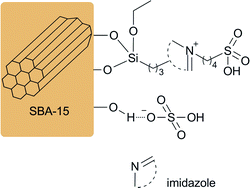
Open Access Article
Open Access ArticleEfficient and reusable SBA-15-immobilized Brønsted acidic ionic liquid for the ketalization of cyclohexanone with glycol
Ruiyun Li ab,
Heyuan Songab,
Guoqin Wangab and
Jing Chen*a
ab,
Heyuan Songab,
Guoqin Wangab and
Jing Chen*a
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000, P. R. China. E-mail: chenj@licp.cas.cn; Fax: +86-931-496-8129; Tel: +86-931-496-8068
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 13th February 2018
Abstract
Ketalization of cyclohexanone with glycol has been carried out using molecular sieve SBA-15 immobilized Brønsted acidic ionic liquid catalyst. The properties of the heterogeneous catalysts were characterized by elemental analysis, Fourier transform infrared (FT-IR) spectra, scanning electron microscopy (SEM), thermogravimetry/differential scanning calorimetry (TG/DSC), and N2 adsorption–desorption (BET). The results suggested that Brønsted acidic ionic liquid [BSmim][HSO4] had been successfully immobilized on the surface of SBA-15 and the catalytic performance evaluation demonstrated that the catalyst BAIL@SBA-15 exhibited excellent catalytic activities in the ketalization of cyclohexanone with glycol. In addition, the effects of reaction temperature, catalyst loading, reaction time, and reactant molar ratio have also been investigated in detail, and a general reaction mechanism for the ketalization of cyclohexanone with glycol was given. The SBA-15 immobilized ionic liquid can be recovered easily and after reusing for 5 times in the ketalization reaction, the catalyst could still give satisfactory catalytic activity.
1. Introduction
Currently, ionic liquids (ILs) are receiving widespread attention as media for a variety of reactions because of their unique properties such as low vapor pressure, high chemical and thermal stability, and adjustable structure.1–4 Compared to conventional acid catalysts, acidic ionic liquids possess several advantages, such as, reactions can be carried out under solvent-free conditions, and the physical and chemical properties of ionic liquids can be tailored by varying cations and anions.5,6 Since 2002 when Cole et al.7 first reported SO3H-functionalized ionic liquids with strong Brønsted acidity, the applications of the Brønsted acidic ionic liquids (BAILs) with alkyl sulfonic acid groups in cations as a replacement for conventional homogenous and heterogeneous acids have received great attention.7–10 And BAILs have been used as acid catalysts for esterification, alkylation, Pechmann reactions, acetalization and oligomerization.11–13Although ionic liquids have become commercially available, several drawbacks, such as unendurable viscosity, high cost and tedious purification procedure of the product, restricted their widespread applications.14 In order to solve problems mentioned above, immobilized ionic liquids catalysts combining the characteristics of ionic liquids, inorganic acids and solid acids had been proposed.15,16
Compared to other well-known acid catalysts, immobilized ILs have several advantages. The most important may be the easily adjustable acidity, and the possibilities given by the selection of support materials.17,18 Recently, rapidly increasing reports have become available describing the application of task specific ionic liquids (TSILs) immobilized on various supports. The immobilized TSILs show additional advantages, such as decreasing consumption of ionic liquids, the facilitation of catalyst separation and recycling from reaction system, lower contamination of product, and the ability of using in gas phase reactions.19 In 2007, Sugimura et al.8 investigated the copolymerization of 1-vinylimidazolium based acidic ionic liquid with styrene and its use as effective and reusable catalyst for acetal formation under mild reaction condition. In 2011, Miao et al.17 reported acetalization of different aldehydes with alcohols catalyzed by SG-[(CH2)3SO3H-HIM]HSO4. The yields range from 85% to 98.5% under condition of 4 wt% catalyst amount, 110 °C. In 2004, a novel micropore zeolite MOR supported acidic ionic liquids was prepared.20 It possesses an excellent performance for ketalization of cyclohexanone with glycol. The conversion of cyclohexanone can reach 69.5%.
More recently, it has reported on the preparations of a series of organic–inorganic hybrid mesoporous materials incorporated with ionic liquids.21 The commonly used solid supports may be subdivided into resins, nanotubes, fibers, zeolites and mesoporous silicas (MSs).22 Mesoporous materials possess the excellent characteristics of stable mesoporous structure, high surface area, controllable pore size, and thermal stability, which make them attractive supports for the preparation of immobilized ionic liquid catalyst.23 Mesoporous materials, mostly ordered mesoporous molecular sieves (MCM-41, SBA-15), have been used as efficient catalysts and supports for many catalytic reactions.24–29 As an appealing type of mesoporous material, SBA-15 has relatively large specific surface area, controlled pore size and pore volumes. Besides, the abundant hydroxyl group on surface of SBA-15 could render it convenient for chemical modification and suitable for specific catalytic applications. Furthermore, the surface of the molecular sieves are sensitive to hydrothermal treatment, and the hydrothermal treatment could be used to activate the surface of SBA-15 and increase its capacity for grafting. Additives NaCl, KCl and NaF etc. are usually used to moderate the wall thickness and the degree of polymerization during post-synthesis hydrothermal treatments of SBA-15.30,31 All these make SBA-15 a potential candidate to act as suitable supports.32–35
In these papers, a series of heterogeneous catalysts, mesoporous materials SBA-15 and amorphous SiO2 immobilized SO3H-functionalized ionic liquid was synthesized. And the surface properties of the obtained catalysts were characterized by elemental analysis, Fourier transform infrared (FT-IR) spectra, scanning electron microscopy (SEM), thermogravimetry/differential scanning calorimetry (TG/DSC) and N2 adsorption–desorption isotherms (BET). Moreover, the effects of reaction parameters such as reaction time, reaction temperature and catalyst loading were studied and the recyclability of the catalyst was also examined. A general reaction mechanism for ketalization of cyclohexanone with glycol was given.
2. Experimental
2.1. Material
Silica gel (35–60 mesh) was purchased from Sigma-Aldrich. SBA-15 was purchased from Nanjing XFNANO Materials Tech Co., Ltd. 3-Chloropropyltriethoxysilane (CPES, purity ≥ 98%), 1,4-butane sultone (purity ≥ 99%) and 1-methylimidazole (purity ≥ 99%) was purchased from Aladdin Reagent Co, Ltd (Shanghai, China). Toluene, anhydrous ethanol, ethyl ether, anhydrous methanol, acetone, sulfuric acid were purchased from Rianlon Chemical Co., Ltd. Cyclohexanone and toluene were dried according to standard operations and stored over 4 Å molecular sieves. Other reagents were of analytical grade and used without any further purification.2.2. Synthesis of [BSmim][HSO4]
[BSmim][HSO4] was synthesized and purified according to the literature.36,37 1,4-Butane sultone (27.23 g, 0.2 mol) was dissolved in toluene, and 1-methylimidazole (16.42 g, 0.2 mol) was added dropwisely. The mixture was stirred at 60 °C for 8 h under vigorous stirring to obtain the white precipitate. The generated white solid zwitterion was filtrated and washed with toluene for three times. After dried in vacuum to remove unreacted materials, the zwitterion was mixed with H2SO4 in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in anhydrous toluene, and stirred magnetically at 60 °C for 8 h. Finally, the mixture was dried in vacuum to form corresponding BAILs.
1 in anhydrous toluene, and stirred magnetically at 60 °C for 8 h. Finally, the mixture was dried in vacuum to form corresponding BAILs.
2.3. Synthesis of SBA-15 immobilized ionic liquid
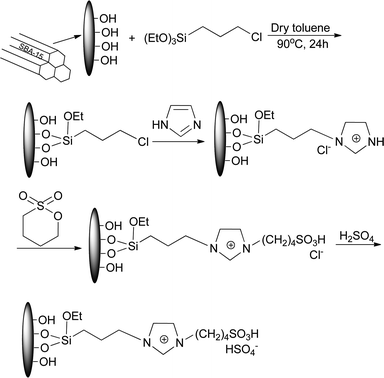
SBA-15 supported acidic ionic liquid was synthesized according to the literature.38 Activated SBA-15 (5 g) was suspended in 50 ml dry toluene and an excess of 3-chloropropyl-triethoxysilane (6 g) was added. The mixture was reacted under stirring for 24 h at 90 °C. Upon the reaction was completed, the products was collected by filtration and washed with toluene and ethyl ether in turn for three times. After dried under vacuum at 70 °C for 8 h, SBA-15-Cl was obtained as a white powder.Subsequently, SBA-15-Cl (all product obtained from the last step) was placed in a reaction flask containing 50 ml toluene and an excess of imidazole (3 g) was added. The mixture was refluxed with stirring for 24 h at 90 °C. Then the products was disposed in the same way as the last step to acquire intermediate [SBA-15-im]Cl. The obtained [SBA-15-im]Cl and 1,4-butane sultone (4.0 g) were added in a 200 ml flask containing 50 ml toluene. The mixture was reacted with magnetic stirring for another 24 h at 100 °C, to undergo a condensation reaction to form the silica chemically bonded sultone. After the same treatment, [SBA-15-Bs-im]Cl was dried under vacuum at 60 °C for 8 h.
Finally, [SBA-15-Bs-im]Cl was added in a round-bottom flask containing 100 ml 1,2-dichloromethane. 1,2-Dichloromethane diluted 2.5 g of sulphuric acid was slowly added into the flask at 0 °C with stirring. The mixture was gradually heated to room temperature and reacted for 48 h. Then, the mixture was washed and filtered with 1,2-dichloromethane and ethyl ether until the pH of the filtrate reached 7.0. The prepared SBA-15 immobilized ionic liquid BAIL@SBA-15 was dried under vacuum at 60 °C for 12 h to remove the residual volatiles and moisture before use (Scheme 1).
2.4. Catalyst characterization
Elemental analysis was performed on an Elementar Vario EL cube analyzer (Elementar Analysensysteme GmbH, Germany) and the grafting amount of ILs on supports was determined by the contents of the N element. Fourier transform infrared (FT-IR) spectra in the frequency range of 4000–400 cm−1 were measured at room temperature on a Nexus 870 spectrometer (Nicolet Instruments Co., USA). Thermogravimetry/differential scanning calorimetry (TG/DSC) was performed on a Netzsch Model STA 449 F3 simultaneous thermal analyzer (Netzsch, Germany) in an air atmosphere. The shape and surface morphology of the catalysts were examined via scanning electron microscopy (SEM) (Model SU8020, Hitachi, Japan). N2 adsorption–desorption isotherms were recorded using a TristarII 3020 instrument (Micromeritics, USA) and pore size distribution curves were calculated from the analysis of desorption branch of the isotherm by the BJH (Barrett–Joyner–Halenda). Before adsorption, samples were degassed at 473 K for 4 h.2.5. Catalytic activity evaluation
Catalytic activity experiments were conducted in a 25 ml round-bottom flask. Immobilized ILs, cyclohexanone and glycol were added quantitatively into the reactor successively. The mixture was heated to a desired temperature and stirred at a speed of 600 rad min−1. Upon reaction completion, the qualitative analysis of products was performed by Agilent 7980A/5975C GC-MS. The products were quantified by GC Smart equipped with a hydrogen flame ionization detector (FID). A capillary column ZB-WAX (30 m × 0.25 mm × 0.25 μm) was used to determine the composition of the samples with nitrogen as the carrier gas at a flow rate of about 3 ml min−1. 1,2-Dichloroethane was used as the internal standard for the quantitative analysis of the products.3. Results and discussion
3.1. FT-IR
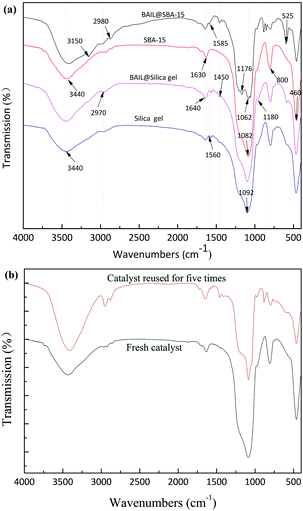
To confirm the immobilization of active component on the mesoporous structure, FT-IR spectroscopic studies were carried out in the frequency range of 4000–400 cm−1. The FT-IR spectra of molecular sieve SBA-15, amorphous SiO2 and immobilized ionic liquids catalysts were shown in Fig. 1(a), respectively. As can be seen from the spectra of BAIL@SBA-15, the characteristic peaks of [BSmim][HSO4] around 3150 cm−1, 2970 cm−1, 1571 cm−1, and 1176 cm−1, 1058 cm−1, 626 cm−1, 525 cm−1 could be clearly observed. They were ascribed to N–H, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the imidazole ring, and S
N stretching vibrations of the imidazole ring, and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching vibrations of the SO3H group and hydrogen sulfate, respectively.39,40 The typical peaks of Si–O–Si on SBA-15 supported ionic liquids could be observed around 460 cm−1, 769 cm−1 and 954 cm−1 and the strong absorbance at 3440 cm−1 and 1630 cm−1 corresponds to the stretching frequency of physical adsorbed water. Hence, the above results confirm that the ILs were successfully immobilized onto the supports by covalent bonds.
O symmetric stretching vibrations of the SO3H group and hydrogen sulfate, respectively.39,40 The typical peaks of Si–O–Si on SBA-15 supported ionic liquids could be observed around 460 cm−1, 769 cm−1 and 954 cm−1 and the strong absorbance at 3440 cm−1 and 1630 cm−1 corresponds to the stretching frequency of physical adsorbed water. Hence, the above results confirm that the ILs were successfully immobilized onto the supports by covalent bonds.
 | ||
| Fig. 1 Fourier transform infrared spectra of (a) molecular sieve and supported catalysts; and (b) the fresh and five times reused catalysts. | ||
3.2. SEM
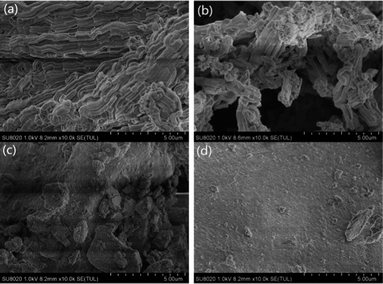
Fig. 2 shows the scanning electron microscopy (SEM) images of the samples. It can be seen from the pictures that samples are amorphous and accumulations of small particles. Compared with the catalyst in Fig. 2(b), the patent material in Fig. 2(a) is smother than the former because of immobilization of the IL. And the ionic liquid [BSmim][HSO4] had been immobilized on the surface of SBA-15.3.3. N2 adsorption–desorption
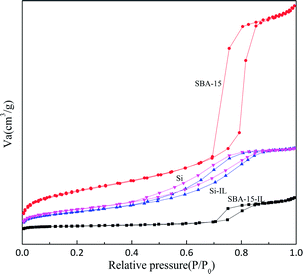
The N2 adsorption–desorption isotherms and pore size distribution of the mesoporous materials and catalysts are depicted in Fig. 3. The textural properties such as the BET surface areas, pore diameter and pore volume derived from the N2 adsorption–desorption measurements are included in Table 1. It is clear that the obtained isotherms are similar to type IV isotherm curve with H1-type hysteresis loop, characteristic of highly ordered mesoporous materials according to the IUPAC classification. Adsorption data were obtained over a relative pressure ranging from 0.01 Pa to 0.99 Pa. The BAIL@SBA-15 exhibits a hysteresis loop and a sharp increase in pore volume adsorbed above a relative pressure (P/P0) at 0.7 Pa.| Samples | BET surface area, SBET (m2 g−1) | Pore volume, Vp (cm3 g−1) | Dp (Å) | Bonding amount (mmol g−1) |
|---|---|---|---|---|
| SBA-15 | 541.8 | 1.3 | 97.2 | 0 |
| BAIL@SBA-15 | 42.9 | 0.2 | 125.2 | 1.5342 |
| Silica gel | 297.1 | 0.5 | 65.0 | 0 |
| BAIL@Silica gel | 262.9 | 0.4 | 73.8 | 0.4418 |
The physical properties of molecular sieve, silica gel and immobilized catalysts are shown in Table 1. Compared to parent mesoporous materials, the surface area of SBA-15 decreased from 541.8 m2 g−1 to 42.9 m2 g−1 with the increasing of pore size from 9.7 nm to 12.5 nm. This is attributed to the immobilization of BAIL onto the framework of molecular sieve. The immobilization of ionic liquids blocked a small fraction of the pores, which leads to the increasing of the average pore size and the aggregation of adjacent plate-like particles. The surface modification is probably a multi-layered process, in which additional ILs molecules interact with those covalent attached to the supports surface. From all the above surface analysis data, it is confirmed that the 3-chloropropyl-triethoxysilane and ionic liquid is modified into the framework of the molecular sieve and amorphous silica.
3.4. TG-DSC
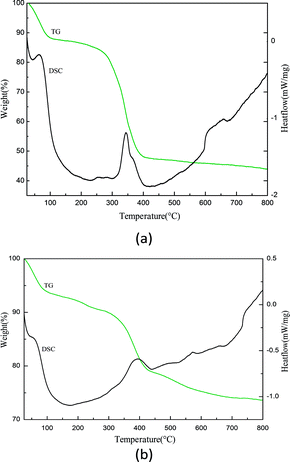
The TG-DSC analysis was employed to investigate the thermal stability of the catalysts. The thermograms of the samples are presented in Fig. 4. The thermogravimetric (TG) curve of BAIL@SBA-15 showed an initial weight loss of ∼10% up to 100 °C, which is attributed to the release of physisorbed water and residual solvent in supported catalysts. A significant weight loss of ∼30% was found in the temperature range of 200–400 °C because of the organic components of ionic liquid and the propyl of 3-chloropropyltriethoxysilane separated from the surface of support. The peaks in the DSC curve also prove this process. Therefore, the catalysts can be operated stably under 100 °C (Table 1).3.5. Ketalization of cyclohexanone with glycol
The catalytic activity of several supported ILs were evaluated on the ketalization of cyclohexanone with glycol under the optimized reaction condition and the results were listed in Table 2. It can be observed that the molecular sieve SBA-15 as catalyst has 22.6% conversion of cyclohexanone (CYC), which demonstrates molecular sieve SBA-15 has relatively poor catalytic activity (Table 2, entry 4). With the successful immobilization of ionic liquid [Bsmim][HSO4] on molecular sieve, the BAIL@SBA-15 gave a drastic increase in the catalytic activity and the conversion of cyclohexanone was 85.2% (Table 2, entry 1). However, there is little inferior to the corresponding homogeneous catalyst of [BSmim][HSO4] (Table 2, entry 3) under the same condition. The heterogeneous catalyst BAIL@SBA-15 can remain high catalytic performance, even though the catalyst loading content of Brønsted acidic ionic liquid was obviously less than the pure BAIL. Therefore, the molecular sieve SBA-15 plays a significant roles on the immobilized catalyst. The cooperative pathway of catalyst system enhances the conversion of cyclohexanone in the ketalization reaction. It could also be found that the SBA-15 supported acidic ionic liquid has much higher activity than the support materials of silica gel.| Entry | Catalyst | Conversion of CYC/(%) | Selectivity/(%) |
|---|---|---|---|
| a Reaction conditions: cyclohexanone (30 mmol), glycol (60 mmol), catalyst (1.3%, based on the mole ratio of cyclohexanone), reaction temperature (50 °C), reaction time 3 h.b The molar ratio of [BSmim][HSO4] is 10%. | |||
| 1 | BAIL@SBA-15 | 85.2 | 100 |
| 2 | BAIL@Silica gel | 79.1 | 100 |
| 3 | [BSmim][HSO4]b | 88.7 | 100 |
| 4 | SBA-15 | 22.6 | 100 |
| 5 | Blank | 0.5 | 100 |
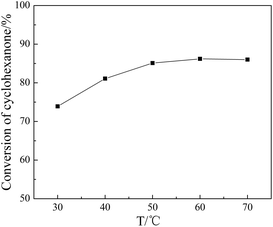
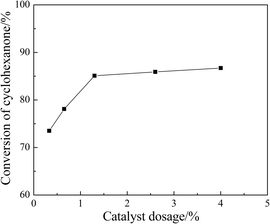
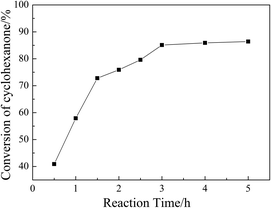
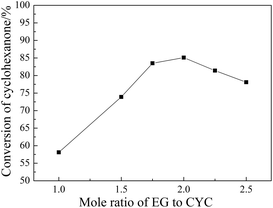
3.6. Influence of reaction parameters on the ketalization
SBA-15 supported acidic ionic liquid was selected as catalyst in the ketalization reaction to optimize the reaction condition since BAIL@SBA-15 showed excellent catalytic activity. The effect of reaction parameters such as reaction temperature, reaction time, catalyst loading, reactant molar ratio were investigated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CYC increased from 1
CYC increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion of cyclohexanone was enhanced from 58.1% to 85.1%. However, further increase of the molar ratio to 2.5
1, the conversion of cyclohexanone was enhanced from 58.1% to 85.1%. However, further increase of the molar ratio to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in a decrease in conversion due to the reduction of catalyst concentration . Thus the most suitable molar ratio of EG
1 resulted in a decrease in conversion due to the reduction of catalyst concentration . Thus the most suitable molar ratio of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CYC was 2
CYC was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.7. Mechanism
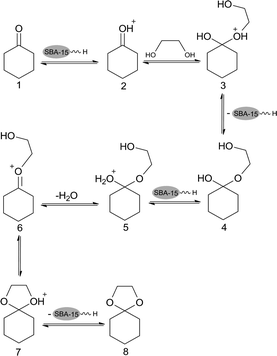
The possible mechanism for hydrogen bond between anion of ionic liquid and support was shown in Scheme 2. The supported ionic liquids catalyst system is likely operated in a cooperative pathway. Cation of ionic liquid was firmly immobilized through the covalent bonds to the surface of the supports and the anion was also not easily lost because of ionic bonding with cation and hydrogen bonding with supports. Therefore, the supported ionic liquid can be reused for several times with small loss in the selectivity and cyclohexanone conversion.Ketalization is a reversible reaction and the possible two-step mechanism for ketalization of cyclohexanone with glycol over BAIL@SBA-15 was consistent with the reported ones.17,41 Scheme 3 envisages the mechanism of the ketalization of cyclohexanone with glycol using SBA-15 supported ionic liquid. In the first step, the cyclohexanone is protonated by the acid site of BAIL@SBA-15 (H+ ions of the catalyst) to produce the intermediate 2 and then activated for nucleophilic addition of O atom of the alcohol to form the hemiacetal 4. Protonation of 4 leads to intermediate 5 which undergoes subsequent dehydration to give 6. It accepted a second molecule of alcohol hydroxyl group to give intermediate 7. This step is also an exothermic reaction, and last, removal of a proton from 7 leads to the formation of the ketal 8.
3.8. Recycling of catalyst
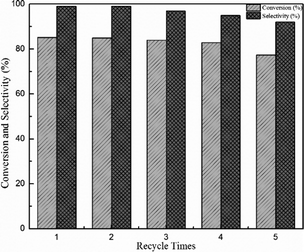
The recovery of catalyst is highly preferable from both environmental and economic points of view. The reusability of the SBA-15 supported ionic liquid was investigated in the ketalization of cyclohexanone with glycol, and the result was shown in Fig. 9. In each cycle, the catalyst was easily recovered by centrifugation and washed with dichloromethane for three times, followed by drying under vacuum before the next run. As shown in Fig. 9, the catalyst BAIL@SBA-15 can be readily recovered and reused five times without significant loss of activity. And the conversion and selectivity were 77.4% and 92.0%, respectively.The recovered catalyst that reused for five times has no obvious change in structure, referring to the FT-IR spectrum in comparison with fresh catalyst listed in Fig. 1(b). Measuring by elemental analysis, the grafting amount of ionic liquid on BAIL@SBA-15 decreased from 1.5342 mmol g−1 to 0.4820 mmol g−1 after being used for 5 times. It was found that the loss of anion HSO4− may be the main reason for the decrease in catalytic activity.
4. Conclusions
In summary, a series of mesoporous materials SBA-15 and amorphous SiO2 supported acidic ionic liquids were synthesized by chemical covalent bond. On the basis of characterization results, the Brønsted acidic ionic liquid [BSmim][HSO4] was proved to be successfully immobilized onto mesoporous materials and the results show that BAIL@SBA-15 is more active in the ketalization reaction. The conversion of cyclohexanone can reach 85.2% and the amount of catalyst is reduced greatly compared with pure ionic liquids. The experiment demonstrated that the optimum reaction conditions for the ketalization of cyclohexanone with glycol is 1.3% of BAIL@SBA-15 as catalyst, initial molar ratio of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CYC was 2 and reaction temperature is 50 °C for 3 h. The possible mechanism for hydrogen bond between anion of ionic liquid and support and general reaction mechanism for ketalization reaction was given. Furthermore, the supported ionic liquids can be easily recovered for five times without a significant loss of catalytic activity.
CYC was 2 and reaction temperature is 50 °C for 3 h. The possible mechanism for hydrogen bond between anion of ionic liquid and support and general reaction mechanism for ketalization reaction was given. Furthermore, the supported ionic liquids can be easily recovered for five times without a significant loss of catalytic activity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 21473225).Notes and references
- Y. Y. Jiang, G. N. Wang, Z. Zhou, Y. T. Wu, J. Geng and Z. B. Zhang, Chem. Commun., 2008, 505–507 RSC.
- D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Gratzel, Angew. Chem., 2008, 47, 1923–1927 CrossRef CAS PubMed.
- A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed.
- D. Fang, K. Gong, Q. Shi and Z. Liu, Catal. Commun., 2007, 8, 1463–1466 CrossRef CAS.
- H. Xing, T. Wang, Z. Zhou and Y. Dai, J. Mol. Catal. A: Chem., 2007, 264, 53–59 CrossRef CAS.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459–2477 CrossRef CAS.
- A. C. Cole and J. L. Jensen, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed.
- R. Sugimura, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2007, 8, 770–772 CrossRef CAS.
- H. H. Wu, F. Yang, P. Cui, J. Tang and M. Y. He, Tetrahedron Lett., 2004, 45, 4963–4965 CrossRef CAS.
- X. Liang and C. Qi, Catal. Commun., 2011, 12, 808–812 CrossRef CAS.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- P. Wasserscheid, M. Sesing and W. Korth, Green Chem., 2002, 4, 134–138 RSC.
- J. P. B. Atef Arfan, Org. Process Res. Dev., 2005, 9, 743–748 CrossRef.
- S. Sahoo, P. Kumar, F. Lefebvre and S. B. Halligudi, Appl. Catal., A, 2009, 354, 17–25 CrossRef CAS.
- C. Feher, E. Krivan, J. Hancsok and R. Skoda-Foeldes, Green Chem., 2012, 14, 403–409 RSC.
- W. Chen, Y. Zhang, L. Zhu, J. Lan, R. Xie and J. You, J. Am. Chem. Soc., 2007, 129, 13879–13886 CrossRef CAS PubMed.
- J. Miao, H. Wan, Y. Shao, G. Gua and B. Xu, J. Mol. Catal. A: Chem., 2010, 348, 77–82 CrossRef.
- P. Sharma and M. Gupta, Green Chem., 2015, 17, 1100–1106 RSC.
- Z. Wu, Z. Li, G. Wu, L. Wang, S. Lu, L. Wang, H. Wan and G. Guan, Ind. Eng. Chem. Res., 2014, 53, 3040–3046 CrossRef CAS.
- Z. M. Li, Y. Zhou, D. J. Tao, W. Huang, X. S. Chen and Z. Yang, RSC Adv., 2014, 4, 12160–12167 RSC.
- H. Jin, M. B. Ansari and S. E. Park, Catal. Today, 2015, 245, 116–121 CrossRef CAS.
- M. Wysocka-Zolopa, I. Zablocka, A. Basa and K. Winkler, Chem. Heterocycl. Compd., 2017, 53, 78–86 CrossRef CAS.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed.
- W. Xie and C. Zhang, Food Chem., 2016, 211, 74–82 CrossRef CAS PubMed.
- B. Lebeau, A. Galarneau and M. Linden, Chem. Soc. Rev., 2013, 42, 3661–3662 RSC.
- K. A. Shah, J. K. Parikh and K. C. Maheria, Catal. Today, 2014, 237, 29–37 CrossRef CAS.
- H. Zhao, N. Yu, Y. Ding, R. Tan, C. Liu, D. Yin, H. Qiu and D. Yin, Microporous Mesoporous Mater., 2010, 136, 10–17 CrossRef CAS.
- S. Udayakumar, S. W. Park, D. W. Park and B. S. Choi, Catal. Commun., 2008, 9, 1563–1570 CrossRef CAS.
- Z. Alothman, Materials, 2012, 5, 2874–2902 CrossRef CAS.
- F. Zhang, Y. Yan, H. Yang, Y. Meng, C. Yu, B. Tu and D. Zhao, J. Phys. Chem. B, 2005, 109, 8723–8732 CrossRef CAS PubMed.
- C. Pirez, A. F. Lee, J. C. Manayil, C. M. A. Parlett and K. Wilson, Green Chem., 2014, 16, 4506–4509 RSC.
- J. N. Appaturi, M. R. Johan, R. J. Ramalingam and H. A. Al-Lohedan, Microporous Mesoporous Mater., 2018, 256, 67–74 CrossRef CAS.
- D. Zhao, J. Sun, Q. Li and G. D. Stucky, Chem. Mater., 2000, 12, 275–279 CrossRef CAS.
- J. Qin, B. Li and D. Yan, Crystals, 2017, 7, 89–100 CrossRef.
- K. Arya, D. S. Rawat and H. Sasai, Green Chem., 2012, 14, 1956–1963 RSC.
- W. Wang, L. Shao, W. Cheng, J. Yang and M. He, Catal. Commun., 2008, 9, 337–341 CrossRef CAS.
- Y. Gu, F. Shi and Y. Deng, Catal. Commun., 2003, 4, 597–601 CrossRef CAS.
- J. Yang, T. Zeng, D. Cai, L. Li, W. Tang, R. Hong and T. Qiu, Asia-Pac. J. Chem. Eng., 2016, 11, 901–909 CrossRef CAS.
- K. Qiao, H. Hagiwara and C. Yokoyama, J. Mol. Catal. A: Chem., 2006, 246, 65–69 CrossRef CAS.
- Z. Xu, H. Wan, J. Miao, M. Han, C. Yang and G. Guan, J. Mol. Catal. A: Chem., 2010, 332, 152–157 CrossRef CAS.
- B. Thomas, S. Prathapan and S. Sugunan, Microporous Mesoporous Mater., 2005, 80, 65–72 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |