Open Access Article
Open Access ArticleRifaximin, a pregnane X receptor (PXR) activator regulates apoptosis in a murine model of breast cancer
Swetlana Gautama,
Priyanka Singha,
Manjari Singha,
Subhadeep Roya,
Jitendra K. Rawata,
Rajnish K. Yadava,
Uma Devib,
Pushpraj S. Guptab,
Shubhini A. Sarafa and
Gaurav Kaithwas *a
*a
aDepartment of Pharmaceutical Sciences, School of Biosciences and Biotechnology, Babasaheb Bhimrao Ambedkar University (A Central University), Vidya vihar, Raebareli Road, Lucknow-226 025, UP, India. E-mail: gauravpharm@hotmail.com
bDepartment of Pharmaceutical Sciences, Faculty of Health Medical Sciences Indigenous and Alternative Medicine, SHIATS-Deemed to be University, Formerly Allahabad Agricultural Institute, Naini, Allahabad, UP, India
First published on 17th January 2018
Abstract
The present study was proposed to investigate the effect of rifaximin (RFX) on methyl nitrosourea (MNU) induced mammary gland carcinoma in albino wistar rats. Animals were randomized and divided among four groups of six animals each. Group I (control 0.9% normal saline, 3 ml kg−1, p.o.); Group II (toxic control, MNU 47 mg kg−1, i.v.); Group III (RFX, 25 mg kg−1, p.o.); Group IV (RFX, 50 mg kg−1, p.o.). Toxicity was induced by single i.v. injection of MNU. MNU treatment was evident with increased alveolar bud count, differentiation score, up-regulated inflammatory enzyme markers (COX, LOX, NO and H2S) and oxidative stress markers (TBAR's, protein carbonyl, SOD, catalase and Ach). The mammary gland surface architecture was studied using SEM, carmine staining and H&E staining. The treatment with RFX elicited noticeable restoration of the overall histological architecture in the experimental animals similar to the control. In the MNU treated toxic group, the levels of oxidative stress markers significantly increased in comparison to the control, which was subsequently restored after RFX treatment. Furthermore, RFX up regulated the levels of caspase 3 and caspase 8, when compared to the MNU treated animals. MNU associated toxicity was also ascertained, when determined for UCHL-1, COX, NF-κBp65, BAD, and BCL-xl expression, while RFX demonstrated modulation of the same.
Introduction
Rifaximin (RFX), is a nonsynthetic antibiotic of the rifamycin group of antibiotics, approved for treatment of traveller's diarrhoea and hepatic encephalopathy. Several clinical trials have revealed that RFX has efficacy toward irritable bowel disease (IBD), presumably as a result of alteration of intestinal microbia.1 The mechanism contributing to the beneficial effects of RFX in IBD is not fully understood. Recently, it was reported that the susceptibility to IBD was strongly associated with genetic variation in the pregnane X receptor gene (PXR), a member of the nuclear receptor family.2 Subsequently, RFX was investigated for its role in the activation of the PXR. PXR-humanized (hPXR), PXR-null, and wild-type mice were treated orally with RFX and rifampicin (a well-established human PXR ligand) and it was reported that RFX mediates activation of human PXR.3PXR is a ligand dependent transcription factor known to regulate xenobiotic and cholesterol metabolism, energy homeostasis, gut mucosal defence and cancer development. Initially, the expression of PXR was reported to be highly tissue specific in liver and intestine.4–7 Later, studies endorsed the PXR expression in mouse kidney, ovary, uterus, human brain and breast tissues.7–10 PXR has also been reported to be highly expressed in certain cancers and promote cell proliferation and chemoresistance,11–13 and potentially contributing to malignancy.12 Moreover, PXR overexpression by stable transfection of hPXR or by pharmacological activation has been reported to inhibit apoptosis in HepG2 cells.14 On the same line, over-expressing constitutively activated PXR or through pharmacological activation by rifampicin has been reported to pro-proliferative and anti-apoptotic in HCT116 (human colon cancer) and LS180 (intestinal human colon adenocarcinoma) cells.15 The studies have given an overall impression of pro-proliferative and anti-apoptotic role of PXR. However, PXR has also been shown to favourably regulate apoptosis, particularly in the tissues that are outside the metabolic realm of the liver and intestine, including tumour tissues of endometrial and breast cancer.16,17
A recent in vitro study has also proposed PXR as a novel mediator of apoptosis via p53-dependent and independent pathways. Ectopic expression of human PXR via stable transfection significantly inhibited the growth and cell proliferation in vitro, suggesting a novel physiological role of PXR in protecting the cells from unregulated oncogenic proliferation.16 On the same line, Verma and colleagues18 reported the inhibition of cell proliferation in the MCF-7 and ZR-75-1 breast cancer cells through structurally and functionally distinct PXR activators. The study also reported that the proliferative inhibition is mediated through cell cycle arrest at the G1/S phase followed by apoptosis mediated through the up-regulated pro-apoptotic genes, CDKN1A (p21), BBC3 (PUMA) and BAX.18 Henceforth, role of PXR, as pro- or anti-apoptotic in breast cancers stands as an important aspect for optimizing breast cancer therapies. The above aspect of PXR activators is further strengthened by the fact that most of the commonly used chemotherapeutic agents (e.g. tamoxifen, taxol, cyclophosphamide, cisplatin) are PXR activators and requires further investigation using appropriate in vivo systems. Considering the above the present study was undertaken to elaborate the effect of RFX (PXR activator) on cellular proliferation and apoptosis against N-methyl nitrosourea (MNU) induced mammary gland carcinoma.
Materials and methods
Drug and chemicals
RFX was procured from Lupin Limited, Mumbai, India. MNU was obtained from Sigma Aldrich Co. St. Louise Mo 63103 USA. The fluorometric assay kits for the estimation of caspase 3 (SC-4263) and caspase 8 (SC-4267) were purchased from Santacruz biotechnology Inc., California, Delaware. All other chemicals were of analytical grade and procured from Genetix Asia Private Limited, New Delhi else otherwise stated in the text.Experimental protocol
Albino wistar female rats of 100–120 g body weight were used for this study. The rats were procured from the central animal house facility. The animals housed in polypropylene cages under controlled conditions (12 h light/dark cycle), with a free access to a standard pellet diet and water ad libitum. They were acclimatized for a period of two weeks prior to the commencement of the experiment. Animals were randomized and divided into 4 groups of 6 animals each. Group I (control 0.9% normal saline, 3 ml kg−1, p.o.); Group II (toxic control, MNU 47 mg kg−1, i.v.); Group III (RFX, 25 mg kg−1, p.o.); Group IV (RFX, 50 mg kg−1, p.o.). Toxicity was induced by single i.v. injection of MNU followed by RFX therapy for 90 days at the dose mentioned above. The blood samples were collected under chloroform anaesthesia through retro orbital plexus in centrifugation tubes. The blood samples were incubated at 37 °C for 1 h and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to collect serum. The serum samples were stored at −20 °C till further use. Animals were sacrificed on the 120th day and subjected to estimation using the methods elaborated in the forthcoming section. Animal experiments were carried out as per the guidelines established by the Department of Animal welfare, Government of India, and was approved by the “institutional animal ethics committee (IAEC)” (approval no.: IAEC/SHIATS/PA16III/SSPG19).
000 rpm for 15 min to collect serum. The serum samples were stored at −20 °C till further use. Animals were sacrificed on the 120th day and subjected to estimation using the methods elaborated in the forthcoming section. Animal experiments were carried out as per the guidelines established by the Department of Animal welfare, Government of India, and was approved by the “institutional animal ethics committee (IAEC)” (approval no.: IAEC/SHIATS/PA16III/SSPG19).
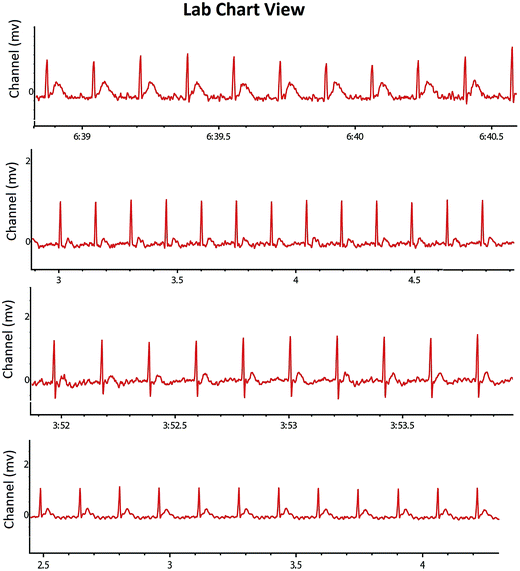
Heart rate variability (HRV)
The animals were anaesthetized using ketamine hydrochloride (100 mg kg−1, i.m.) and diazepam (5 mg kg−1, i.m.) in combination on 119th day and subsequently mounted on a wax tray. The platinum hook electrodes were placed on the skin of the dorsal and ventral thorax to record the ECG signal. The electrodes were connected to Bio-amplifier (ML-136) (AD Instruments, Australia) and channel PowerLab (ML-826) (AD Instruments, Australia) to convert analogue to digital signals. The ECG signals were saved on the hard disk and analysed offline using Labchart Pro-8 (AD Instruments, Australia). HRV analysis was conducted on multiple segments of continuous ECG signals perceived in the preceding section. Firstly, all the raw signals were inspected manually to ensure that all the R waves are detected correctly. Subsequently, HR was calculated by plotting the number of R waves per unit time. Following the same, time and frequency domain parameters of HRV were calculated using the Lab chart Pro-8 (AD Instruments, Australia).19Carmine staining of whole mounts mammary gland
Mammary gland tissues from each group were assessed for their morphological changes using carmine staining. The tissues were fixed by putting the slides into Carney's fixative solution for two days. Slides were washed with 70% ethanol and stained with a carmine alum solution for two days. Subsequently, stained tissues were rehydrated using increasing concentration of ethanol (70%, 95%, 100%) followed by xylene for two days. Dehydrated slides were observed under a light microscope at 4× (N120, BR Biochem Life Sciences, New Delhi, India).20 Mammary gland differentiation (DF) was assessed by scoring the number of alveolar buds (ABs) type 1 and type 2. The score values (0–5) from AB1 and AB2 were added to a final DF score (0–10).21Scanning electron microscopy (SEM)
Fresh small unfixed pieces of the mammary gland tissues were taken from the each group. Tissue blocks were treated in eagle's balanced solution containing 100 μg ml−1 collagenase and 2.5 TRU (turbidity reduction unit) per ml of hyaluronidase containing 0.1 M sodium phosphate buffer (pH 5.3) and 0.15 M sodium chloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 30 min at 37 °C. After digestion, the tissue blocks rinsed in the balanced solution, fixed in 4% glutaraldehyde in 0.1 M cacodylate/HCl buffer (pH 7.2) at room temperature for 3 h and placed in 8 N HCl for 30–70 min at 60 °C. After HCl digestion, the blocks were rinsed three times in distilled water to remove the acid. All the specimens were dehydrated with increasing acetone concentration 70%, 90%, 95%, and 100% for 45 min and examined under SEM at 1000× (JEOL JSM-6490LV).22
1) for 30 min at 37 °C. After digestion, the tissue blocks rinsed in the balanced solution, fixed in 4% glutaraldehyde in 0.1 M cacodylate/HCl buffer (pH 7.2) at room temperature for 3 h and placed in 8 N HCl for 30–70 min at 60 °C. After HCl digestion, the blocks were rinsed three times in distilled water to remove the acid. All the specimens were dehydrated with increasing acetone concentration 70%, 90%, 95%, and 100% for 45 min and examined under SEM at 1000× (JEOL JSM-6490LV).22
Morphological evaluation
Mammary gland tissues were appraised histopathologically using haematoxylin and eosin (H&E) staining. The tissues were fixed in paraformaldehyde for overnight, succeeded by 70% isopropanol overnight. Tissues were further exposed to augmenting concentration of isopropanol (70%, 90%, and 100%) consequently superseded by dehydration with 100% xylene. The tissues were embedded in paraffin wax and the blocks were prepared. 5 μm sections were prepared using microtome followed by staining with H&E. The sections were visualized and photographed at 40× using digital biological microscope (N120, BR-Biochem Life Sciences, New Delhi, India).23Biochemical estimation
The mammary gland tissues (10% w/v) were homogenized in 0.15 M KCl and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatants were scrutinized for biochemical parameters including thiobarbituric acid reactive substances (TBAR's), superoxide dismutase (SOD), catalase, protein carbonyl and acetylcholine (Ach) using the methods established in our laboratory.24–27
000 rpm. The supernatants were scrutinized for biochemical parameters including thiobarbituric acid reactive substances (TBAR's), superoxide dismutase (SOD), catalase, protein carbonyl and acetylcholine (Ach) using the methods established in our laboratory.24–27
Caspase-3 and caspase-8
Caspase-3 and caspase-8 fluorometric assays were performed using the methods elaborated in the literature. The assay was carried out in 96-well plate. Equal volumes of serum sample from both control and experimental animals were diluted with reaction buffer and DTT was added to a final concentration of 10 mM. To the reactant mixture 5 μl of IETD-AFC/DEVD-AFC substrate was added and incubated for 1 h at 37 °C. Free AFC levels formed were measured in a plate reader with a 400 nm excitation and a 505 nm emission. The results of experimental samples were compared with control and expressed as fluorescence units per mg of protein.28,29Western blotting
