou and J. Y. Zhu, BioEnergy Res., 2013, 6, 476–485 CrossRef CAS.
- Y. Zhu, W. Wang, Y. Wang and Y. Jin, BioResources, 2015, 10, 7361–7371 CAS.
- H. Lou, J. Zhu, T. Q. Lan, H. Lai and X. Qiu, ChemSusChem, 2013, 6, 919–927 CrossRef CAS PubMed.
- Z. Wang, J. Zhu, Y. Fu, M. Qin, Z. Shao, J. Jiang and F. Yang, Biotechnol. Biofuels, 2013, 6, 156 CrossRef CAS PubMed.
- T. K. Ghose, Pure Appl. Chem., 1987, 59, 257–268 CrossRef CAS.
- C. Lai, M. Tu, Q. Yong and S. Yu, RSC Adv., 2015, 5, 97966–97974 RSC.
- M. M. Bradford, Anal. Biochem., 1976, 25, 248–256 CrossRef.
- C. A. Haynes, E. Sliwinsky and W. Norde, J. Colloid Interface Sci., 1994, 164, 394–409 CrossRef CAS.
- Y. Uraki, N. Ishikawa, M. Nishida and Y. Sano, J. Wood Sci., 2001, 47, 301–307 CrossRef CAS.
- H. Nonaka, A. Kobayashi and M. Funaoka, J. Wood Chem. Technol., 2014, 34, 169–177 CrossRef CAS.
- Y. Lu, B. Yang, D. Gregg, J. N. Saddler and S. D. Mansfield, Appl. Biochem. Biotechnol., 2002, 98–100, 641–654 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |