Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective removal of lead ions from aqueous solutions using 1,8-dihydroxyanthraquinone (DHAQ) functionalized graphene oxide; isotherm, kinetic and thermodynamic studies†
Mohammad Khazaei ab,
Simin Nasseri
ab,
Simin Nasseri *bc,
Mohammad Reza Ganjalide,
Mehdi Khoobi
*bc,
Mohammad Reza Ganjalide,
Mehdi Khoobi f,
Ramin Nabizadehb,
Elham Gholibegloog and
Shahrokh Nazmarab
f,
Ramin Nabizadehb,
Elham Gholibegloog and
Shahrokh Nazmarab
aDepartment of Environmental Health Engineering, School of Public Health and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
bDepartment of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, P. O. Box: 14155-6446, Tehran, Iran. E-mail: naserise@tums.ac.ir; Fax: +98 2188950188; Tel: +98 2188954914
cCenter for Water Quality Research, Institute for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran
dCenter of Excellence in Electrochemistry, Faculty of Chemistry, University of Tehran, Tehran, Iran
eBiosensor Research Center, Endocrinology & Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
fDepartment of Pharmaceutical Biomaterials and Medical Biomaterials Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
gDepartment of Chemistry, Faculty of Science, University of Zanjan, Zanjan, Iran
First published on 2nd February 2018
Abstract
An anthraquinone – graphene structure was fabricated and applied for the removal of lead(II) from aqueous solution. The equilibrium occurred in about 10 min revealing the high adsorption rate at the beginning of the process. The maximum Pb(II) adsorption capacity of the Fe3O4@DHAQ_GO nanocomposite was about 283.5 mg g−1 that was observed at 323 K and pH 5.5. The Pb(II) adsorption ability increased with the increasing pH. The isotherm and kinetic studies indicated that the Sips isotherm model and the linear form of the pseudo-second kinetic model had a better fit with the experimental results. The positive value of ΔH0 indicated endothermic interactions between Pb(II) and Fe3O4@DHAQ_GO. The negative ΔG0 indicated that the reactions are spontaneous with a high affinity for Pb(II). The positive ΔS0 values indicated increasing randomness at the solid–solute interface during the adsorption process. The selective removal of Pb(II) by the nanocomposite confirms the presence of higher-affinity binding sites for Pb(II) than Cd(II), Co(II), Zn(II), and Ni(II) ions. Furthermore, the Fe3O4@DHAQ_GO nanocomposite revealed an excellent preferential adsorbent for Pb(II) spiked in drinking water samples containing natural ion matrices. EDTA-2NA 0.01 N was found to be a better elution agent than HCl 0.1 M for the nanocomposite regeneration. After five adsorption/desorption cycles using EDTA-2NA 0.01 N, more than 84% of the adsorbed Pb(II) was still desorbed in 30 min. Capturing sub-ppm initial concentrations of Pb(II) and the capability to selectively remove lead from drinking water samples make the Fe3O4@DHAQ_GO nanocomposite practically convenient for water treatment purposes. High adsorption capacity and facile chemical synthesis route are the other advancements.
1. Introduction
Lead ions are a severe environmental concern and can contaminate drinking water resources.1,2 The maximum contaminant level (MCL) of Pb2+ for drinking water is 10 ppb set by EPA and national standard organizations.3,4 The strict limitations on discharge effluents containing Pb2+ into natural water bodies are due to the high toxicity potential for vital organs such as brain and kidney.2Different methods are currently applied for the removal of high concentrations of lead ion that can be found in industrial wastewaters;5–8 whereas only a few methods e.g. using functionalized adsorbents9,10 and membrane technologies11 can be adapted for the capturing of low concentrations (around 1 ppm) commonly occurring in drinking water sources. Furthermore, avoiding alteration of the natural ion matrices of drinking waters during the removal of a certain target contaminant is a consideration especially for membrane-based water treatment technologies.12,13 New generation adsorbents such as graphene oxide and carbon nanotubes show metal adsorption capacities much more than those of traditional adsorbents.14 For example, the ordinary adsorption capacity of activated carbon is less than 70 mg g−1, whereas graphene oxide nanosheets are capable of reaching an adsorption capacity of 4000 mg g−1.15
Graphene oxide is an emerging carbon-based nanomaterial that has revealed the promising adsorptive properties. Despite the graphene (G) and reduced graphene oxide (RGO), the graphene oxide (GO) creates a highly stable aqueous dispersion.16 This property leads to increase the effective contacts with target contaminants without vigorous mechanical mixing. The dispersability properties of GO is attributed to the plenty hydrophilic functional groups covering the GO flakes.17 The GO flake surface contains various functional groups including epoxy and hydroxide, whereas the edge of flakes mainly contains the carboxylic groups.18
In recent years, using Pb2+ selective membrane electrodes (ISE) have been extensively studied with different active materials to determine lead ion concentration in water and wastewater.19,20 The active materials are mainly consisting of ligands or Schiff bases, which are known as ionophores.21 It has been revealed that some ionophores such as anthraquinone,22–24 methacrylate,25 and nucleic acids26 have the selective affinity to lead ion. The main drawback regarding to the most of ionophores is their hydrophobic nature which makes them unusable to create aqueous solution for the lead ion removal.27 Using GO flakes as the aqueous dispersion agents can provide an appropriate platform for the attachment of ionophores and producing a water dispersible GO-ionophore composite.
1,8-Dihydroxyanthraquinone (DHAQ), namely Dantron is a dye intermediate and a medicine.27,28 Furthermore, some works report the high affinity of DHAQ as a ligand to form stable complexes with Pb2+.20,24 In this study, DHAQ was used as an ionophore agent in the structure of Fe3O4@SiO2–GO to form the Fe3O4@DHAQ_GO nanocomposite and aimed to have Pb2+ selective removal property from aqueous solutions.
2. Materials and methods
2.1. Materials
Graphite powder (particle size 20 μm), tetraethyl orthosilicate (TEOS), (3-aminopropyl) triethoxysilane (APTES), n-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC·HCl), and 1,8-dihydroxyanthraquinone (DHAQ) were purchased from Sigma-Aldrich, Ltd. Co. All other chemicals such as sodium nitrate (NaNO3), potassium permanganate (KMnO4), sulfuric acid (H2SO4), hydrochloric acid (HCl), hydrogen peroxide aqueous solution (H2O2), iron chloride hexahydrate (FeCl3, 6H2O), and iron chloride tetrahydrate (FeCl2, 4H2O) were of reagent grade and used without further purification.2.2. Preparation of Fe3O4@SiO2_GO
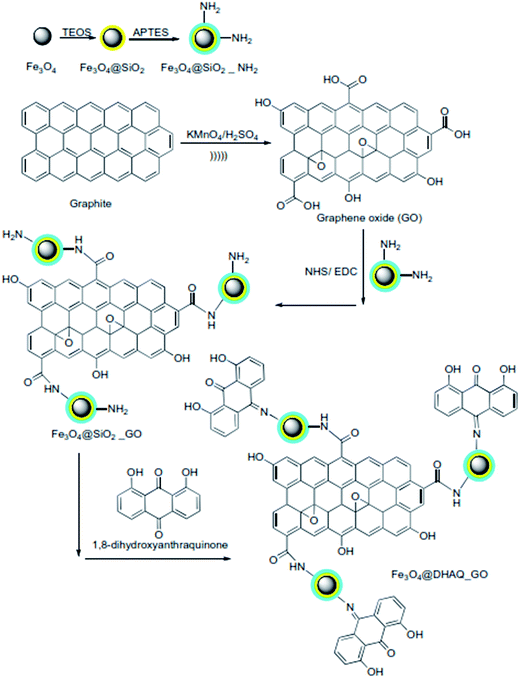
Our previous work reported the fabrication of graphene oxide (GO), Fe3O4 magnetic nanoparticles, Fe3O4@SiO2_NH2 nanoparticles, and Fe3O4@SiO2_GO nanocomposite.1 The preparation of GO was based on using sulfuric acid as digestion agent, and H2O2 for the oxidation of graphite.29 Co-precipitation method was used to prepare Fe3O4 magnetic nanoparticles.30 Then, NH2-groups were applied as linkers to create covalent bonds between Fe3O4 magnetic nanoparticles and GO. Consequently, covering APTES and TEOS on the Fe3O4 magnetic nanoparticles produces Fe3O4@SiO2_NH2.31,32 Finally, a condensation reaction between the carboxylic groups (COO–) of GO and the amine groups (NH2–) of Fe3O4@SiO2 was prepared for the fabrication of Fe3O4@SiO2_GO nanocomposite.322.3. Preparation of Fe3O4@DHAQ_GO
200 mg DHAQ was added into 50 mL DMF followed by mild stirring for 3 hours. Then, 200 mg EDS and 100 mg NHS were added and pH was adjusted between 4 to 6 followed by vigorous mixing for 2 hours at room temperature. After that, 0.5 g Fe3O4@SiO2_GO was dispersed into the mixture and mixing was continued up to 12 hours. Finally, dispersed solid was separated via centrifuge (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min), washed with deionized water, and dried to obtain Fe3O4@DHAQ_GO. Schematic of the synthesis path applied for the fabrication of Fe3O4@DHAQ_GO nanocomposite was presented in Fig. 1. As revealed, 1,8-dihydroxyanthraquinone attaches to amine group linked with Fe3O4 nanoparticle.
000 rpm, 10 min), washed with deionized water, and dried to obtain Fe3O4@DHAQ_GO. Schematic of the synthesis path applied for the fabrication of Fe3O4@DHAQ_GO nanocomposite was presented in Fig. 1. As revealed, 1,8-dihydroxyanthraquinone attaches to amine group linked with Fe3O4 nanoparticle.
2.4. Instrumentation
The prepared nanocomposite was characterized applying SEM (MIRA3, TESCAN®, Czech), AFM (SPM, VEECO®, USA), FTIR (Spectrum One, Perkin-Elmer®, USA), XRD (Philips®, Netherlands), UV-Visible spectrophotometer (Perkin-Elmer®, USA), TEM (EM900, Zeiss®, Germany), TGA (TGA 4000, Perkin-Elmer®, USA), and pHpzc. The initial and final concentration of Hg(II) were measured by using an ICP-OES (ARCOS, SPECTRO®, Germany). pH was adjusted by using a MITEC-965 (micra®, India) pH meter. A thermostatic shaker (Innova 4340, Eppendorf, Germany) was used to study the batch experiments.2.5. Characterization
A Hitachi-S4160 scanning microscope were used to provide SEM images (Tokyo, Japan). The AFM measurements were obtained by using a Nanoscope V multimode atomic force microscope (Veeco Instruments, USA). Samples prepared for the AFM measurements contained dispersions of GO/methanol solutions (70 mg mL−1) smeared on a fresh mica surface and allowed drying in the air.332.6. Adsorption experiments
A typical adsorption experiment was established by adding 10 mg Fe3O4@DHAQ_GO into a 100 mL solution containing Pb2+ ions at room temperature. Varied initial concentrations of Pb2+, from 1 mg L−1 to 10 mg L−1, were used and for all the Pb2+ aliquots, the pH value was kept on 7 applying buffer solutions. The mixing rate was constant at 150 rpm for the all solutions.An external magnetic field was used for the removal of adsorbent after the adsorption time. The equilibrium adsorption capacity (qe, mg g−1) of Pb2+ was determined by the following equation.
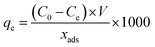 | (1) |
A Spectro Arcos ICP-optical emission spectrometer (SPECTRO Analytical Instruments, Kleve, Germany) was used for the measurement of Pb2+ concentrations.
The parameters of isotherm and kinetic equations were determined by applying Solver engine of Microsoft Excel spreadsheet software34 based on nonlinear forms of the equations.
2.7. Selectivity study
Two independent studies were conducted to investigate the capability of Fe3O4@DHAQ_GO nanocomposite for the selective capturing of Pb2+ from water: binary ion study; including aliquots contained binary ion matrices (Pb2+/Cu2+, Pb2+/Cd2+, Pb2+/Zn2+, and Pb2+/Co2+) and selective removal of Pb2+ from natural water samples; including drinking water samples spiked with Pb2+ ions. The concentration of metal ions was measured by using ICP-OES. The distribution coefficient Kd (mL g−1), selectivity coefficient k, and the relative selectivity coefficient k were determined by eqn (2)–(4), respectively.
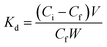 | (2) |
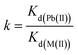 | (3) |
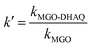 | (4) |
2.8. Desorption and regeneration
Pb2+ in solution (25 mL, 2.45 mg L−1) was adsorbed onto Fe3O4@DHAQ_GO (30 mg L−1) at pH 7 for 1 h and then the adsorbents were separated by applying an external magnetic field and the residual quantity of metal ions was determined by ICP-OES. After that, the adsorbents were regenerated in 25 mL Erlenmeyer flask containing 10 mL 0.02 mol L−1 eluent to completely leach metal ions at room temperature for 6 h. The concentration of metal ions released from adsorbent into the aqueous phase was measured by ICP-OES. Desorption ratio (D) was determined by using the following equation:
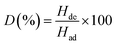 | (5) |
3. Results and discussion
It is well known that various derivatives of anthraquinone are able to form stable complexes with a variety of metal ions in some non-aqueous solvents35,36 and anthraquinone–lead(II) complexes are among the most stable ones.37,38 Applying the graphene oxide provides the active sites for the anthraquinone that can be covalently bonded and produced a hydrophilic property which is appropriate for the adsorption of Pb2+ in the aqueous solution.3.1. Characterization studies
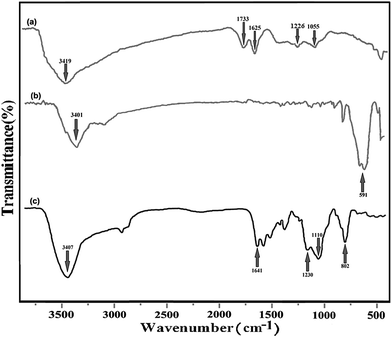
The FT-IR spectra for GO, Fe3O4@SiO2–GO, and Fe3O4@DHAQ_GO are presented in Fig. 2. The stretchings C–O (1055 cm−1), C–OH (1226 cm−1), C–O carbonyl (1733 cm−1), and O–H hydroxide (3419 cm−1)39–41 can be observed in the FT-IR spectrum of GO (Fig. 2(a)). The stretching assigned to the adsorbed water molecules is observed at 1621 cm−1 assigning also to the skeletal vibrations of un-oxidized graphite.42,43In Fig. 2(b), the spectrum of Fe3O4@SiO2–GO is depicted. It shows the vibration of Fe–O stretching at 591 cm−1 and an intense stretching around 3400 cm−1. Although, it can be attributed to the remaining water on the surfaces of Fe3O4 nanoparticles.44
Fig. 2(c) depicts the FT-IR spectrum of Fe3O4@DHAQ_GO. As shown, a vibration is observed at 3401 cm−1 assigning to the N–H stretching. Furthermore, the peak at 1733 cm−1, observed in Fig. 2(a), is disappeared and a new wide peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching is appeared at 1641 cm−1. The vibration of C–N stretching is appeared at 1230 cm−1.45 The obvious peaks at 802 and 1110 cm−1 can be attributed to the Si–O vibrations. The FTIR spectra confirmed that APTES functionalized Fe3O4 has been bonded covalently to GO nanosheets via the amide linkage.46
N stretching is appeared at 1641 cm−1. The vibration of C–N stretching is appeared at 1230 cm−1.45 The obvious peaks at 802 and 1110 cm−1 can be attributed to the Si–O vibrations. The FTIR spectra confirmed that APTES functionalized Fe3O4 has been bonded covalently to GO nanosheets via the amide linkage.46
Fig. S1† depicts field emission SEM images of GO, Fe3O4@SiO2–GO, and Fe3O4@DHAQ_GO nanoparticles. From Fig. S1(a),† it can be observed that GO is partially transparent and 2- or 3-layered graphene oxides are formed.47,48 From Fig. S1(b),† the spherical Fe3O4@SiO2–NH2 nanoparticles having 50–60 nm diameters are identified, which finally have been enveloped by GO layers producing aggregated morphologies of Fe3O4@DHAQ_GO as shown in Fig. S1(c)†.
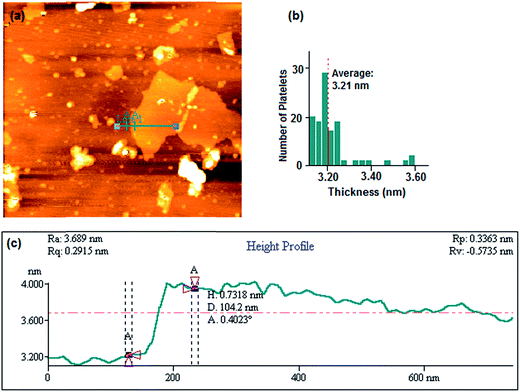
Fig. 3 illustrates the tapered mode AFM topography scan. A single platelet of GO laid on a freshly cleaved mica surface can be observed in Fig. 3(a) and (b) represents a frequency histogram of platelets thicknesses having the mean thickness of 3.21 nm. Height profile of the green line (Line 1 in Fig. 3(a)) presents a height of 0.732 nm in cross-section A–A as shown in Fig. 3(c).
Fig. S2† presents thermal gravimetric analysis (TGA) of Fe3O4 magnetic nanoparticles, Fe3O4@SiO2–GO, Fe3O4@DHAQ_GO, and graphene oxide. As revealed, major weight losses were occurred between 150 and 350 °C attributing to CO, CO2 released from labile functional groups.48,49 Slower rate of mass loss was detected between 350 and 650 °C assigning to the removal of some stable oxygenated functional groups. Similar trends of weight loss were observed in Fe3O4@SiO2–GO and Fe3O4@DHAQ_GO. The Fe3O4@DHAQ_GO weight loss was 13.5% more than those of Fe3O4@SiO2–GO in 740 °C attributing to the presence of 1,8-dihydroxyanthraquinone in the structure of Fe3O4@DHAQ_GO.50
Fig. S3† shows the XRD patterns of GO and Fe3O4@SiO2–GO. GO sharp diffraction peaks observable at 2θ = 12.24° and 42.83° are attributed to the reflections of (002) and (101), respectively. Furthermore, six typical peaks at about 2θ = 30.4, 35.6, 43.1, 54.1, 57.7 and 62.5° are observed for Fe3O4@SiO2–GO, attributing to indices (220), (311), (400), (422), (511) and (440), respectively. Appropriate match of intensities and positions of above mentioned diffraction peaks confirming by pure magnetite JCPDS card (75-1610).51 As represented in XRD patterns corresponding to Fe3O4@SiO2–GO, the reflection peak (002) belonging to GO was disappeared. It is assumed that the GO sheets cover the Fe2O3 nanoparticles and it hinders the stacking of sheets to create a crystalline arrangement.52
The vibration sample magnetization (VSM) was used to determine the magnetic characteristics of fabricated materials contained Fe3O4. Fig. S4† shows that the maximum saturation magnetizations of Fe3O4 NPs, Fe3O4-APTES, GO@SiO2–Fe3O4, and Fe3O4@DHAQ_GO were 53.2, 40.1, 19.7, and 13.5 emu g−1, respectively. Decreasing the maximum saturation magnetizations can be ascribed to the Fe3O4 nanoparticles covering consecutively by APTES, SiO2–GO, and DHAQ.
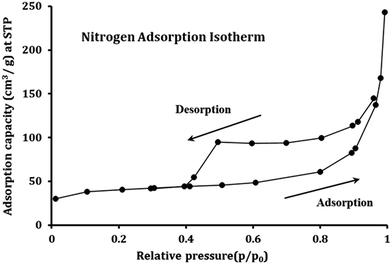
Fig. 4 presents the nitrogen adsorption isotherm of Fe3O4@DHAQ_GO nanocomposite. The surface area of 215 m2 g−1 was obtained that is relatively lowered than those reported for pristine GO.53 It seems that the agglomeration of Fe3O4 NPs and GO nanosheets tend to an shrinking effect on the GO nanosheets causing the decrease of free surface area48 as observed in Fig. S1.† The average pore size of Fe3O4@DHAQ_GO was determined to be about 9.26 nm identifying the mesopore structure of the adsorbent.
3.2. Adsorption experiments
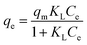 | (6) |
| qe = KfC1/neF | (7) |
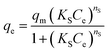 | (8) |
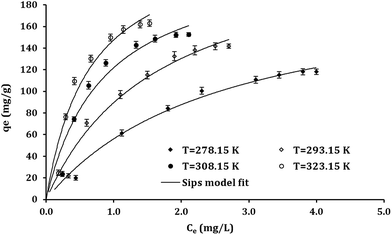
As represented from Table 1, the R2 values indicated that Sips model has better fit with the experimental results then Langmuir and Freundlich models. Fig. 5 depicts the nonlinear functions of Sips model fitted to the experimental points obtained from the batch studies in different temperatures.
| T (K) | qexp (mg g−1) | Langmuir | Freundlich | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL | RL2 | nF | KF | RF2 | qm (mg g−1) | KS | nS | R2 | ||
| 278 | 118 | 200.7 | 0.38 | 0.98 | 1.7 | 55.5 | 0.97 | 119.1 | 0.76 | 1.61 | 0.99 |
| 293 | 141 | 239.4 | 0.59 | 0.96 | 1.7 | 85.3 | 0.96 | 142.5 | 1.24 | 1.69 | 0.98 |
| 308 | 152 | 225.3 | 1.17 | 0.93 | 2.01 | 114 | 0.93 | 151.6 | 2.12 | 2.36 | 0.99 |
| 323 | 163 | 243.1 | 1.54 | 0.93 | 1.96 | 142 | 0.93 | 164.3 | 3.06 | 2.38 | 0.99 |
The Sips equation containing three parameters having the capability to apply for both the homogeneous and heterogeneous systems.56 The surface heterogeneity of adsorbent should be considered if the deviation of nS values from 1 is observed.55,57 However, the Sips isotherm reach a constant level at high concentrations while a pattern of Freundlich model can be observed at low concentrations.57
As revealed from Table 1, the Pb2+ adsorption capacities of Fe3O4@DHAQ_GO nanocomposite were increased by the increasing of temperature assigning to decrease water viscosity along with the increasing of Pb2+ collisions between the sites of nanocomposite and Pb2+ ions. The maximum adsorption capacities (qm) obtained by Langmuir isotherm were overestimated (e.g. 243.1 in 323 K) while those of Sips model (e.g. 164.3 in 323 K) represents a good fit to the experimental data (also, see Fig. 5) which can be due to the heterogeneity characteristic considered in the Sips model.58 Increasing the deviations of nS and nF values from unity can be assigned to develop the nanocomposite surface heterogeneity over raising the temperature.57
| qt = qe(1 − exp(−k1t)) | (9) |
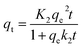 | (10) |
Fig. 6 illustrates fitting the non-linear forms of pseudo-second kinetic model to the experimental points. As shown, the equilibrium was took place sooner for the batch systems underwent lower Pb2+ initial concentrations.
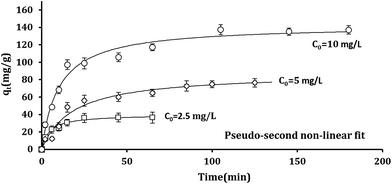 | ||
| Fig. 6 Nonlinear forms of pseudo-second kinetic model fitted on experimental points at different Pb2+ initial concentrations (adsorbent dosage 100 mg g−1; volume of solution 100 mL; pH 7; T = 298 K). | ||
Table S1† presents kinetic parameters of Pb2+ removal obtained by using the non-linear forms of pseudo-first and pseudo-second kinetic models (eqn (9) and (10)). As found in Table S1,† according to the R2 values, the pseudo-second model has better fit to the experimental points and K2 are increased by increasing the temperature, both are the evidences favor the chemisorption occurring.61–63
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (11) |
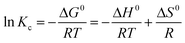 | (12) |
| T | KL (L g−1) | qm (mg g−1) | ΔG0 (kJ mol−1) | ΔS0 (J mol−1 K) | ΔH0 (kJ mol−1) |
|---|---|---|---|---|---|
| 278 | 0.388 | 200.7 | −13.79 | 135.97 | 24.07 |
| 293 | 0.598 | 239.4 | −15.58 | — | — |
| 308 | 1.178 | 225.3 | −18.12 | — | — |
| 323 | 1.546 | 243.1 | −19.73 | — | — |
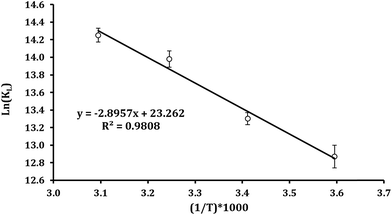
Enthalpy (ΔH0) and entropy (ΔS0) can be determined by plotting ln(Kc) versus 1/T as revealed in Fig. 7. Furthermore, (ΔH0) and (ΔS0) can obtained be from the slope and intercept of linear form of eqn (12), respectively.64–66
Table 2 represents that ΔG0 has negative amounts assigning to different temperatures. So, it can be concluded that Pb2+ adsorption on Fe3O4@DHAQ_GO nanocomposite proceeds spontaneously.
Fan et al. (2013) found that obtained ΔG0 is ranged from −10.26 to −16.24 kJ mol−1 at 303–323 K.67 Also, Kumar et al. (2014) reported that the changes of free energy ΔG0 at 298 K are −6.46 kJ mol−1. As shown in Table 2, ΔG0 is −19.73 kJ mol−1 at 323 K having an appropriate agreement with the findings of Fan et al. (2013). Similar finding were reported by other researchers.68–70
As represented in Table 2, increasing the temperature tends to lower values assigned to ΔG0 confirming that the adsorption is more efficient at the higher temperatures.71,72 The enthalpy (ΔH0) value was 24.07 kJ mol−1 having the positive value of ΔH0 that indicates the endothermic nature of the adsorption. The entropy (ΔS0) was obtained with a positive value proving the increase of randomness during Pb2+ adsorption process.73,74
3.3. Selectivity study
Two independent studies were conducted to evaluate the selectivity properties of Fe3O4@DHAQ_GO nanocomposite for the separation of Pb2+ ions from aqueous ion matrices. The first one was capturing Pb2+ ions from four different aqueous solutions so that each solution contains Pb2+ and one other divalent metal ion. Consequently, four binary ion matrices were prepared, including Pb2+/Cu2+, Pb2+/Cd2+, Pb2+/Zn2+, and Pb2+/Co2+.The second study was conducted for the assessment selective removal of Pb2+ in drinking water samples containing natural ion matrices. Certain amounts of Pb2+ ion were spiked into 30 different drinking water samples collected from various groundwater sources. Batch experiments were conducted based on the optimized values of variables pH, dosage, temperature, and the initial concentration.
| Metal ion | Fe3O4@DHAQ_GO | GO@Fe3O4 | K | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | kd (L g−1) | KFe3O4@DHAQ_GO | qe (mg g−1) | kd (L g−1) | kGO@SiO2–Fe3O4 | ||
| Pb2+ | 132 | 41.88 | 19.37 | 47 | 8.14 | 0.99 | 19.66 |
| Cu2+ | 15 | 2.16 | 50 | 8.26 | |||
| Pb2+ | 133 | 36.81 | 31.89 | 37 | 5.74 | 1.34 | 23.72 |
| Ni2+ | 9 | 1.15 | 30 | 4.27 | |||
| Pb2+ | 123 | 34.73 | 19.41 | 24 | 3.67 | 0.71 | 27.20 |
| Co2+ | 12 | 1.79 | 32 | 5.15 | |||
| Pb2+ | 119 | 32.38 | 15.09 | 21 | 3.21 | 0.58 | 26.09 |
| Cd2+ | 15 | 2.15 | 35 | 5.54 | |||
| Model elementsa | Coefficient | Standard error | T value | Pr(>|t|) | P-value |
|---|---|---|---|---|---|
| a Multiple R2: 0.81, adjusted R2: 0.73. | |||||
| Intercept | 105.47 | 5.23 | 20.15 | 0.0001 | 0.001 |
| NO3− | 0.70 (a) | 0.43 | 1.62 | 0.120 | 0.1 |
| SO42− | 0.17 (b) | 0.05 | 2.8 | 0.009 | 0.001 |
| Cl− | 0.31 (c) | 0.08 | 3.66 | 0.001 | 0.001 |
| HCO3− | 0.15 (d) | 0.04 | 3.51 | 0.002 | 0.001 |
| Na+ | −0.29 (e) | 0.14 | −1.95 | 0.064 | 0.05 |
| K+ | −0.47 (f) | −0.27 | −1.75 | 0.093 | 0.05 |
| Mg2+ | −1.15 (g) | −0.29 | −3.89 | 0.0008 | 0.001 |
| Ca2+ | −0.73 (h) | −0.15 | −4.69 | 0.0001 | 0.001 |
3.4. Desorption and regeneration
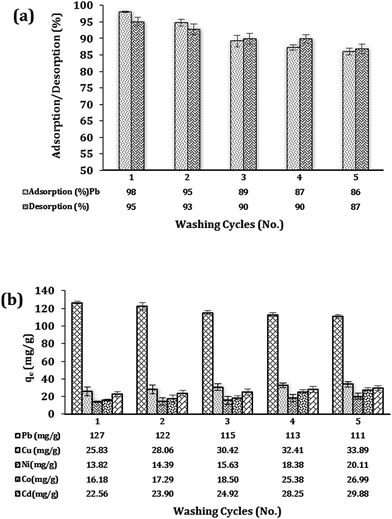
Fig. 8(a) depicts the repeated adsorption/desorption of Pb2+ ions using batch experiments exposed with Fe3O4@DHAQ_GO nanocomposite in single ion aqueous solution. As shown, after 5 consecutive regeneration steps, the nanocomposite could remove 86 percent of Pb2+ ions so that only 12 percent of removal loss was observed.Fig. 8(b) shows the results of Fe3O4@DHAQ_GO regeneration study in an aqueous ion matrix consisting of five divalent metals. This experiment aims to investigate the presence of four coexistence ions (Cu2+, Ni2+, Co2+, Cd2+) in the case of their effect on lead removal and to assess the capability of the nanocomposite for the retaining of lead adsorption capacity after several washing steps in the presence of other cations. As observed, the removal capacities of Pb2+ ion was remained more than 111 mg g−1 over five regeneration steps using the desorption agent EDTA-2NA 0.01 N. Furthermore, increasing the sorption capacity assigned to the four coexistence ions were almost negligible confirming the notable stability of the nanocomposite structure over several regeneration experiments.
The stability of the Pb2+ and Fe3O4@DHAQ_GO complex was confirmed via the adsorption/desorption experiments. The conventional methods for evaluating the regeneration and reusability of adsorbents are according to the consecutive adsorption/desorption steps in batch volumes containing deionized water solution inoculated with the target pollutant. Consequently, the effects of coexistence ions are neglected, especially when the reusability of adsorbents having selectivity properties is considered.77
Here, we put forward a facile approach to investigate the reusability of Fe3O4@DHAQ_GO in aqueous ion matrices containing different competitor divalent cations (Fig. 8). Yu et al. reported applying EDTA-2Na 0.015 N as washing agent over three cycles regeneration steps. Results showed the notable interference of Cd2+ (ref. 78) while, in our work, the minimum interfering of the coexistence cations was observed.
4. Conclusions
In this work, a novel hydrophilic nanocomposite based on GO was synthesized comprising an anthraquinone derivative having selective removal capability for lead. Fe3O4 nanoparticles was used as a magnetic agent to facilitate the separation of nanocomposite from aqueous solution. Also, GO was used as a dispersible platform to obtain the hydrophilic property for the nanocomposite and preparing enough surface area to proceed the adsorptive mechanisms. The morphology and structure of the obtained adsorbent was characterized by UV-Vis, FT-IR, SEM, XRD, and TGA. The synthesis rout was simple and DHAQ was an environmental friendly compound without toxic effect. The selectivity characteristics of the nanocomposite was evaluated through two different methods including controlled ion matrices and the natural ion matrices obtained from drinking water samples. Furthermore, the regeneration and reusability studies were conducted in the presence of coexistence ions. It seems that Fe3O4@DHAQ_GO nanocomposite can be a promising selective removal agent for the removal of lead from polluted waters and industrial discharges.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was part of a PhD dissertation of the first author and has been financially supported by a grant (No. 28232-27-01-94) from Tehran University of Medical Sciences, Tehran, Iran. The authors would like to express their thanks to the Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences for their collaboration.References
- M. Khazaei, S. Nasseri, M. R. Ganjali, M. Khoobi, R. Nabizadeh, A. H. Mahvi, S. Nazmara and E. Gholibegloo, J. Environ. Health Sci. Eng., 2016, 14, 1 CrossRef PubMed
.
- WHO, Journal, 2003.
- EPA, Drinking Water Contaminants, http://www.epa.gov/safewater/contaminants/index.html, 1/30/2010., (accessed 9/13/2015, 2015).
- ISIRI, Journal, 2014.
- H. Karimi, International Journal of Water and Wastewater Treatment, 2017, 3 Search PubMed
.
- Q. Li, T. Liu and P. Deng, in, Characterization of Minerals, Metals, and Materials, Springer, 2015, pp. 667–674 Search PubMed
.
- R. Kamaraj, P. Ganesan and S. Vasudevan, Int. J. Environ. Sci. Technol., 2015, 12, 683–692 CrossRef CAS
.
- S. Vasudevan, J. Lakshmi and G. Sozhan, Environ. Sci. Pollut. Res., 2012, 19, 2734–2744 CrossRef CAS PubMed
.
- E. Aliyari, M. Alvand and F. Shemirani, Anal. Methods, 2015, 7, 7582–7589 RSC
.
