Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4†
Qingdong Qina,
Yahong Liua,
Xuchun Lib,
Tian Suna and
Yan Xu *a
*a
aSchool of Civil Engineering, Southeast University, Nanjing 210096, China. E-mail: xuxucalmm@seu.edu.cn; Fax: +86 25 83790757; Tel: +86 25 83790757
bSchool of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou 310018, China
First published on 3rd January 2018
Abstract
To facilitate rapid dye removal in oxidation processes, copper ferrite (CuFe2O4) was isothermally reduced in a H2 flow and used as a magnetically separable catalyst for activation of hydrogen peroxide (H2O2). The physicochemical properties of the reduced CuFe2O4 were characterized with several techniques, including transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy and magnetometry. In the catalytic experiments, reduced CuFe2O4 showed superior catalytic activity compared to raw CuFe2O4 for the removal of methylene blue (MB) due to its relatively high surface area and loading Fe0/Cu0 bimetallic particles. A limited amount of metal ions leached from the reduced CuFe2O4 and these leached ions could act as homogeneous Fenton catalysts in MB degradation. The effects of experimental parameters such as pH, catalyst dosage and H2O2 concentration were investigated. Free radical inhibition experiments and electron spin resonance (ESR) spectroscopy revealed that the main reactive species was hydroxyl radical (˙OH). Moreover, reduced CuFe2O4 could be easily separated by using an external magnet after the reaction and remained good activity after being recycled five times, demonstrating its promising long-term application in the treatment of dye wastewater.
1. Introduction
The textile industry produces a large amount of wastewater that is extremely harmful to humans and the environment since it contains a high concentration of dyes and a variety of recalcitrant organic compounds. Therefore, many treatment processes such as physical separation, chemical oxidation and biological degradation have been widely employed for the removal of dyes from wastewater.1 Among the different water treatment approaches tested so far, advanced oxidation processes (AOPs) have shown great potential for the treatment of industrial wastewaters.2 AOPs are characterized by hydroxyl radical (˙OH) with a redox potential of 2.80 eV, which can react with almost all recalcitrant organic compounds. As one of the most effective AOPs, Fenton process has unique advantages due to its simple generation of ˙OH by a reaction between Fe2+ and H2O2, low cost and environmental benignity.3 However, the application of traditional homogeneous Fenton processes is limited by the requirement of low solution pH (<4) and the formation of ferric hydroxide sludge during wastewater treatment. Therefore, alternative catalysts for the Fenton reaction are pursued to overcome the aforementioned drawbacks of the Fe2+/H2O2 system.Recently, heterogeneous Fenton-like processes have been investigated as a more practical and efficient alternative technique for removing recalcitrant organic pollutants.4 Many iron based catalysts, such as Fe2O3,5 Fe3O4,6 α-FeOOH7 and CuFe2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8,9 have been applied to activate H2O2 into reactive radicals for the degradation of organic pollutants in water. In particular, CuFe2O4, a kind of magnetic material with cubic structure, has received considerably higher attention in water treatment due to its high magnetic permeability, excellent chemical and mechanical stability.10 Feng et al. prepared CuFe2O4 nanoparticles as a heterogeneous Fenton-like catalyst to degrade sulfanilamide and found that the pseudo-first-order rate constant was 5.9 × 10−3 min−1.8 Wang et al. synthesized mesoporous CuFe2O4 as a heterogeneous Fenton-like catalyst to degrade imidacloprid and reported that the apparent reaction rate constant was 1.7 × 10−2 min−1.9 However, CuFe2O4 seems to present weak catalytic activity due to its low electron transfer rate, which limits the practical application of heterogeneous Fenton-like catalyst.
8,9 have been applied to activate H2O2 into reactive radicals for the degradation of organic pollutants in water. In particular, CuFe2O4, a kind of magnetic material with cubic structure, has received considerably higher attention in water treatment due to its high magnetic permeability, excellent chemical and mechanical stability.10 Feng et al. prepared CuFe2O4 nanoparticles as a heterogeneous Fenton-like catalyst to degrade sulfanilamide and found that the pseudo-first-order rate constant was 5.9 × 10−3 min−1.8 Wang et al. synthesized mesoporous CuFe2O4 as a heterogeneous Fenton-like catalyst to degrade imidacloprid and reported that the apparent reaction rate constant was 1.7 × 10−2 min−1.9 However, CuFe2O4 seems to present weak catalytic activity due to its low electron transfer rate, which limits the practical application of heterogeneous Fenton-like catalyst.
Zero-valent Fe (Fe0) has proved to be an efficient catalyst for the heterogeneous Fenton-like reaction due to the generation of ferrous iron by the corrosion of metal iron.11,12 In addition, Fe0 as an electrons donor could reduce Fe3+ to Fe2+, which could be able to accelerate the formation of ˙OH.13 Nevertheless, Fe0 trends to aggregate and forms large particles due to strong anisotropic dipolar interactions, which leads to a decrease in surface area and ultimately a lower catalytic activity.14 Therefore, it is essential to anchor and immobilize Fe0 onto supports to prevent aggregation. Several studies have supported Fe0 on Fe3O4 surface to enhance organic compounds degradation.13–16 These results showed a significant increase in activity for the oxidation of organics due to a thermodynamically favorable electron transfer from Fe0 to Fe3O4 producing active Fe2+ species. More recently, to achieve better catalytic activity, iron–copper bimetallic catalyst system has also attracted increasing attention.17,18 The combination of copper with iron exhibits an improved catalytic activity due to the synergic effects of two-metal redox couples. For instance, Wang et al. synthesized iron–copper bimetallic nanoparticles embedded within ordered mesoporous carbon (CuFe–MC) and observed a greater catalytic activity of CuFe–MC than those of Fe–MC and Cu–MC.17 The reactions in Fenton-like system with iron–copper bimetallic nanoparticles were described by the following equations:17,18
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe0 + 2H+ → Fe0 + 2H+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + H2 Fe2+ + H2
| (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe0 + H2O2 + 2H+ → Fe0 + H2O2 + 2H+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + 2H2O Fe2+ + 2H2O
| (2) |
2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu0 + H2O2 + 2H+ → 2 Cu0 + H2O2 + 2H+ → 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ + 2H2O Cu+ + 2H2O
| (3) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + H2O2 → Fe2+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ˙OH + OH− Fe3+ + ˙OH + OH−
| (4) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + H2O2 → Fe3+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + ˙O2H + H+ Fe2+ + ˙O2H + H+
| (5) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ + H2O2 → Cu+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu2+ + ˙OH + OH− Cu2+ + ˙OH + OH−
| (6) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu2+ + H2O2 → Cu2+ + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ + ˙O2H + H+ Cu+ + ˙O2H + H+
| (7) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + Fe3+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ → Cu+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + Fe2+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu2+ Cu2+
| (8) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe0 + 2 Fe0 + 2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ → 3 Fe3+ → 3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ Fe2+
| (9) |
Therefore, to increase the catalytic activity of CuFe2O4, the surface modification of CuFe2O4 by introducing Fe0 and Cu0 was proposed in this study.
In this work, we used H2 to reduce CuFe2O4 and obtained zero-valent iron–copper bimetallic particles on the surface of CuFe2O4. The reduced CuFe2O4 was then used as Fenton-like catalyst. The overarching goal of this study was to develop a powerful candidate of heterogeneous Fenton-like catalyst. Methylene blue (MB) was selected as a model compound for dyes. The common influencing parameters on MB degradation were comprehensively investigated. The magnetic separation and regeneration of reduced CuFe2O4 were performed. Finally, the possible catalytic mechanism was also discussed.
2. Materials and methods
2.1. Materials
H2O2 (30%, w/w) was of analytical grade and was supplied by Sinopharm Chemical Reagent Co. (Shanghai, China). Other chemicals (analytical grade) used in the study were purchased from Sigma-Aldrich and used without further purification. All solutions were prepared using 18 MΩ deionized H2O at neutral pH (Millipore, USA). The stock solutions containing 250 mg L−1 of MB were freshly prepared by dissolving appropriate amounts of MB and kept in the dark.2.2. Preparation of reduced CuFe2O4
The CuFe2O4 was synthesized in classical alkaline medium using conventional literature recipes.19 In brief, 0.025 mol CuCl2·2H2O and 0.05 mol FeCl3·6H2O were dissolved together in 100 mL of deionized H2O, and then 75 mL NaOH solution (4 M) was added dropwise, followed by heating at 90 °C. The black precipitate was homogenized by vigorous stirring for 2 h at 90 °C and then washed by deionized water several times, until the water pH did not change. Finally, the CuFe2O4 was filtrated and dried at 70 °C overnight followed by calcination in flowing air at 400 °C for 4 h.The reduced CuFe2O4 was prepared by thermal treatment at 400 °C in a quartz tube under H2 (99.99%) flow (30 mL min−1) for 4 h with a heating rate of 10 °C min−1. After reduction, the material was cooled down under H2 flow to room temperature and was transferred to a sample vial and kept sealed under nitrogen atmosphere prior to use.
2.3. Characterization
Transmission electron micrograph (TEM) of the samples was taken on a Hitachi H-8100 TEM, operated at 200 kV. Powder X-ray diffraction (XRD) patterns were recorded on a Philips PW1710 diffractometer using Cu Kα radiation. Nitrogen adsorption–desorption isotherms were measured at 77 K on a Micromeritics ASAP 2020 sorptometer, with the samples outgassed for 16 h at 110 °C and 10−6 Torr prior to measurement. X-ray photoelectron spectroscopy (XPS) of the above mentioned samples were recorded on a spectrometer (Perkin-Elmer PHI-5300/ESCA, USA) with an Al Kα X-ray source. All the binding energies were referenced to the neutral C 1s peak at 284.6 eV to compensate for the surface charging effects. The XPS results were collected as binding energy forms and fitted using a curve-fitting program (XPSPEAK41 software).2.4. Experimental procedure
Batch degradation experiments of MB were carried out in a 100 mL conical flask at 25 °C in the dark. The initial concentration of MB was 50 mg L−1, and the total volume of reaction solution was 50 mL. The reaction suspension was prepared by adding the required amount of catalyst into 50 mL solution that had been adjusted to the desired pH value by 0.1 M HNO3. A known concentration of H2O2 was added to the solution to initiate the reaction. Samples were taken at set intervals using a 1 mL syringe, and quenched with excess methanol. To evaluate the contribution of homogeneous Fenton catalyzed by the leaching Fe and Cu ions on the MB degradation, experiment was carried out as follows: after mixing reduced CuFe2O4 at solution pH 3.2 for 25 min and removing the catalyst by filtration, MB and H2O2 were then added into the filtrate. The reusability of the catalyst was evaluated by collecting the catalyst with a magnet, washing with deionized water, drying the used catalyst under vacuum, and using it for the next reaction under similar experimental conditions. The catalytic activities were calculated by the concentration of MB (C/C0), where C0 and C were the MB concentrations at initial and time t, respectively.3. Results and discussion
3.1. Characterization
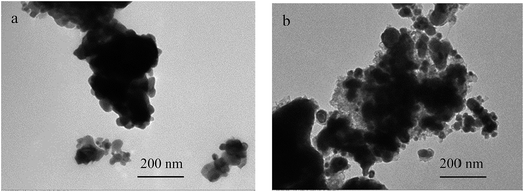
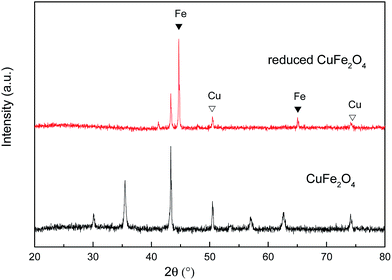
The transmission electron microscope (TEM) images of the CuFe2O4 and reduced CuFe2O4 are shown in Fig. 1. It can be seen that CuFe2O4 had a relatively smooth nonporous surface and reduced CuFe2O4 had fluffy appearance. The specific surface area (SSA) of CuFe2O4 and reduced CuFe2O4 was obtained from the N2 adsorption–desorption isotherms (Fig. S1a†). It can be seen that both the isotherms could be classified as type IV based on the IUPAC classification scheme. The SSA was measured to be 15.6 m2 g−1 for CuFe2O4 and 51.8 m2 g−1 for reduced CuFe2O4, respectively. The pore size distribution showed that there was a significant increase in volume of pores ranged from 2 to 8 nm after H2 reduction (Fig. S1b†).The X-ray diffraction patterns of CuFe2O4 and reduced CuFe2O4 are presented in Fig. 2. In the pattern of the CuFe2O4, all of the diffraction peaks matched well with the standard XRD pattern (PDF #77-0010), which indicated that the prepared CuFe2O4 had great purity. In the pattern of the reduced CuFe2O4, the (110) and (200) diffractions of Fe (PDF #99-0064) and (200) and (220) diffractions of Cu (PDF #70-3039) can be observed simultaneously, indicating the successful loading of Fe0 and Cu0 bimetallic particles in the reduced CuFe2O4.
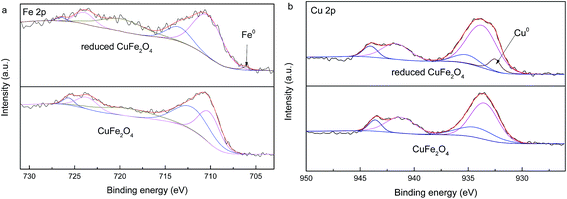
Surface elemental composition of CuFe2O4 and reduced CuFe2O4 is analyzed by the use of XPS (Fig. 3). In Fig. 3a, the binding energy at 711 eV and 725 eV can be ascribed to Fe 2p3/2 and Fe 2p1/2 according to previous study.20 The presence of the peak around 710.1 and 712.0 eV (for CuFe2O4) suggested that Fe3+ existed in two coordination environments where A-sites at higher binding energy and B-sites at lower binding energy, respectively.21 After redox reaction, the presence of a Fe0 peak with weak intensity at 706.1 eV was further evidence for the loading of Fe0 in the reduced CuFe2O4.22 For the XPS of Cu 2p regions (Fig. 3b), the peak at binding energy of 932.5 eV for the reduced CuFe2O4 was assigned to Cu0, which further confirmed the formation of Cu0 phase in the reduced CuFe2O4.23 The surface of the reduced CuFe2O4 samples contained 2.2% Fe0 and 10.6% Cu0 based on XPS analysis.
3.2. Catalytic activity of reduced CuFe2O4
Batch experiments were conducted to compare the removal efficiencies of MB by various processes. As shown in Fig. 4, CuFe2O4 exhibited relatively low catalytic activity and only 20% of MB was decoloured after 25 min. By contrast, MB was rapidly degraded by reduced CuFe2O4 and greater than 74% of MB was destructed within 25 min at 0.5 mM H2O2 and 0.1 g L−1 catalyst dosage. Meanwhile, H2O2 alone showed no remarkable degradation of MB and less than 7% of MB was adsorbed onto reduced CuFe2O4. The kinetic data were then fitted to a pseudo-first-order kinetic model (C = C0e−kt). The Fenton-like reaction rate k (min−1) was calculated to be 0.007 (R2 = 0.87) and 0.055 (R2 = 0.98) min−1 for CuFe2O4 and reduced CuFe2O4, respectively. These results clearly indicate that the catalytic activity of CuFe2O4 is significantly enhanced after H2 reduction. One possible reason was the increased SSA, which provided more active sites for H2O2 decomposition and produced more reactive oxidants such as ˙OH. The other possible reason was the introduction of Fe0 and Cu0, which could facilitate the decomposition of H2O2 into ˙OH and accelerate electron transfer from Fe0 and/or Cu0 to CuFe2O4.16,24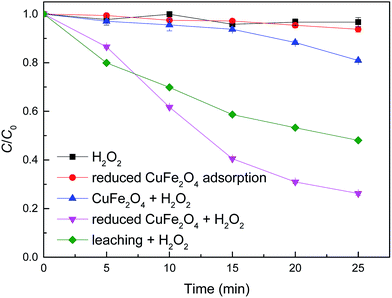 | ||
| Fig. 4 Comparison of the removal efficiency of MB with different catalytic systems. Conditions: [H2O2] = 0.5 mM, catalyst dosage = 0.1 g L−1, [MB] = 50 mg L−1, initial pH = 3.2 ± 0.1. | ||
To better understand the contribution of homogeneous Fenton reaction catalyzed by the leaching Fe and Cu ions on the MB degradation, experiment in homogeneous systems was carried out by removing reduced CuFe2O4 catalyst after vigorous agitation for 25 min. As shown in Fig. 4, the removal of MB after a reaction period of 25 min in the homogeneous Fenton-like reaction system was 52%. By contrast, the removal of MB catalyzed by reduced CuFe2O4 at 25 min was 74%. These results suggest that the removal of MB was attributed by both the homogeneous and heterogeneous Fenton-like reactions. Similar results were also obtained by Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles, which assumed that the removal of 2,4-dichlorophenol was partially attributed to the bulk homogeneous Fenton reaction due to the dissolved Fe ions.6 The leached ions were determined in our study and the concentrations of total dissolved Fe and Cu were 0.49 and 1.09 mg L−1 after a reaction period of 25 min.
A comparison was carried out between the reaction rate constant of reduced CuFe2O4 with those reported in previous studies.25–31 Based on the obtained results (Table S1†), it seems that the proposed heterogeneous Fenton-like system leads to a high efficiency for MB degradation.
3.3. Effects of parameters on MB degradation
The degradation of organic pollutants was usually influenced by pH, catalyst dosage and H2O2 concentration. The degradation of MB over time under different experimental conditions was evaluated.Fig. 5 shows the effect of pH on the removal of MB by reduced CuFe2O4. It can be seen that pH had a distinct influence on the removal of MB by Fenton-like reaction. The relatively slow degradation of MB was observed at pH values of 4.5 and 6.0, while a lower pH induced a higher kinetic rate. The increased oxidation efficiency at lower pH values can be attributed to the higher oxidation potential of ˙OH and the more dissolved fraction of iron species.12 It was also proposed that acidic conditions were favourable for the stability of H2O2 and were beneficial for the generation of ˙OH and the formation of metal oxide-pollutant inner–sphere complexes that will promote reaction.6 On the other hand, as Fe0 and Cu0 were loaded on the surface of reduced CuFe2O4, the lower pH will favor the generation of Fe2+ and Cu+ (eqn (1)–(3)), which could promote the decomposition of H2O2 into ˙OH (eqn (4) and (6)).32
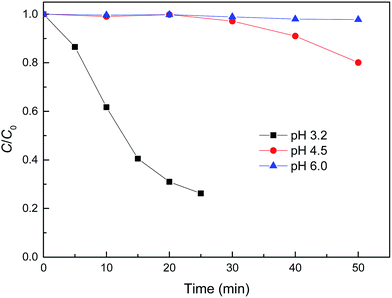 | ||
| Fig. 5 Effect of initial pH on MB degradation by reduced CuFe2O4. Conditions: [H2O2] = 0.5 mM, [reduced CuFe2O4] = 0.1 g L−1, [MB] = 50 mg L−1. | ||
Fig. 6 shows the degradation of MB by reduced CuFe2O4 at different catalyst dosages. It can be observed that the removal of MB by degradation increased along with increasing catalyst dosages. The degradation of MB achieved 59.4% with 0.05 g L−1 catalyst dosage, and up to 73.8% when the catalyst dosage increased to 0.1 g L−1. This finding was likely attributed to the increased amount of active sites on the solid catalyst surface, which was expected to accelerate the reactions of H2O2 decomposition. Moreover, increasing catalyst dosage could result in a higher iron dissolution, and ultimately producing more radicals. However, when reduced CuFe2O4 addition increased to 0.2 g L−1, the degradation of MB was not further enhanced, probably due to the agglomeration of particles and the scavenging of ˙OH or other radicals by present iron species through undesirable reactions.12
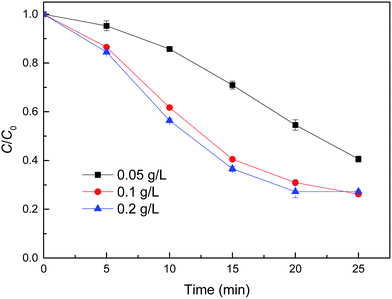 | ||
| Fig. 6 Effect of catalyst dose on MB degradation by reduced CuFe2O4. Conditions: [H2O2] = 0.5 mM, [MB] = 50 mg L−1, initial pH = 3.2 ± 0.1. | ||
The effect of H2O2 concentration on the removal of MB using reduced CuFe2O4 was also investigated (Fig. 7). It was observed clearly that the degradation of MB was gradually accelerated with H2O2 concentration increasing from 0.2 to 1.0 mM. This positive correlation could be related to the accelerated generation of oxidizing intermediates that were responsible for MB oxidation, when considering that H2O2 alone had a negligible degradation effect on MB. It was proposed that H2O2 is the precursor in the reaction with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ generating ˙OH.33 With sufficient H2O2, the amount of ˙OH generated will be enhanced, leading to a high removal efficiency of MB.
Fe2+ generating ˙OH.33 With sufficient H2O2, the amount of ˙OH generated will be enhanced, leading to a high removal efficiency of MB.
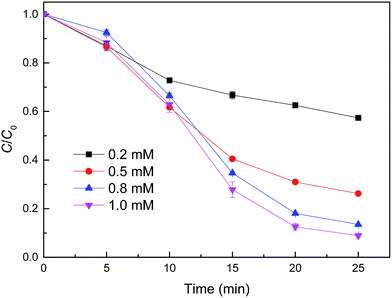 | ||
| Fig. 7 Effect of H2O2 concentration on MB degradation by reduced CuFe2O4. Conditions: [reduced CuFe2O4] = 0.1 g L−1, [MB] = 50 mg L−1, initial pH = 3.2 ± 0.1. | ||
3.4. Stability and reusability of reduced CuFe2O4
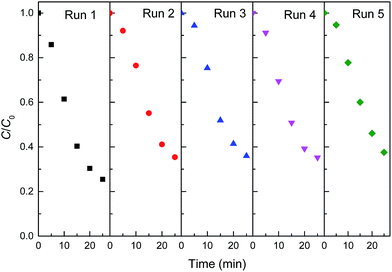
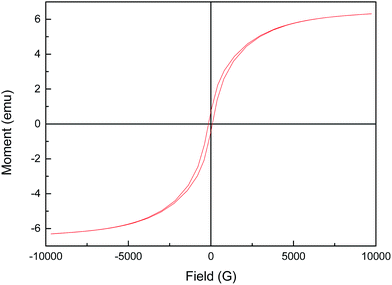
Stability is an important factor for the application of catalyst in heterogeneous Fenton-like reaction. From the view of practical application, the long term stability of reduced CuFe2O4 is crucial. Therefore, the leaching characteristic and the activity variation of reduced CuFe2O4 in cycles are especially concerned. The leaching ions were determined and the concentration of total dissolved iron and copper were 0.49 and 1.09 mg L−1 after 25 min reaction. This phenomenon partially confirmed the stability of catalyst. Successive experiments were performed to evaluate the possibility of reduced CuFe2O4 reuse. As shown in Fig. 8, it was observed that reduced CuFe2O4 remained a high catalytic activity after five consecutive runs. The XRD pattern of the catalyst after five cycles showed that crystal phase of the used catalyst was almost the same as that of the fresh catalyst (Fig. S2†). These results demonstrate that reduced CuFe2O4 has a high stability and a good reusability after the recycling tests, suggesting the feasibility of the reduced CuFe2O4/H2O2 system for a longer reaction time.The magnetization curves of reduced CuFe2O4 were investigated by vibrating sample magnetometer (VSM) at 25 °C and the results were illustrated in Fig. 9. The magnetic hysteresis curve revealed that reduced CuFe2O4 was ferromagnetic and had a magnetic saturation of about 6.3 emu g−1, which ensured that the catalyst could be easily separated by a magnet and reused from aqueous solution.
3.5. The enhanced reaction mechanism
In order to discriminate the active species in the reduced CuFe2O4/H2O2 system for MB degradation, tert-butyl alcohol (TBA) was used as the scavenger of ˙OH in this study. As shown in Fig. 10, the degradation efficiency of MB decreased from 75% without inhibitor to 34 and 3% with the addition of 1 and 10 mM TBA, respectively. There was almost no MB degradation with the addition of 10 mM TBA during the reaction, indicating that the ˙OH produced by H2O2 in the Fenton-like reaction was scavenged. These results indicate that MB is mainly decomposed by the attack of ˙OH.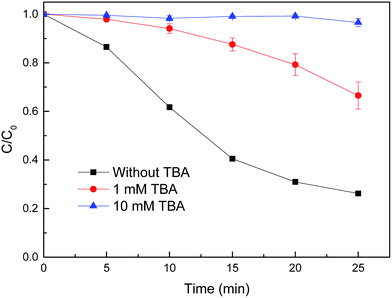 | ||
| Fig. 10 Effect of TBA on MB degradation by reduced CuFe2O4. Conditions: [H2O2] = 0.5 mM, [reduced CuFe2O4] = 0.1 g L−1, [MB] = 50 mg L−1, initial pH = 3.2 ± 0.1. | ||
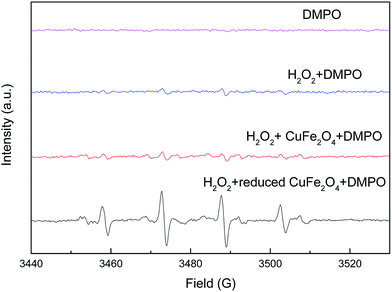
To ascertain the reaction mechanism, electron spin resonance (ESR) spectroscopy was performed by using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as trapping agent to examine ˙OH produced in the heterogeneous Fenton-like reaction. As shown in Fig. 11, the ESR spectrum in the presence of catalysts displayed a 4-fold characteristic peak of DMPO–˙OH adduct with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The intensity of DMPO–˙OH peaks by using reduced CuFe2O4 as catalyst was much stronger than CuFe2O4, demonstrating a high catalytic activity in the reduced CuFe2O4/H2O2 system. Therefore, it can be concluded that reduced CuFe2O4 could effectively activate H2O2 to generate ˙OH and the ˙OH was the predominant active radical in the heterogeneous Fenton-like reaction.
1. The intensity of DMPO–˙OH peaks by using reduced CuFe2O4 as catalyst was much stronger than CuFe2O4, demonstrating a high catalytic activity in the reduced CuFe2O4/H2O2 system. Therefore, it can be concluded that reduced CuFe2O4 could effectively activate H2O2 to generate ˙OH and the ˙OH was the predominant active radical in the heterogeneous Fenton-like reaction.
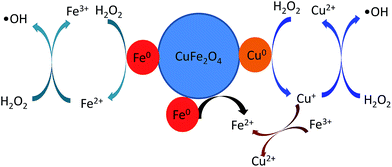
The above results have shown that the ˙OH is the major active radical in the heterogeneous Fenton-like processes. Moreover, the enhanced catalytic activity is displayed by catalyst of reduced CuFe2O4, which shows high MB removal efficiency. The Fenton-like reaction rate k (min−1) was calculated to be 0.007 and 0.055 min−1 for CuFe2O4 and reduced CuFe2O4, respectively. When normalized by SSA, the k/SSA values indicate a higher MB removal efficiency for the reduced CuFe2O4 than for the CuFe2O4. This suggests that Fe0 and Cu0 bimetallic particles loaded in the reduced CuFe2O4 play important role in the heterogeneous Fenton reaction. According to all above experimental results, a possible mechanism for reduced CuFe2O4 degradation of MB has been proposed. In the first step, the H2O2 molecules can be adsorbed on reduced CuFe2O4 and react with the metallic particles. Specifically, the Fe0 can be oxidized to Fe2+ via a two electron transfer (eqn (2)) and Cu0 can be oxidized to Cu+ via a one electron transfer from the particles surface to H2O2 (eqn (3)).18,34 These oxidation reactions were further confirmed by the results of XPS (Fig. S3†). The peak of Fe0 disappeared after reaction. The atomic ratio of Cu0 decreased from 10.6% in the fresh catalyst to 4.9% in the used catalyst.
Then, Fe2+ on the surface participate in the reaction by activating H2O2 molecules to produce ˙OH according to the Haber–Weiss mechanism (eqn (4) and (5)).33,35 Similarly, Cu+ on the surface can also participate in the reaction by activating H2O2 molecules to generate ˙OH (eqn (6)).17,18 On the other hand, the loading of Fe0 could act as an electron transfer agent during reaction, which could easily reduce Fe3+ species in the CuFe2O4 phase to regenerate the active Fe2+ species (eqn (9)).13,15 Since the standard reduction potential of Fe3+/Fe2+ is 0.77 V and Cu2+/Cu+ is 0.17 V, the redox cycles of Fe3+/Fe2+ and Cu2+/Cu+ are also proposed (eqn (8)).17,18 Therefore, based on above analysis, a possible enhanced reaction mechanism of MB degradation by reduced CuFe2O4 is proposed as illustrated in Fig. 12.
4. Conclusions
Reduced CuFe2O4 was synthesized and used as a heterogeneous Fenton-like catalyst, which exhibited much higher catalytic activity towards the degradation of MB in the presence of H2O2 compared with raw CuFe2O4. The use of 0.1 g L−1 reduced CuFe2O4 induced nearly 74% of MB degradation within 25 min in the presence of 0.5 mM H2O2 at initial pH 3.2. The characterization and experimental results suggested that the high catalytic activity was attributed to the high surface area and the presence of Fe0/Cu0 bimetallic particles on the surface of reduced CuFe2O4. The reusability tests indicated that reduced CuFe2O4 was relatively stable and could be reused several times as a Fenton-like catalyst. Moreover, reduced CuFe2O4 displayed a superparamagnetic property, which allowed them to be easily separated and collected in practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China [51408119 and 41671468], The Science and Technology Project of Zhejiang Province (2017C33036) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors would like to thank Analytical Center of NIGLAS for making this study possible by making laboratory facilities available.References
- A. Ahmad, S. H. Mohd-Setapar, C. S. Chuong, A. Khatoon, W. A. Wani, R. Kumar and M. Rafatullah, RSC Adv., 2015, 5, 30801–30818 RSC.
- J. L. Wang and L. J. Xu, Crit. Rev. Environ. Sci. Technol., 2012, 42, 251–325 CrossRef CAS.
- P. V. Nidheesh, R. Gandhimathi and S. T. Ramesh, Environ. Sci. Pollut. Res., 2013, 20, 2099–2132 CrossRef CAS PubMed.
- R. S. Ribeiro, A. M. T. Silva, J. L. Figueiredo, J. L. Faria and H. T. Gomes, Appl. Catal., B, 2016, 187, 428–460 CrossRef CAS.
- N. Panda, H. Sahoo and S. Mohapatra, J. Hazard. Mater., 2011, 185, 359–365 CrossRef CAS PubMed.
- L. J. Xu and J. L. Wang, Appl. Catal., B, 2012, 123, 117–126 CrossRef.
- X. Y. Li, Y. Huang, C. Li, J. M. Shen and Y. Deng, Chem. Eng. J., 2015, 260, 28–36 CrossRef CAS.
