Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of ZnO@TiO2 nanotubes heterostructured film by thermal decomposition and their photocatalytic performances†
Yulong Liao *ab,
Kaibin Zhanga,
Xiaoyi Wanga,
Dainan Zhanga,
Yuanxun Lia,
Hua Sua,
Huaiwu Zhanga and
Zhiyong Zhonga
*ab,
Kaibin Zhanga,
Xiaoyi Wanga,
Dainan Zhanga,
Yuanxun Lia,
Hua Sua,
Huaiwu Zhanga and
Zhiyong Zhonga
aState Key Laboratory of Electronic Thin Film and Integrated Devices, University of Electronic Science and Technology of China, Chengdu 610054, China. E-mail: yulong.liao@uestc.edu.cn; Fax: +86-028-83202556; Tel: +86-028-83201440
bCenter for Applied Chemistry, University of Electronic Science and Technology of China, Chengdu 610054, China
First published on 20th February 2018
Abstract
TiO2 nanotubes (NTs) arrays prepared by anodic oxidation were modified with ZnO particles and their morphology and photocatalytic properties were investigated. A simple thermal decomposition process was involved in the modification method. Zinc acetate solution was filled into the TiO2 NTs arrays, and ZnO@TiO2 heterojunction films were formed after the thermal treatment. The morphology and catalytic properties of the heterojunction films could be manipulated by the concentration of zinc acetate solution. Compared to TiO2 NTs arrays, the ZnO@TiO2 heterojunction films with an optimized concentration of zinc acetate showed enhanced catalytic performances. Their photocatalytic activities were discussed with respect to the formation of ZnO@TiO2 heterojunctions and enforced charge separation. This study demonstrates a simple method to prepare ZnO nanoparticles@TiO2 NT heterojunction films, which is promising for other environmental and energy related applications.
Introduction
In recent years, the environment has been deteriorated due to pollution, while energy innovation is imminent. With such a trend, nanostructured semiconductors have scope for development and are in demand.1,2 Nanoscale metal oxides can be applied in numerous areas, such as in clean and recyclable energy.3–6 Among the nano-metal oxides, ZnO and TiO2 were widely adopted as photoelectric material and applied in the photovoltaic (PV) and photoelectrochemical (PEC) methods. TiO2 can not only be used as a catalyst for the treatment of water pollution, but also as a new type of solar cell materials.7,8 Ordered TiO2 array can produce higher charge collection efficiency owing to a direct conduction path for electrons when used as photo electrodes.9–11 In order to avoid recycling problems, TiO2 nanomaterials in powder form were gradually being eliminated, while TiO2 NTs have attracted increasing attention. TiO2 NTs arrays have several advantages as photocatalysts, such as simple synthetic craft, cheap cost and high stability.12,13 Nanotubes have a greater specific surface area than bulk materials and therefore, have a higher adsorption capacity. In particular, if these NTs can be filled with inorganic, organic, metal or magnetic nanoparticles in nano-scale, it will greatly improve their photoelectric, electromagnetic, and catalytic performances. In the field of pollution monitoring, metal oxides such as TiO2 and ZnO have received the attention of researchers due to their attractive photocatalytic performance. Pure TiO2 NTs have limited photocatalytic activity without visible light response, as reported in former studies,14,15 owing to their wide band gap (Eg ∼ 3.3 eV) and high recombination rate of photogenerated electron–hole pairs.16–20 TiO2 NTs need to be decorated or modified to achieve better performance. ZnO stands out as a modifier material. ZnO is one of the most promising and demanding functional materials for its good flexibility in synthesis and morphology.21–23For photocatalytic reaction, the conduction band edge of ZnO is more negative than TiO2, which makes ZnO an ideal material for enhancing the photoelectrochemical performance by coupling with TiO2.24–26 ZnO and TiO2 have similar bandgap energies (TiO2 ∼ 3.3 eV, ZnO ∼ 3.4 eV). Their level structures are mutually satisfied for constitution of heterogeneous structural materials.27–29 Heterogeneous ZnO@TiO2 nano-composites have exhibited excellent performances partly due to the high reactivity of TiO2 and the high electron mobility of ZnO, which improve the process of electrons and holes transfer between the corresponding conduction and valence bands.30–34 Compared to pure TiO2 NTs arrays, heterogeneous ZnO@TiO2 NTs arrays are expected to have a greater light-response and higher collection efficiency.
