Open Access Article
Open Access ArticleOne-pot three component synthesis of 5-allyl-1,2,3-triazoles using copper(I) acetylides†
Parigi Raghavendar Reddy,
Lianji Cui and
Jae-Sang Ryu *
*
College of Pharmacy & Graduate School of Pharmaceutical Sciences, Ewha Womans University, 52 Ewhayeodae-gil, Seodaemun-Gu, Seoul 03760, Republic of Korea. E-mail: ryuj@ewha.ac.kr
First published on 12th January 2018
Abstract
One-pot three-component reactions using copper(I) acetylide, azide, allyl iodide, and NaOH have been developed. The reactions proceed smoothly at room temperature to afford 5-allyl-1,2,3-triazoles, which can be further transformed into a variety of 1,2,3-triazole-fused bi-/tricyclic scaffolds. This method offers the most efficient, convenient, and practical route towards useful polycyclic scaffolds in moderate to excellent yields.
Introduction
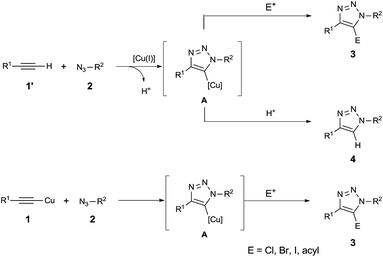
1,2,3-Triazoles1 are very important heterocycles in chemistry and biology.2 Synthetic molecules containing a 1,2,3-triazole scaffold exhibit diverse biological activities, which have drawn the attention of medicinal chemists in the drug discovery field. Currently, there are several 1,2,3-triazole-containing medicines on the market, and the number of potential pharmaceuticals based on these scaffolds keeps increasing.3 Beyond the drug market, 1,2,3-triazoles are utilized in a variety of areas including bioconjugation,4 polymer and materials science,5 and related areas6 including supramolecular chemistry,7 DNA labeling8 and oligonucleotide synthesis.9 Such wide applications of 1,2,3-triazoles are due to their facile synthesis through Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC), so-called ‘click chemistry’. Since it was first introduced by Sharpless and co-workers, CuAAC has been rapidly adopted as a universal coupling process. However, in terms of substrate scope, CuAAC is restricted to terminal alkynes, leading to 1,4-disubstituted 1,2,3-triazoles, and the one-pot synthetic methods for the fully substituted 1,2,3-triazoles are still relatively few.10In one-pot three-component reactions used to obtain 5-halo 1,2,3-triazoles, an electrophile X+ (X = Cl, Br, and I) is added into the CuAAC reaction to trap X+ with an in situ generated 5-copper(I) 1,2,3-triazole intermediate A (Scheme 1).11 However, the result is usually the formation of a mixture of the desired 5-substituted 1,2,3-triazole 3 and the byproduct 1,4-disubstituted 1,2,3-triazole 4, which is generated from a competitive protonation of the 5-copper(I) 1,2,3-triazole intermediate A.12 This competitive protonation is accelerated by a proton source, provided from the terminal alkyne substrate in the normal CuAAC. Therefore, this problem cannot be avoided in the presence of terminal alkyne substrates or protic polar solvents. Recently, this drawback was smartly overcome by using copper(I) acetylide instead of a terminal alkyne in the halogenation12 and acylation, by Y. Hu's group.13
 | ||
| Scheme 1 Cu(I)-catalyzed azide–alkyne cycloaddition methods for the synthesis of 5-substituted 1,2,3-triazoles. | ||
Copper(I) acetylides are highly crystalline polymeric complexes, (RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCu)n.14 They are stable to air, water, acid/base and heat15 so they can be kept on the shelf for several months without the quality deteriorating. In the absence of exogenous ligands or additives, copper(I) phenylacetylide, PhC
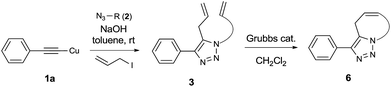
CCu)n.14 They are stable to air, water, acid/base and heat15 so they can be kept on the shelf for several months without the quality deteriorating. In the absence of exogenous ligands or additives, copper(I) phenylacetylide, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCu, is not effective under typical CuAAC conditions:13 It did not undergo a cycloaddition with azide and did not provide a 1,2,3-triazole product. However, when it was combined with both azide and acyl chloride, it quickly provided 5-acyl-1,2,3-triazoles.13 The presence of acyl chloride accelerated the cycloaddition of copper(I) phenylacetylide and azide.13 This intriguing result motivated us to investigate one-pot three-component reactions using copper(I) phenylacetylide. We are particularly interested in 5-allyl-1,2,3-triazoles as precursors for the development of new anticancer agents. However, efficient methods for the synthesis of 5-allyl-1,2,3-triazoles are rare.10a,b Interestingly, copper(I) acetylide has never been used directly as a substrate for the synthesis of 5-allyl-1,2,3-triazoles, although it is known to be a key intermediate. Herein, we report one-pot three-component reactions for the synthesis of 5-allyl-1,2,3-triazoles from copper(I) acetylides.
CCu, is not effective under typical CuAAC conditions:13 It did not undergo a cycloaddition with azide and did not provide a 1,2,3-triazole product. However, when it was combined with both azide and acyl chloride, it quickly provided 5-acyl-1,2,3-triazoles.13 The presence of acyl chloride accelerated the cycloaddition of copper(I) phenylacetylide and azide.13 This intriguing result motivated us to investigate one-pot three-component reactions using copper(I) phenylacetylide. We are particularly interested in 5-allyl-1,2,3-triazoles as precursors for the development of new anticancer agents. However, efficient methods for the synthesis of 5-allyl-1,2,3-triazoles are rare.10a,b Interestingly, copper(I) acetylide has never been used directly as a substrate for the synthesis of 5-allyl-1,2,3-triazoles, although it is known to be a key intermediate. Herein, we report one-pot three-component reactions for the synthesis of 5-allyl-1,2,3-triazoles from copper(I) acetylides.
Results and discussion
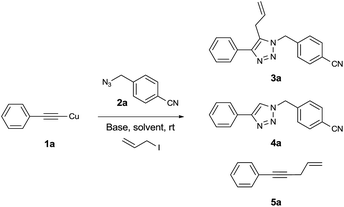
To assess the feasibility of the tandem CuAAC–allylation reaction, we started to investigate one-pot three component reactions in the presence of copper(I) phenylacetylide (1a), 4-cyanobenzyl azide (2a), allyl iodide, and base (Table 1). In preliminary screening of reaction conditions, we obtained the desired product 5-allyl-1,2,3-triazole 3a and a byproduct enyne 5a with 47% and 42% yield respectively after 4 h at room temperature when 1.5 equivalents of cyanobenzyl azide (2a), 3 equivalents of allyl iodide, and 2 equivalents of Et3N were employed (entry 1). The yield of the desired product 3a increased to 60% and the yield of the byproduct enyne 5a decreased to 36% when the amount of allyl iodide increased to 4 equivalents (entry 2). The use of Et3N appeared to decompose the stable polymeric complex with the structure [(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCu)2]n into a lower polymeric or a more reactive monomeric structure. As soon as Et3N was added, the heterogeneous reaction mixture became clear and rapidly yielded both an undesired byproduct as well as the desired product. However, replacing Et3N with pyridine, quinine, or inorganic bases such as Na3PO4, K3PO4, Na2CO3, K2CO3, Cs2CO3, NaOH, or KOH did not change the heterogeneity of the reaction. The copper(I) phenylacetylide (1a) remained suspended in CH2Cl2, which not only slowed reaction progress but also significantly reduced byproduct formation. The reaction using quinine increased the yield of desired product 3a up to 74%, but interestingly 2% of a protonated byproduct 4a was isolated instead of the allyl alkyne byproduct 5a (entry 3). The reaction using pyridine did not significantly increase the yield compared to the reaction of Et3N. However, the formation of byproducts 4a and 5a was not observed (entry 4). Similarly, the reactions using inorganic bases also generated 5-allyl-1,2,3-triazole 3a as a sole product (entries 5–11). Hydroxide bases (KOH, NaOH: entries 10 and 11) are better than phosphate bases (Na3PO4, K3PO4: entries 5 and 6) or carbonate bases (Na2CO3, K2CO3, Cs2CO3: entries 7–9) in terms of yield. Among bases used, the best result was obtained with NaOH (84%, entry 10). It appears the use of base is essential for the reaction. Without a base, 5-allyl-1,2,3-triazole 3a was isolated with only a moderate yield (52%, entry 12). The role of NaOH is not clear, and it is still under investigation. It is also noteworthy that the byproduct 1,4-disubstituted 1,2,3-triazole 4a, which is generated from a competitive protonation of 5-copper(I) 1,2,3-triazole intermediate A, was not detected in reactions using copper(I) phenylacetylide (1a) with the exception of quinine.
CCu)2]n into a lower polymeric or a more reactive monomeric structure. As soon as Et3N was added, the heterogeneous reaction mixture became clear and rapidly yielded both an undesired byproduct as well as the desired product. However, replacing Et3N with pyridine, quinine, or inorganic bases such as Na3PO4, K3PO4, Na2CO3, K2CO3, Cs2CO3, NaOH, or KOH did not change the heterogeneity of the reaction. The copper(I) phenylacetylide (1a) remained suspended in CH2Cl2, which not only slowed reaction progress but also significantly reduced byproduct formation. The reaction using quinine increased the yield of desired product 3a up to 74%, but interestingly 2% of a protonated byproduct 4a was isolated instead of the allyl alkyne byproduct 5a (entry 3). The reaction using pyridine did not significantly increase the yield compared to the reaction of Et3N. However, the formation of byproducts 4a and 5a was not observed (entry 4). Similarly, the reactions using inorganic bases also generated 5-allyl-1,2,3-triazole 3a as a sole product (entries 5–11). Hydroxide bases (KOH, NaOH: entries 10 and 11) are better than phosphate bases (Na3PO4, K3PO4: entries 5 and 6) or carbonate bases (Na2CO3, K2CO3, Cs2CO3: entries 7–9) in terms of yield. Among bases used, the best result was obtained with NaOH (84%, entry 10). It appears the use of base is essential for the reaction. Without a base, 5-allyl-1,2,3-triazole 3a was isolated with only a moderate yield (52%, entry 12). The role of NaOH is not clear, and it is still under investigation. It is also noteworthy that the byproduct 1,4-disubstituted 1,2,3-triazole 4a, which is generated from a competitive protonation of 5-copper(I) 1,2,3-triazole intermediate A, was not detected in reactions using copper(I) phenylacetylide (1a) with the exception of quinine.
| Entry | Base | Solvent | Time (h) | Yieldb (%) | ||
|---|---|---|---|---|---|---|
| 3a | 4a | 5a | ||||
| a Reaction conditions: 1a (65.8 mg, 400 μmol), 2a (94.9 mg, 600 μmol), base (800 μmol), allyl iodide (146 μL, 1.60 mmol), solvent (1 mL). All reactions were carried out under Ar.b Isolated yields.c 1.20 mmol of allyl iodide was used. | ||||||
| 1c | Et3N | CH2Cl2 | 4 | 47 | 0 | 42 |
| 2 | Et3N | CH2Cl2 | 4 | 60 | 0 | 36 |
| 3 | Quinine | CH2Cl2 | 24 | 74 | 2 | 0 |
| 4 | Pyridine | CH2Cl2 | 24 | 63 | 0 | 0 |
| 5 | Na3PO4 | CH2Cl2 | 24 | 47 | 0 | 0 |
| 6 | K3PO4 | CH2Cl2 | 24 | 45 | 0 | 0 |
| 7 | Na2CO3 | CH2Cl2 | 24 | 62 | 0 | 0 |
| 8 | K2CO3 | CH2Cl2 | 21 | 71 | 0 | 0 |
| 9 | Cs2CO3 | CH2Cl2 | 24 | 67 | 0 | 0 |
| 10 | NaOH | CH2Cl2 | 24 | 84 | 0 | 0 |
| 11 | KOH | CH2Cl2 | 24 | 83 | 0 | 0 |
| 12 | — | CH2Cl2 | 24 | 52 | 0 | 0 |
| 13 | NaOH | Dioxane | 24 | 89 | 0 | 0 |
| 14 | NaOH | THF | 24 | 85 | 0 | 0 |
| 15 | NaOH | Toluene | 24 | 94 | 0 | 0 |
| 16 | NaOH | CH3CN | 24 | 68 | 0 | 0 |
Next, we investigated the solvent effect on the yield of Cu(I)-catalyzed azide–alkyne cycloaddition–allylation reactions of copper(I) acetylides in the presence of NaOH. The reactions were effective in various solvents including CH2Cl2, dioxane, THF, toluene, and CH3CN (entries 13–16). Considering yield of the product, toluene (entry 15) was the best solvent for the reaction. On the basis of Table 1, we chose NaOH as a base and the relatively nonpolar solvent, toluene, for further study.
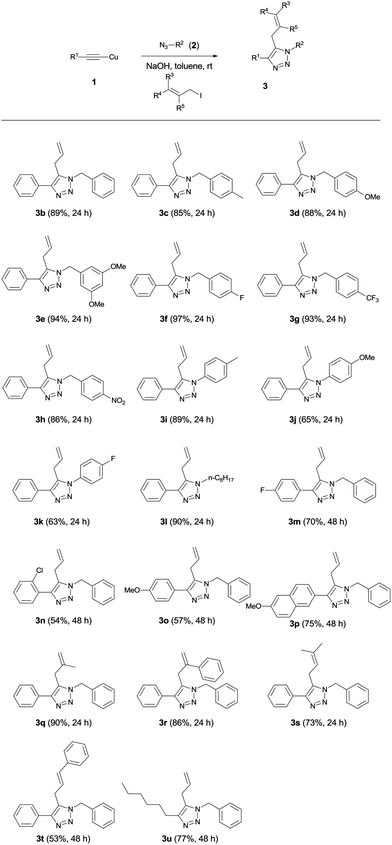
Once we had established optimal reaction conditions, we examined the scope of one-pot three-component reactions (Table 2). We applied the copper(I) acetylide system to various azides in toluene at room temperature. All reactions using copper(I) acetylides in Table 2 smoothly furnished 5-allyl-1,4-disubstituted 1,2,3-triazoles 3b–u and a trace amount of enyne byproduct 5b–u at room temperature. As anticipated, the 1,4-disubstituted 1,2,3-triazoles 4, which are unavoidable in the typical CuAAC reactions, were not detected. First, we explored the reaction scope with benzyl azides. The reaction yields were independent of the electronic nature of azides. Substrates 2c–e bearing electron-donating substituents and substrates 2f–h bearing electron-withdrawing substituents underwent the one-pot three-component reactions very smoothly, and provided the corresponding 5-allyl-1,4-disubstituted 1,2,3-triazoles 3c–h at room temperature, in good to excellent yields (85–97%). The reaction scope was not limited to only benzyl azides, but was also compatible with aryl azides 2i–k and aliphatic azide 2l. In addition, a variety of copper(I) arylacetylides were also tested. Regardless of the electronic influence of substituents on copper(I) arylacetylides, desired products 3m–p were obtained in moderate to good yields (54–75%). It is worth noting that this method is not limited to the use of simple allyl iodide. Other various substituted allyl iodides could be used for one-pot three-component reactions, and produced the corresponding 5-allyl-1,4-disubstituted 1,2,3-triazoles 3q–t with yields in the range of 53–90%. Delightfully, the reaction scope was also expandable to copper(I) alkylacetylide, which afforded 4-alkyl 1,2,3-triazole 3u.
| a Reaction conditions: 1 (400 μmol), 2 (600 μmol), NaOH (32.0 mg, 800 μmol), allyl iodide (1.60 mmol), toluene (1 mL). All reactions were carried out under Ar. |
|---|
 |
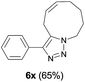
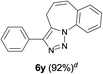
Finally, we studied further transformations of 5-allyl-1,4-disubstituted 1,2,3-triazoles into various fused polyheterocycles in order to demonstrate the synthetic utility of the 5-allyl-1,4-disubstituted 1,2,3-triazoles as versatile building blocks (Table 3). The alkene-tethered 5-allyl-1,2,3-triazoles 3v–x were synthesized based on Cu(I)-catalyzed azide–alkyne cycloaddition–allylation reactions of copper(I) acetylides in good yields (entries 1–3, 65–80%) and these were converted into fused 1,2,3-triazoles 6v–x by ring-closing metathesis reactions, which were effective for the synthesis of 7-, 8- and 9-membered fused 1,2,3-triazoles with good yields (entries 1–3, 65–87%). The transformations were also applicable to substrates bearing styrene-type alkenes 3y and 3z. The ring-closing metathesis of 3y and 3z proceeded smoothly to afford fused tricyclic 1,2,3-triazoles (entries 4 and 5, 70–92%).
| Entry | Yield of 3c | Yield of 6c |
|---|---|---|
| a Reaction conditions: 1a (65.8 mg, 400 μmol), 2 (600 μmol), NaOH (32.0 mg, 800 μmol), allyl iodide (146 μL, 1.60 mmol), toluene (1 mL). All reactions were carried out under Ar.b Reaction conditions: 3 (200 μmol), Grubbs' 1st generation catalyst (5 mol%), CH2Cl2 (9.3 mL).c Isolated yields.d 180 μmol of 3 was used.e 150 μmol of 3 and Hoveyda-Grubbs' 2nd generation catalyst (5 mol%) were used. | ||
| 1 |  |
 |
| 2 |  |
 |
| 3 | 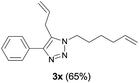 |
 |
| 4 | 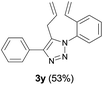 |
 |
| 5 | 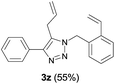 |
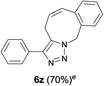 |
On the basis of previous mechanistic studies16 and our own observation, we speculate that Cu(I) acetylide 1 coordinates with the azide 2 to form the intermediate I-1 and following cyclization leads to the metallocycle intermediate I-2. The 6-membered Cu(I)-intermediate contracts to the Cu(I)-1,2,3-triazole intermediate I-3, which is readily trapped by allyl iodide to yield the desired 5-allyl-1,4-disubstituted 1,2,3-triazole 3 (Scheme 2).
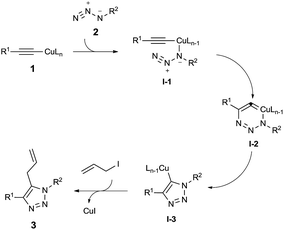 | ||
| Scheme 2 Plausible mechanism of Cu(I)-mediated one-pot three component synthesis of 5-allyl-1,2,3-triazoles. | ||
Conclusions
In conclusion, we have developed a novel one-pot three component reaction method for the synthesis of 5-allyl-1,4-disubstituted 1,2,3-triazoles from copper(I) acetylides. The 5-allyl-1,4-disubstituted 1,2,3-triazoles were produced via 1,3-dipolar cycloaddition followed by in situ trapping of the C(sp2)–Cu intermediate. The byproduct 1,4-disubstituted 1,2,3-triazole 4, which is generated from a competitive protonation of 5-copper(I) 1,2,3-triazole intermediate A, was not isolated. The present method was successfully applied to achieve production of synthetically useful heterocycles. This domino reaction is characterized by mild conditions and no protonated byproduct formation, and allows for the efficient construction of functionalized fused polycyclic 1,2,3-triazoles.Experimental
General
All reactions were performed in oven-dried glassware fitted with glass stoppers under positive pressure of Ar with magnetic stirring, unless otherwise noted. Air- and moisture-sensitive liquids and solutions were transferred via syringe or stainless-steel cannula. TLC was performed on 0.25 mm E. Merck silica gel 60 F254 plates and visualized under UV light (254 nm) or by staining with cerium ammonium molybdenate (CAM), potassium permanganate (KMnO4) or p-anisaldehyde. Flash chromatography was performed on E. Merck 230–400 mesh silica gel 60. Reagents were purchased from commercial suppliers, and used without further purification unless otherwise noted. Solvents were distilled from proper drying agents (CaH2 or Na wire) under Ar atmosphere at 760 mmHg. NMR spectra were recorded at 24 °C. Chemical shifts are expressed in ppm relative to TMS (1H, 0 ppm), CDCl3 (1H, 7.26 ppm; 13C, 77.2 ppm), and C6H5F (19F, −113.15 ppm); coupling constants are expressed in Hz. High resolution mass spectra (HRMS) were obtained by electrospray ionization (ESI, TOF) or electron ionization (EI, magnetic sector). Infrared spectra were recorded with peaks reported in cm−1.General procedure for the Cu(I)-catalyzed azide–alkyne cycloaddition–allylation reactions
Copper(I) acetylide (400 μmol) was placed in a 25 mL one-arm roundbottom flask. A solution of azide (600 μmol) in anhydrous toluene (1 mL), allyl iodide (268 mg, 1.60 mmol) and NaOH (32.0 mg, 800 μmol) were sequentially added to the reaction mixture. The resulting suspension was stirred at room temperature for 24 h or 48 h as indicated in Table 1. The mixture was filtered and washed with CH2Cl2 (20 mL). To scavenge Cu, polymer-bound ethylenediaminetriacetic acid acetamide (3.0–4.0 mmol g−1, 200 mg) was added to the filtrate, and stirred for 2 h. The polymer was filtered off and the filtrate was concentrated by rotary evaporation. Purification of crude residue by column chromatography yielded 3a–z. The reaction of 3y was carried out for 3 days at room temperature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (113 mg, 94%). Mp = 113–115 °C. 1H NMR (400 MHz, CDCl3): δ 7.68–7.63 (m, 4H), 7.45–7.41 (m, 2H), 7.38–7.34 (m, 1H), 7.28 (d, J = 8.4 Hz, 2H), 5.83 (m, 1H), 5.57 (s, 2H), 5.15 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.88 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.2, 140.3, 132.9, 132.1, 131.0, 130.4, 128.9, 128.3, 128.0, 127.3, 118.3, 118.2, 112.6, 51.4, 27.1. HRMS (ESI) m/z calculated for C19H17N4 [M + H]+ 301.1448, found 301.1451. IR (KBr film): ν 3061, 2229, 1639, 1506, 920, 820, 777, 699 cm−1.
1 hexane/EtOAc). White solid (113 mg, 94%). Mp = 113–115 °C. 1H NMR (400 MHz, CDCl3): δ 7.68–7.63 (m, 4H), 7.45–7.41 (m, 2H), 7.38–7.34 (m, 1H), 7.28 (d, J = 8.4 Hz, 2H), 5.83 (m, 1H), 5.57 (s, 2H), 5.15 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.88 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.2, 140.3, 132.9, 132.1, 131.0, 130.4, 128.9, 128.3, 128.0, 127.3, 118.3, 118.2, 112.6, 51.4, 27.1. HRMS (ESI) m/z calculated for C19H17N4 [M + H]+ 301.1448, found 301.1451. IR (KBr film): ν 3061, 2229, 1639, 1506, 920, 820, 777, 699 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (97.9 mg, 89%). 1H NMR (400 MHz, CDCl3): δ 7.70–7.67 (m, 2H), 7.44–7.40 (m, 2H), 7.36–7.30 (m, 4H), 7.20–7.18 (m, 2H), 5.83 (m, 1H), 5.54 (s, 2H), 5.15 (dm, J = 12.0 Hz, 1H), 4.92 (dm, J = 15.2 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.0, 135.1, 132.4, 131.5, 130.4, 129.1, 128.8, 128.5, 128.0, 127.3, 117.9, 52.1, 27.1. HRMS (ESI) m/z calculated for C18H18N3 [M + H]+ 276.1495, found 276.1501. IR (KBr film): ν 3063, 3033, 1639, 1608, 1496, 918, 800, 764 cm−1
1 hexane/EtOAc). Pale yellow liquid (97.9 mg, 89%). 1H NMR (400 MHz, CDCl3): δ 7.70–7.67 (m, 2H), 7.44–7.40 (m, 2H), 7.36–7.30 (m, 4H), 7.20–7.18 (m, 2H), 5.83 (m, 1H), 5.54 (s, 2H), 5.15 (dm, J = 12.0 Hz, 1H), 4.92 (dm, J = 15.2 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.0, 135.1, 132.4, 131.5, 130.4, 129.1, 128.8, 128.5, 128.0, 127.3, 117.9, 52.1, 27.1. HRMS (ESI) m/z calculated for C18H18N3 [M + H]+ 276.1495, found 276.1501. IR (KBr film): ν 3063, 3033, 1639, 1608, 1496, 918, 800, 764 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (97.9 mg, 85%). Mp = 42–44 °C. 1H NMR (400 MHz, CDCl3): δ 7.69–7.66 (m, 2H), 7.44–7.39 (m, 2H), 7.36–7.31 (m, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 5.84 (m, 1H), 5.49 (s, 2H), 5.17 (dq, J = 10.4 Hz, 2.0 Hz, 1H), 4.93 (dq, J = 17.2 Hz, 2.0 Hz, 1H), 3.45 (m, 2H), 2.33 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 146.0, 138.3, 132.5, 132.1, 131.5, 130.4, 129.8, 128.8, 128.0, 127.3, 117.9, 52.0, 27.2, 21.3. HRMS (ESI) m/z calculated for C19H20N3 [M + H]+ 290.1652, found 290.1657. IR (KBr film): ν 3056, 3031, 1639, 1495, 1360, 919, 792, 716 cm−1.
1 hexane/EtOAc). White solid (97.9 mg, 85%). Mp = 42–44 °C. 1H NMR (400 MHz, CDCl3): δ 7.69–7.66 (m, 2H), 7.44–7.39 (m, 2H), 7.36–7.31 (m, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 5.84 (m, 1H), 5.49 (s, 2H), 5.17 (dq, J = 10.4 Hz, 2.0 Hz, 1H), 4.93 (dq, J = 17.2 Hz, 2.0 Hz, 1H), 3.45 (m, 2H), 2.33 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 146.0, 138.3, 132.5, 132.1, 131.5, 130.4, 129.8, 128.8, 128.0, 127.3, 117.9, 52.0, 27.2, 21.3. HRMS (ESI) m/z calculated for C19H20N3 [M + H]+ 290.1652, found 290.1657. IR (KBr film): ν 3056, 3031, 1639, 1495, 1360, 919, 792, 716 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (107 mg, 88%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.65 (m, 2H), 7.43–7.39 (m, 2H), 7.35–7.31 (m, 1H), 7.15 (dm, J = 9.6 Hz, 2H), 6.86 (dm, J = 9.6 Hz, 2H), 5.84 (m, 1H), 5.46 (s, 2H), 5.16 (dq, J = 10.4 Hz, 2.0 Hz, 1H), 4.92 (dq, J = 16.8 Hz, 2.0 Hz, 1H), 3.78 (s, 3H), 3.46 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 159.7, 146.0, 132.5, 131.5, 130.3, 128.9, 128.8, 128.0, 127.3, 127.1, 117.8, 114.5, 55.4, 51.7, 27.1. HRMS (ESI) m/z calculated for C19H20N3O [M + H]+ 306.1601, found 306.1595. IR (KBr film): ν 3063, 3005, 1639, 1514, 1250, 1032, 921, 825, 715 cm−1
1 hexane/EtOAc). Pale yellow liquid (107 mg, 88%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.65 (m, 2H), 7.43–7.39 (m, 2H), 7.35–7.31 (m, 1H), 7.15 (dm, J = 9.6 Hz, 2H), 6.86 (dm, J = 9.6 Hz, 2H), 5.84 (m, 1H), 5.46 (s, 2H), 5.16 (dq, J = 10.4 Hz, 2.0 Hz, 1H), 4.92 (dq, J = 16.8 Hz, 2.0 Hz, 1H), 3.78 (s, 3H), 3.46 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 159.7, 146.0, 132.5, 131.5, 130.3, 128.9, 128.8, 128.0, 127.3, 127.1, 117.8, 114.5, 55.4, 51.7, 27.1. HRMS (ESI) m/z calculated for C19H20N3O [M + H]+ 306.1601, found 306.1595. IR (KBr film): ν 3063, 3005, 1639, 1514, 1250, 1032, 921, 825, 715 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (126 mg, 94%). Mp = 80–82 °C. 1H NMR (400 MHz, CDCl3): δ 7.64–7.61 (m, 2H), 7.38–7.34 (m, 2H), 7.27 (tt, J = 6.4 Hz, 1.2 Hz, 1H), 6.33 (t, J = 2.0 Hz, 1H), 6.27 (d, J = 2.0 Hz, 2H), 5.79 (m, 1H), 5.39 (s, 2H), 5.10 (dq, J = 12.0 Hz, 2.0 Hz, 1H), 4.88 (dq, J = 18.8 Hz, 2.0 Hz, 1H), 3.67 (s, 6H), 3.40 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 161.4, 145.9, 137.3, 132.4, 131.4, 130.5, 128.7, 127.9, 127.2, 117.8, 105.3, 100.0, 55.5, 52.1, 27.1. HRMS (ESI) m/z calculated for C20H22N3O2 [M + H]+ 336.1707, found 336.1708. IR (KBr film): ν 3003, 2938, 1598, 1206, 1066, 921, 777 cm−1.
1 hexane/EtOAc). White solid (126 mg, 94%). Mp = 80–82 °C. 1H NMR (400 MHz, CDCl3): δ 7.64–7.61 (m, 2H), 7.38–7.34 (m, 2H), 7.27 (tt, J = 6.4 Hz, 1.2 Hz, 1H), 6.33 (t, J = 2.0 Hz, 1H), 6.27 (d, J = 2.0 Hz, 2H), 5.79 (m, 1H), 5.39 (s, 2H), 5.10 (dq, J = 12.0 Hz, 2.0 Hz, 1H), 4.88 (dq, J = 18.8 Hz, 2.0 Hz, 1H), 3.67 (s, 6H), 3.40 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 161.4, 145.9, 137.3, 132.4, 131.4, 130.5, 128.7, 127.9, 127.2, 117.8, 105.3, 100.0, 55.5, 52.1, 27.1. HRMS (ESI) m/z calculated for C20H22N3O2 [M + H]+ 336.1707, found 336.1708. IR (KBr film): ν 3003, 2938, 1598, 1206, 1066, 921, 777 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (114 mg, 97%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.66 (m, 2H), 7.44–7.40 (m, 2H), 7.34 (tt, J = 6.4 Hz, 1.2 Hz, 1H), 7.20 (dd, J = 8.8 Hz, 5.6 Hz, 2H), 7.03 (t, J = 8.8 Hz, 2H), 5.84 (m, 1H), 5.50 (s, 2H), 5.16 (dm, J = 10.0 Hz, 1H), 4.91 (dm, J = 16.4 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 162.8 (d, JC–F = 246.0 Hz), 146.1, 132.3, 131.3, 130.9 (d, JC–F = 3.1 Hz), 130.3, 129.3 (d, JC–F = 7.8 Hz), 128.9, 128.1, 127.3, 118.0, 116.1 (d, JC–F = 21.7 Hz), 51.4, 27.1. 19F NMR (376 MHz, CDCl3): δ −113.1. HRMS (ESI) m/z calculated for C18H17FN3 [M + H]+ 294.1401, found 294.1405. IR (KBr film): ν 3066, 3011, 1640, 1512, 1015, 920, 715, 698 cm−1.
1 hexane/EtOAc). Pale yellow liquid (114 mg, 97%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.66 (m, 2H), 7.44–7.40 (m, 2H), 7.34 (tt, J = 6.4 Hz, 1.2 Hz, 1H), 7.20 (dd, J = 8.8 Hz, 5.6 Hz, 2H), 7.03 (t, J = 8.8 Hz, 2H), 5.84 (m, 1H), 5.50 (s, 2H), 5.16 (dm, J = 10.0 Hz, 1H), 4.91 (dm, J = 16.4 Hz, 1H), 3.45 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 162.8 (d, JC–F = 246.0 Hz), 146.1, 132.3, 131.3, 130.9 (d, JC–F = 3.1 Hz), 130.3, 129.3 (d, JC–F = 7.8 Hz), 128.9, 128.1, 127.3, 118.0, 116.1 (d, JC–F = 21.7 Hz), 51.4, 27.1. 19F NMR (376 MHz, CDCl3): δ −113.1. HRMS (ESI) m/z calculated for C18H17FN3 [M + H]+ 294.1401, found 294.1405. IR (KBr film): ν 3066, 3011, 1640, 1512, 1015, 920, 715, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (128 mg, 93%). Mp = 63–65 °C. 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 8.0 Hz, 2H), 7.36–7.29 (m, 3H), 5.84 (m, 1H), 5.57 (s, 2H), 5.15 (d, J = 10.0 Hz, 1H), 4.90 (d, J = 17.2 Hz, 1H), 3.45 (dm, J = 3.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 146.1, 139.1 (q, JC–F = 1.5 Hz), 132.1, 131.2, 130.8 (q, JC–F = 32.5 Hz), 130.5, 128.8, 128.1, 127.6, 127.3, 126.1 (q, JC–F = 3.9 Hz), 124.0 (q, JC–F = 270.9 Hz), 118.0, 51.4, 27.1. 19F NMR (376 MHz, CDCl3): δ −63.1. HRMS (ESI) m/z calculated for C19H17N3F3 [M + H]+ 344.1369, found 344.1374. IR (KBr film): ν 3064, 1640, 1496, 1125, 824, 779, 699 cm−1.
1 hexane/EtOAc). Light yellow solid (128 mg, 93%). Mp = 63–65 °C. 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 8.0 Hz, 2H), 7.36–7.29 (m, 3H), 5.84 (m, 1H), 5.57 (s, 2H), 5.15 (d, J = 10.0 Hz, 1H), 4.90 (d, J = 17.2 Hz, 1H), 3.45 (dm, J = 3.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 146.1, 139.1 (q, JC–F = 1.5 Hz), 132.1, 131.2, 130.8 (q, JC–F = 32.5 Hz), 130.5, 128.8, 128.1, 127.6, 127.3, 126.1 (q, JC–F = 3.9 Hz), 124.0 (q, JC–F = 270.9 Hz), 118.0, 51.4, 27.1. 19F NMR (376 MHz, CDCl3): δ −63.1. HRMS (ESI) m/z calculated for C19H17N3F3 [M + H]+ 344.1369, found 344.1374. IR (KBr film): ν 3064, 1640, 1496, 1125, 824, 779, 699 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (110 mg, 86%). Mp = 93–95 °C. 1H NMR (400 MHz, CDCl3): δ 8.19 (dm, J = 8.8 Hz, 2H), 7.67 (dm, J = 8.8 Hz, 2H), 7.44–7.41 (m, 2H), 7.37–7.33 (m, 3H), 5.84 (m, 1H), 5.61 (s, 2H), 5.14 (dm, J = 12.0 Hz, 1H), 4.89 (dm, J = 17.2 Hz, 1H), 3.47 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 148.0, 146.2, 142.2, 132.1, 131.0, 130.5, 128.9, 128.3, 128.2, 127.3, 124.3, 118.2, 51.1, 27.1. HRMS (ESI) m/z calculated for C18H17N4O2 [M + H]+ 321.1346, found 321.1348. IR (KBr film): ν 3081, 1608, 1520, 1495, 1347, 805, 734 cm−1.
1 hexane/EtOAc). Light yellow solid (110 mg, 86%). Mp = 93–95 °C. 1H NMR (400 MHz, CDCl3): δ 8.19 (dm, J = 8.8 Hz, 2H), 7.67 (dm, J = 8.8 Hz, 2H), 7.44–7.41 (m, 2H), 7.37–7.33 (m, 3H), 5.84 (m, 1H), 5.61 (s, 2H), 5.14 (dm, J = 12.0 Hz, 1H), 4.89 (dm, J = 17.2 Hz, 1H), 3.47 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 148.0, 146.2, 142.2, 132.1, 131.0, 130.5, 128.9, 128.3, 128.2, 127.3, 124.3, 118.2, 51.1, 27.1. HRMS (ESI) m/z calculated for C18H17N4O2 [M + H]+ 321.1346, found 321.1348. IR (KBr film): ν 3081, 1608, 1520, 1495, 1347, 805, 734 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (98.3 mg, 89%). Mp = 106–108 °C. 1H NMR (400 MHz, CDCl3): δ 7.82 (d, J = 8.4 Hz, 2H), 7.48–4.37 (m, 5H), 7.33 (d, J = 8.4 Hz, 2H), 5.91 (m, 1H), 5.19 (d, J = 9.6 Hz, 1H), 4.93 (d, J = 17.2 Hz, 1H), 3.57 (dm, J = 4.0 Hz, 2H), 2.45 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 145.4, 140.1, 134.1, 133.4, 131.4, 131.1, 130.1, 128.9, 128.1, 127.3, 125.5, 118.2, 27.7, 21.4. HRMS (ESI) m/z calculated for C18H18N3 [M + H]+ 276.1495, found 276.1501. IR (KBr film): ν 2919, 1517, 992, 934, 710 cm−1.
1 hexane/EtOAc). White solid (98.3 mg, 89%). Mp = 106–108 °C. 1H NMR (400 MHz, CDCl3): δ 7.82 (d, J = 8.4 Hz, 2H), 7.48–4.37 (m, 5H), 7.33 (d, J = 8.4 Hz, 2H), 5.91 (m, 1H), 5.19 (d, J = 9.6 Hz, 1H), 4.93 (d, J = 17.2 Hz, 1H), 3.57 (dm, J = 4.0 Hz, 2H), 2.45 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 145.4, 140.1, 134.1, 133.4, 131.4, 131.1, 130.1, 128.9, 128.1, 127.3, 125.5, 118.2, 27.7, 21.4. HRMS (ESI) m/z calculated for C18H18N3 [M + H]+ 276.1495, found 276.1501. IR (KBr film): ν 2919, 1517, 992, 934, 710 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light brown solid (76.0 mg, 65%). Mp = 97–99 °C. 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 7.2 Hz, 2H), 7.47–7.34 (m, 5H), 7.03 (d, J = 9.2 Hz, 2H), 5.91 (m, 1H), 5.19 (dd, J = 10.0 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 17.6 Hz, 1.2 Hz, 1H), 3.88 (s, 3H), 3.56 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 160.6, 133.4, 131.4, 129.4, 128.9, 128.8, 128.0, 127.2, 127.1, 118.1, 114.7, 114.6, 55.8, 27.7. HRMS (ESI) m/z calculated for C18H18N3O [M + H]+ 292.1444, found 292.1449. IR (KBr film): ν 3081, 1639, 1508, 1260, 1030, 835, 714 cm−1.
1 hexane/EtOAc). Light brown solid (76.0 mg, 65%). Mp = 97–99 °C. 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 7.2 Hz, 2H), 7.47–7.34 (m, 5H), 7.03 (d, J = 9.2 Hz, 2H), 5.91 (m, 1H), 5.19 (dd, J = 10.0 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 17.6 Hz, 1.2 Hz, 1H), 3.88 (s, 3H), 3.56 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 160.6, 133.4, 131.4, 129.4, 128.9, 128.8, 128.0, 127.2, 127.1, 118.1, 114.7, 114.6, 55.8, 27.7. HRMS (ESI) m/z calculated for C18H18N3O [M + H]+ 292.1444, found 292.1449. IR (KBr film): ν 3081, 1639, 1508, 1260, 1030, 835, 714 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (70.0 mg, 63%). Mp = 121–123 °C. 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 7.2 Hz, 2H), 7.54–7.48 (m, 2H), 7.48–7.44 (m, 2H), 7.40–7.36 (m, 1H), 7.24 (t, J = 8.4 Hz, 2H), 5.92 (m, 1H), 5.21 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.57 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 163.3 (d, JC–F = 249.2 Hz), 145.6, 133.2, 132.6 (d, JC–F = 3.1 Hz), 131.2 (d, JC–F = 6.2 Hz), 128.9, 128.3, 127.7 (d, JC–F = 9.3 Hz), 127.3, 126.1, 118.4, 116.7 (d, JC–F = 22.5 Hz), 27.7. 19F NMR (376 MHz, CDCl3): δ −110.9. HRMS (EI) m/z calculated for C17H14FN3 [M]+ 279.1171, found 279.1172. IR (KBr film): ν 3082, 1514, 1244, 992, 846, 721, 701 cm−1.
1 hexane/EtOAc). Light yellow solid (70.0 mg, 63%). Mp = 121–123 °C. 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 7.2 Hz, 2H), 7.54–7.48 (m, 2H), 7.48–7.44 (m, 2H), 7.40–7.36 (m, 1H), 7.24 (t, J = 8.4 Hz, 2H), 5.92 (m, 1H), 5.21 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.57 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 163.3 (d, JC–F = 249.2 Hz), 145.6, 133.2, 132.6 (d, JC–F = 3.1 Hz), 131.2 (d, JC–F = 6.2 Hz), 128.9, 128.3, 127.7 (d, JC–F = 9.3 Hz), 127.3, 126.1, 118.4, 116.7 (d, JC–F = 22.5 Hz), 27.7. 19F NMR (376 MHz, CDCl3): δ −110.9. HRMS (EI) m/z calculated for C17H14FN3 [M]+ 279.1171, found 279.1172. IR (KBr film): ν 3082, 1514, 1244, 992, 846, 721, 701 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (107 mg, 90%). 1H NMR (400 MHz, CDCl3): δ 7.68 (dm, J = 8.4 Hz, 2H), 7.42 (tm, J = 8.4 Hz, 2H), 7.35–7.31 (m, 1H), 5.96 (m, 1H), 5.20 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.96 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 4.23 (t, J = 7.2 Hz, 2H), 3.58 (m, 2H), 1.92 (quin, J = 7.2 Hz, 2H), 1.40–1.26 (m, 10H), 0.87 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 145.3, 132.9, 131.7, 129.9, 128.8, 127.9, 127.3, 117.8, 48.3, 31.8, 30.3, 29.3, 29.2, 27.2, 26.8, 22.7, 14.2. HRMS (ESI) m/z calculated for C19H28N3 [M + H]+ 298.2278, found 298.2278. IR (KBr film): ν 2954, 2926, 1640, 1495, 1361, 765, 698 cm−1.
1 hexane/EtOAc). Pale yellow liquid (107 mg, 90%). 1H NMR (400 MHz, CDCl3): δ 7.68 (dm, J = 8.4 Hz, 2H), 7.42 (tm, J = 8.4 Hz, 2H), 7.35–7.31 (m, 1H), 5.96 (m, 1H), 5.20 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.96 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 4.23 (t, J = 7.2 Hz, 2H), 3.58 (m, 2H), 1.92 (quin, J = 7.2 Hz, 2H), 1.40–1.26 (m, 10H), 0.87 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 145.3, 132.9, 131.7, 129.9, 128.8, 127.9, 127.3, 117.8, 48.3, 31.8, 30.3, 29.3, 29.2, 27.2, 26.8, 22.7, 14.2. HRMS (ESI) m/z calculated for C19H28N3 [M + H]+ 298.2278, found 298.2278. IR (KBr film): ν 2954, 2926, 1640, 1495, 1361, 765, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (81.6 mg, 70%). Mp = 79–81 °C. 1H NMR (400 MHz, CDCl3): δ 7.65 (dd, J = 8.8 Hz, 5.6 Hz, 2H), 7.36–7.30 (m, 3H), 7.20–7.18 (m, 2H), 7.11 (t, J = 8.8 Hz, 2H), 5.81 (m, 1H), 5.53 (s, 2H), 5.15 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.90 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.42 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 162.7 (d, JC–F = 245.3 Hz), 145.2, 135.0, 132.2, 130.2, 129.2, 129.1 (d, JC–F = 8.5 Hz), 128.5, 127.6 (d, JC–F = 3.1 Hz), 127.4, 118.0, 115.8 (d, JC–F = 20.9 Hz), 52.2, 27.1. 19F NMR (376 MHz, CDCl3): δ −110.9. HRMS (ESI) m/z calculated for C18H17FN3 [M + H]+ 294.1401, found 294.1404. IR (KBr film): ν 3081, 1640, 1510, 1224, 992, 841, 764, 698 cm−1.
1 hexane/EtOAc). White solid (81.6 mg, 70%). Mp = 79–81 °C. 1H NMR (400 MHz, CDCl3): δ 7.65 (dd, J = 8.8 Hz, 5.6 Hz, 2H), 7.36–7.30 (m, 3H), 7.20–7.18 (m, 2H), 7.11 (t, J = 8.8 Hz, 2H), 5.81 (m, 1H), 5.53 (s, 2H), 5.15 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.90 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.42 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 162.7 (d, JC–F = 245.3 Hz), 145.2, 135.0, 132.2, 130.2, 129.2, 129.1 (d, JC–F = 8.5 Hz), 128.5, 127.6 (d, JC–F = 3.1 Hz), 127.4, 118.0, 115.8 (d, JC–F = 20.9 Hz), 52.2, 27.1. 19F NMR (376 MHz, CDCl3): δ −110.9. HRMS (ESI) m/z calculated for C18H17FN3 [M + H]+ 294.1401, found 294.1404. IR (KBr film): ν 3081, 1640, 1510, 1224, 992, 841, 764, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (66.7 mg, 54%). 1H NMR (400 MHz, CDCl3): δ 7.47–7.42 (m, 2H), 7.38–7.29 (m, 5H), 7.18 (d, J = 8.0 Hz, 2H), 5.65 (m, 1H), 5.58 (s, 2H), 5.05 (dd, J = 10.0 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 3.27 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 144.1, 135.0, 134.1, 132.6, 132.4, 132.2, 130.4, 130.1, 129.8, 129.1, 128.4, 127.2, 126.9, 117.9, 52.3, 27.5. HRMS (EI) m/z calculated for C18H16ClN3 [M]+ 309.1031, found 309.1033. IR (KBr film): ν 3064, 3032, 1639, 1497, 920, 760, 736, 694 cm−1.
1 hexane/EtOAc). Pale yellow liquid (66.7 mg, 54%). 1H NMR (400 MHz, CDCl3): δ 7.47–7.42 (m, 2H), 7.38–7.29 (m, 5H), 7.18 (d, J = 8.0 Hz, 2H), 5.65 (m, 1H), 5.58 (s, 2H), 5.05 (dd, J = 10.0 Hz, 1.2 Hz, 1H), 4.92 (dd, J = 16.8 Hz, 1.2 Hz, 1H), 3.27 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 144.1, 135.0, 134.1, 132.6, 132.4, 132.2, 130.4, 130.1, 129.8, 129.1, 128.4, 127.2, 126.9, 117.9, 52.3, 27.5. HRMS (EI) m/z calculated for C18H16ClN3 [M]+ 309.1031, found 309.1033. IR (KBr film): ν 3064, 3032, 1639, 1497, 920, 760, 736, 694 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (70.0 mg, 57%). 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 8.8 Hz, 2H), 7.36–7.30 (m, 3H), 7.20–7.18 (m, 2H), 6.96 (d, J = 8.8 Hz, 2H), 5.82 (m, 1H), 5.52 (s, 2H), 5.13 (dd, J = 12.0 Hz, 1.6 Hz, 1H), 4.91 (dd, J = 17.2 Hz, 1.6 Hz, 1H), 3.83 (s, 3H), 3.42 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 159.6, 145.9, 135.3, 132.5, 129.8, 129.1, 128.6, 128.4, 127.3, 124.1, 117.8, 114.3, 55.5, 52.2, 27.2. HRMS (ESI) m/z calculated for C19H20N3O [M + H]+ 306.1601, found 306.1604. IR (KBr film): ν 2932, 1616, 1508, 1251, 1015, 836, 727, 696 cm−1.
1 hexane/EtOAc). Pale yellow liquid (70.0 mg, 57%). 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 8.8 Hz, 2H), 7.36–7.30 (m, 3H), 7.20–7.18 (m, 2H), 6.96 (d, J = 8.8 Hz, 2H), 5.82 (m, 1H), 5.52 (s, 2H), 5.13 (dd, J = 12.0 Hz, 1.6 Hz, 1H), 4.91 (dd, J = 17.2 Hz, 1.6 Hz, 1H), 3.83 (s, 3H), 3.42 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 159.6, 145.9, 135.3, 132.5, 129.8, 129.1, 128.6, 128.4, 127.3, 124.1, 117.8, 114.3, 55.5, 52.2, 27.2. HRMS (ESI) m/z calculated for C19H20N3O [M + H]+ 306.1601, found 306.1604. IR (KBr film): ν 2932, 1616, 1508, 1251, 1015, 836, 727, 696 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (106 mg, 75%). Mp = 113–115 °C. 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 0.8 Hz, 1H), 7.83 (dd, J = 8.4 Hz, 1.6 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.38–7.31 (m, 3H), 7.23–7.21 (m, 2H), 7.17–7.14 (m, 2H), 5.87 (m, 1H), 5.57 (s, 2H), 5.18 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.98 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.93 (s, 3H), 3.52 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 158.1, 146.2, 135.2, 134.2, 132.5, 130.4, 129.9, 129.2, 129.1, 128.5, 127.4 (2C), 126.8, 126.1, 126.0, 119.3, 118.0, 105.8, 55.5, 52.2, 27.3. HRMS (EI) m/z calculated for C23H21N3O 355.1687, found 355.1685. IR (KBr film): ν 2936, 2840, 1634, 1600, 1263, 1029, 726, 695 cm−1.
1 hexane/EtOAc). Light yellow solid (106 mg, 75%). Mp = 113–115 °C. 1H NMR (400 MHz, CDCl3): δ 8.04 (d, J = 0.8 Hz, 1H), 7.83 (dd, J = 8.4 Hz, 1.6 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.38–7.31 (m, 3H), 7.23–7.21 (m, 2H), 7.17–7.14 (m, 2H), 5.87 (m, 1H), 5.57 (s, 2H), 5.18 (dd, J = 10.4 Hz, 1.2 Hz, 1H), 4.98 (dd, J = 17.2 Hz, 1.2 Hz, 1H), 3.93 (s, 3H), 3.52 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 158.1, 146.2, 135.2, 134.2, 132.5, 130.4, 129.9, 129.2, 129.1, 128.5, 127.4 (2C), 126.8, 126.1, 126.0, 119.3, 118.0, 105.8, 55.5, 52.2, 27.3. HRMS (EI) m/z calculated for C23H21N3O 355.1687, found 355.1685. IR (KBr film): ν 2936, 2840, 1634, 1600, 1263, 1029, 726, 695 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (104 mg, 90%). Mp = 56–58 °C. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.39–7.28 (m, 4H), 7.19 (dd, J = 7.6, 1.6 Hz, 2H), 5.51 (s, 2H), 4.90 (t, J = 1.2 Hz, 1H), 4.44 (s, 1H), 3.32 (s, 2H), 1.77 (d, J = 0.4 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 146.1, 140.3, 135.0, 131.4, 130.6, 128.9, 128.7, 128.3, 127.8, 127.2, 127.1, 112.8, 52.0, 30.9, 22.9. HRMS (ESI) m/z calculated for C19H20N3 [M + H]+ 290.1652, found 290.1654. IR (KBr film): ν 3063, 3031, 1605, 1496, 1251, 890, 770, 729 cm−1.
1 hexane/EtOAc). Light yellow solid (104 mg, 90%). Mp = 56–58 °C. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.39–7.28 (m, 4H), 7.19 (dd, J = 7.6, 1.6 Hz, 2H), 5.51 (s, 2H), 4.90 (t, J = 1.2 Hz, 1H), 4.44 (s, 1H), 3.32 (s, 2H), 1.77 (d, J = 0.4 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 146.1, 140.3, 135.0, 131.4, 130.6, 128.9, 128.7, 128.3, 127.8, 127.2, 127.1, 112.8, 52.0, 30.9, 22.9. HRMS (ESI) m/z calculated for C19H20N3 [M + H]+ 290.1652, found 290.1654. IR (KBr film): ν 3063, 3031, 1605, 1496, 1251, 890, 770, 729 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Colorless liquid (120 mg, 86%). 1H NMR (400 MHz, CDCl3): δ 7.69 (dt, J = 7.2, 1.6 Hz, 2H), 7.41 (t, J = 7.2 Hz, 2H), 7.37–7.28 (m, 9H), 7.22–7.13 (m, 2H), 5.52 (s, 2H), 5.47 (t, J = 1.6 Hz, 1H), 4.65 (t, J = 2.0 Hz, 1H), 3.82 (t, J = 2.0 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 146.4, 142.6, 139.7, 134.9, 131.2, 130.3, 129.0, 128.8, 128.6, 128.4, 128.3, 128.0, 127.4, 127.1, 125.7, 114.3, 52.2, 28.5. HRMS (ESI) m/z calculated for C24H22N3 [M + H]+ 352.1808, found 352.1807. IR (KBr film): ν 3058, 3032, 1954, 1628 cm−1.
1 hexane/EtOAc). Colorless liquid (120 mg, 86%). 1H NMR (400 MHz, CDCl3): δ 7.69 (dt, J = 7.2, 1.6 Hz, 2H), 7.41 (t, J = 7.2 Hz, 2H), 7.37–7.28 (m, 9H), 7.22–7.13 (m, 2H), 5.52 (s, 2H), 5.47 (t, J = 1.6 Hz, 1H), 4.65 (t, J = 2.0 Hz, 1H), 3.82 (t, J = 2.0 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 146.4, 142.6, 139.7, 134.9, 131.2, 130.3, 129.0, 128.8, 128.6, 128.4, 128.3, 128.0, 127.4, 127.1, 125.7, 114.3, 52.2, 28.5. HRMS (ESI) m/z calculated for C24H22N3 [M + H]+ 352.1808, found 352.1807. IR (KBr film): ν 3058, 3032, 1954, 1628 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (89.0 mg, 73%). Mp = 86–88 °C. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.38–7.28 (m, 4H), 7.16 (d, J = 6.4 Hz, 2H), 5.54 (s, 2H), 4.93 (m, 1H), 3.41 (d, J = 6.4 Hz, 2H), 1.65 (d, J = 1.6 Hz, 3H), 1.62 (d, J = 0.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 145.1, 135.2, 135.1, 132.6, 131.6, 128.9, 128.6, 128.1, 127.7, 127.4, 127.0, 118.5, 52.0, 25.5, 22.3, 18.1. HRMS (ESI) m/z calculated for C20H22N3 [M + H]+ 304.1808, found 304.1811. IR (KBr film): ν 3025, 1654, 1602, 1494, 1249, 968, 728, 708 cm−1.
1 hexane/EtOAc). White solid (89.0 mg, 73%). Mp = 86–88 °C. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.38–7.28 (m, 4H), 7.16 (d, J = 6.4 Hz, 2H), 5.54 (s, 2H), 4.93 (m, 1H), 3.41 (d, J = 6.4 Hz, 2H), 1.65 (d, J = 1.6 Hz, 3H), 1.62 (d, J = 0.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 145.1, 135.2, 135.1, 132.6, 131.6, 128.9, 128.6, 128.1, 127.7, 127.4, 127.0, 118.5, 52.0, 25.5, 22.3, 18.1. HRMS (ESI) m/z calculated for C20H22N3 [M + H]+ 304.1808, found 304.1811. IR (KBr film): ν 3025, 1654, 1602, 1494, 1249, 968, 728, 708 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). White solid (74.5 mg, 53%). Mp = 100–102 °C. 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.2 Hz, 2H), 7.43 (t, J = 7.2 Hz, 2H), 7.38–7.26 (m, 6H), 7.25–7.18 (m, 5H), 6.21 (d, J = 16.0 Hz, 1H), 6.12 (dt, J = 16.0, 5.2 Hz, 1H), 5.58 (s, 2H), 3.61 (dd, J = 5.2, 1.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.9, 136.3, 135.0, 132.5, 131.3, 130.5, 129.0, 128.7, 128.6, 128.3, 129.9, 127.8, 127.3, 127.2, 126.2, 123.6, 52.2, 26.3. HRMS (ESI) m/z calculated for C24H21N3 [M + H]+ 352.1808, found 352.1812. IR (KBr film): ν 3082, 3025, 1955, 1654, 727 cm−1.
1 hexane/EtOAc). White solid (74.5 mg, 53%). Mp = 100–102 °C. 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.2 Hz, 2H), 7.43 (t, J = 7.2 Hz, 2H), 7.38–7.26 (m, 6H), 7.25–7.18 (m, 5H), 6.21 (d, J = 16.0 Hz, 1H), 6.12 (dt, J = 16.0, 5.2 Hz, 1H), 5.58 (s, 2H), 3.61 (dd, J = 5.2, 1.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.9, 136.3, 135.0, 132.5, 131.3, 130.5, 129.0, 128.7, 128.6, 128.3, 129.9, 127.8, 127.3, 127.2, 126.2, 123.6, 52.2, 26.3. HRMS (ESI) m/z calculated for C24H21N3 [M + H]+ 352.1808, found 352.1812. IR (KBr film): ν 3082, 3025, 1955, 1654, 727 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Colorless oil (87.2 mg, 77%). 1H NMR (400 MHz, CDCl3): δ 7.36–7.27 (m, 3H), 7.12 (dd, J = 8.0 Hz, 2.0 Hz, 2H), 5.72–5.58 (m, 1H), 5.46 (s, 2H), 5.07 (dq, J = 10.4 Hz, 1.6 Hz, 1H), 4.89 (dq, J = 16.8 Hz, 1.6 Hz, 1H), 3.22 (dt, J = 5.6 Hz, 1.6 Hz, 2H), 2.60 (t, J = 7.6 Hz, 2H), 1.66 (quintet, J = 6.4 Hz, 2H), 1.41–1.22 (m, 6H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, DMSO-d6): δ 144.8, 136.1, 133.3, 130.4, 128.6, 127.8, 127.2, 116.7, 50.6, 30.9, 28.9, 28.2, 25.9, 24.2, 22.0, 13.8. HRMS (ESI) m/z calculated for C18H25N3 [M + H]+ 284.2121, found 284.2127. IR (KBr film): ν 3065, 3033, 2928, 1640, 1456, 992, 729 cm−1.
1 hexane/EtOAc). Colorless oil (87.2 mg, 77%). 1H NMR (400 MHz, CDCl3): δ 7.36–7.27 (m, 3H), 7.12 (dd, J = 8.0 Hz, 2.0 Hz, 2H), 5.72–5.58 (m, 1H), 5.46 (s, 2H), 5.07 (dq, J = 10.4 Hz, 1.6 Hz, 1H), 4.89 (dq, J = 16.8 Hz, 1.6 Hz, 1H), 3.22 (dt, J = 5.6 Hz, 1.6 Hz, 2H), 2.60 (t, J = 7.6 Hz, 2H), 1.66 (quintet, J = 6.4 Hz, 2H), 1.41–1.22 (m, 6H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, DMSO-d6): δ 144.8, 136.1, 133.3, 130.4, 128.6, 127.8, 127.2, 116.7, 50.6, 30.9, 28.9, 28.2, 25.9, 24.2, 22.0, 13.8. HRMS (ESI) m/z calculated for C18H25N3 [M + H]+ 284.2121, found 284.2127. IR (KBr film): ν 3065, 3033, 2928, 1640, 1456, 992, 729 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (76.8 mg, 80%). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 6.8 Hz, 2H), 7.42 (t, J = 6.8 Hz, 2H), 7.33 (tt, J = 6.8 Hz, 1.2 Hz, 1H), 5.97 (m, 1H), 5.78 (m, 1H), 5.21 (dq, J = 10.0 Hz, 0.8 Hz, 1H), 5.14–5.08 (m, 2H), 4.94 (dq, J = 17.2 Hz, 0.8 Hz, 1H), 4.29 (t, J = 7.2 Hz, 2H), 3.58 (m, 2H), 2.70 (q, J = 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 133.5, 132.8, 131.6, 130.1, 128.8, 127.9, 127.3, 118.2, 117.9, 47.5, 34.4, 27.2. HRMS (ESI) m/z calculated for C15H18N3 [M + H]+ 240.1495, found 240.1502. IR (KBr film): ν 3080, 1640, 1495, 918, 766, 699 cm−1.
1 hexane/EtOAc). Pale yellow liquid (76.8 mg, 80%). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 6.8 Hz, 2H), 7.42 (t, J = 6.8 Hz, 2H), 7.33 (tt, J = 6.8 Hz, 1.2 Hz, 1H), 5.97 (m, 1H), 5.78 (m, 1H), 5.21 (dq, J = 10.0 Hz, 0.8 Hz, 1H), 5.14–5.08 (m, 2H), 4.94 (dq, J = 17.2 Hz, 0.8 Hz, 1H), 4.29 (t, J = 7.2 Hz, 2H), 3.58 (m, 2H), 2.70 (q, J = 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 133.5, 132.8, 131.6, 130.1, 128.8, 127.9, 127.3, 118.2, 117.9, 47.5, 34.4, 27.2. HRMS (ESI) m/z calculated for C15H18N3 [M + H]+ 240.1495, found 240.1502. IR (KBr film): ν 3080, 1640, 1495, 918, 766, 699 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (77.7 mg, 77%). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.41 (t, J = 7.2 Hz, 2H), 7.33 (t, J = 7.2 Hz, 1H), 5.95 (m, 1H), 5.79 (m, 1H), 5.20 (d, J = 10.0 Hz, 1H), 5.07 (d, J = 10.0 Hz, 1H), 5.03 (d, J = 17.2 Hz, 1H), 4.95 (d, J = 17.2 Hz, 1H), 4.23 (t, J = 7.2 Hz, 2H), 3.57 (m, 2H), 2.14 (q, J = 7.2 Hz, 2H), 2.04 (quin, J = 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 136.8, 132.8, 131.6, 130.0, 128.7, 127.8, 127.2, 117.8, 116.1, 47.4, 30.7, 29.1, 27.1. HRMS (ESI) m/z calculated for C16H20N3 [M + H]+ 254.1652, found 254.1660. IR (KBr film): ν 3079, 2979, 1640, 1495, 916, 765, 700 cm−1.
1 hexane/EtOAc). Pale yellow liquid (77.7 mg, 77%). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 2H), 7.41 (t, J = 7.2 Hz, 2H), 7.33 (t, J = 7.2 Hz, 1H), 5.95 (m, 1H), 5.79 (m, 1H), 5.20 (d, J = 10.0 Hz, 1H), 5.07 (d, J = 10.0 Hz, 1H), 5.03 (d, J = 17.2 Hz, 1H), 4.95 (d, J = 17.2 Hz, 1H), 4.23 (t, J = 7.2 Hz, 2H), 3.57 (m, 2H), 2.14 (q, J = 7.2 Hz, 2H), 2.04 (quin, J = 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 136.8, 132.8, 131.6, 130.0, 128.7, 127.8, 127.2, 117.8, 116.1, 47.4, 30.7, 29.1, 27.1. HRMS (ESI) m/z calculated for C16H20N3 [M + H]+ 254.1652, found 254.1660. IR (KBr film): ν 3079, 2979, 1640, 1495, 916, 765, 700 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Pale yellow liquid (69.5 mg, 65%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.65 (m, 2H), 7.43–7.39 (m, 2H), 7.34–7.31 (m, 1H), 5.95 (m, 1H), 5.76 (m, 1H), 5.20 (dd, J = 10.0 Hz, 0.8 Hz, 1H), 5.04–4.98 (m, 2H), 4.94 (dd, J = 8.8 Hz, 0.8 Hz, 1H), 4.23 (t, J = 7.6 Hz, 2H), 3.57 (m, 2H), 2.10 (q, J = 7.6 Hz, 2H), 1.94 (quin, J = 7.6 Hz, 2H), 1.47 (quin, J = 7.6 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 138.0, 132.8, 131.6, 129.9, 128.8, 127.8, 127.3, 117.8, 115.3, 48.0, 33.2, 29.5, 27.1, 25.9. HRMS (ESI) m/z calculated for C17H22N3 [M + H]+ 268.1808, found 268.1814. IR (KBr film): ν 3079, 2978, 1640, 1495, 914, 769, 698 cm−1.
1 hexane/EtOAc). Pale yellow liquid (69.5 mg, 65%). 1H NMR (400 MHz, CDCl3): δ 7.68–7.65 (m, 2H), 7.43–7.39 (m, 2H), 7.34–7.31 (m, 1H), 5.95 (m, 1H), 5.76 (m, 1H), 5.20 (dd, J = 10.0 Hz, 0.8 Hz, 1H), 5.04–4.98 (m, 2H), 4.94 (dd, J = 8.8 Hz, 0.8 Hz, 1H), 4.23 (t, J = 7.6 Hz, 2H), 3.57 (m, 2H), 2.10 (q, J = 7.6 Hz, 2H), 1.94 (quin, J = 7.6 Hz, 2H), 1.47 (quin, J = 7.6 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 145.3, 138.0, 132.8, 131.6, 129.9, 128.8, 127.8, 127.3, 117.8, 115.3, 48.0, 33.2, 29.5, 27.1, 25.9. HRMS (ESI) m/z calculated for C17H22N3 [M + H]+ 268.1808, found 268.1814. IR (KBr film): ν 3079, 2978, 1640, 1495, 914, 769, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light yellow solid (60.8 mg, 53%). Mp = 81–83 °C. 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 7.6 Hz, 2H), 7.74 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.44–7.36 (m, 2H), 7.31 (d, J = 7.6 Hz, 1H), 6.18 (dd, 17.6 Hz, 11.2 Hz, 1H), 5.73 (d, J = 17.6 Hz, 1H), 5.72–5.65 (m, 1H), 5.27 (d, J = 10.8 Hz, 1H), 5.02 (d, J = 10.0 Hz, 1H), 4.81 (d, J = 16.4 Hz, 1H), 3.43 (d, J = 5.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 144.9, 135.5, 133.7, 132.8, 132.3, 131.3, 130.9, 130.7, 128.9, 128.5, 128.1, 127.2 (2C), 126.4, 118.2, 117.9, 27.5. HRMS (ESI) m/z calculated for C19H18N3 [M + H]+ 288.1495, found 288.1492. IR (KBr film): ν 3063, 2964, 1640, 1494, 991, 769, 698 cm−1.