- Y. Feng, C. Z. Liao and K. M. Shih, Chemosphere, 2016, 154, 573–582 CrossRef CAS PubMed.
- Y. B. Wang, H. Y. Zhao, M. F. Li, J. Q. Fan and G. H. Zhao, Appl. Catal., B, 2014, 147, 534–545 CrossRef CAS.
- D. H. K. Reddy and Y. S. Yun, Coord. Chem. Rev., 2016, 315, 90–111 CrossRef CAS.
- D. H. Bremner, A. E. Burgess, D. Houllemare and K. C. Namkung, Appl. Catal., B, 2006, 63, 15–19 CrossRef CAS.
- L. J. Xu and J. L. Wang, J. Hazard. Mater., 2011, 186, 256–264 CrossRef CAS PubMed.
- F. C. C. Moura, M. H. Araujo, R. C. C. Costa, J. D. Fabris, J. D. Ardisson, W. A. A. Macedo and R. M. Lago, Chemosphere, 2005, 60, 1118–1123 CrossRef CAS PubMed.
- R. Sharma, S. Bansal and S. Singhal, RSC Adv., 2015, 5, 6006–6018 RSC.
- D. Wan, W. B. Li, G. H. Wang, L. L. Lu and X. B. Wei, Sci. Total Environ., 2017, 574, 1326–1334 CrossRef CAS PubMed.
- R. C. C. Costa, F. C. C. Moura, J. D. Ardisson, J. D. Fabris and R. M. Lago, Appl. Catal., B, 2008, 83, 131–139 CrossRef CAS.
- Y. B. Wang, H. Y. Zhao and G. H. Zhao, Appl. Catal., B, 2015, 164, 396–406 CrossRef CAS.
- J. Wang, C. Liu, J. S. Li, R. Luo, X. R. Hu, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, Appl. Catal., B, 2017, 207, 316–325 CrossRef CAS.
- F. Qi, W. Chu and B. B. Xu, Chem. Eng. J., 2015, 262, 552–562 CrossRef CAS.
- A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564–1574 CrossRef CAS.
- C. Reitz, C. Suchomski, J. Haetge, T. Leichtweiss, Z. Jaglicic, I. Djerdj and T. Brezesinski, Chem. Commun., 2012, 48, 4471–4473 RSC.
- P. L. Wang, X. Zhou, Y. G. Zhang, L. P. Yang, K. K. Zhi, L. L. Wang, L. T. Zhang and X. F. Guo, RSC Adv., 2017, 7, 26983–26991 RSC.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2015, 176, 249–265 CrossRef.
- K. Y. Li, Y. Q. Zhao, C. S. Song and X. W. Guo, Appl. Surf. Sci., 2017, 425, 526–534 CrossRef CAS.
- J. S. Zhang, T. J. Yao, C. C. Guan, N. X. Zhang, H. Zhang, X. Zhang and J. Wu, J. Colloid Interface Sci., 2017, 505, 130–138 CrossRef CAS PubMed.
- S. T. Yang, W. Zhang, J. R. Xie, R. Liao, X. L. Zhang, B. W. Yu, R. H. Wu, X. Y. Liu, H. L. Li and Z. Guo, RSC Adv., 2015, 5, 5458–5463 RSC.
- S. C. Hsieh and P. Y. Lin, J. Nanopart. Res., 2012, 14, 10 CrossRef.
- Z. L. Shi, X. X. Wang and S. H. Yao, Chin. J. Inorg. Chem., 2015, 31, 696–702 CAS.
- Q. Wang, S. L. Tian and P. Ning, Ind. Eng. Chem. Res., 2014, 53, 643–649 CrossRef CAS.
- T. Shahwan, S. Abu Sirriah, M. Nairat, E. Boyaci, A. E. Eroglu, T. B. Scott and K. R. Hallam, Chem. Eng. J., 2011, 172, 258–266 CrossRef CAS.
- W. Z. Tang and R. Z. Chen, Chemosphere, 1996, 32, 947–958 CrossRef CAS.
- S. S. Lin and M. D. Gurol, Environ. Sci. Technol., 1998, 32, 1417–1423 CrossRef CAS.
- C. Lee, C. R. Keenan and D. L. Sedlak, Environ. Sci. Technol., 2008, 42, 4921–4926 CrossRef CAS PubMed.
- W. P. Kwan and B. M. Voelker, Environ. Sci. Technol., 2003, 37, 1150–1158 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12488k |
| This journal is © The Royal Society of Chemistry 2018 |