Thus far, ZnO@TiO2 heterojunctions have been successfully prepared by hydrothermal process,35 spin coating,36 electrospinning and sol–gel processes.37 In this study, a new synthesis approach is proposed: TiO2 NT micro-containers template thermal decomposition method. TiO2 NTs arrays were prepared by anodic oxidation method and filled with various concentrations of zinc acetate solution. This method is found to be extremely simple and efficient. The TiO2 nanotubes simply need to be immersed in zinc acetate solution; then, the acetate is volatilized after annealing and ZnO nanoparticles are formed inside the TiO2 NT micro-containers. The morphology and composition of the obtained ZnO@TiO2 heterojunction films were investigated by SEM (scanning electron microscopy), TEM (transmission electron microscopy), and XRD (X-ray diffraction). The photocatalytic performances of ZnO@TiO2 heterojunction films were also further evaluated by degradation of methyl orange solution.
Experimental section
2.1 Fabrication of TiO2 NTs arrays
The titanium dioxide nanotubes were prepared by anodic oxidation reaction38,39 on pure titanium sheets with the size of 1.5 cm × 5 cm. The cut sheets were cleaned and soaked in ethanol for sonication. Electrolyte is required for the electrochemical reaction. As the reaction medium, the electrolyte must have corrosion property and the capability to provide oxygen ions. The electrolyte solution consists of 98% by volume of ethylene glycol and 2% by volume of deionized water with the addition of 0.3% by mass of ammonium fluoride. Two titanium sheets were selected as anode and cathode, which were connected to the positive and negative electrodes of the power supply, respectively. At the same time, the two electrodes separated by 2.5 cm were immersed in the electrolyte solution. The constant voltage between the two titanium sheets was 55 V and the reaction lasted for 2 h. Magnetic stirring was maintained during the reaction. TiO2 NTs arrays grew on the anode titanium sheet. After the reaction, TiO2 NTs arrays were rinsed and soaked in alcohol for 12 h. The nanotubes were dried in an oven at 80 °C. Then, it was annealed in a sintering furnace at a heating rate of 2 °C per minute and held for two hours after reaching 450 °C.2.2 Fabrication of ZnO@TiO2 heterojunction films
TiO2 NTs arrays with metal oxide modification can achieve better performance. ZnO was selected as the metal oxide for modification in this study. The synthesis method is simple and efficient, which mainly involves a thermal decomposition process, which is schematically shown in Fig. 1. Zinc acetate was dissolved in deionized water at a concentration gradient of 0.2 mol L−1, 0.4 mol L−1, 0.6 mol L−1, 0.8 mol L−1, and 1.0 mol L−1. The prepared TiO2 NTs arrays were immersed in different concentrations of zinc acetate solution, while a large number of bubbles appeared on the film, which indicated that the solution entered the tube. After the bubbles disappeared, the sheets were taken out. The soaked TiO2 NTs arrays were gently wiped with a filter paper to remove the excess solution (if the excess solution on the titanium sheet is not removed, zinc oxide may cover the surface of the NTs arrays and thus affect their photocatalytic performance). The subsequent step is annealing, at a temperature of 400 °C for 2 h, followed by natural cooling. As shown in Fig. 1, the TiO2 NTs were first used as containers to load the zinc acetate solution; then, they acted as nanoreactors for the thermal decomposition of zinc acetate. In the end, ZnO nanoparticles were formed inside those TiO2 NTs and finally, ZnO@TiO2 heterojunction films were obtained. Other experimental details are shown in ESI.† Different concentrations of zinc acetate solution were used during the fabrication process and thus, various ZnO@TiO2 heterojunction films were obtained. For the simplicity of presentation, we denoted the ZnO@TiO2 heterojunction films as ZnO-0.2 (0.4, 0.6, 0.8, and 1), which corresponds to the TiO2 NTs arrays film impregnated with 0.2 (0.4, 0.6, 0.8, and 1) mol L−1 zinc acetate solution.2.3 Photocatalytic performances of ZnO@TiO2 heterojunction films
The photocatalytic activity of ZnO@TiO2 heterojunction films were characterized by photodegradation of organic dyes in water. Methyl orange at a concentration of 10−4 mol L−1 was used the indicator for testing, which acts as an organic pollutant. The ZnO@TiO2 heterojunction films (cut to fixed size: 1.5 cm × 3 cm) were placed in a quartz cup filled with methyl orange solution (5 mL). They were magnetically stirred in the dark for 1 h to attain adsorption–desorption equilibrium. Then, they were irradiated by UV light with a power of 28 W and a light intensity of 10.5 mW cm−2. The methyl orange solution was sampled every half an hour. The above photodegradation tests were carried out on the heterojunctions separately. The concentration of the sampled methyl orange solution was measured using a spectrophotometer.2.4 Characterization
For the observation of TiO2 NTs morphology and structure, a scanning electron microscope (SEM, JSM-7000F, JEOL Inc. Japan) was used. For the determination of crystallization degree of TiO2 and the ZnO crystallization in heterojunctions, X-ray diffraction (XRD) technique was used. For the comparison between TiO2 NTs arrays' and ZnO@TiO2 heterojunction films' ability to degrade organic pollutants under ultraviolet light, a spectrophotometer (JASCO V-570 UV/VIS/NIR) was used.Results and discussion
TiO2 NTs generally do not exhibit effective photocatalytic activity if they are amorphous. Hence, in this study, the TiO2 NTs arrays film produced by anodic oxidation were annealed for crystallization before loading ZnO crystals. X-ray diffraction patterns of the pure TiO2 NTs arrays and the ZnO@TiO2 heterojunction films are shown in Fig. 2. It can be seen that the pure TiO2 NTs arrays were well crystallized. The diffraction peaks at 25.2°, 36.9°, 37.8°, 48.1°, 53.89°, 55.0°, 62.68°, 75.0°, and 76.0° were attributed to the (101), (103), (004), (200), (105), (211), (204) (215), and (301) planes of anatase phase, respectively. Moreover, the minor diffraction peaks at 40.1° and 53.0° were attributed to (101) and (102) planes of Ti, respectively. These results indicate that the TiO2 NTs arrays were crystallized into anatase phase before they were used as containers or reactors to load ZnO. Compared with pure TiO2, the diffraction peaks of ZnO were observed in ZnO@TiO2 heterojunction films. New peaks at 31.7°, 34.4°, and 36.2° could be assigned to the (100), (002) and (101) planes of hexagonal ZnO, respectively.40 The lattice parameters of the ZnO can be obtained by calculation. As a hexagonal structure, the lattice parameters of ZnO are calculated as: a = b = 0.3253 nm, c = 0.5129 nm which are close to the reported values: a = b = 0.3249 nm and c = 0.5206 nm.41 It can be concluded from the XRD results that zinc acetate was thermally decomposed inside the TiO2 NTs arrays and hence, the ZnO@TiO2 heterojunction films were successfully obtained. XRD patterns of various ZnO@TiO2 heterojunctions are shown in ESI Fig. S2.† When zinc acetate solution at low concentration was used, the ZnO particles in the nanotubes were low in number and the diffraction peaks were not easily observed. | ||
| Fig. 2 XRD pattern of TiO2 NTs arrays and ZnO@TiO2 heterojunctions. S1 stands for ZnO@TiO2 heterojunctions and S2 stands for TiO2 NTs arrays. | ||
Microscopic morphology of the TiO2 NTs can be well observed by SEM. The microstructure of pure TiO2 nanotubes and nanotubes modified with ZnO are shown in Fig. 3(a). The as-prepared pure TiO2 NTs arrays are closely ordered with minute voids between the tubes; the nozzles are spherical in shape and the diameter of the tubes is approximately the same with an average of about 80 nm. The tube wall is about 10 nm thick. The morphology of the TiO2 NTs arrays film impregnated with low concentration zinc acetate solution has hardly been affected. At a concentration of 0.2 mol L−1 zinc acetate solution, as shown in Fig. 3(b), the red circles indicate the ZnO decorative particles, which are coated along the tube wall at the part of the nozzle. The open tube-mouth morphology is not affected. With the increase in concentration of zinc acetate, the nozzles of the TiO2 NTs are occupied by ZnO blocks and the nozzle area became smaller. When the concentration of zinc acetate solution was 0.6 mol L−1, it can be observed from Fig. 3(c) that most of the nanotubes were wide open, some nanotubes were covered, and the ZnO particles aggregated to form large blocks deposited around the nozzles. Fig. 3(d) shows that the tubular structure is almost invisible and replaced by the mesh structure and attached ZnO particles when the concentration of zinc acetate was 1 mol L−1. The above SEM results indicate that the ZnO@TiO2 heterojunctions can be prepared by a simple thermal decomposition method and the ZnO particles were directly observed through SEM. The loading amount of ZnO is directly related to the increase in the concentration of zinc acetate. With an appropriate amount of ZnO particles, the morphology of TiO2 nanotubes will not be affected. However, on continuously increasing the loading amount of ZnO, the nozzle was gradually filled until it was completely covered and the open-tube mouth morphology and tubular structure disappeared.
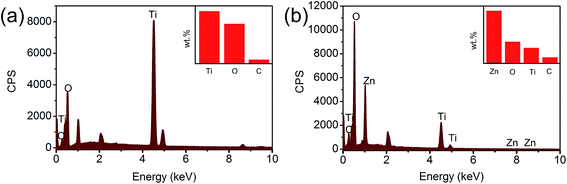
In order to further confirm the preparation of the ZnO@TiO2 heterojunction films, energy-dispersive spectrometer (EDS) was adopted for elemental analysis. Fig. 4(a) shows the EDS results of pure TiO2 NTs arrays. The characteristic peaks of only Ti, O and a small amount of C could be observed, indicating the formation of anatase TiO2 NTs. Fig. 4(b) shows the EDS results of the ZnO-0.8 heterojunction films, in which the characteristic peak of Zn is clearly observed. The inset of Fig. 4(b) is the calculated elements proportion, in which Zn is shown as the main element of the film since EDS is a surface analytic technology. More elemental descriptions are listed in ESI Tables S1 and S2.† The main elements in TiO2 nanotubes were Ti and O, whose atom percentages were 27.94% and 65.17%, respectively. In ZnO@TiO2 heterojunction, the atomic percentage of Zn reached 27% and the mass percentage reached 55%. Nevertheless, the EDS results confirm that ZnO were successfully loaded in the TiO2 NTs arrays.
The photocatalytic properties of the as-prepared ZnO@TiO2 heterojunction films were evaluated by degradation of methyl orange solution. The methyl orange solution acts as the probe organic pollutant with an initial concentration of 10−4 mol L−1 (C0). The heterojunction films were immersed into the methyl orange solution for photodegradation. Then, the methyl orange solution was sampled every 30 min. The concentration of methyl orange (labeled as C) was measured with the increase in degradation time. The decomposition degree of methyl orange can be expressed as C/C0. The environment for photodegradation of methyl orange is shown in ESI Fig. S1.† As shown in Fig. 5, methyl orange could also be subjected to photocatalytic degradation by the pure TiO2 NTs arrays since the TiO2 NTs arrays are essentially photocatalysts. For the ZnO@TiO2 heterojunction films, samples ZnO-0.2, ZnO-0.4, and ZnO-0.6 exhibited enhanced photocatalytic performances. In the first 60 min, the enhanced photocatalytic activities were in the order of sample ZnO-0.4, sample ZnO-0.2, and sample ZnO-0.6. After 90 min, sample ZnO-0.6 exhibited the best performance. Finally, after 120 min, all the above three samples had shown improved photocatalytic activities as compared to pure TiO2 NTs arrays. Moreover, the degradation kinetics, as shown in Fig. 5, indicated that samples ZnO-0.8 and ZnO-1 had deteriorated photocatalytic activities as compared to pure TiO2 NTs arrays.
