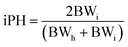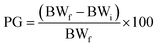Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deconvoluting the dual hypoglycemic effect of wedelolactone isolated from Wedelia calendulacea: investigation via experimental validation and molecular docking†
Vikas Kumar a,
Kalicharan Sharmab,
Bahar Ahmedb,
F. A. Al-Abbasic,
Firoz Anwarc and
Amita Verma
a,
Kalicharan Sharmab,
Bahar Ahmedb,
F. A. Al-Abbasic,
Firoz Anwarc and
Amita Verma *d
*d
aNatural Product Drug Discovery Laboratory, Department of Pharmaceutical Sciences, Faculty of Health Sciences, Sam Higginbottom Institute of Agriculture, Technology & Sciences, Allahabad, Uttar Pradesh, India – 211007
bDepartment of Pharmaceutical Chemistry, SPER, Jamia Hamdard, New Delhi-110062, India
cDepartment of Biochemistry, King Abdulaziz University, Jeddah-21589, Kingdom of Saudi Arabia
dBioorganic & Medicinal Chemistry Research Laboratory, Department of Pharmaceutical Sciences, Faculty of Health Sciences, Sam Higginbottom University of Agriculture, Technology & Sciences, Allahabad-211007, Uttar Pradesh, India. E-mail: amitaverma.dr@gmail.com; amita.verma@shiats.edu.in
First published on 18th May 2018
Abstract
Wedelia calendulacea has a long history of use in the Indian Ayurvedic System of Medicine for the treatment, prevention, and cure of a diverse range of human diseases such as diabetes obesity, and other metabolic diseases. A wide range of chemical constituents, such as triterpenoid saponin, kauren diterpene, and coumestans, has been isolated from the plant. Conversely, no published literature is available in relation to the isolation of wedelolactone (WEL) for its anti-diabetic effect. The aim of the present study was to isolate the bioactive phyto-constituent from Wedelia calendulacea and to scrutinize the antidiabetic effect with its possible mechanism of action. The structure of the isolated compound was elucidated by different spectroscopy techniques. Proteins, such as dipeptidyl peptidase-4 (DPPIV), glucose transporter 1 (GLUT1), and peroxisome proliferator-activated receptors-γ (PPARγ), were also subjected to in silico docking. Later, this isolated compound was scrutinized against α-glucosidase and α-amylase enzyme activity along with an oral glucose tolerance test (OGTT) for estimation of glucose utilization. Streptozotocin (STZ) was used for the induction of type II diabetes mellitus (DM) in Wistar rats. The rats were divided into different groups and received the WEL (5, 10, and 20 mg kg−1, b.w.) and glibenclamide (2.5 mg kg−1, b.w.) for 28 days. The blood glucose level (BGL), plasma insulin, and body weight were determined at regular time intervals. The serum lipid profile hypolipidemic effect for the different antioxidant markers and hepatic tissue markers were scrutinized along with an inflammatory mediator to deduce the possible mechanism. With the help of spectroscopy techniques, the isolated compound was identified as wedelolactone. In the docking study, WEL showed docking scores of −6.17, −9.43, and −7.66 against DPP4, GLUTI, and PRARY, respectively. WEL showed the inhibition of α-glucosidase (80.65%) and α-amylase (93.83%) and suggested an effect on postprandial hyperglycemia. In the OGTT, WEL significantly (P < 0.001) downregulated the BGL, a marker for better utilization of drugs. In the diabetes model, WEL reduced the BGL and enhanced the plasma insulin and body weight. It also significantly (P < 0.001) modulated the lipid profile; this suggested an anti-hyperlipidemia effect. WEL significantly (P < 0.001) distorted the hepatic tissue, acting as an antioxidant marker in a dose-dependent manner. WEL significantly (P < 0.001) downregulated the C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6) level. On the basis of the available results, we can conclude that WEL can be an alternative drug for the treatment of type II DM either by inhibiting the production of inflammatory mediator or by the downregulation of oxidative stress.
Introduction
Diabetes mellitus (DM) is a metabolic disease exemplified through the upregulation of BGL causing either an expansion of insulin deficiency or insulin resistance. DM is connected with the development of macrovascular complications, which can further turn and progress into cardiovascular disorders. Nowadays, due to changes in lifestyles, increased obesity, and an aging population, the frequency and occurrence of diabetes is rising.1,2 According to the World Health Organization (WHO), millions of people suffer from diabetes, with the number of cases increasing every year, especially in the middle-income countries. Epidemiological studies have suggested that type II diabetes has a higher disease frequency compared to type I diabetes.3 Only 10% of patients suffer from type I diabetes, while 90% suffer from type II diabetes among all types of diabetes.4,5 A cost evaluation from a recent review study showed that the cost of diabetes treatment is more than the US$ 827 billion, annually. A comparison of the life expectancy for diabetic patients shows it is 10 years less than for non-diabetics. If the pace of this disease maintains the same, it could reach 360–380 million people affected globally by 2025–2030. Therefore, the diabetes disease is a global burden and requires prompt attention. According to the Indian Task Force on Diabetes Care in India, there is a 9% incidence of diabetes in urban areas and 3% in rural areas.6,7Various investigations have suggested that enzymes, namely DPPIV, α-amylase, protein tyrosine phosphatase 1B (PTP1B), and α-glucosidase, can be targeted for the treatment of type II DM. DPPIV is considered as the prime target for glycemic control, and works by inactivation of the glucagon inhibitory peptide, glucagon-like peptide (GLP-1), and incretin hormones. DPP-4 inhibition not only affects the incretins breakdown, but also influences the cytokines and chemokines. A continuous inhibition of DPPIV has a beneficial effect on decreasing HbA1c and postprandial glucose levels.8,9 The prolonged inhibition of DPPIV enzymes suggests a novel mechanism to treat type II DM. Such an inhibition is a target for these enzymes for the activation of glucose transport GLUT4 in cell plasma membranes, activation of AMP kinase, and upregulation of the glucose-6P and glycogen stores. Moreover, researchers have diverted their research toward the inhibition of PTP1B, which circulate the insulin signaling via dephosphorylating the phosphotyrosine residue on insulin receptors.8,10
Nowadays, synthetic drugs and insulin therapy are used for the treatment of type II DM. Conversely, these types of treatment have their own side effects, such as weight gain, dropsy, and drug resistance. Due to the limitation of available drugs for the treatment of DM and the limitations in accessing public health systems in low-income communities, patients are attracted to alternative therapies with fewer side complications. According to reports in the ancient literature, more than 800 medicinal plants have been reported to have antidiabetic activity, while ethnopharmacological survey reports show that more than 1200 medicinal plants are used for diabetes mellitus.11,12 Presently, alternative systems of therapies are gaining more popularity due to their high margin of safety profiles. A lot of medicinal plants are available for the treatment of DM. A few of these plants have already been systematically and scientifically screened for antidiabetic potential.
There exist 65 species of Wedelia distributed in warm and tropical temperate regions, such as India, China, Ceylon, Japan, and Burma. Wedelia calendulacea (Compositae) is a perennial herb commonly cultivated in marshy and wet places in Uttar Pradesh, Arunachal Pradesh, and Assam in India and is also found in the coastal areas of Japan and the rest of the World.13 Traditionally, the plant is used as a deobstruent, cholagogue, and in the treatment of hepatics and its disorders. It's also used in the Ayurvedic system of medicine as a Hepatoprotective drug. The Vaidyas (Ayurvedic physician in Tamilnadu) and other traditional physicians use this plant in the treatment of various liver disorders in the form of a liver tonic.13,14 The leaves are used in the treatment of cephalalgia, cough, and skin disease in the form of a cream and tonic. Further, it is also used for its effect on swelling, the central nervous system, coughs, baldness, skin disease, and diarrhea.15,16
Norwedelolactone and norwedelic acid have also been isolated from the plant,17 while three bisdesmosidic oleanolic acid saponins have been isolated from the plant (fresh plant), one of which is a new bisdesmosidic oleanolic acid saponin. The second saponin has been identified as a known ginsenoside. The structure of the third saponin has not been fully worked out but it appears to be a bisdesmosidic oleanolic acid glycoside with a β-D-glucosidal moiety at position-28 of oleanolic acid.
No detailed anti-diabetic effect of isolated wedelolactone from Wedelia calendulacea had been previously scrutinized against STZ-induced type II DM. Therefore, in the current study, we investigated the anti-diabetic effect of isolated wedelolactone to find the possible mechanism of action. In the current study, we hypothesized that wedelolactone produced its anti-diabetic effect to either inhibit the production of inflammatory mediator or by downregulation of oxidative stress.
Material and methods
Plant material
The plant material Wedelia calendulacea was collected from the market and authenticated by Dr M. P. Sharma, Department of Botany, Jamia Hamdard, and the prepared herbarium was submitted to the department for further reference.Extraction and isolation
The collected Wedelia calendulacea plant material was dried in the shade and crushed to produce a coarse powder. The obtained powder was subjected to methanol for further extraction. The ethanolic extract was further fractionated with petroleum ether, chloroform, and methanol.18The methanolic extract was used for column chromatography in a silica gel (60–120 mesh) slurry and the column was packed with petroleum ether. The packed column was eluted via using different polarity solvents (pet ether, chloroform, and methanol).
Dry lab study (in silico docking)
A molecular docking study was carried out using the Schrodinger software suite (Maestro, version 9.4). The build panel was used to prepare the 3D sketched format of the ligand and docking was used for preparing the ligrep application. For the molecular docking study, the protein (PDB ID: 2G63, 5EQG and 2PRG) was selected from the protein data bank, followed by removing the solvent and adding the hydrogen, for minimization of the presence of the standard ligand via the preparation of a protein wizard. For validation of the docking study, the standard (glibenclamide) was re-docked to calculate the RMSD.In vitro α-glucosidase activity
The inhibitory α-glucosidase effect of WEL was scrutinized by using the reported model of Ahmed et al. (2014) and Kumar et al. (2015)19,20 with minor modification. A 96-well microtiter plate was used for estimation of the inhibition of α-glucosidase enzymes. The different concentrations of WEL were mixed with the enzyme (20 μl) solution, containing 0.8 U ml−1 of α-glucosidase in 0.01 M PBS, and then incubated at room temperature for 15 min. To terminate the reaction, 0.2 M Na2CO3 was mixed in PBS (0.1 M) and the absorbance of the mixture was estimated at 405 nm. The results are presented herein as a percentage of the inhibition of α-glucosidase and α-amylase via using the following formula:α-Amylase activity
In order to scrutinize the effect of WEL, α-amylase enzymes activity was assessed using the reported model of Ali et al. (2006)21 with minor modification. Briefly, the test sample (WEL and acarbose) was mixed and pre-incubated with sodium phosphate (20 mM; pH = 6.7) for 5 min and the final volume was made up to 2 ml via adding the starch (2% w/v) and the mixture was then incubated again for 5 min at room temperature. After the incubation period, di-nitro salicylic acid (1 ml) was added. The reaction mixture was kept in a boiling water bath for 5 min. After that, the reaction mixture was cooled down in an ice bath and again deionized water was added and the absorbance of the reaction mixture was estimated at 540 nm. The percentage of inhibition was determined via using the pre-determined formula.Animals
Wistar rats (150–180 g, body weight; same age) were used for the current experimentation study. The rats were kept in single polyethylene cages and retained under standard laboratory conditions (40–60% humidity; 22 ± 5 °C and 12/12 h day/night cycle). The rats received a standard diet (food chew), purchased from Hindustan Liver Limited, Mumbai, India, and received water ad libitum. The current research protocol was approved by the Institutional Animal Ethical Committee (IAEC) as per the instruction for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (IAEC/SHUATS/PA/2017XII/SVS03).Oral glucose tolerance test (OGTT test)
Kumar et al. and Ahmed et al.'s methods was used for the oral glucose tolerance test with minor modification.19,22 Swiss albino Wistar rats were grouped in seven groups:Group I: normal and treated with saline only,
Group II: normal and treated with WEL (20 mg kg−1),
Group III: glucose and treated with saline only (1 ml kg−1),
Group IV: glucose and treated with WEL (5 mg kg−1),
Group V: glucose and treated with WEL (10 mg kg−1),
Group VI: glucose and treated with WEL (20 mg kg−1),
Group VII: glucose and treated with glibenclamide (2.5 mg kg−1).
The blood glucose levels of all the groups rats were determined at 0 h and after that, all the groups rats received glucose (2 mg kg−1), except NC and NC received WEL (20 mg kg−1). Blood samples of all the groups rats were withdrawn at regular intervals (0, 30 60, 90, and 120 min) to determine the blood glucose level (BGL).
Experimental protocol
The rats were grouped as following, with each group containing 6 rats:Group I: normal and treated with saline only (1 ml kg−1 per day) for 28 days,
Group II: normal and treated with WEL (20 mg kg−1 per day) for 28 days,
Group III: diabetic and treated with saline only (1 ml kg−1 per day) for 28 days,
Group IV–VI: diabetic rats treated with WEL (5, 10, and 20 mg kg−1 per day) for 28 days,
Group VII: diabetic and treated with glibenclamide (2.5 mg kg−1 per day) for 28 days.
The rats received the above-discussed treatment for 28 days. The body weight and food intake were monitored at regular intervals. The BGL and plasma insulin levels were also determined at a regular interval.23
Ponderal homogeneity index (iPH) and ponderal grain (PG)
The ponderal homogeneity index and the ponderal gain were estimated by using the formula:where BWi = initial body weight, BWh = highest body weight.
where BWi = body weight (initial), BWf = body weight (final).
Biochemical parameters
Blood samples were collected by retro-orbital plexus puncture, and then centrifuged (5000 rpm) for 15 min to collect the serum for estimation of blood glucose, plasma level, and other parameters. The hepatic parameters, namely alkaline phosphatase (AST), aspartate aminotransferase (AST), alanine transaminase (ALT); serum renal parameters, including blood urea nitrogen (BUN), total protein, creatinine, albumin; lipid parameters, such as total cholesterol (TC), high density lipoprotein (HDL), triglyceride (TG), very low density lipoprotein (VLDL) and, low density lipoprotein (LDL), were scrutinized using commercially available diagnostic kits. The atherogenic index and coronary risk index were scrutinized as per the given formula:Antioxidant parameters
The antioxidant parameters, such as malonaldehyde (MDA), glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT), were determined by the reported method of Anwar et al., Afzal et al., Khan et al., Kumar et al., and Verma et al., with minor modification.24–31Inflammatory and pro-inflammatory mediators
Pro-inflammatory cytokines, namely interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and inflammatory mediators, such as C-reactive protein (CRP), were also estimated as per the instructions in the available commercial kits.Statistical analysis
In the experimental study, all the data are reported with the mean SEM and analysis of variance (ANOVA) via using the Graph Pad Prism software version 5.0 (U. S. A), where P values such as p < 0.05, p < 0.01, and p < 0.001 are considered as significant.Results
Characterization of compound WC-1
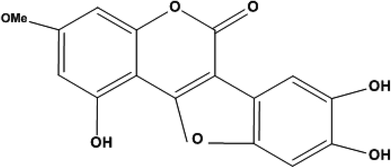
Elution of the column with CHCl3–MeOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) yielded WEL (Fig. 1) as a light green amorphous powder, 95 mg (Fig. 2).
5) yielded WEL (Fig. 1) as a light green amorphous powder, 95 mg (Fig. 2).
Rf: 0.58 (CHCl3–MeOH: 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
M.p.: >300 °C.
UV: λmax 352, 304, 294, 249 nm (ESI Fig. 1†).
IR (KBr): νmax 3305 (OH), 1717 (δ-lactone carbonyl), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1200 (C–O, furan), 1060 (C–O, phenolic), 1448, 1319, 1149, 953, 863 cm−1 (ESI Fig. 2†).
C), 1200 (C–O, furan), 1060 (C–O, phenolic), 1448, 1319, 1149, 953, 863 cm−1 (ESI Fig. 2†).
1D NMR (DMSO): shown in Table 1 (ESI Fig. 3, 4 and Table 1† shows the NMR data).
| Position | 1H NMR | 13C NMR |
|---|---|---|
| 1 | — | — |
| 2 | — | 161 |
| 3 | — | 154 |
| 4 | — | 100.8 |
| 5 | — | 153 |
| 6 | 6.38 (d, J = 2.0) | 97.1 |
| 7 | — | 158 |
| 8 | 6.38 (d, J = 2.0) | 97.6 |
| 9 | — | 95.8 |
| 10 | 7.08 s | 91.9 |
| 11 | — | 143.8 |
| 12 | — | 142.7 |
| 13 | 7.26 s | 103 |
| 14 | — | 157 |
| 15 | 3.73 s | 54.2 |
| — | 8.98 brs at 11,12 (OH) | — |
| — | 10.58 s at 5 (OH) | — |
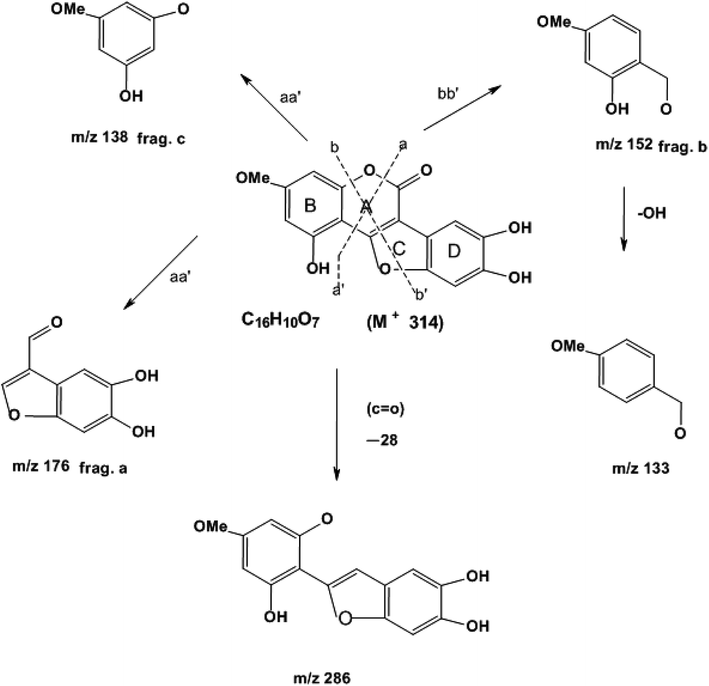
EIMS (probe) 70 eV, m/z % (rel. int): 314 (M)+ (C16H10O7) (100), 286 (15), 176 (70), 152 (20), 138 (58) (ESI Fig. 5†).
Dry lab activity (in silico docking)
Molecular docking studies were carried by the automated docking protocol. Fig. 3 shows the binding poses of the WEL and glibenclamide. As can be observed, the docking program positioned compounds WEL to make hydrogen bonds with His740 and arg125 and also makes pi–pi stacking with tyr666 against a backbone of DPP4 protein. This gives a better interaction with the backbone of the DPP4 protein due to its hydrophilic interaction and pi–pi interaction with the amino acid residue of DPP4 protein. We further validated the interaction via superimposing our ligand WEL with glibenclamide and observed that the binding orientation of our ligand was almost similar to WEL (Table 2).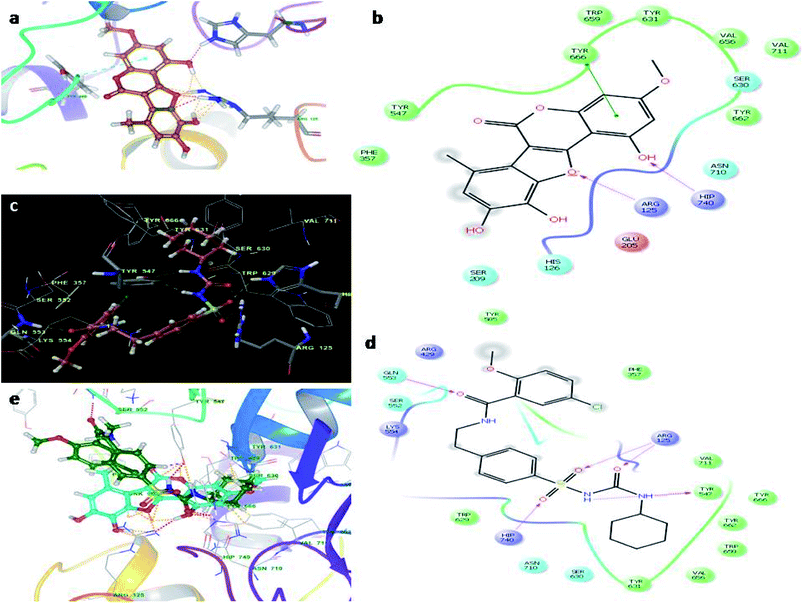 | ||
| Fig. 3 Molecular docking studies of wedelolactone and glibenclamide against DPP4 catalytic site (PDBID-2G63). (a) Binding interaction of WEL, (b) Ligplot of WEL against DPP4 catalytic site, (c) binding interaction of glibenclamide against DPP4 catalytic site, (d) Ligplot of glibenclamide against DPP4 and (e) superimposition of WEL with glibenclamide against DPP4 catalytic domain. | ||
| S. no | Ligands | Docking score | ||
|---|---|---|---|---|
| DPP4 | GLUT1 | PPARY | ||
| 1 | Wedelolactone | −6.23 | −8.98 | −7.98 |
| 2 | Glibenclamide | −6.05 | −8.29 | −6.73 |
Fig. 4 illustrates the binding pose of the WEL and glibenclamide ligands. The docking program positioned compounds WEL to make hydrogen bonds with Gln283, Asn288, and Asn415 against the backbone of the GLUT1 protein. Here, a better interaction with the backbone of GLUT1 protein was observed due hydrophilic interactions and pi–pi interactions with the amino acid residue of the GLUT1 protein. We further validated the interaction by superimposing our ligand (WEL) with the glibenclamide (standard) ligand and observed that the binding orientation of our ligand was similar to that of WEL. Fig. 5 reveals the binding poses of the WEL and glibenclamide ligands. Fig. 5 also shows the docking positioned compounds where WEL makes hydrogen bonds with arg288 and tyr327 and also gives pi–pi stacking with Arg288 against the backbone of the PPARY protein. Herein, WEL gives better interaction with the backbone of PPARY protein because it exhibits a hydrophilic interaction and pi–pi interaction with the amino acid residue of the PPARY protein. We further validated the interaction by superimposing our ligand (WEL) with the glibenclamide (standard) ligand and observed that the binding orientation of our ligand was similar to the WEL.
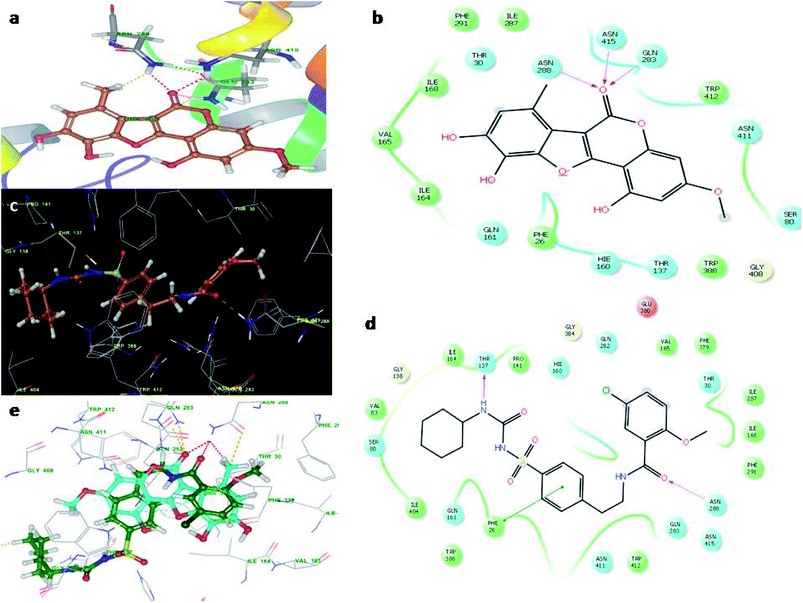 | ||
| Fig. 4 Molecular docking studies of wedelolactone and glibenclamide against GLUT1 catalytic site (PDBID-5EQG). (a) Binding interaction of WEL, (b) Ligplot WELagainst GLUT1 catalytic site, (c) Binding interaction of glibenclamide against GLUT1 catalytic site, (d) Ligplot of glibenclamide against GLUT1 and (e) superimposition of WEL with glibenclamide against GLUT1 catalytic domain. | ||
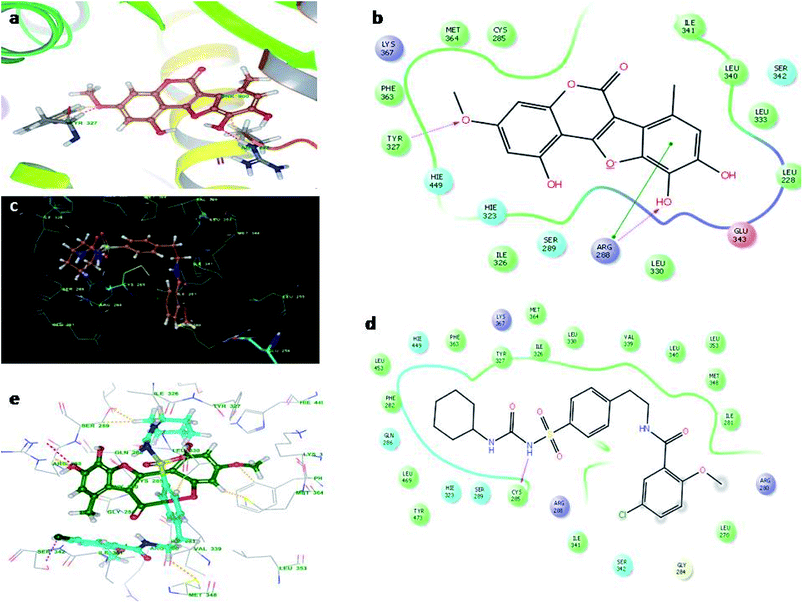 | ||
| Fig. 5 Molecular docking studies of wedelolactone and glibenclamide PPARY catalytic site (PDBID-2PRG). (a) Binding interaction of WEL, (b) WEL against PPARY catalytic site, (c) binding interaction of glibenclamide against PPARY catalytic site, (d) Ligplot of glibenclamide against PPARY and (e) superimposition of WEL with glibenclamide against PPARY catalytic domain. | ||
Effect of WEL on α-glucosidase and α-amylase activity
Table 3 shows the effect of WEL on the α-glucosidase and α-amylase enzyme activity. WEL exhibited an inhibition activity on α-glucosidase (80.65 ± 2.13) and α-amylase (93.83 ± 1.82) enzymes. Acarbose also showed the inhibition of α-glucosidase (80.65 ± 2.13) and α-amylase (42.23 ± 1.02) enzymes.| S. no | Sample | % inhibition | |
|---|---|---|---|
| α-Glucosidase | α-Amylase | ||
| 1 | Wedelolactone | 80.65 ± 2.13 | 93.83 ± 1.82 |
| 2 | Acarbose | 48.92 ± 1.23 | 42.23 ± 1.02 |
Effect of WEL on the oral glucose tolerance test (OGTT)
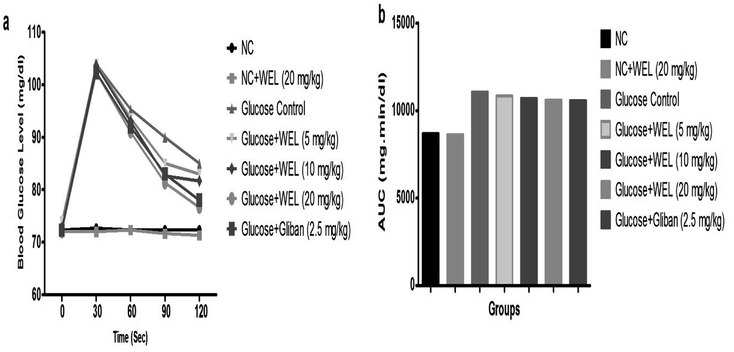
The OGTT was executed for the estimation of postprandial hyperglycemia. During the OGTT experimental study, the blood glucose of NC and the NC received WEL (20 mg kg−1) exhibited an almost constant BGL at the end of the experimental study. The glucose control group rats showed an upregulation of BGL at the end of the study (Fig. 6). The BGL of the glucose control group rats increased till the 90 min after the glucose administration and then slightly decreased in the last 30 min. But WEL treatment showed a reduced BGL from the starting time and this continued to decrease the BGL till the end of the experimental study in a concentration-dependent manner. The result suggests the WEL-treated rats had better glucose utilization.Effect of WEL on body weight
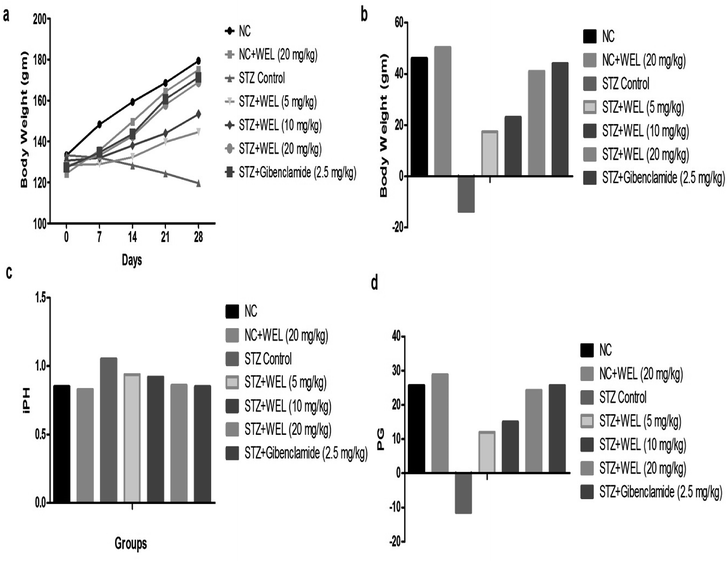
The NC and NC treated WEL group rats demonstrated an augmented body weight as compared to STZ-induced type II DM rats. On the contrary, the STZ-treated rats treated with WEL illustrated a significantly (p < 0.001) augmented body weight in a concentration-dependent manner (Fig. 7a). The NC group rats showed an increase of 1.64 g gain body weight per day throughout the experimental study. NC group rats treated with WEL (20 mg kg−1) revealed a 1.78 g gain in body weight per day till the end of the experimental study. STZ-induced type II DM rats showed a 0.49 g reduced body weight per day. STZ-induced rats treated with WEL showed a gain in body weight of 0.62, 082, and 1.46 g per day at doses of 5, 10, and 20 mg kg−1. Glibenclamide-treated drugs showed a growth in gain rate of 1.56 g per day as compared to the initial body weight (Fig. 7b).The ponderal homogeneity index (iPH) of NC and NC receiving WEL (20 mg kg−1) showed values of 0.85 and 0.83, respectively, which were considerably increased in the STZ-induced type II DM group rats. WEL treatment to STZ-induced type II DM rats showed a downregulation of the iPH level at a dose-dependent manner, and WEL (20 mg kg−1) showed an iPH almost near to the NC group (Fig. 7c). The ponderal grain (PG) was found to be almost equal in the NC and NC rats treated with WEL (20 mg kg−1), while the STZ-induced type II DM group exhibited the opposite trend of PG, which was decreased to −11.53 g in a dose-dependent treatment manner with WEL for the upregulation of PG. The same result was found in the glibenclamide-treated group rats (Fig. 7d).
Effect of WEL on blood glucose and insulin level
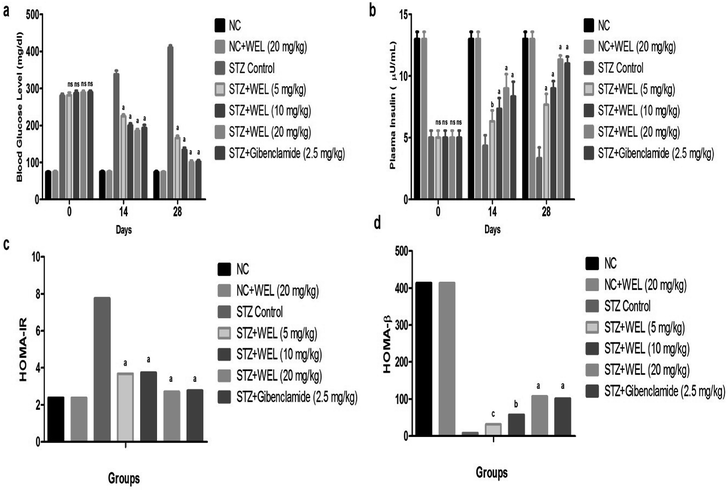
The BGL of the NC and NC received treated group rats showed initial BGLs of 74 ± 1.32 and 74.67 ± 1.89; and were almost the same at 74.33 ± 1.65 and 74.23 ± 1.59 at the end of the experimental study. The initial BGL of the STZ-induced type II DM group rats was 280 ± 4.83, which was significant at 410.64 ± 5.34 till the end of the study. The STZ-induced type II DM group rats treated with WEL (5, 10, and 20 mg kg−1) showed BGLs of 281.67 ± 3.45, 287.21 ± 4.93, and 288 ± 3.84 mg dl−1 at day 0, which were significantly (P < 0.001) reduced to 165.67 ± 2.57, 134 ± 3.42, and 100 ± 2.87 mg dl−1 at day 28, respectively. These results suggest the antidiabetic effect of WEL via reducing the BGL at the end of the experimental study (Fig. 8a).The plasma insulin level of the NC and NC received WEL (20 mg kg−1) groups had almost similar levels at 13 ± 0.98 and 13 ± 0.84 at day 0 and 13 ± 0.67 and 13 ± 0.49 at day 28. STZ-induced type II DM group rats showed the decreased plasma insulin level of 5 ± 0.64 (μU ml−1) due to toxicity induced in the pancreas tissue. WEL (5, 10, and 20 mg kg−1)-treated group rats showed plasma insulin levels of 5.03 ± 0.73, 5.08 ± 0.62 and 5.09 ± 0.93 (μU ml−1) at day 0, which were significantly (P < 0.001) increased to 7.65 ± 0.94, 9.01 ± 0.73, and 11.45 ± 0.83 (μU ml−1) at day 28 in a dose-dependent manner. On the other hand, the STZ-induced type II DM group rats treated with glibenclamide showed a significant (P < 0.001) increase in plasma insulin level from 5.06 ± 0.43 to 11 ± 0.83 (μU ml−1), which suggested an antidiabetic effect. This effect occurred due to the improved dysfunction of pancreatic β-cells (Fig. 8b).
HOMA-IR was increased and HOMA-β was decreased in the STZ-induced type II DM group rats as compared to NC and NC received WEL (20 mg kg−1) group rats. WEL treatment showed a reduced level of HOMA-IR (Fig. 8c) and enhanced level of HOMA-β (Fig. 8d) as compared to the STZ-induced type II DM group rats, which suggested an improvement of the insulin level and an antidiabetic effect.
Effect of WEL on antioxidant parameters
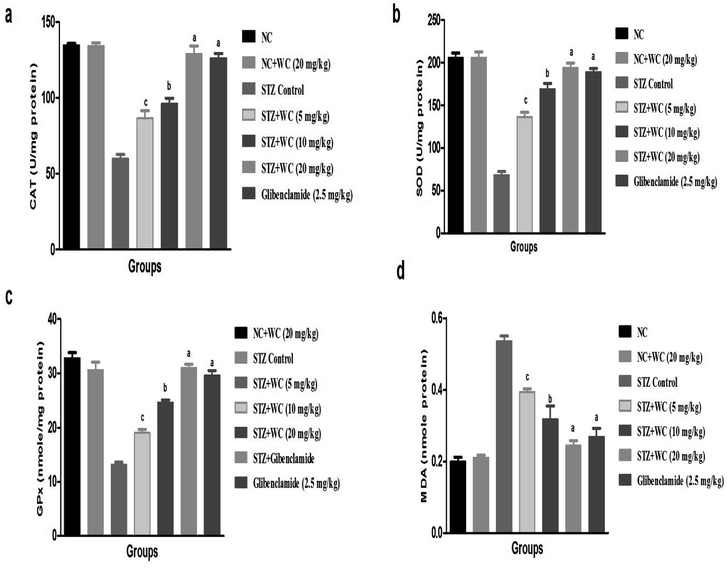
Fig. 6 clearly shows the effect of WEL on the STZ-induced type II DM group rats. NC (134.6 ± 4.32 U mg−1 protein) and NC (134 ± 5.19 U mg−1 protein) group rats treated with WEL exhibited similar levels of antioxidant markers. STZ-induced type II DM rats showed a reduction in the level of CAT (59.8 ± 3.87 U mg−1 protein) in comparison with the NC group rats. WEL (5, 10, and 20 mg kg−1) treatment showed a significantly (P < 0.001) increased (86.6 ± 3.93, 96.2 ± 3.23, and 129 ± 4.92 U mg−1 protein) level of CAT as compared to the STZ-induced type II DM group rats (Fig. 9a).A similar trend was observed in the SOD (68 ± 2.46 U mg−1 protein) (Fig. 9b) and GPx (13.2 ± 1.06 U mg−1 protein) antioxidant marker in the STZ-induced type II DM group rats, while WEL treatment showed the upregulation of SOD (193.8 ± 4.91 U mg−1 protein) and GPx (31.02 ± 1.16 U mg−1 protein) antioxidant marker levels as compared to the STZ-induced type II DM group. A same improvement of the SOD (188.6 ± 5.12 U mg−1 protein) and GPx (29.6 ± 1.82 U mg−1 protein) antioxidant marker was observed in the glibenclamide group rats (Fig. 9c).
In terms of the MDA level, STZ-induced type II DM group rats showed an increased level of MDA (0.536 ± 0.08 nmole protein), which was significantly (P < 0.001) reduced by WEL treatment (0.244 ± 0.02 nmole protein). Glibenclamide-treated rats showed an increased MDA level (Fig. 9d).
Effect of WEL on hepatic enzyme parameters
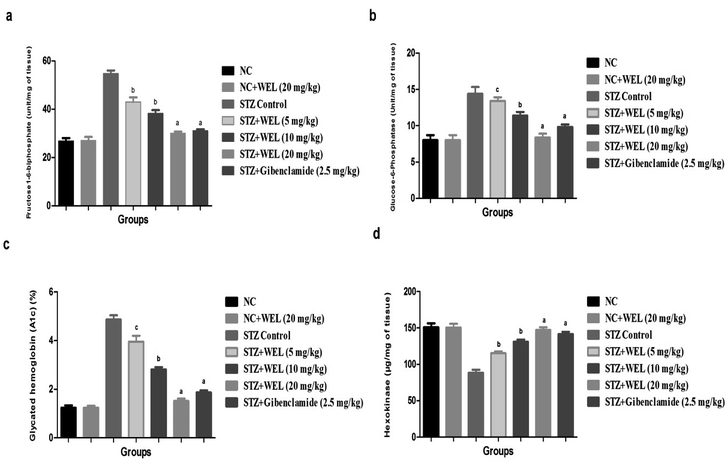
Fig. 10 illustrates the effect of WEL on the normal and STZ-induced type II DM group rats. STZ-induced type II DM group rats showed the upregulation of fructose1-6-biphosphate, glucose-6-phosphatase, and glycated hemoglobin, and downregulation of the hexokinase level as compared to NC and NC rats treated with WEL (20 mg kg−1) (Fig. 7). WEL treatment significantly (P < 0.001) altered the level of diabetic parameters in a dose-dependent manner. Glibenclamide showed a modulation of the level of the diabetic parameters in comparison with the STZ-induced type II DM group rats.Effect of WEL on lipid profile
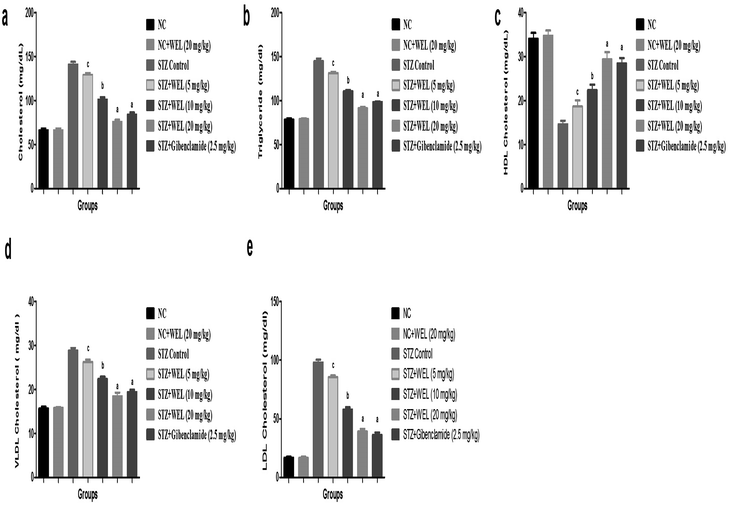
Fig. 11 reveals the effect on the normal control and WEL on the lipid profile. Alteration of the lipid profile is a crucial parameter, which occurs due to diabetes. STZ-induced type II DM group rats showed an increased level of triglycerides, total cholesterol, LDL, and VLDL, and reduced HDL level at the end of the experimental study as compared to the normal control and normal control treated with WEL (20 mg kg−1) (Fig. 11). STZ-induced type II DM group rats treated with WEL showed an alteration of the lipid profile and showed the effect of WEL against lipid abnormality.Effect of WEL on the coronary risk index and atherogenic index
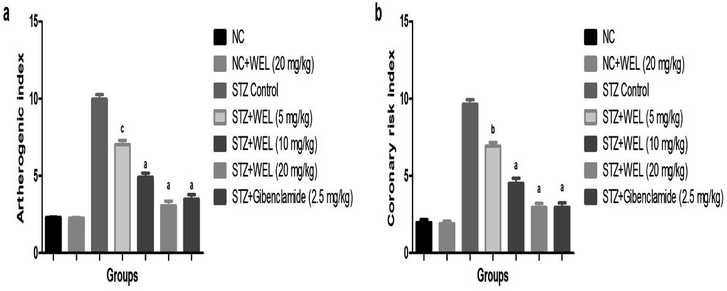
Coronary risk factor and an atherogenic index were amplified in the STZ-induced type II DM rats, while the dose-dependent treatment of WEL showed a reduction in the level of coronary risk factor and the atherogenic index in a concentration-dependent manner and suggested a cardio-protective effect of WEL (Fig. 12).Effect of WEL on inflammatory markers
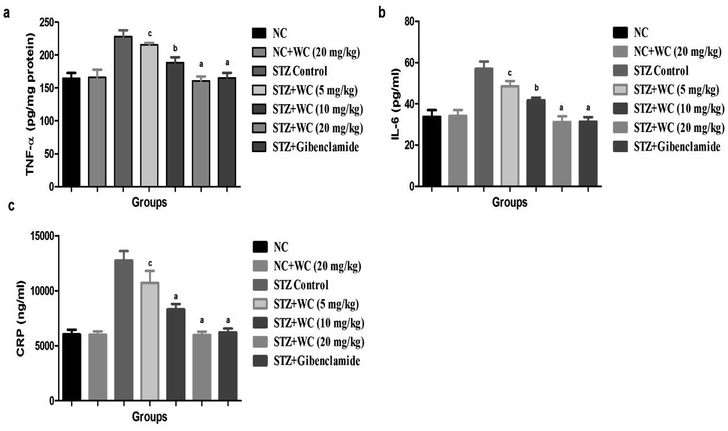
Inflammatory markers, such as TNF-α, IL-16, and CRP, were estimated in the normal control and WEL-treated group rats. The level of TNF-α was upregulated in the STZ-induced type II DM group as compared to the NC and NC treated rats with WEL (20 mg kg−1). A level of TNF-α almost double was found in the STZ-induced type II DM group, which was significantly (P < 0.001) downregulated by the WEL in a dose-dependent manner (Fig. 13a).The same downregulation trend was found in the IL-6 and CRP levels of the STZ-induced type II DM group as compared to the NC and NC treated rats with WEL (20 mg kg−1), which was significantly (P < 0.001) reduced by the WEL. On the other hand, glibenclamide significantly (P < 0.001) reduced the level of the above-discussed inflammatory mediators (Fig. 13b and c).
Discussion
Compound wedelolactone (WEL) was obtained as a green amorphous powder corresponding to a molecular formula of C16H10O7, as established by different spectroscopy techniques, such as DEPT spectra, mass spectrum (M+ 314), and 13C NMR chromatographic observation of TLC for the isolated compound suggested a bright blue fluorescence and generated a green color with FeCl3. In the UV spectrum, we found a few peaks at 249, 294, 304, and 352 nm. The IR spectrum showed peaks at 3305 cm−1, suggesting the presence of a hydroxyl group, δ-lactone carbonyl (1717 cm−1), and a double bond (1619 cm−1). The 13C NMR and 1H NMR showed 16 carbon atoms for the molecule, consisting of eleven quaternary, four methane, and one methoxy carbon atoms (in total C16H10). The 1H NMR spectrum illustrated two proton doublets at δH = 6.38 attributable to positions 6 and 8, while other singlets at δH = 7.26 (δc = 91.9) and δH = 7.26 (δc = 103) were also obtained in the spectrum, which could be assigned to positions 10 and 13 of the ring D. The 13C NMR spectrum exhibited peaks in the downfield region at δc = 153, 143.8, and 142.7 due to carbons at positions 5, 11, and 12 containing the hydroxyl groups. The 1H NMR spectrum also exhibited a broad signal of two protons due to hydroxy groups at positions 11 and 12, and a singlet of one proton at δH = 10.58, which was further downfield due to hydrogen bonds with the oxygen of ring C, and thus was assignable to position 5. The 1H NMR spectrum demonstrated a three protons singlet at δH = 3.73 (δc = 54.2) due to a methoxyl group assignable at C-7. Other peaks in the 13C NMR and 1H NMR also provided support for the proposed structure of wedelolactone.The mass fragmentation pattern revealed prominent peaks at m/z 314 due to a molecular ion peak, m/z 286 due to removal of a carbonyl group of ring A, m/z 176 (frag. a) and m/z 138 (frag. c) from the rupture of ring A by aa′, and m/z 152 (frag. b) by the rupture of ring A and ring C by bb′. Other peaks in the spectrum were also in good agreement with the structure. Thus on the basis of the above peaks, we elucidated the structure of wedelolactone as 5-hydroxy-7-methoxy-11,12-dihydroxy-benzofurano coumarin.
Streptozotocin (STZ), a nitrosourea compound obtained from the soil microbe (Streptomyces achromogenes), has been widely exploited for the induction of type I (high dose) and type II (low dose) diabetes mellitus in an experimental model of animals. STZ-induced type I and type II DM due to a specific alkylating potency. Due to the presence of a methyl nitrosourea moiety, it induces cytotoxicity into the pancreatic islet of β-cells in the experimental animals via the induction of DNA methylation and resultant damage to the DNA.1,2,32 Damaging DNA further activated the poly(ADP-ribose) polymerase, starting the depletion of NAD+. It also acts as a nitric acid donor in pancreatic cells, which boosts the production of superoxide radical fragmentation into the islet cells, with the resultant death of pancreatic β-cells via necrosis and apoptosis. STZ exerts a diabetogenic effect via damaging the pancreatic β-cells, imitating DM.1,2,32 In the current experimental study, wedelolactone (coumestan) isolated from the Wedelia calendulacea, possessed various types of pharmacological benefits. Various categories of oral synthetic hypoglycemic drugs, including sulphonylureas, α-glucosidase, biguanides, thiazolidinediones, and a lot more are available in the market for the treatment of DM. In the current experimental study, we selected glibenclamide (sulphonylureas class of drug), which has a mechanism on the insulin secretion.
α-Glucosidase and α-amylase (carbohydrate hydrolyzing enzymes) are responsible for postprandial hyperglycemia. Acarbose inhibitor, an inhibitor of α-glucosidase and α-amylase, acts via blocking the activity of both enzymes leading to downregulation of the polysaccharides breakdown and thereby downregulating the boosting post-prandial in BGL and additionally inducing weight loss.33 α-Amylase initiates the process by the digestion of carbohydrates through the breakdown of 1,4 glycosidic linkages of polysaccharides to disaccharides, which further convert into monosaccharides via α-glucosidase enzymes, which leads to postprandial hyperglycemia.34 WEL showed an inhibition of enzymatic activity, suggesting the therapeutic targets for type II DM. The synthetic drug showed a higher inhibition of acarbose inhibitor of α-amylase activity; conversely, the use of the synthetic drug is limited due to their specific side effects, namely meteorism, distention, diarrhea, and abdominal distention, which occur due to the excessive reduction of α-amylase. The previous investigations suggested that a strong inhibition of α-glucosidase and mild reduction of α-amylase exhibited by various plant-based compounds could be responsible for there being fewer side effects.35 Therefore, the inhibitory effect of α-glucosidase and α-amylase are considered as one of the important mechanism to control hyperglycemia via delaying the digestion of carbohydrates, which continues to downregulate postprandial hyperglycemia. The same result was observed by the WEL via the inhibition of α-glucosidase and α-amylase activity, which suggests it could be used as an alternative anti-diabetic drug with less side effects.
Insulin resistance plays a significant phase in metabolic syndrome, representing the major risk factor for the expansion of diabetes mellitus and glucose intolerance.36 Downregulation of the insulin resistance postpones the augmentation of type II DM and its complications. The OGTT test was performed for scrutinizing the effect of the drug on the utilization of glucose. In the OGTT test, glucose disposal was observed to be considerably upregulated and downregulated for blood glucose, and it was observed for up to 6 h.2,37 OGTT control group rats exhibited increased BGL till 6 h. On the contrary, WEL treatment group rats had reduced BGL, suggesting the better utilization of glucose in the treated rats.1 Glibenclamide-treated group rats showed similar results, and so on the basis of a comparison of the results, we can conclude that WEL has a similar effect to glibenclamide on the OGTT rats via the downregulation of insulin resistance.
STZ-induced DM rats served as hyperglycemia interconnected with the downregulation and discharge of insulin secretion. This occurs due to damage of pancreatic β-cells by STZ-induced toxicity in the pancreas. STZ-induced DM rats showed a boosted level of fasting BGL due to the damage of pancreatic β-cells.1,32 The oral administration of WEL and glibenclamide showed an improvement in insulin secretion from existing and regenerated β-cells of the pancreas.
STZ-induced DM rats showed weight loss throughout the experimental study. The reduced body weight was observed in the rats due to amplifying the muscle destruction and degradation of structural proteins.38 Preclinical trials on WEL suggested an improvement in body weight may be due to reversing gluconeogenesis, glycogenolysis, and proteolysis.
Insulin plays a significant role in the metabolism of liver glycogen via the regulation of glycogenesis, which controls the above metabolism by standardizing the conversion of the dependent form of glucose-6-phosphate into the independent form of glucose-6-phosphate of glucose synthase.39 Glycogen synthetase enzyme increases glycogenesis, which plays a key role during the expansion of diabetes via converting glycogen into glucose, resulting also in boosting the blood glucose concentration. During diabetes, an increased number of glycosylation proteins is observed. Skeletal muscle maximum utilizes the glycogen as an express reflection of insulin activity, as insulin enhances intracellular glycogen deposition via a reduction of glycogen phosphorylase and by boosting glycogen synthase.40,41 Glycosylated hemoglobin is considered to be a significant marker of glycemic control. STZ-induced DM rats showed reduced liver glycogen activity, which was significantly (p < 0.001) enhanced by WEL treatment in a dose-dependent manner, indicating the reduced glucose synthetase enzymes during treatment. The possible mechanism of action may be due to a reduction in the absorption of glucose from the intestine or by a reduction of neoglucogenesis similar to with the standard drugs.
The liver plays a crucial role in the synthesis of glucose metabolism and postprandial hyperglycemia. In the liver, glucose is utilized to renovate the glucose into glucose-6-phosphatase with the help of hexokinase. The insufficiency of hexokinase results in a reduction of glycolysis and a significant downregulation of glucose utilization for energy production.42,43 Glucose-6-phosphatase converts fats into carbohydrates and during the type II DM augments the level of glucose-6-phosphatase, boosting the turning of fats into carbohydrates and starting deposition into the hepatic and renal tissue and inhibiting the action of hexokinase. Hexokinase, glucose-6-phosphatase and fructose-1-6-phosphate enzymes play key roles in the gluconeogenic pathway.44,45 Glucose-6-phosphatase and fructose-1-6-phosphate levels reach extended levels due to the boost in the synthesis of enzymes, which results in the upregulatation of glucose production via the liver during diabetes. STZ-induced type II DM group rats showed a reduced level of hexokinase and increased level of glucose-6-phosphatase and fructose-1-6-phosphate, which was dose-dependently altered by the WEL, with an almost similar result observed in the glibenclamide-treated group rats. The possible mechanism of action of this may be attributed to enhanced glycolysis and reduced gluconeogenesis.44,45 Glucose-6-phosphate dehydrogenase is considered as the first regulatory enzyme of the pentose phosphate pathway. STZ-induced type II DM group rats showed the reduction of glucose-6-phosphate dehydrogenase activity, possibly due to the reduction of hexose monophosphate shunt (HMP), therefore reducing the generation of equivalents, including NADH and NADPH, which are necessary for the glutathione reductase and thereby GSH activity in tissues. The reduced activity of NADPH in the cells makes them more susceptible to inducing oxidative damage46. STZ-induced type II DM group rats demonstrated a reduced activity of glucose-6-phosphate dehydrogenase, which was dose-dependently boosted by the WEL. The possible mechanism for this may be the increased production of hydrogen, which further binds to the NADP+ and produces the NADPH, which further induces the synthesis of fats from the carbohydrate and decreases the plasma glucose to a considerable extent. According to Ahmed et al., the plasma insulin level is controlled by the reduced activity of glucose-6-phosphatase and fructose-1-6-phosphate, and the same results were found in our study, which suggests that WEL increased the plasma insulin level via the reduced activity of glucose-6-phosphatase and fructose-1-6-phosphate.
Mitochondrial dysfunctions have been linked to the progression of various types of pathologies, such as diabetes. Mitochondrial abnormalities may contribute to metabolic deformity and emanate insulin resistance and increase the chance of developing diabetes. Generally, mitochondrials generate the energy for cells and work as the powerhouse of the cell. In an inefficient endogenous antioxidant system, this occurs due to a low consumption of ATP production/oxygen, which leads to the production of superoxide anions.47,48 The generation of superoxide radicals increases the oxidative stress load and leads to the formation of reactive oxygen species (ROS). ROS formation may boost the production of pro-inflammatory cytokines and the rate of mutagenesis. Mitochondrial generation of ATP occurs less in cells, which further increases the amount of lipids and glucose in the cell. Previously published literature suggest that insulin resistance is connected to the reduced mitochondrial oxidative enzymes, mitochondrial number, and reduced ATP synthesis in human muscle. In the current experimental investigation, we showed the dual mechanism action as either an antioxidant or anti-inflammatory mechanism.48–50 The anti-inflammatory potential of WEL gives support to our hypothesis. Several previous published literature reports have suggested that the free radical/reactive oxygen species/reactive nitrogen species start the production of oxidative stress, which plays a significant role in the expansion of hyperglycemia and its complications. A variety of mechanisms are involved in the production of ROS during diabetes, including glucose auto-oxidation, lipid peroxidation (LPO), production of glycation products, and protein glycation.50–52 STZ induces toxicity in the pancreatic β-cells and starts the secretion of insulin and increases the overload of ROS/free radicals. Decreased levels of endogenous antioxidants then start the generation of superoxide (O2) and hydrogen peroxide (H2O2) and induce the tissue and cellular damage, causing the diverse diabetes conditions. SOD and CAT are considered as the significant primary endogenous antioxidant enzymes. Superoxide radicals damage the cellular membrane and biological structure of cells. SOD converts the superoxide into H2O2. Another endogenous antioxidant CAT switches H2O2 into hydroxyl radicals and converts this in tissue to highly reactive hydroxyl radicals.22 Polyunsaturated fatty acids play a significant role in the production of ROS, whereby ROS react with the cell membrane and all biological substances and induce lipid peroxidation. The upregulation of the LPO level impairs the membrane-bound enzyme activity via reducing the membrane fluidity. Researchers are now targeting the estimation of the MDA level (as an indicator of LPO) for estimation of an increased overload of LPO during type II DM.1,2 STZ-induced type II DM group rats showed an amplified level of MDA, which was significantly (P < 0.001) downregulated by the WEL, suggesting an anti-diabetic effect.
In addition to hyperglycemia, high levels of TC and TG can be found in the blood of type II DM due to a change of lifestyle and high intake of junk foods and fats. The lipid abnormality occurs in diabetic patients due to the insensitivity of the peripheral tissue of insulin.20,53 Insulin plays an imperative role in the downregulation of hormone-sensitive lipase enzyme and in the activation of lipoprotein lipase. Previous literature suggests that diminution of free cholesterol and free acids via pancreatic cholesterol esterase and pancreatic lipase downregulate the hyperlipidemia linked with the DM. STZ-induced type II DM group rats showed increased levels of total cholesterol and LDL, which was significantly (p < 0.001) reduced by the WEL in a dose-dependent manner. The possible mechanism of action may be due to the inhibition of pancreatic cholesterol esterase and pancreatic lipase. During the DM, the deposition of free fatty acids is increased in the peripheral tissue and boosts the level of triglycerides in the serum.
The inflammatory mediator and inflammation reaction play a crucial role in the development of diabetes and its complications. Inflammation reaction decreases insulin sensitivity and reduces the sensitivity of lipase tissue. In normal conditions, an inflammatory marker, such as TNF-α and CRP, can be found in a low amount in the circulation, leading to a low grade of inflammation vascular damage.1 Normally, CRP secreted from the hepatic tissue plays an important role in the secretion of TNF-α and IL-6. But several researchers suggested that an augmented level of CRP is directly linked with insulin resistance, obesity, glucose tolerance, BGL, and the etiology of type II DM; the same result was observed in the STZ-induced type II DM group, and WEL treatment significantly (P < 0.001) decreased the CRP level.54,55 The regeneration of pancreatic β-cells suggests the antidiabetic effect of WEL. During diabetes, an increased level of TNF-α showed the effect on the insulin receptor and decreased the insulin sensitivity, while IL-6 shows the effect on the glucose via altering the insulin receptor, IRS, glut-4, which increase the incidence of insulin resistance. In our study, we observed increased levels of TNF-α and IL-6, which suggests the resistance of the insulin level in the STZ-induced type II DM group, while the WEL treatment demonstrated the reduced level of an inflammatory mediator. The improved insulin secretion provides support for our hypothesis.
Conclusion
The result of the current experimental study suggested the antihyperglycemic effect and in vitro inhibition of DPPIV suggesting the antidiabetic effect of WEL. Several previous studies showed that standard drugs, such as glibenclamide, have several side effects, such as headache, nausea, and low blood sugar level, when taken in a higher amount. Based on our results, we can conclude that WEL might be useful in the treatment and prevention of diabetes and its complications, such as insulin resistance and spoiled glucose tolerance with a DPP-IV inhibitory effect.Conflicts of interest
None, all authors declare none conflict of interest.Abbreviations
| WEL | Wedelolactone |
| DPPIV | Dipeptidyl peptidase-4 |
| GLUT1 | Glucose transporter 1 |
| PPARγ | Peroxisome proliferator-activated receptors-γ |
| OGTT | Oral glucose tolerance test |
| STZ | Streptozotocin |
| DM | Diabetes mellitus |
| CRP | C-reactive protein |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin 6 |
| PTP1B | Protein tyrosine phosphatase 1B |
| BGL | Blood glucose level |
| iPH | Ponderal homogeneity index |
| PG | Ponderal grain |
| AST | Alkaline phosphatase |
| AST | Aspartate aminotransferase |
| ALT | Alanine transaminase |
| BUN | Blood urea nitrogen |
| TP | Total protein |
| TC | Total cholesterol |
| HDL | High density lipoprotein |
| TG | Triglyceride |
| VLDL | Very low density lipoprotein, |
| LDL | Low density lipoprotein |
| MDA | Malonaldehyde |
| GPx | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
References
- V. Kumar, P. C. Bhatt, G. Kaithwas, M. Rashid, F. A. Al-abbasi, J. A. J. Khan, F. Anwar and A. Verma, Beni-Suef Univ. J. Basic Appl. Sci., 2016, 5, 255–276 CrossRef.
- V. Kumar, D. Ahmed, A. Verma, F. Anwar, M. Ali and M. Mujeeb, BMC Complementary Altern. Med., 2013, 13, 273 CrossRef PubMed.
- C. Pelletier, S. Dai, K. C. Roberts, A. Bienek, J. Onysko and L. Pelletier, Chronic Dis. Inj. Can., 2012, 33, 47–52 Search PubMed.
- P. Drouin, J. F. Blickle, B. Charbonnel, E. Eschwege, P. J. Guillausseau, P. F. Plouin, J. M. Daninos, N. Balarac, J. P. Sauvanet and D. O. F. Diabetes, Diabetes Care, 2009, 32, S62–S67 CrossRef PubMed.
- F. Zaccardi, D. R. Webb, T. Yates and M. J. Davies, Postgrad. Med. J., 2016, 92, 63–69 CrossRef PubMed.
- M. M. Ortegon, W. K. Redekop and L. W. Niessen, Diabetes Care, 2004, 27, 901–907 CrossRef PubMed.
- K. Gu, C. C. Cowie and M. I. Harris, Diabetes Care, 1998, 21, 1138–1145 CrossRef PubMed.
- D. J. Drucker and M. A. Nauck, Lancet, 2006, 368, 1696–1705 CrossRef.
- E. J. Verspohl, Pharmacol. Ther., 2009, 124, 113–138 CrossRef PubMed.
- L. Liu, J. Liu, W. T. Wong, X. Y. Tian, C. W. Lau, Y. X. Wang, G. Xu, Y. Pu, Z. Zhu, A. Xu, K. S. L. Lam, Z. Y. Chen, C. F. Ng, X. Yao and Y. Huang, Hypertension, 2012, 60, 833–841 CrossRef PubMed.
