Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Core–shell microspheres for the ultrafast degradation of estrogen hormone at neutral pH†
Katherine Villa a,
Jemish Parmar
a,
Jemish Parmar ab,
Diana Vilela
ab,
Diana Vilela ab and
Samuel Sánchez
ab and
Samuel Sánchez *abc
*abc
aInstitute for Bioengineering of Catalonia (IBEC), The Barcelona Institute of Science and Technology, Baldiri Reixac 10-12, 08028 Barcelona, Spain. E-mail: ssanchez@ibecbarcelona.eu; Tel: +34 934 020 558
bMax Planck Institute for Intelligent Systems Institution, Heisenbergstraße 3, 70569 Stuttgart, Germany
cInstitució Catalana de Recerca i Estudis Avancats (ICREA), Pg. Lluís Companys 23, 08010, Barcelona, Spain
First published on 5th February 2018
Abstract
In the past few years there has been growing concern about human exposure to endocrine disrupting chemicals. This kind of pollutants can bioaccumulate in aquatic organisms and lead to serious health problems, especially affecting child development. Many efforts have been devoted to achieving the efficient removal of such refractory organics. In this regard, a novel catalyst based on the combination of α-FeOOH and MnO2@MnCO3 catalysts has been developed by up-scalable techniques from cheap precursors and tested in the photo-Fenton-like degradation of an endocrine disruptor. Almost total degradation of 17α-ethynylestradiol hormone was achieved after only 2 min of simulated solar irradiation at neutral pH. The outstanding performance of FeOOH@MnO2@MnCO3 microspheres was mainly attributed to a larger generation of hydroxyl radicals, which are the primary mediators of the total oxidation for this hormone. This work contributes to the development of more cost-effective systems for the rapid and efficient removal of persistent organic pollutants present in sewage plant effluents under direct solar light.
Introduction
Industrial wastewater effluents from pharmaceuticals, agriculture, cosmetics, and personal care products usually contain a high amount of refractory organic pollutants.1 Among them, endocrine disrupting chemicals are considered a major concern for the aquatic environment and human beings, because of their ability to interact with the cell receptors of the body and mimic hormone-like activity. The presence of endocrine disruptors in water can lead to many health issues related to the reproductive system and immune disorders, even in very low concentrations.2 Considering that these toxic chemicals cannot be totally degraded by the classical bacteria-assisted water treatment methods and their tendency to accumulate in natural water and aquatic animals,3 it is essential to explore alternative catalytic approaches for their efficient removal in the water cleaning process.One of the most powerful methods for this purpose is the Fenton reaction,3–5 which involves the generation of highly oxidizing species, known as hydroxyl radicals (HO˙), through the reaction between a dissolved iron salt (Fe2+) and hydrogen peroxide (H2O2), which can degrade all the organic molecules to CO2 and water. The performance of Fenton reaction can be improved by the so-called photo-Fenton degradation, where UV-visible light is used to generate extra reactive radicals from the photolysis of H2O2, and enhance the regeneration of the Fe2+ catalyst.6,7 Thus, the possibility of using solar light as energy source is an attractive way to transform it in a sustainable and more efficient technology.
Nevertheless, a real application of this advanced oxidation process is still hampered, mainly by the intrinsic limitations of the Fenton catalysts related to the requirement of an acidic pH. This entails extra costs associated with neutralization steps and further recovery of the formed iron sludges.
To address these problems, extensive recent research has focused on the development of heterogeneous iron-based catalysts that can activate H2O2 at neutral pH and facilitate recovery after the treatment process.8–12 However, the efficiency of these heterogeneous Fenton-like catalysts is usually found to be lower than its homogeneous counterpart. Thus, the design of heterogeneous catalysts that can effectively harvest solar light and decompose H2O2 into HO˙ without the need for a pH change is crucial for the practical application of solar Fenton-based wastewater treatments.
Among the catalysts reported for heterogeneous photo-Fenton-like reaction, amorphous iron oxide FeOOH (α-goethite) has shown an outstanding performance, even though its applicability has been limited by the slow Fe3+/Fe2+ cycle rates.13–15 Alternatively, other transition metal oxides, such as CeO2, CuO and MnO2, have also been reported as active materials for Fenton-like oxidation treatments.16–18 Among them, MnO2 (a non-toxic and cheap material) has not been extensively studied for the oxidation reaction of organic pollutants, despite being an efficient catalyst for the decomposition of H2O2.19,20
Here, we investigate for the first time the photo-Fenton-like degradation of 17α-ethynylestradiol hormone (EE2) using FeOOH@MnO2@MnCO3 core–shell catalyst. This material was synthesized by a facile surface oxidation of spherical MnCO3 crystal template to MnO2@MnCO3 and subsequent coating of α-FeOOH nanoparticles on it. Since MnO2 facilitates the fast decomposition of H2O2 and iron cycling, the combination of α-FeOOH and MnO2 results in a great enhancement of the overall catalytic process. Additionally, the performance of this material can be improved by using mainly visible light, which is the major component of the solar radiation. The outcome of this research would contribute to the feasibility of the photo-Fenton-like technology by using Fe/Mn microspheres-based system for the treatment of contaminated water with industrial pollutants.
Experimental
Catalyst preparation
MnCO3 microparticles were synthesized by mixing 70 mL of 0.014 M MnSO4 solution (ACS reagent ≥99.0%, Sigma-Aldrich) with 0.79 g of NH4CO3 (BioUltra ≥99.5%, Sigma-Aldrich) at room temperature for 15 h.21 A schematic illustration of the synthesis procedure of α-FeOOH@MnO2@MnCO3 microspheres is shown in Fig. 1. A core–shell structure of MnO2@MnCO3 was prepared by mixing 0.09 g of the as-synthesized MnCO3 with 6.0 mL of aqueous solution of 0.032 M KMnO4 (ACS reagent ≥99.0%, Sigma-Aldrich) for 4 h. The sample was collected by centrifugation and washed with Milli-Q water. Afterwards, the MnO2@MnCO3 sample was dispersed in 30 mL of glycerol–water mixture by sonication for 20 min. FeOOH was grown on the MnO2@MnCO3 microparticles by adding 0.166 g of FeSO4·7H2O (ACS reagent ≥99.0%, Sigma-Aldrich) to that mixture and stirring for 10 min. Afterwards, the black resulting suspension was transferred into a 50 mL Teflon-lined stainless-steel autoclave and heated at 120 °C for 24 h. The dark orange precipitate was collected by centrifugation and washed sequentially with water and ethanol, and dried at 60 °C for 6 h. A control synthesis experiment was carried out by adding the iron precursor (in the absence of MnO2@MnCO3 microparticles) to confirm that the iron oxide phase grown by the hydrothermal reaction consists of α-FeOOH.For the sake of simplicity, the FeOOH@MnO2@MnCO3 material has been labeled as Fe/MnO2@MnCO3.
Catalyst characterization
The crystal structure of the as-synthesized samples was determined by X-ray powder diffraction (XRD) using a Bruker D8 Advance diffractometer equipped with a Cu Kα radiation (1.5417 Å) source, a LYNXEYE super speed detector and a Ni filter. X-ray diffraction data was collected in the 2θ range between 20° and 80° using a scan rate of 0.05° by 2 s. The light absorption properties were measured by using a UV-Vis diffuse reflectance spectrophotometer (Perkin Elmer Lambda 950 UV-Vis) with a wavelength range of 250–800 nm. A Zeiss Auriga microscope equipped with energy-dispersive X-ray (EDX) detector was used to perform scanning electron microscopy (SEM) and EDX analysis.X-ray photoelectron spectroscopy (XPS) from SPECS system (Germany) was used to identify Mn and Fe oxidation states on the samples. The instrument was equipped with XR50 duel anode source (Al operated at 150 W) and a Phoibos MCD-9 detector. All measurements were done under the vacuum (pressure 5 × 10−9 mbar) and the hemispherical analyzer was set at the pass energy 25 eV while the high resolution spectra step size was set at 0.1 eV. Casa XPS program (Casa Software Ltd., UK) was used for the data analysis.
Photo-Fenton set-up
The degradation of an aqueous solution of EE2 was carried out in a 10 mL capacity cylindrical glass vessel. The catalyst slurries were magnetically stirred during the reaction. For the photo-Fenton-like experiments, a 300 W high pressure UV-visible lamp (Ultravitalux Osram, 280–780 nm) was used to simulate solar irradiation. In a typical experiment, 6 mL of the 0.5 ppm of EE2 solution containing 0.0015 g of catalyst was placed in the reactor. Since the main aim of this study was to evaluate the catalytic activity at near-neutral pH (6.7), no acidic or basic pH adjustment was performed.Prior to illumination, the suspension was sonicated in the dark for 30 min to reach adsorption–desorption equilibrium. After that, the lamp was turned on and H2O2 was added to initiate the reaction. Liquid samples were periodically taken out, immediately centrifuged, and then analyzed by high performance liquid chromatography (Acquity) coupled to a triple quadrupole mass spectrometer LC/MS/MS (API 3000). The mobile phase consisted of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v ultrapure water
50 v/v ultrapure water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile mixture and the injection volume of the sample was 10 μL. In the case of the MB degradation, the concentration was monitored by measuring its absorbance at 664 nm (λmax) during the reaction by using a UV-Vis spectrophotometer (Specord 50 Plus).
acetonitrile mixture and the injection volume of the sample was 10 μL. In the case of the MB degradation, the concentration was monitored by measuring its absorbance at 664 nm (λmax) during the reaction by using a UV-Vis spectrophotometer (Specord 50 Plus).
A blank test without catalyst (only H2O2 + light irradiation) was also performed to evaluate the contribution of the photolysis of H2O2, which involves the generation of hydroxyl radicals, on the oxidation of our target molecule (EE2). Additionally, a test with catalyst under light irradiation and no H2O2 was carried out to estimate the photocatalytic influence on the resulting performance.
The hydroxyl radical generation during the photo-Fenton-like reaction was identified by following a fluorescence method, using 5 × 10−4 M terephthalic acid (TA) as a chemical trap of HO˙. The excitation wavelength was 320 nm and the fluorescence emission spectra were acquired from 350–600 nm using a multimode microplate reader (Infinite M200 PRO). The experimental conditions were the same as those described above for the irradiation tests and H2O2 concentration was 0.1 M.
Results and discussion
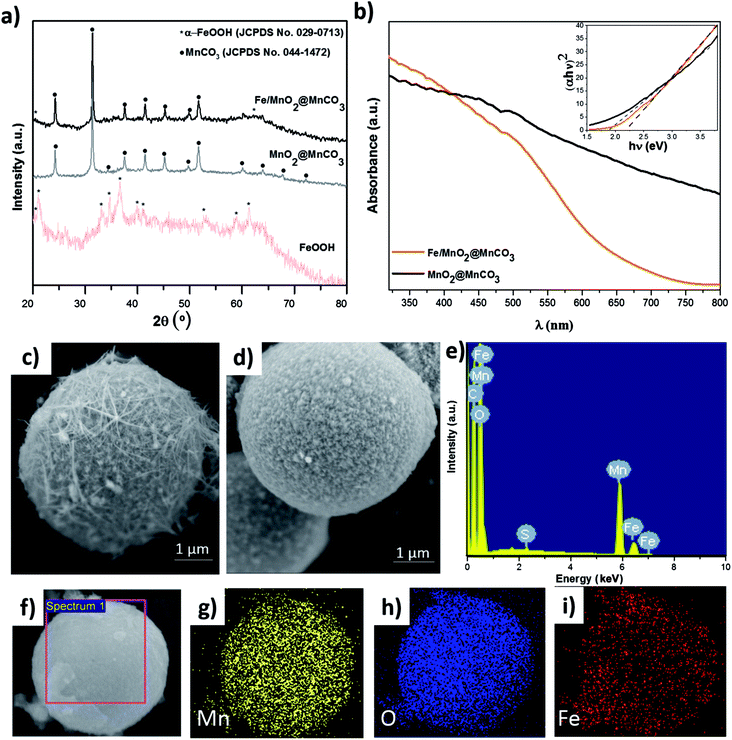
To study the composition and structure of the catalysts, different characterization techniques such as XRD, UV-Vis and SEM/EDX were used (Fig. 2). As observed in XRD patterns displayed in Fig. 2a, MnO2@MnCO3 only presents diffraction peaks corresponding to the crystalline structure of MnCO3 (JCPDS no. 044-1472). Since the intensity of the core peaks was too high, the external shell of MnO2 was not appreciated, which is in agreement with previously reported works.20,21 The XRD patterns of the iron oxide obtained by the hydrothermal reaction indicate the formation of α-goethite FeOOH phase (JCPDS no. 029-0713). In the case of Fe/MnO2@MnCO3 microparticles, the diffraction peaks are very similar to those of bare MnO2@MnCO3, being also difficult to detect the peaks associated with MnO2. However, an additional peak at around 20.1° and a shoulder at approximately 62.1° can be attributed to the α-FeOOH phase from the surface of Fe/MnO2@MnCO3 microspheres.A slight shift in the optical absorption of bare MnO2@MnCO3 catalyst was observed in the UV-Vis diffuse reflectance spectra after being loaded with α-FeOOH (Fig. 2b). The band gap energy (Eg) of the materials was calculated from the absorption data by using the Tauc plots (see inset, Fig. 2b), where (absorbance energy)2 is plotted against energy.22 The estimated values of 1.9 and 2.2 eV for MnO2@MnCO3 and Fe/MnO2@MnCO3 catalysts, respectively, confirm that the optical absorption properties of these Mn-based materials lie in the visible region. Considering a real application under direct solar light, this is advantageous in comparison with other widely studied materials such as TiO2, which can only be activated by ultraviolet light.23,24
SEM images are shown in Fig. 2c and d. MnO2@MnCO3 microparticles consist of a spherical shape with an approximate diameter of 4.5 μm and a fibrous surface. It is worth noting that due to their large size compared to nanometric particles, these microparticles can be easily recovered from the solution after each catalytic reaction. In the case of the Fe/MnO2@MnCO3, the spherical shape, size, and roughness were preserved (Fig. 2d). However, due to the formation of the external α-FeOOH coating on the surface, it presents a higher homogeneity than MnO2@MnCO3. The EDX spectrum confirmed the presence of Fe in the Fe/MnO2@MnCO3 catalyst structure (Fig. 2e), in agreement with XRD characterization. Furthermore, EDX-mapping showed the uniform distribution of Fe on the catalyst surface (Fig. 2f–i).
The chemical states of Fe and Mn in Fe/MnO2@MnCO3 sample were investigated by XPS. Fig. 3a shows the XPS spectrum of Fe 2p. The complex multiplet-split of Fe 2p regions is typical of Fe3+ compounds.25 The two main peaks at 712.75 and 726.14 eV can be assigned to Fe 2p3/2 and Fe 2p1/2 signals from FeOOH. Additionally, the satellite peak of Fe 2p3/2 at 720.83 eV would confirm the presence of Fe3+ ions in the composite.26,27 In the case of Mn 2p (Fig. 3b), two main peaks at 643.73 and 655.55 eV are distinguished, corresponding to Mn 2p3/2 and Mn 2p1/2, respectively. These two spin–orbit components have a separation of approximately 12 eV, evidencing the presence of Mn4+ from the MnO2 material.26 An additional Mn 2p3/2 satellite peak at 648.19 eV can be attributed to the presence of MnO (MnOOH oxide),25 resulting from the hydrothermal treatment in the presence of FeOOH precursors. This fact evidences a mixture of valence states (Mn3+ and Mn4+) in the Fe/MnO2@MnCO3 catalyst. The XPS O 1s region shows three contributions at 530.12, 531.93 and 533.70 eV (Fig. 3c). The first one can be attributed to the oxygen atoms in the lattice of metal–oxygen structure. The second one at 531.93 eV corresponds to the metal-hydroxyl bond and the latter at 533.70 eV is assigned to the water adsorbed on the catalyst surface.28–30 Finally, the spectrum from C 1s display a high signal observed at 291.8 eV (Fig. 3d) may be assigned to the carbonate groups (CO32−) present in the sample, due to the composite core (MnCO3).
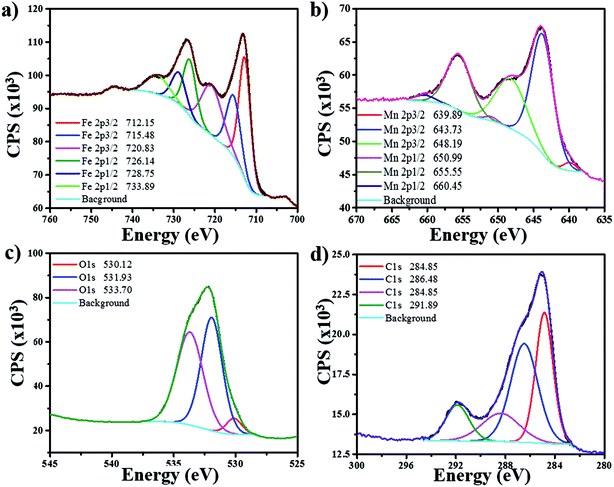 | ||
| Fig. 3 XPS spectra of Fe/MnO2@MnCO3 catalyst for its different elements: (a) iron (Fe), (b) manganese (Mn), (c) oxygen (O), (d) carbon (C). | ||
A preliminary screening of the bare catalysts and the composite was carried out at neutral pH using methylene blue (a common dye waste). As presented in Fig. S1,† the performance of FeOOH catalyst is almost negligible, whereas Fe/MnO2@MnCO3 composite shows a remarkable Fenton-like activity compared to MnO2@MnCO3 material. Based on this, FeOOH photocatalyst was not considered for further degradation experiments.
The photo-Fenton-like degradation of EE2, as an example of a persistent endocrine disrupting pollutant, was studied in an aqueous suspension containing the as-synthesized MnO2@MnCO3 and Fe/MnO2@MnCO3 catalysts in the presence of H2O2, under UV-visible light irradiation. Considering that endocrine disrupting chemicals are usually found at low concentrations in sewage plant effluents, and taking into account that even at those low levels they are toxic,31,32 we selected a 0.5 ppm EE2 concentration for the degradation tests.
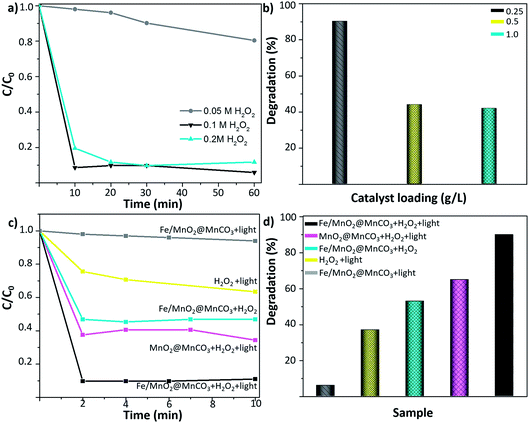
Initially, the concentration of H2O2 was optimized. As shown in Fig. 4a, the degradation of 0.5 ppm EE2 enhanced from 10 to 90% by increasing the H2O2 concentration from 0.05 M to 0.1 M. However, a further increase to 0.2 M did not lead to an additional improvement of the degradation rates, evidencing that a plateau was reached. Therefore, 0.1 M H2O2 was selected as the optimal minimum concentration for further experiments. Afterwards, to select the optimal amount of catalyst, different catalyst loadings from 0.25 to 1 g L−1 were tested (Fig. 4b). The highest degradation value was obtained by using the lowest amount of catalyst (0.25 g L−1). This fact can be attributed to the severe light scattering effect provoked by larger catalyst concentrations, which reduces the number of available photons able to reach each catalyst particle,33,34 resulting in a decrease of the radical species generation and a subsequent decrease in the degradation efficiency of the EE2 hormone.
Fig. 4c and d show the degradation kinetics of 0.5 ppm EE2 compound after 10 min of UV-visible irradiation. Control experiments consisting of the irradiation of Fe/MnO2@MnCO3 (without H2O2) and 0.1 M H2O2 (without catalyst) were carried out under the optimized reaction conditions. It was found that the photocatalytic activity of Fe/MnO2@MnCO3 without H2O2 only contributed to around 6% of the degradation after 10 min. Furthermore, it is well-known that the irradiation of H2O2 with UV-visible irradiation also leads to hydroxyl radicals generation by photolysis.35 Nonetheless, under those conditions the degradation yield was also low and slow. It is worth highlighting that the high pressure lamp used in this work (maximum intensity at 365 nm) cannot lead to the photodegradation of the synthetic hormone.36 When the photo-Fenton tests were performed in the presence of MnO2@MnCO3 and Fe/MnO2@MnCO3 catalysts and 0.1 M H2O2, the degradation rates were noticeably enhanced after only two minutes of light irradiation. This result shows that the presence of the catalyst is required to improve the decomposition of H2O2 and accelerate the degradation rate.
The MnO2@MnCO3 catalyst showed a 62% EE2 degradation within 2 min of the photo-Fenton-like reaction, and that maximum degradation yield remained constant over time, suggesting that the catalyst is rapidly consuming all the H2O2 to generate O2. Moreover, unlike the Fe-based catalysts, MnO2 is more prone to generate superoxide and hydroperoxy radicals,37 which have a lower oxidation potential than the hydroxyl radicals.38
As shown in Fig. 4d, the highest degradation yield was achieved with the Fe/MnO2@MnCO3 microspheres. This improvement in the photo-Fenton performance might be explained by multiple factors, such as the synergistic effect arising from the activity of manganese and iron metal oxides in the photo-Fenton-like reaction. In addition, it has been reported that Fe-based catalysts are not efficient in activating H2O2 at high pH values,39–42 while MnO2 is able to efficiently decompose H2O2 molecules even at neutral pH.37 Due to this combination, the resulting Fe/MnO2@MnCO3 composite achieved more than 90% degradation of the hormone at near-neutral pH (6.7) in just 2 min of light irradiation. Given the limit of quantification of the equipment, degradations above 90% could not be measured reliably (see ESI, Fig. S2†).
Furthermore, this approach was compared to previous works about Fenton-like and photo-Fenton-like degradation of EE2. For instance, Park et al. reported a 90% degradation of 0.02 ppb of this hormone after 8 h of Fenton-like reaction with Ag nanoparticles at pH 4,43 while in our study, a 53% degradation (0.5 ppm EE2 concentration) value was obtained with our material after 10 min of Fenton-like reaction at near-neutral pH. Even in a homogeneous photo-Fenton reaction, which is usually considered more efficient than the heterogeneous one, 80% degradation was reported after 160 min of UV-visible irradiation at pH 3.44 Thus, Fe/MnO2@MnCO3 catalyst is a promising material for the ultrafast degradation of endocrine disruptor chemicals in contaminated water without the need of an acidic pH.
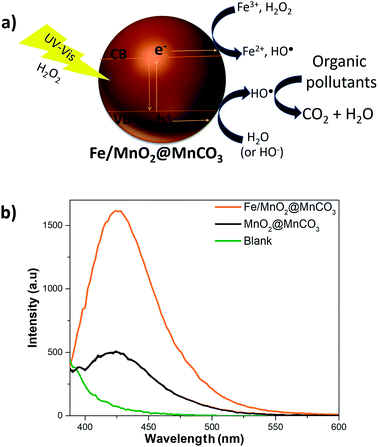
Although the mechanism of the photo-Fenton-like reaction is still not fully understood, it is widely accepted that it involves mainly the generation of hydroxyl radicals (highly reactive species), which are the key players in the oxidation of organic compounds to CO2 and water.45 As observed in Fig. 5a, after activation of the Fe/MnO2@MnCO3 catalyst with light energy higher than ∼2.2 eV, electron (e−) and hole (h+) pairs are generated. Then, these photo-generated holes can react with water or hydroxide ions adsorbed on the catalyst surface to produce hydroxyl radicals. In addition, the electrons can contribute to enhancing the reduction of Fe3+ to Fe2+ ions (limiting step), and react with H2O2 to form additional HO˙ radicals. Moreover, Mn4+ and Mn3+ ions help to the recycling of Fe2+ to Fe3+ species by electron transfer. Finally, the reduced Mn2+ ions are oxidized again by the H2O2 adsorbed on the catalyst surface.46
Ohko et al.47 studied the photocatalytic degradation mechanism of 17β-estradiol (E2) over TiO2 photocatalyst. 10ε-17β-Dihydroxy-1,4-estradien-3-one and testosterone-like compounds were detected as intermediary products, which were further oxidized to CO2. The whole oxidation pathway was mainly mediated by hydroxyl radicals, starting from the oxidation of the phenol group. Since the molecular configuration of EE2 (steroid structure with a phenol group) is similar to E2 hormone, it can be expected that its degradation mechanism follows the same pathway.
Consequently, to evaluate the radical species produced during the photo-Fenton-like reaction, we examined the formation of hydroxyl radicals using TA to specifically trap these reactive species, and produce 2-hydroxyterephthalic acid (HTA), which exhibits fluorescence emission at 425 nm.48,49 The fluorescence spectra corresponding to the formation of HTA in the presence of the catalysts are shown in Fig. 5b. The signal intensity associated with the formation of HO˙ species was almost 3 times higher in the presence of Fe/MnO2@MnCO3 in comparison with bare MnO2@MnCO3. This result clearly confirms that the α-FeOOH coating promotes a higher production of hydroxyl radicals on MnO2@MnCO3 microparticles, resulting in an enhancement of the degradation of EE2 hormone.
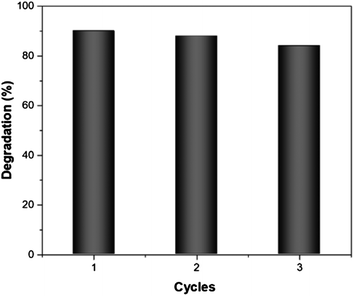
Finally, the reusability of Fe/MnO2@MnCO3 catalyst was studied in the photo-Fenton-like degradation of EE2 after 10 min of simulated solar irradiation under 3 consecutive cycles. After each test, the sample was recovered by centrifugation and reused with a fresh solution of the hormone. As can be seen in Fig. 6, the catalytic activity decreased by only 6% between the first and third cycle. This result might be due to a blockage of the active sites by the intermediaries formed during the degradation process that remain adsorbed on the catalyst surface.50,51 Based on these results, it seems fair to suggest that this material exhibits a good reusability, which along with its low-cost and up-scalable synthesis, results in a cost-effective catalyst for the efficient degradation of organic pollutants from industry wastewaters.
Conclusions
To sum up, we have developed a novel cost-effective and reusable composite based on iron/manganese microspheres, which has demonstrated to be an efficient heterogeneous catalyst for the fast photo-Fenton-like degradation of 17α-ethynylestradiol hormone, under simulated solar irradiation. Considering that its synthesis process involves precipitation reaction at room temperature and hydrothermal steps from cheap precursors, this material can be easily obtained at a large scale. The synergy between FeOOH and MnO2 significantly improves the resulting performance due to a fast activation of H2O2 and a larger generation of hydroxyl radicals, thus achieving more than 90% degradation of the hormone within 2 minutes of reaction at near-neutral pH. Moreover, the relatively large size of the resulting composite facilitates the recovery step after each catalytic reaction. Besides this, since the light absorption of Fe/MnO2@MnCO3 catalyst lies in the visible region, this material can be successfully used for the practical application of solar driven Fenton-like systems for the treatment of industrial organic pollutants, without the need of an acidic pH.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Program (FP7/20072013)/ERC grant agreement no. 311529 (LT-NRBS) and ERC-2015-PoC/Project ID: 713608 (MICROCLEANERS). D. V. acknowledges financial support provided by the European Commission under Horizon 2020s Marie Skłodowska-Curie Actions COFUND scheme [Grant Agreement No. 712754] and by the Severo Ochoa programme of the Spanish Ministry of Economy and Competitiveness [Grant SEV-2014-0425 (2015–2019)]. S. S. thanks the Spanish MINECO for grants CTQ2015-68879-R (MICRODIA). The authors thank S. Murcia-López and T. Andreu from the Catalan Institute for Energy Research for the structural characterization of the materials. The authors also thank L. Soler for the help with XPS. Authors thank CERCA Programme/Generalitat de Catalunya.Notes and references
- A. J. Ebele, M. Abou-Elwafa Abdallah and S. Harrad, Emerg. Contam., 2017, 3, 1–16 CrossRef.
- D. Montes-Grajales, G. J. L. Bernardes and J. Olivero-Verbel, Chem. Res. Toxicol., 2016, 29, 150–161 CrossRef CAS PubMed.
- T. Basile, A. Petrella, M. Petrella, G. Boghetich, V. Petruzzelli, S. Colasuonno and D. Petruzzelli, Ind. Eng. Chem. Res., 2011, 50, 8389–8401 CrossRef CAS.
- L. Soler and S. Sánchez, Nanoscale, 2014, 6, 7175–7182 RSC.
- W. Gao and J. Wang, ACS Nano, 2014, 8, 3170–3180 CrossRef CAS PubMed.
- J. Guzmán, R. Mosteo, M. P. Ormad and J. L. Ovelleiro, J. Agric. Food Chem., 2015, 63, 391–397 CrossRef PubMed.
- C. Sirtori, A. Zapata, I. Oller, W. Gernjak, A. Agüera and S. Malato, Environ. Sci. Technol., 2009, 43, 1185–1191 CrossRef CAS PubMed.
- M. Zhang, Q. Yao, W. Guan, C. Lu and J.-M. Lin, J. Phys. Chem. C, 2014, 118, 10441–10447 CAS.
- W. Luo, L. Zhu, N. Wang, H. Tang, M. Cao and Y. She, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- X. Yang, X. Xu, J. Xu and Y. Han, J. Am. Chem. Soc., 2013, 135, 16058–16061 CrossRef CAS PubMed.
- L. Xu and J. Wang, Environ. Sci. Technol., 2012, 46, 10145–10153 CrossRef CAS PubMed.
- J. Katuri, X. Ma, M. M. Stanton and S. Sánchez, Acc. Chem. Res., 2016, 50, 2–11 CrossRef PubMed.
- X. Qian, M. Ren, Y. Zhu, D. Yue, Y. Han, J. Jia and Y. Zhao, Environ. Sci. Technol., 2017, 51, 3993–4000 CrossRef CAS PubMed.
- J. Xu, Y. Li, B. Yuan, C. Shen, M. Fu, H. Cui and W. Sun, Chem. Eng. J., 2016, 291, 174–183 CrossRef CAS.
- J. He, W. Ma, J. He, J. Zhao and J. C. Yu, Appl. Catal., B, 2002, 39, 211–220 CrossRef CAS.
- A. Gogoi, M. Navgire, K. C. Sarma and P. Gogoi, Chem. Eng. J., 2017, 311, 153–162 CrossRef CAS.
- H. Lee, H.-J. Lee, J. Seo, H.-E. Kim, Y. K. Shin, J.-H. Kim and C. Lee, Environ. Sci. Technol., 2016, 50, 8231–8238 CrossRef CAS PubMed.
- E.-J. Kim, D. Oh, C.-S. Lee, J. Gong, J. Kim and Y.-S. Chang, Catal. Today, 2017, 282, 71–76 CrossRef CAS.
- R. B. Valim, M. C. Santos, M. R. V. Lanza, S. A. S. Machado, F. H. B. Lima and M. L. Calegaro, Electrochim. Acta, 2012, 85, 423–431 CrossRef CAS.
- M. Safdar, T. D. Minh, N. Kinnunen and J. Jänis, ACS Appl. Mater. Interfaces, 2016, 8, 32624–32629 CAS.
- J. B. Fei, Y. Cui, X. H. Yan, W. Qi, Y. Yang, K. W. Wang, Q. He and J. B. Li, Adv. Mater., 2008, 20, 452–456 CrossRef.
- J. Tauc and A. Menth, J. Non-Cryst. Solids, 1972, 8, 569–585 CrossRef.
- R. J. Ramírez, C. A. P. Arellano, A. A. Á. Gallegos, A. E. J. González and S. S. Martínez, J. Photochem. Photobiol., A, 2015, 305, 51–59 CrossRef.
- J. Parmar, S. Jang, L. Soler, D.-P. Kim and S. Sánchez, Lab Chip, 2015, 15, 2352–2356 RSC.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- Y. Lv, H. Che, A. Liu, J. Mu, C. Dai, X. Zhang, Y. Bai, G. Wang and Z. Zhang, J. Appl. Electrochem., 2017, 47, 433–444 CrossRef CAS.