 | ||
| Fig. 5 Degradation kinetics of methyl orange solution by TiO2 NTs films and various types of ZnO@TiO2 heterojunction films. | ||
The above photocatalytic results indicate that the ZnO@TiO2 heterojunction films have improved degradation performances than that of the pure TiO2 NTs arrays when an appropriate amount of ZnO nanoparticles were loaded (or an appropriate concentration of zinc acetate solution was used). The mechanism of the ZnO@TiO2 heterojunction films with enhanced photocatalytic degradation is demonstrated in Fig. 6. In particular, titanium dioxide (TiO2), a wide-band-gap semiconductor (∼3.2 eV), is useful for organic degradation. When TiO2 was appropriately irradiated, electron–hole pairs or excitons could be generated by incoming photons, which either migrate to the material surface or recombine and dissipate the energy as heat. Those on the surface can then participate in redox reactions (react with O2 to form O2−) and generate reactive oxygen species (ROS),42,43 while holes oxidize OH− to yield hydroxyl radicals (OH˙).44 These radicals play an important role in reacting with the methyl orange molecules and decompose them into CO2 and H2O. Before ZnO and TiO2 contact, the relative positions of their conduction bands and valence bands are shown in Fig. 6(b). ZnO and TiO2 have a similar bandgap value and only ultraviolet radiation can excite the electrons. When ZnO was loaded to the TiO2 NTs arrays and formed ZnO@TiO2 heterojunctions, photogenerated electron–hole separation occurs on both TiO2 and ZnO separately under ultraviolet light. The heterojunction between TiO2 and ZnO led to the crossing of their energy levels. The photogenerated electrons transferred from the ZnO conduction band to the TiO2 conduction band and the holes moved from TiO2 valence band to ZnO valence band as shown in Fig. 6(c). Photogenerated electrons will be separated in this case, while the recombination will be suppressed,45 resulting in accelerating the formation of ROS as shown in Fig. 6(a). These highly reactive ROS reacted with the methyl orange molecules and decomposed them into CO2 and H2O. Photocatalytic testing indicated that with an appropriate zinc acetate concentration (ZnO-0.2, ZnO-0.4, and ZnO-0.6), the role of ZnO modification is positive for the ZnO@TiO2 heterojunction films. SEM results have shown that the ZnO is attached only to the tube wall without clogging the nozzle. Compared with pure TiO2 NTs, the deteriorated activities of samples ZnO-0.8 and ZnO-1 were attributed to the excessive loaded ZnO nanoparticles. SEM results have also clearly shown that the overdose of ZnO crystals block the entire tube-mouth and surface of the TiO2 NTs arrays (see Fig. 3(d)). Without open-tube mouths, both the MO molecules transportation and photo-generated ROS diffusion will be restricted. Therefore, there should be a balance between ZnO loading mass and retaining the open tube-mouth configuration for achieving the best photocatalytic activity of the ZnO@TiO2 heterojunction films. Since the above discussion is consistent with XRD, SEM, EDS, and photocatalytic results, we believe that the optimized ZnO@TiO2 heterojunction films exhibited enhanced photocatalytic activities as compared to pure TiO2 NTs arrays.
Conclusions
Compact and ordered TiO2 NTs arrays were used as substrates and modified with a certain amount of ZnO crystalline particles. After a simple thermal decomposition process, a series of ZnO@TiO2 heterojunctions films were successfully prepared. The heterojunction obtained by this method is simple and efficient. The zinc acetate was transferred to the inner walls and periphery of the nanotubes when the TiO2 NTs arrays were used as nano-containers and nano-reactors. The morphological and crystal properties of the final ZnO@TiO2 heterojunctions films could be tuned by using different concentrations of zinc acetate. SEM results indicated that an excessive concentration of zinc acetate blocked the TiO2 NT mouth morphology, destroyed the tubular structures, and deteriorated the photocatalytic activities. The heterojunction obtained using zinc acetate with concentrations of 0.4 and 0.6 mol L−1 had a much improved photocatalytic effect as compared to TiO2 NTs arrays, which could be attributed to the separation of electron–hole pairs and the inhibition of recombination. The ZnO@TiO2 heterojunctions films prepared in this study are expected to be used as efficient recyclable photocatalysts or used in other environmental and energy related areas.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National R&D Program of China under No. 2017YFA0207400 & 2016YFA0300801, National Natural Science Foundation of China under No. 51502033, 61734002 & 61571079, International Cooperation Projects under Grant No. 2015DFR50870, and the Science and Technology Project of Sichuan Province No. 2017JY0002.References
- F. X. Xiao, ACS Appl. Mater. Interfaces, 2012, 4, 7054–7062 Search PubMed.
- Y. H. Chiu and Y. J. Hsu, Nano Energy, 2017, 31, 286–295 CrossRef CAS.
- W. T. Sun, Y. Yu, H. Y. Pan, X. F. Gao, Q. Chen and L. M. Peng, J. Am. Chem. Soc., 2008, 130, 1124–1125 CrossRef CAS PubMed.
- Z. H. Zhang, M. F. Hossain and T. Takahashi, Int. J. Hydrogen Energy, 2010, 35, 8528–8535 CrossRef CAS.
- M. K. Tian, W. J. Peng, B. W. Shang, W. L. Tao and Y. Xiao, Int. J. Hydrogen Energy, 2012, 37, 12827–12832 CrossRef CAS.
- Z. He, C. Kim, L. H. Lin, T. H. Jeon, S. Lin, X. C. Wang and W. Choi, Nano Energy, 2017, 42, 58–68 CrossRef CAS.
- Y. L. Liao, H. W. Zhang, W. X. Que, P. Zhong, F. M. Bai, Z. Y. Zhong, Q. Y. Wen and W. H. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 6463–6466 CAS.
- M. Y. Chen and Y. J. Hsu, Nanoscale, 2013, 5, 363–368 RSC.
- K. Zhu, N. R. Neale, A. Miedaner and A. J. Frank, Nano Lett., 2007, 7, 69–74 CrossRef CAS PubMed.
- W. Haotong and Y. Bai, Acta Polym. Sin., 2011, 939–949 Search PubMed.
- J. Y. Zhang and F. X. Xiao, J. Mater. Chem. A, 2017, 5, 23681–23693 CAS.
- Y. L. Xie, Z. X. Li, Z. G. Xu and H. L. Zhang, Electrochem. Commun., 2011, 13, 788–791 CrossRef CAS.
- Z. L. He, W. X. Que, P. Sun and J. B. Ren, ACS Appl. Mater. Interfaces, 2013, 5, 12779–12783 CAS.
- M. G. Hosseini, S. Zeynali, M. M. Momeni and R. Najjar, J. Appl. Polym. Sci., 2012, 124, 4671–4677 CAS.
- X. Y. Chen, J. H. Li, Z. H. Sun, X. Fang, Z. P. Wei, F. Fang, X. Y. Chu, S. Li and X. H. Wang, J. Alloys Compd., 2013, 571, 114–117 CrossRef CAS.
- M. M. Momeni, Y. Ghayeb and M. Davarzadeh, J. Electroanal. Chem., 2015, 739, 149–155 CrossRef CAS.
- Q. F. Gui, D. L. Yu, S. Y. Zhang, H. P. Xiao, C. Y. Yang, Y. Song and X. F. Zhu, J. Solid State Electrochem., 2014, 18, 141–148 CrossRef CAS.
- M. Hosseini, M. M. Momeni and M. Faraji, J. Appl. Electrochem., 2010, 40, 1421–1427 CrossRef CAS.
- P. Rai, S. M. Majhi, Y. T. Yu and J. H. Lee, RSC Adv., 2015, 5, 76229–76248 RSC.
- M. Mo, M. Zheng, J. S. Tang, Q. Lu and Y. Y. Xun, J. Mater. Sci., 2014, 49, 877–885 CrossRef CAS.
- J. P. Deng, M. Q. Wang, J. Liu, X. H. Song and Z. Yang, J. Colloid Interface Sci., 2014, 418, 277–282 CrossRef CAS PubMed.
- H. Y. Guan, W. P. Xu, X. Li, H. Peng, Y. Feng, J. Zhang and C. N. Li, Mater. Lett., 2016, 163, 69–71 CrossRef CAS.
- Y. H. Dong, T. Liu, X. Song, L. H. Dong, Z. W. Guo and Y. Y. Shen, Micro Nano Lett., 2014, 9, 552–555 CAS.
- F. Zhu, C. P. Li, M. N. Ha, Z. F. Liu, Q. S. Guo and Z. Zhao, J. Mater. Sci., 2016, 51, 4639–4649 CrossRef CAS.
- W. H. Lin, Y. H. Chiu, P. W. Shao and Y. J. Hsu, ACS Appl. Mater. Interfaces, 2016, 8, 32754–32763 CAS.
- Y. C. Chen, Y. C. Pu and Y. J. Hsu, J. Phys. Chem. C, 2012, 116, 2967–2975 CAS.
- T. G. Ulusoy, A. Ghobadi and A. K. Okyay, J. Mater. Chem. A, 2014, 2, 16867–16876 CAS.
- F. Kayaci, S. Vempati, C. Ozgit-Akgun, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 5735–5745 RSC.
- S. Hernandez, V. Cauda, A. Chiodoni, S. Dallorto, A. Sacco, D. Hidalgo, E. Celasco and C. F. Pirri, ACS Appl. Mater. Interfaces, 2014, 6, 12153–12167 CAS.
- A. K. Chakraborty and M. A. Kebede, J. Cluster Sci., 2012, 23, 247–257 CrossRef CAS.
- J. F. Lei, X. P. Li, W. S. Li, F. Q. Sun, D. S. Lu and Y. L. Lin, J. Solid State Electrochem., 2012, 16, 625–632 CrossRef CAS.
- A. Vomiero, G. Jimenez, C. Baratto, E. Comini, I. Concina, G. Faglia, M. Falasconi, M. Ferroni, I. Kholmanov, N. Poli, A. Ponzoni, S. Todros, G. Sberveglieri and IEEE, in 2009 34th IEEE Photovoltaic Specialists Conference, IEEE, New York, 2009, vol. 1–3, p. 841 Search PubMed.
- L. Zhang, H. B. Wu, B. Liu and X. W. Lou, Energy Environ. Sci., 2014, 7, 1013–1017 Search PubMed.
- F. X. Xiao and B. Liu, Nanoscale, 2017, 9, 17118–17132 RSC.
- M. Aliofkhazraei, N. Ali and P. Lund, Int. J. Hydrogen Energy, 2017, 42, 20868 CrossRef CAS.
- M. Y. Xu, H. L. Niu, J. J. Huang, J. M. Song, C. J. Mao, S. Y. Zhang, C. F. Zhu and C. L. Chen, Appl. Surf. Sci., 2015, 351, 374–381 CrossRef CAS.
- G. Marci, V. Augugliaro, M. J. Lopez-Munoz, C. Martin, L. Palmisano, V. Rives, M. Schiavello, R. J. D. Tilley and A. M. Venezia, J. Phys. Chem. B, 2001, 105, 1033–1040 CrossRef CAS.
- Y. B. Xie and D. G. Fu, J. Appl. Electrochem., 2010, 40, 1281–1291 CrossRef CAS.
- Z. L. He, W. X. Que, Y. C. He, J. X. Hu, J. Chen, H. M. A. Javed, Y. N. Ji, X. N. Li and D. Fei, Ceram. Int., 2013, 39, 5545–5552 CrossRef CAS.
- H. E. Byrne, W. L. Kostedt, J. M. Stokke and D. W. Mazyck, J. Non-Cryst. Solids, 2009, 355, 525–530 CrossRef CAS.
- A. N. Hattori, A. Ono and H. Tanaka, Nanotechnology, 2011, 22, 5 CrossRef PubMed.
- J. W. Zhou, M. Zhang and Y. F. Zhu, Phys. Chem. Chem. Phys., 2014, 16, 17627–17633 RSC.
- Y. B. Fu, L. D. Zhang and J. Y. Zheng, Sci. China, Ser. B: Chem., 2006, 49, 238–245 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- G. X. Song, F. Xin and X. H. Yin, J. Colloid Interface Sci., 2015, 442, 60–66 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13222k |
| This journal is © The Royal Society of Chemistry 2018 |