- S. K. Ghadiri, S. Nasseri, R. Nabizadeh, M. Khoobi, S. Nazmara and A. H. Mahvi, J. Mol. Liq., 2017, 242, 1111–1117 CrossRef CAS
.
- A. Gholami, A. Moghadassi, S. Hosseini, S. Shabani and F. Gholami, J. Ind. Eng. Chem., 2014, 20, 1517–1522 CrossRef CAS
.
- R. Rezaee, S. Nasseri, A. Mahvi, R. Nabizadeh, S. Mousavi, A. Rashidi, A. Jafari and S. Nazmara, J. Environ. Health Sci. Eng., 2015, 13, 1–11 CrossRef CAS PubMed
.
- C. J. Madadrang, H. Y. Kim, G. Gao, N. Wang, J. Zhu, H. Feng, M. Gorring, M. L. Kasner and S. Hou, ACS Appl. Mater. Interfaces, 2012, 4, 1186–1193 CAS
.
- P. Ganesan, R. Kamaraj, G. Sozhan and S. Vasudevan, Environ. Sci. Pollut. Res., 2013, 20, 987–996 CrossRef CAS PubMed
.
- W. Li, S. Gao, L. Wu, S. Qiu, Y. Guo, X. Geng, M. Chen, S. Liao, C. Zhu and Y. Gong, Sci. Rep., 2013, 3, 2125 CrossRef PubMed
.
- J. Kim, L. J. Cote and J. Huang, Acc. Chem. Res., 2012, 45, 1356–1364 CrossRef CAS PubMed
.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed
.
- J. Paredes, S. Villar-Rodil, A. Martinez-Alonso and J. Tascon, Langmuir, 2008, 24, 10560–10564 CrossRef CAS PubMed
.
- M. J. Gismera, M. T. Sevilla and J. R. Procopio, Anal. Sci., 2006, 22, 405 CrossRef CAS PubMed
.
- N. Tavakkoli, Z. Khojasteh, H. Sharghi and M. Shamsipur, Anal. Chim. Acta, 1998, 360, 203–208 CrossRef CAS
.
- M. Rezayi, R. Karazhian, Y. Abdollahi, L. Narimani, S. B. T. Sany, S. Ahmadzadeh and Y. Alias, Sci. Rep., 2014, 4 CAS
.
- M. Barzegar, M. F. Mousavi, H. Khajehsharifi, M. Shamsipur and H. Sharghi, IEEE Sens. J., 2005, 5, 392–397 CrossRef CAS
.
- X.-G. Li, X.-L. Ma and M.-R. Huang, Talanta, 2009, 78, 498–505 CrossRef CAS PubMed
.
- A. Goswami and A. K. Singh, Talanta, 2002, 58, 669–678 CrossRef CAS PubMed
.
- T. Alizadeh, N. Hamidi, M. R. Ganjali and F. Rafiei, J. Environ. Chem. Eng., 2017, 5, 4327–4336 CrossRef CAS
.
- X. Niu, Y. Zhong, R. Chen, F. Wang, Y. Liu and D. Luo, Sens. Actuators, B, 2018, 255, 1577–1581 CrossRef CAS
.
- Z. Zhang, D. Shi, H. Ding, H. Zheng and H. Chen, Int. J. Environ. Sci. Technol., 2015, 12, 3351–3362 CrossRef CAS
.
- Y. Shen, Q. Xu, D. Gao and H. Shi, Ozone: Sci. Eng., 2017, 39, 219–232 CrossRef CAS
.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef
.
- H. Hamadi, M. Gholami and M. Khoobi, International Journal of Heterocyclic Chemistry, 2011, 1, 23–34 Search PubMed
.
- P. Tartaj and C. J. Serna, J. Am. Chem. Soc., 2003, 125, 15754–15755 CrossRef CAS PubMed
.
- F. He, J. Fan, D. Ma, L. Zhang, C. Leung and H. L. Chan, Carbon, 2010, 48, 3139–3144 CrossRef CAS
.
- L. J. Cote, F. Kim and J. Huang, J. Am. Chem. Soc., 2008, 131, 1043–1049 CrossRef PubMed
.
- M. A. Hossain, H. H. Ngo and W. Guo, J. Water Sustainability, 2013, 3, 223–237 CAS
.
- F.-y. Wu, M.-h. Hu, Y.-m. Wu, X.-f. Tan, Y.-q. Zhao and Z.-j. Ji, Spectrochim. Acta, Part A, 2006, 65, 633–637 CrossRef PubMed
.
- M. M. Hoffman, A. B. Kryjevski, A. G. Sykes and D. S. Kilin, Mol. Phys., 2016, 114, 562–575 CAS
.
- S. Riahi, M. Mousavi, M. Shamsipur and H. Sharghi, Electroanalysis, 2003, 15, 1561–1565 CrossRef CAS
.
- H. Reza Pouretedal, A. Forghaniha, H. Sharghi and M. Shamsipur, Synth. React. Inorg. Met.-Org. Chem., 1998, 31, 2591–2605 Search PubMed
.
- G. I. Titelman, V. Gelman, S. Bron, R. L. Khalfin, Y. Cohen and H. Bianco-Peled, Carbon, 2005, 43, 641–649 CrossRef CAS
.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342–3347 CrossRef CAS
.
- S. D. Abkenar, M. Khoobi, R. Tarasi, M. Hosseini, A. Shafiee and M. R. Ganjali, J. Environ. Eng., 2014, 141, 04014049 CrossRef
.
- F. Cataldo, Fullerenes, Nanotubes, Carbon Nanostruct., 2003, 11, 1–13 CrossRef CAS
.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed
.
- X. Yang, Y. Wang, X. Huang, Y. Ma, Y. Huang, R. Yang, H. Duan and Y. Chen, J. Mater. Chem., 2011, 21, 3448–3454 RSC
.