- S. Mentreddy, A. Mohamed and A. Rimando, Proc. Assoc. Adv. Ind. Crop. Conf., 2005, 341–353 Search PubMed.
- N. H. Parikh, P. K. Parikh and C. Kothari, Chin. J. Nat. Med., 2014, 12, 335–344 Search PubMed.
- A. K. Sharma, K. K. Anand, P. Pushpangadan, B. K. Chandan, C. L. Chopra, Y. S. Prabhakar and N. P. Damodaran, J. Ethnopharmacol., 1989, 25, 93–102 CrossRef PubMed.
- T. Prakash, N. R. Rao and A. H. M. Viswanatha Swamy, Phytomedicine, 2008, 15, 959–970 CrossRef PubMed.
- A. Verma, B. Ahmed and M. H. Masoodi, Indian Drugs, 2010, 47, 51–54 Search PubMed.
- D. Hegde, R. Khosa and R. Goel, Anc. Sci. Life, 1994, 14, 77–81 Search PubMed.
- T. R. Govindachari and M. S. Premila, Phytochemistry, 1985, 24, 3068–3069 CrossRef.
- A. Verma, D. Singh, F. Anwar, P. C. Bhatt, F. Al-Abbasi and V. Kumar, Inflammopharmacology, 2017, 26(1), 133–146 CrossRef PubMed.
- D. Ahmed, V. Kumar, A. Verma, G. S. Shukla and M. Sharma, SpringerPlus, 2015, 4, 315 CrossRef PubMed.
- D. Ahmed, V. Kumar, M. Sharma and A. Verma, BMC Complementary Altern. Med., 2014, 14, 155 CrossRef PubMed.
- H. Ali, P. J. Houghton and A. Soumyanath, J. Ethnopharmacol., 2006, 107, 449–455 CrossRef PubMed.
- V. Kumar, F. Anwar, D. Ahmed, A. Verma, A. Ahmed, Z. a. Damanhouri, V. Mishra, P. W. Ramteke, P. C. Bhatt and M. Mujeeb, BMC Complementary Altern. Med., 2014, 14, 76 CrossRef PubMed.
- V. Kumar, P. C. Bhatt, M. Rahman, D. K. Patel, N. Sethi, A. Kumar, N. K. Sachan, G. Kaithwas, F. A. Al-abbasi, F. Anwar and A. Verma, BMC Complementary Altern. Med., 2016, 16, 510 CrossRef PubMed.
- F. Anwar, G. Mushtaq, I. Kazmi, M. Afzal, R. Khan, F. A. Al-Abbasi, A. Ahmad and V. Kumar, RSC Adv., 2015, 5, 68385–68391 RSC.
- R. Khan, I. Kazmi, M. Afzal, F. A. Al Abbasi, G. Mushtaq, A. Ahmad, V. Kumar and F. Anwar, RSC Adv., 2015, 5, 67996–68002 RSC.
- V. Kumar, F. A. Al-Abbasi, D. Ahmed, A. Verma, M. Mujeeb and F. Anwar, Food Funct., 2015, 6, 1652–1666 Search PubMed.
- A. Verma, P. C. Bhatt, G. kaithwas, N. Sethi, M. Rashid, Y. Singh, M. Rahman, F. Al-Abbasi, F. Anwar and V. Kumar, Inflammopharmacology, 2016, 24, 233–251 CrossRef PubMed.
- V. Kumar, F. Anwar, A. Verma and M. Mujeeb, J. Food Sci. Technol., 2014, 52, 3402–3411 Search PubMed.
- F. Anwar, F. A. Al-Abbasi, P. C. Bhatt, A. Ahmad, N. Sethi and V. Kumar, Toxicol. Res., 2015, 4, 1308–1323 RSC.
- V. Kumar, F. A. Al-Abbasi, A. Verma, M. Mujeeb and F. Anwar, Toxicol. Res., 2015, 4, 1072–1084 RSC.
- M. Afzal, I. Kazmi, R. Khan, P. Rana, V. Kumar, F. A. Al-Abbasi, M. A. Zamzami and F. Anwar, Arch. Biochem. Biophys., 2017, 623–624, 58–63 CrossRef PubMed.
- D. Ahmed, V. Kumar, A. Verma, P. S. Gupta, H. Kumar, V. Dhingra, V. Mishra and M. Sharma, BMC Complementary Altern. Med., 2014, 14, 243 CrossRef PubMed.
- D. Gomathi, M. Kalaiselvi and C. Uma, Evaluation, 2012, 3, 226–229 Search PubMed.
- X. Yao, L. Zhu, Y. Chen, J. Tian and Y. Wang, Food Chem., 2013, 139, 59–66 CrossRef PubMed.
- G. Kim, Y. Kwon and H. Jang, J. Med. Food, 2011, 14, 712–717 CrossRef PubMed.
- J. A. Shin, J. H. Lee, S. Y. Lim, H. S. Ha, H. S. Kwon, Y. M. Park, W. C. Lee, M. Il Kang, H. W. Yim, K. H. Yoon and H. Y. Son, J. Diabetes Invest., 2013, 4, 334–343 CrossRef PubMed.
- D. Ahmed, V. Kumar, A. Verma, G. S. Shukla and M. Sharma, SpringerPlus, 2015, 4, 315 CrossRef PubMed.
- V. Kumar, D. Ahmed, P. S. Gupta, F. Anwar and M. Mujeeb, BMC Complementary Altern. Med., 2013, 13, 222 CrossRef PubMed.
- A. Von Wilamowitz-Moellendorff, R. W. Hunter, M. García-Rocha, L. Kang, I. López-Soldado, L. Lantier, K. Patel, M. W. Peggie, C. Martínez-Pons, M. Voss, J. Calbó, P. T. W. Cohen, D. H. Wasserman, J. J. Guinovart and K. Sakamoto, Diabetes, 2013, 62, 4070–4082 CrossRef PubMed.
- J. Jensen, P. I. Rustad, A. J. Kolnes and Y. C. Lai, Front. Physiol., 2011, 2, 112 Search PubMed.
- J. Jensen, E. Jebens, E. O. Brennesvik, J. Ruzzin, M. A. Soos, E. M. L. Engebretsen, S. O'Rahilly and J. P. Whitehead, Am. J. Physiol.: Endocrinol. Metab., 2006, 290, E154–E162 CrossRef PubMed.
- M. König, S. Bulik and H.-G. Holzhütter, PLoS Comput. Biol., 2012, 8, e1002577 Search PubMed.
- D. Gitenay, C. Wiel, H. Lallet-Daher, D. Vindrieux, S. Aubert, L. Payen, H. Simonnet and D. Bernard, Cell Death Dis., 2014, 5, e1089 CrossRef PubMed.
- S. Heikkinen, M. Pietilä, M. Halmekytö, S. Suppola, E. Pirinen, S. S. Deeb, J. Jänne and M. Laakso, J. Biol. Chem., 1999, 274, 22517–22523 CrossRef PubMed.
- P. Enes, S. Panserat, S. Kaushik and A. Oliva-Teles, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2008, 149, 80–86 CrossRef PubMed.
- C. M. Grant, L. P. Collinson, J. H. Roe and I. W. Dawes, Mol. Microbiol., 1996, 21, 171–179 Search PubMed.
- J. Szendroedi, E. Phielix and M. Roden, Nat. Rev. Endocrinol., 2011, 8, 92–103 CrossRef PubMed.
- A. P. Rolo and C. M. Palmeira, Toxicol. Appl. Pharmacol., 2006, 212, 167–178 CrossRef PubMed.
- C. Bonnard, A. Durand, S. Peyrol, E. Chanseaume, M. A. Chauvin, B. Morio, H. Vidal and J. Rieusset, J. Clin. Invest., 2008, 118, 789–800 Search PubMed.
- M. Valko, C. J. Rhodes, J. Moncol, M. Izakovic and M. Mazur, Chem.-Biol. Interact., 2006, 160, 1–40 CrossRef PubMed.
- E. Wright Jr., J. L. Scism-Bacon and L. C. Glass, Int. J. Clin. Pract., 2006, 60, 308–314 CrossRef PubMed.
- J. W. Baynes, Diabetes, 1991, 40, 405–412 CrossRef PubMed.
- D. Ahmed, M. Sharma, V. Kumar, H. K. Bajaj and A. Verma, J. Food Sci. Technol., 2014, 52, 5427–5441 CrossRef PubMed.
- E. J. Brunner, M. Kivimäki, D. R. Witte, D. A. Lawlor, G. D. Smith, J. A. Cooper, M. Miller, G. D. O. Lowe, A. Rumley, J. P. Casas, T. Shah, S. E. Humphries, A. D. Hingorani, M. G. Marmot, N. J. Timpson and M. Kumari, PLoS Med., 2008, 5, 1278–1286 Search PubMed.
- J.-P. Bastard, M. Maachi, C. Lagathu, M. J. Kim, M. Caron, H. Vidal, J. Capeau and B. Feve, Eur. Cytokine Network, 2006, 17, 4–12 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12568b |
| This journal is © The Royal Society of Chemistry 2018 |