1 hexane/EtOAc). Light yellow solid (60.8 mg, 53%). Mp = 81–83 °C. 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 7.6 Hz, 2H), 7.74 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.44–7.36 (m, 2H), 7.31 (d, J = 7.6 Hz, 1H), 6.18 (dd, 17.6 Hz, 11.2 Hz, 1H), 5.73 (d, J = 17.6 Hz, 1H), 5.72–5.65 (m, 1H), 5.27 (d, J = 10.8 Hz, 1H), 5.02 (d, J = 10.0 Hz, 1H), 4.81 (d, J = 16.4 Hz, 1H), 3.43 (d, J = 5.2 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 144.9, 135.5, 133.7, 132.8, 132.3, 131.3, 130.9, 130.7, 128.9, 128.5, 128.1, 127.2 (2C), 126.4, 118.2, 117.9, 27.5. HRMS (ESI) m/z calculated for C19H18N3 [M + H]+ 288.1495, found 288.1492. IR (KBr film): ν 3063, 2964, 1640, 1494, 991, 769, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Light brown liquid (66.6 mg, 55%). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 6.8 Hz, 2H), 7.50 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.03 (dd, J = 16.8 Hz, 10.8 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 5.80 (m, 1H), 5.67 (dd, J = 17.2 Hz, 0.8 Hz, 1H), 5.63 (s, 2H), 5.42 (dd, J = 12.0 Hz, 0.8 Hz, 1H), 5.13 (d, J = 10.4 Hz, 1H), 4.88 (d, J = 17.2 Hz, 1H), 3.41 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.0, 136.7, 133.4, 132.3, 132.1, 131.4, 130.7, 128.9, 128.6, 128.4, 128.0, 127.5, 127.3, 126.8, 118.2, 117.8, 49.8, 27.1. HRMS (ESI) m/z calculated for C20H20N3 [M + H]+ 302.1652, found 302.1646. IR (KBr film): ν 3062, 3033, 1639, 1608, 1495, 919, 771, 699 cm−1.
1 hexane/EtOAc). Light brown liquid (66.6 mg, 55%). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 6.8 Hz, 2H), 7.50 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.03 (dd, J = 16.8 Hz, 10.8 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 5.80 (m, 1H), 5.67 (dd, J = 17.2 Hz, 0.8 Hz, 1H), 5.63 (s, 2H), 5.42 (dd, J = 12.0 Hz, 0.8 Hz, 1H), 5.13 (d, J = 10.4 Hz, 1H), 4.88 (d, J = 17.2 Hz, 1H), 3.41 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 146.0, 136.7, 133.4, 132.3, 132.1, 131.4, 130.7, 128.9, 128.6, 128.4, 128.0, 127.5, 127.3, 126.8, 118.2, 117.8, 49.8, 27.1. HRMS (ESI) m/z calculated for C20H20N3 [M + H]+ 302.1652, found 302.1646. IR (KBr film): ν 3062, 3033, 1639, 1608, 1495, 919, 771, 699 cm−1.Experimental procedure for ring closing metathesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 7.2 Hz, 2H), 7.44 (t, J = 7.2 Hz, 2H), 7.35 (t, J = 7.2 Hz, 1H), 5.75–5.73 (m, 2H), 4.71 (t, J = 6.0 Hz, 2H), 3.70–3.69 (m, 2H), 2.55–2.52 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 144.7, 133.2, 131.5, 129.0, 128.8, 127.9, 127.8, 123.2, 47.9, 27.9, 25.5. HRMS (ESI) m/z calculated for C13H14N3 [M + H]+ 212.1182, found 212.1185. IR (KBr film): ν 3058, 1660, 1624, 1498, 772, 699 cm−1.
1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 7.2 Hz, 2H), 7.44 (t, J = 7.2 Hz, 2H), 7.35 (t, J = 7.2 Hz, 1H), 5.75–5.73 (m, 2H), 4.71 (t, J = 6.0 Hz, 2H), 3.70–3.69 (m, 2H), 2.55–2.52 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 144.7, 133.2, 131.5, 129.0, 128.8, 127.9, 127.8, 123.2, 47.9, 27.9, 25.5. HRMS (ESI) m/z calculated for C13H14N3 [M + H]+ 212.1182, found 212.1185. IR (KBr film): ν 3058, 1660, 1624, 1498, 772, 699 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 7.6 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 1H), 5.79–5.67 (m, 2H), 4.53 (t, J = 5.2 Hz, 2H), 3.64 (d, J = 4.8 Hz, 2H), 1.90–1.82 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 144.4, 132.9, 131.7, 128.8, 128.4, 127.9, 127.8, 127.1, 47.2, 27.2, 24.4, 23.1. HRMS (ESI) m/z calculated for C14H16N3 [M + H]+ 226.1339, found 226.1336. IR (KBr film): ν 2929, 1665, 1626, 1447, 733, 698 cm−1.
1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 7.6 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 1H), 5.79–5.67 (m, 2H), 4.53 (t, J = 5.2 Hz, 2H), 3.64 (d, J = 4.8 Hz, 2H), 1.90–1.82 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 144.4, 132.9, 131.7, 128.8, 128.4, 127.9, 127.8, 127.1, 47.2, 27.2, 24.4, 23.1. HRMS (ESI) m/z calculated for C14H16N3 [M + H]+ 226.1339, found 226.1336. IR (KBr film): ν 2929, 1665, 1626, 1447, 733, 698 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Mp = 201–203 °C. 1H NMR (400 MHz, CDCl3): δ 7.68 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.36 (t, J = 7.6 Hz, 1H), 5.81 (m, 1H), 5.66 (m, 1H), 4.51 (t, J = 6.0 Hz, 2H), 3.61 (d, J = 7.6 Hz, 2H), 2.35–2.30 (m, 2H), 2.01 (quin, J = 6.4 Hz, 2H), 1.67–1.61 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 145.2, 131.7, 131.5, 131.0, 128.9, 128.0, 127.6, 126.3, 45.8, 25.9, 25.1, 23.0, 21.9. HRMS (ESI) m/z calculated for C15H18N3 [M + H]+ 240.1495, found 240.1498. IR (KBr film): ν 2959, 2932, 1491, 733, 720, 697 cm−1.
1 hexane/EtOAc). Mp = 201–203 °C. 1H NMR (400 MHz, CDCl3): δ 7.68 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.36 (t, J = 7.6 Hz, 1H), 5.81 (m, 1H), 5.66 (m, 1H), 4.51 (t, J = 6.0 Hz, 2H), 3.61 (d, J = 7.6 Hz, 2H), 2.35–2.30 (m, 2H), 2.01 (quin, J = 6.4 Hz, 2H), 1.67–1.61 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 145.2, 131.7, 131.5, 131.0, 128.9, 128.0, 127.6, 126.3, 45.8, 25.9, 25.1, 23.0, 21.9. HRMS (ESI) m/z calculated for C15H18N3 [M + H]+ 240.1495, found 240.1498. IR (KBr film): ν 2959, 2932, 1491, 733, 720, 697 cm−1.6x′: TLC: Rf 0.17 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 4H), 7.42 (t, J = 7.2 Hz, 4H), 7.34 (t, J = 7.2 Hz, 2H), 6.00–5.91 (m, 2H), 5.39–5.35 (m, 2H), 5.21 (d, J = 10.4 Hz, 2H), 4.95 (d, J = 17.2 Hz, 2H), 4.25–4.22 (m, 4H), 3.59–3.57 (m, 4H), 2.09–2.01 (m, 4H), 1.96–1.89 (m, 4H), 1.43 (quin, J = 7.6 Hz, 4H). 13C NMR (100 MHz, CDCl3): δ 145.2, 132.8, 131.4, 130.3, 130.1, 128.9, 128.0, 127.4, 117.9, 48.2, 32.0, 29.6, 27.2, 26.6. HRMS (ESI) m/z calculated for C33H39N6 [M + H]+ 507.3231, found 507.3232. IR (KBr film): ν 2925, 1660, 1600, 1495, 917, 770, 698 cm−1.
1 hexane/EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.2 Hz, 4H), 7.42 (t, J = 7.2 Hz, 4H), 7.34 (t, J = 7.2 Hz, 2H), 6.00–5.91 (m, 2H), 5.39–5.35 (m, 2H), 5.21 (d, J = 10.4 Hz, 2H), 4.95 (d, J = 17.2 Hz, 2H), 4.25–4.22 (m, 4H), 3.59–3.57 (m, 4H), 2.09–2.01 (m, 4H), 1.96–1.89 (m, 4H), 1.43 (quin, J = 7.6 Hz, 4H). 13C NMR (100 MHz, CDCl3): δ 145.2, 132.8, 131.4, 130.3, 130.1, 128.9, 128.0, 127.4, 117.9, 48.2, 32.0, 29.6, 27.2, 26.6. HRMS (ESI) m/z calculated for C33H39N6 [M + H]+ 507.3231, found 507.3232. IR (KBr film): ν 2925, 1660, 1600, 1495, 917, 770, 698 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Mp = 96–98 °C. 1H NMR (400 MHz, CDCl3): δ 8.15 (dd, J = 7.6 Hz, 1.2 Hz, 1H), 7.71 (d, J = 7.6 Hz, 2H), 7.50–7.41 (m, 4H), 7.41–7.36 (m, 2H), 6.65 (d, J = 10.4 Hz, 1H), 6.18 (m, 1H), 3.51 (d, J = 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 142.2, 135.0, 134.9, 130.9, 130.8, 130.2, 129.0, 128.8, 128.7, 128.2, 128.1, 127.9, 127.6, 124.0, 21.2. HRMS (ESI) m/z calculated for C17H14N3 [M + H]+ 260.1182, found 260.1179. IR (KBr film): ν 2919, 1700, 1600, 1491, 992, 772, 699 cm−1.