- Y. Xu, Z. Liu, X. Zhang, Y. Wang, J. Tian, Y. Huang, Y. Ma, X. Zhang and Y. Chen, Adv. Mater., 2009, 21, 1275–1279 CrossRef CAS
.
- Y. Li, X.-Y. Wang, X.-P. Jiang, J.-J. Ye, Y.-W. Zhang and X.-Y. Zhang, J. Nanopart. Res., 2015, 17, 1–12 CrossRef
.
- G. Zhao, X. Ren, X. Gao, X. Tan, J. Li, C. Chen, Y. Huang and X. Wang, Dalton Trans., 2011, 40, 10945–10952 RSC
.
- M. Khazaei, S. Nasseri, M. R. Ganjali, R. Nabizadeh, A. H. Mahvi, E. Gholibegloo, M. Khoobi and S. Nazmara, Desalin. Water Treat., 2017, 83, 144–158 CrossRef
.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS
.
- G. A. Broker, R. P. A. Bettens and E. R. T. Tiekink, CrystEngComm, 2008, 10, 879–887 RSC
.
- E. Aliyari, M. Alvand and F. Shemirani, RSC Adv., 2016, 6, 64193–64202 RSC
.
- M. Tarlani Azar, M. Leili, F. Taherkhani and A. Bhatnagar, Desalin. Water Treat., 2016, 57, 24430–24443 CrossRef CAS
.
- S. Stankovich, D. A. Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed
.
- E. Repo, J. K. Warchol, T. A. Kurniawan and M. E. T. Sillanpää, Chem. Eng. J., 2010, 161, 73–82 CrossRef CAS
.
- L. Guo, G. Li, J. Liu, P. Yin and Q. Li, Ind. Eng. Chem. Res., 2009, 48, 10657–10663 CrossRef CAS
.
- E. Repo, J. K. Warchoł, A. Bhatnagar and M. Sillanpää, J. Colloid Interface Sci., 2011, 358, 261–267 CrossRef CAS PubMed
.
- E. Repo, Doctor of Science, Lappeenranta University of Technology Laboratory of Green Chemistry, 2011 Search PubMed
.
- Y. Ma, P. La, W. Lei, C. Lu and X. Du, Desalin. Water Treat., 2016, 57, 5004–5012 CrossRef CAS
.
- A. Dalvand, E. Gholibegloo, M. R. Ganjali, N. Golchinpoor, M. Khazaei, H. Kamani, S. S. Hosseini and A. H. Mahvi, Environ. Sci. Pollut. Res., 2016, 23, 16396–16405 CrossRef CAS PubMed
.
- S. Vasudevan and J. Lakshmi, Water Sci. Technol.: Water Supply, 2011, 11, 142–150 CrossRef CAS
.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS
.
- R. Kamaraj, D. J. Davidson, G. Sozhan and S. Vasudevan, J. Taiwan Inst. Chem. Eng., 2014, 45, 2943–2949 CrossRef CAS
.
- S. Vasudevan, J. Lakshmi, R. Kamaraj and G. Sozhan, Asia-Pac. J. Chem. Eng., 2013, 8, 162–171 CrossRef CAS
.
- Z.-Y. Yao, J.-H. Qi and L.-H. Wang, J. Hazard. Mater., 2010, 174, 137–143 CrossRef CAS PubMed
.
- S. Vasudevan and J. Lakshmi, Can. J. Chem. Eng., 2012, 90, 1017–1026 CrossRef CAS
.
- M. Naushad, S. Vasudevan, G. Sharma, A. Kumar and Z. ALOthman, Desalin. Water Treat., 2016, 57, 18551–18559 CrossRef CAS
.
- L. Fan, C. Luo, M. Sun, X. Li and H. Qiu, Colloids Surf., B, 2013, 103, 523–529 CrossRef CAS PubMed
.
- H. Hadi Najafabadi, M. Irani, L. Roshanfekr Rad, A. Heydari Haratameh and I. Haririan, RSC Adv., 2015, 5, 16532–16539 RSC
.
- Y. Zhang, L. Yan, W. Xu, X. Guo, L. Cui, L. Gao, Q. Wei and B. Du, J. Mol. Liq., 2014, 191, 177–182 CrossRef CAS
.
- R. Kamaraj, D. J. Davidson, G. Sozhan and S. Vasudevan, RSC Adv., 2015, 5, 39799–39809 RSC
.
- E. Unuabonah, K. Adebowale and B. Olu-Owolabi, J. Hazard. Mater., 2007, 144, 386–395 CrossRef CAS PubMed
.
- R. Kamaraj, A. Pandiarajan, S. Jayakiruba, M. Naushad and S. Vasudevan, J. Mol. Liq., 2016, 215, 204–211 CrossRef CAS
.
- Y.-H. Li, Z. Di, J. Ding, D. Wu, Z. Luan and Y. Zhu, Water Res., 2005, 39, 605–609 CrossRef CAS PubMed
.
- S. Vasudevan and J. Lakshmi, Environ. Eng. Sci., 2012, 29, 563–572 CrossRef CAS
.
- L. Zhu, Z. Zhu, R. Zhang, J. Hong and Y. Qiu, J. Environ. Sci., 2011, 23, 1955–1961 CrossRef CAS
.
- R. Msaadi, S. Ammar, M. M. Chehimi and Y. Yagci, Eur. Polym. J., 2017, 89, 367–380 CrossRef CAS
.
- J. Yu, J. Zheng, Q. Lu, S. Yang, X. Zhang, X. Wang and W. Yang, Colloid Polym. Sci., 2016, 294, 1585–1598 CAS
.
- J. Yu, J. Zheng, Q. Lu, S. Yang, X. Wang, X. Zhang and W. Yang, Iran. Polym. J., 2016, 25, 1009–1019 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13603j |
| This journal is © The Royal Society of Chemistry 2018 |