- A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564–1574 CrossRef CAS.
- M. Huang, Y. Zhang, F. Li, Z. Wang, Alamusi, N. Hu, Z. Wen and Q. Liu, Sci. Rep., 2014, 4, 4518 CrossRef PubMed.
- S. Zhan, D. Zhu, M. Qiu, H. Yu and Y. Li, RSC Adv., 2015, 5, 29353–29361 RSC.
- H.-Q. Wang, J. Chen, S.-J. Hu, X.-H. Zhang, X.-P. Fan, J. Du, Y.-G. Huang and Q.-Y. Li, RSC Adv., 2015, 5, 72495–72499 RSC.
- C. Baronti, R. Curini, G. D'Ascenzo, A. Di Corcia, A. Gentili and R. Samperi, Environ. Sci. Technol., 2000, 34, 5059–5066 CrossRef CAS.
- S. Jobling, M. Nolan, C. R. Tyler, G. Brighty and J. P. Sumpter, Environ. Sci. Technol., 1998, 32, 2498–2506 CrossRef CAS.
- R. Jiang, H.-Y. Zhu, J.-B. Li, F.-Q. Fu, J. Yao, S.-T. Jiang and G.-M. Zeng, Appl. Surf. Sci., 2016, 364, 604–612 CrossRef CAS.
- K. Villa, X. Domènech, S. Malato, M. I. Maldonado and J. Peral, Int. J. Hydrogen Energy, 2013, 38, 12718–12724 CrossRef CAS.
- S. Goldstein, D. Aschengrau, Y. Diamant and J. Rabani, Environ. Sci. Technol., 2007, 41, 7486–7490 CrossRef CAS PubMed.
- B. Liu, F. Wu and N. Deng, J. Hazard. Mater., 2003, 98, 311–316 CrossRef CAS PubMed.
- S.-H. Do, B. Batchelor, H.-K. Lee and S.-H. Kong, Chemosphere, 2009, 75, 8–12 CrossRef CAS PubMed.
- K. Krumova and G. Cosa, in Singlet Oxygen, 2016, pp. 1–21 Search PubMed.
- J. Zheng, Z. Gao, H. He, S. Yang and C. Sun, Chemosphere, 2016, 150, 40–48 CrossRef CAS PubMed.
- Q. Xia, Z. Jiang, J. Wang and Z. Yao, Catal. Commun., 2017, 100, 57–61 CrossRef CAS.
- J. Parmar, D. Vilela, E. Pellicer, D. Esqué-de los Ojos, J. Sort and S. Sánchez, Adv. Funct. Mater., 2016, 26, 4152–4161 CrossRef CAS.
- L. Soler, V. Magdanz, V. M. Fomin, S. Sanchez and O. G. Schmidt, ACS Nano, 2013, 7, 9611–9620 CrossRef CAS PubMed.
- C. M. Park, J. Heo and Y. Yoon, Chemosphere, 2017, 168, 617–622 CrossRef CAS PubMed.
- X. Feng, S. Ding, J. Tu, F. Wu and N. Deng, Sci. Total Environ., 2005, 345, 229–237 CrossRef CAS PubMed.
- K. Villa, S. Murcia-López, T. Andreu and J. R. Morante, Catal. Commun., 2015, 58, 200–203 CrossRef CAS.
- Z. Fang, K. Zhang, J. Liu, J. Fan and Z. Zhao, Water Sci. Eng., 2017 DOI:10.1016/j.wse.2017.10.005.
- Y. Ohko, K. Iuchi, C. Niwa, T. Tatsuma, T. Nakashima, T. Iguchi, Y. Kubota and A. Fujishima, Environ. Sci. Technol., 2002, 36, 4175–4181 CrossRef CAS PubMed.
- G. Mendoza-Damián, F. Tzompantzi, A. Mantilla, R. Pérez-Hernández and A. Hernández-Gordillo, Appl. Clay Sci., 2016, 121–122, 127–136 CrossRef.
- S. Bharathkumar, M. Sakar, R. Vinod K. and S. Balakumar, Phys. Chem. Chem. Phys., 2015, 17, 17745–17754 RSC.
- K. Kaur, R. K. Wanchoo and A. P. Toor, Ind. Eng. Chem. Res., 2015, 54, 3285–3292 CrossRef CAS.
- H. Chen, W. Liu and Z. Qin, Catal. Sci. Technol., 2017, 7, 2236–2244 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: degradation of MB (20 μg ml−1) after 2 h of reaction and Fig. S2: calibration curve of 17α-ethynylestradiol hormone. See DOI: 10.1039/c7ra11705a |
| This journal is © The Royal Society of Chemistry 2018 |