1 hexane/EtOAc). Mp = 96–98 °C. 1H NMR (400 MHz, CDCl3): δ 8.15 (dd, J = 7.6 Hz, 1.2 Hz, 1H), 7.71 (d, J = 7.6 Hz, 2H), 7.50–7.41 (m, 4H), 7.41–7.36 (m, 2H), 6.65 (d, J = 10.4 Hz, 1H), 6.18 (m, 1H), 3.51 (d, J = 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 142.2, 135.0, 134.9, 130.9, 130.8, 130.2, 129.0, 128.8, 128.7, 128.2, 128.1, 127.9, 127.6, 124.0, 21.2. HRMS (ESI) m/z calculated for C17H14N3 [M + H]+ 260.1182, found 260.1179. IR (KBr film): ν 2919, 1700, 1600, 1491, 992, 772, 699 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/EtOAc). Mp = 159–161 °C. 1H NMR (400 MHz, CDCl3): δ 7.58–7.54 (m, 3H), 7.45–7.38 (m, 3H), 7.37–7.35 (m, 2H), 7.30 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 10.4 Hz, 1H), 6.03 (dt, J = 10.4 Hz, 7.6 Hz, 1H), 5.49 (s, 2H), 3.30 (d, J = 8.0 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 144.8, 139.2, 134.0, 131.5, 131.3, 130.9, 129.3, 128.8, 128.7, 128.6, 128.2, 128.0, 127.7, 126.2, 53.4, 22.6. HRMS (ESI) m/z calculated for C18H16N3 [M + H]+ 274.1339, found 274.1336. IR (KBr film): ν 3025, 2360, 1494, 995, 754, 735, 699 cm−1.
1 hexane/EtOAc). Mp = 159–161 °C. 1H NMR (400 MHz, CDCl3): δ 7.58–7.54 (m, 3H), 7.45–7.38 (m, 3H), 7.37–7.35 (m, 2H), 7.30 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 10.4 Hz, 1H), 6.03 (dt, J = 10.4 Hz, 7.6 Hz, 1H), 5.49 (s, 2H), 3.30 (d, J = 8.0 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 144.8, 139.2, 134.0, 131.5, 131.3, 130.9, 129.3, 128.8, 128.7, 128.6, 128.2, 128.0, 127.7, 126.2, 53.4, 22.6. HRMS (ESI) m/z calculated for C18H16N3 [M + H]+ 274.1339, found 274.1336. IR (KBr film): ν 3025, 2360, 1494, 995, 754, 735, 699 cm−1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A2A2A01004511).Notes and references
- S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef PubMed.
- For selected references, see: (a) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef PubMed; (b) J. Hou, X. Liu, J. Shen, G. Zhao and P. G. Wang, Expert Opin. Drug Discovery, 2012, 7, 489 CrossRef PubMed; (c) L. Liang and D. Astruc, Coord. Chem. Rev., 2011, 255, 2933 CrossRef; (d) V. Aragao-Leoneti, V. Campo, A. S. Gomes, R. A. Field and I. Carvalho, Tetrahedron, 2010, 66, 9475 CrossRef; (e) J. Lahann, Click Chemistry for Biotechnology and Materials Science, Wiley, Chichester, 2009 Search PubMed.
- K. M. Faraz, V. Garima, A. Wasim, M. Akranth, A. M. Mumtaz, A. Mymoona, H. Asif, H. S. Misbahul, S. Mohammad and H. S. Rashiduddin, Int. J. Drug Dev. Res., 2017, 9, 22 Search PubMed.
- (a) J. F. Lutz, Angew. Chem., Int. Ed., 2008, 47, 2182 CrossRef CAS PubMed; (b) J. F. Lutz and H. G. Borner, Prog. Polym. Sci., 2008, 33, 1 CrossRef; (c) R. J. Pieters, D. T. S. Rijkers and R. M. J. Liskamp, QSAR Comb. Sci., 2007, 26, 1181 CrossRef CAS; (d) A. J. Dirks, J. Cornelissen, F. L. van Delft, J. C. M. van Hest, R. J. M. Nolte, A. E. Rowan and F. Rutjes, QSAR Comb. Sci., 2007, 26, 1200 CrossRef CAS; (e) J. M. Baskin and C. R. Bertozzi, QSAR Comb. Sci., 2007, 26, 1211 CrossRef; (f) C. M. Salisbury and B. F. Cravatt, QSAR Comb. Sci., 2007, 26, 1229 CrossRef CAS.
- (a) H. Nandivada, X. W. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS; (b) D. Fournier, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1369 RSC; (c) P. L. Golas and K. Matyjaszewski, QSAR Comb. Sci., 2007, 26, 1116 CrossRef CAS; (d) W. H. Binder and C. Kluger, Curr. Org. Chem., 2006, 10, 1791 CrossRef.
- (a) J. F. Lutz and H. Schlaad, Polymer, 2008, 49, 817 CrossRef CAS; (b) P. Wu and V. V. Fokin, Aldrichimica Acta, 2007, 40, 7 CAS; (c) K. A. Kalesh, P. Y. Yang, R. Srinivasan and S. Q. Yao, QSAR Comb. Sci., 2007, 26, 1135 CrossRef CAS; (d) F. Santoyo-Gonz lez and F. Hernandez-Mateo, Top. Heterocycl. Chem., 2007, 7, 133 Search PubMed; (e) V. D. Bock, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2005, 51 Search PubMed; (f) Z. Zhou and C. J. Fahrni, J. Am. Chem. Soc., 2004, 126, 8862 CrossRef CAS PubMed; (g) W. G. Lewis, F. G. Magallon, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2004, 126, 9152 CrossRef CAS PubMed; (h) K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill and Q. Wang, Org. Lett., 2004, 6, 4603 CrossRef CAS PubMed.
- For selected reviews, see: (a) O. S. Miljanic, W. R. Dichtel, I. Aprahamian, R. D. Rohde, H. D. Agnew, J. R. Heath and J. F. Stoddart, QSAR Comb. Sci., 2007, 26, 1165 CrossRef CAS; (b) I. Aprahamian, O. S. Miljanic, W. R. Dichtel, K. Isoda, T. Yasuda, T. Kato and J. F. Stoddart, Bull. Chem. Soc. Jpn., 2007, 80, 1856 CrossRef CAS; (c) A. B. Braunschweig, W. R. Dichtel, O. S. Miljanic, M. A. Olson, J. M. Spruell, S. I. Khan, J. R. Heath and J. F. Stoddart, Chem.–Asian J., 2007, 2, 634 CrossRef CAS PubMed; for selected papers see: (d) W. B. Zhang, Y. Tu, R. Ranjan, R. M. VanHorn, S. Leng, J. Wang, M. J. Polce, C. Wesdemiotis, R. P. Quirk, G. R. Newkome and S. Z. D. Cheng, Macromolecules, 2008, 41, 515 CrossRef CAS; (e) J. S. Marois, K. Cantin, A. Desmarais and J. F. Morin, Org. Lett., 2008, 10, 33 CrossRef CAS PubMed; (f) V. Aucagne, K. D. Haenni, D. A. Leigh, P. J. Lusby and D. B. Walker, J. Am. Chem. Soc., 2006, 128, 2186 CrossRef CAS PubMed; (g) R. K. O'Reilly, M. J. Joralemon, C. J. Hawker and K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5203 CrossRef; (h) D. D. Diaz, K. Rajagopal, E. Strable, J. Schneider and M. G. Finn, J. Am. Chem. Soc., 2006, 128, 6056 Search PubMed.
- J. Gierlich, G. A. Burley, P. M. E. Gramlich, D. M. Hammond and T. Carell, Org. Lett., 2006, 8, 3639 CrossRef CAS PubMed.
- A. Nuzzi, A. Massi and A. Dondoni, QSAR Comb. Sci., 2007, 26, 1191 CAS.
- (a) X. Deng, J. Liang, B. B. Allison, C. Dvorak, H. McAllister, B. M. Savall and N. S. Mani, J. Org. Chem., 2015, 80, 11003 CrossRef CAS PubMed; (b) W. S. Brotherton, R. J. Clark and L. Zhu, J. Org. Chem., 2012, 77, 6443 CrossRef CAS PubMed; (c) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Synlett, 2012, 23, 2179 CrossRef CAS; (d) V. Malnuit, M. Duca, A. Manout, K. Bougrin and R. Benhida, Synlett, 2009, 2123 CAS; (e) L. Li, G. Zhang, A. Zhu and L. Zhang, J. Org. Chem., 2008, 73, 3630 CrossRef CAS PubMed; (f) D. Yang, N. Fu, Z. Liu, Y. Li and B. Chen, Synlett, 2007, 278 CAS; (g) Y.-M. Wu, J. Deng, Y. L. Li and Q.-Y. Chen, Synthesis, 2005, 1314 CrossRef CAS.
- (a) D. N. Barsoum, C. J. Brassard, J. H. A. Deeb, N. Okashah, K. Sreenath, J. T. Simmons and L. Zhu, Synthesis, 2013, 45, 2372 CrossRef CAS; (b) L. Li, R. Li, A. Zhu, G. Zhang and L. Zhang, Synlett, 2011, 874 CrossRef; (c) J. C. Morris, J. Chiche, C. Grellier, M. Lopez, L. F. Bornaghi, A. Maresca, C. T. Supuran, J. Pouyssegur and S.-A. Poulsen, J. Med. Chem., 2011, 54, 6905 CrossRef CAS PubMed; (d) N. W. Smith, B. P. Polenz, S. B. Johnson and S. V. Dzyuba, Tetrahedron Lett., 2010, 51, 550 CrossRef CAS; (e) V. Malnuit, M. Duca, A. Manout, K. Bougrin and R. Benhida, Synlett, 2009, 2123 CAS; (f) L. Li, G. G. Zhang, A. Zhu and L. Zhang, J. Org. Chem., 2008, 73, 3630 CrossRef CAS PubMed; (g) N. Joubert, R. F. Schinazib and L. A. Agrofoglio, Tetrahedron, 2005, 61, 11744 CrossRef CAS; (h) Y. Wu, J. Deng, Y. Li and Q.-Y. Chen, Synthesis, 2005, 1314 CrossRef CAS.
- B. Wang, J. Zhang, X. Wang, N. Liu, W. Chen and Y. Hu, J. Org. Chem., 2013, 78, 10519 CrossRef CAS PubMed.
- B. Wang, N. Liu, C. Shao, Q. Zhang, X. Wang and Y. Hu, Adv. Synth. Catal., 2013, 355, 2564 CrossRef CAS.
- (a) C. Shao, X. Wang, Q. Zhang, S. Luo, J. Zhao and Y. Hu, J. Org. Chem., 2011, 76, 6832 CrossRef CAS PubMed; (b) C. Shao, X. Wang, J. Xu, J. Zhao, Q. Zhang and Y. Hu, J. Org. Chem., 2010, 75, 7002 CrossRef CAS PubMed; (c) C. Shao, G. Cheng, D. Su, J. Xu, X. Wang and Y. Hu, Adv. Synth. Catal., 2010, 352, 1587 CrossRef CAS; (d) S. S. Y. Chui, M. F. Y. Ng and C.-M. Che, Chem.–Eur. J., 2005, 11, 1739 CrossRef CAS PubMed.
- E. C. Royer, M. C. Barral, V. Moreno and A. Santos, J. Inorg. Nucl. Chem., 1981, 43, 705 CrossRef CAS.
- (a) M. S. Singh, S. Chowdhury and S. Koley, Tetrahedron, 2016, 72, 5257 CrossRef CAS; (b) Y. L. Angell and K. Burgess, Chem. Soc. Rev., 2007, 36, 1674 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for 3a–z and 6v–z. See DOI: 10.1039/c7ra12889d |
| This journal is © The Royal Society of Chemistry 2018 |