Protein samples were prepared from the mammary gland tissue through acetone precipitation and quantified by using the Bradford reagent.30 SDS-PAGE analysis was performed following the principles of Laemmli with slight modifications.31 Briefly, protein samples were mixed with sample buffer (125 mM Tris–HCl, pH 6.8, 20% glycerol, 4% SDS, 0.05% bromophenol blue, 10% 2-mercaptoethanol). A 30 μg of protein sample was allowed to resolve through 12% polyacrylamide gel using SDS-PAGE (GX-SCZ2+, Genetix Biotech Asia Pvt. Ltd, New Delhi). The proteins as resolved through SDS-PAGE were transferred to a PVDF membrane (IPVH 00010 Millipore, Bedford, MA USA) using semi-dry transfer (GX-ZY3, Genetix Biotech Asia Pvt. Ltd, New Delhi). Subsequently, membrane was blocked with 3% BSA and 3% not fat milk in TBST for 2 h and incubated overnight with primary antibody against UCHL-1(MA1-83428) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution), (2876 cell signalling), COX (PA5-17614), NF-κBp65 (MA5-1616) (1
2000 dilution), (2876 cell signalling), COX (PA5-17614), NF-κBp65 (MA5-1616) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000), BCL-xl (MA5-15142), BAD (SC-8044) (9292 cell signalling) and β-actin (MA5-15739-HRP) (Pierce, Thermo scientific) (1
2000), BCL-xl (MA5-15142), BAD (SC-8044) (9292 cell signalling) and β-actin (MA5-15739-HRP) (Pierce, Thermo scientific) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution). The membrane was washed with TBST thrice and incubated with HRP conjugated rat antimouse secondary antibody (31
300 dilution). The membrane was washed with TBST thrice and incubated with HRP conjugated rat antimouse secondary antibody (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430, 1
430, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilutions) (Pierce Thermo Scientific) at room temperature for 2 h. The signals were detected using an enhanced chemiluminescence substrate (Western Bright ECL HRP substrate, Advansta, Melanopark, California, US). The quantification of protein was done through densiometric digital analysis of protein bands using Image J software.31,32
5000 dilutions) (Pierce Thermo Scientific) at room temperature for 2 h. The signals were detected using an enhanced chemiluminescence substrate (Western Bright ECL HRP substrate, Advansta, Melanopark, California, US). The quantification of protein was done through densiometric digital analysis of protein bands using Image J software.31,32
Statistical analysis
All data were presented as mean ± SD and analysed by one-way ANOVA followed by Bonferroni test and for the possible significance identification between the various groups. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered as statistically significant. Statistical analysis was performed using Graph pad prism software (5.02).Results
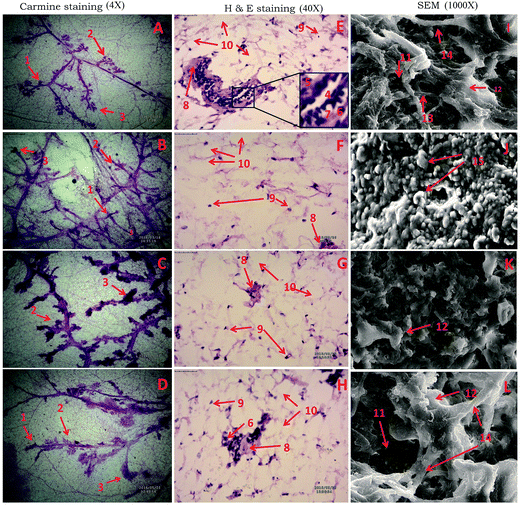
The results revealed non-significant inflation in the AB (9.25 ± 1.58) and lobule score (2.63 ± 1.06) with MNU treatment which was dose dependently curtailed down with the RFX treatment. Similar pattern of significant negating effects were evident in the RFX while scrutinizing the DF score in the whole mounts of the mammary gland (Table 1) (Fig. 1A–D). H&E staining of the mammary gland tissue was evident with myoepithelial cells, cuboidal epithelial cells, loose connective tissue (LCT), dense connective tissue (DCT), lymphocytes, adipocytes and duct in the control animals. The MNU treatment distorted the normal architecture with loss of LCT and DCT along with myoepithelial cells, cuboidal epithelial cells. RFX treatment restored the normal architecture in the dose dependent manner (Fig. 1E–H). The mammary gland tissues were subsequently scrutinized through scanning electron microscopy. The control tissue was very well characterized with the presence capillary network along with collagenous covering. MNU treatment was recorded to have nodules formation representing cellular proliferation. Treatment with RFX helped to restore the collagenous covering and capillary network in the mammary gland (Fig. 1).| Groups | AB1 | AB2 | AB (AB1 + AB2) | Lobules | DF.SCORE 1 (AB1 + AB2+ lobules) | DF.SCORE 2 (lobules/AB1 + AB2) |
|---|---|---|---|---|---|---|
| a (Values are presented as mean ± SD), each group contains 6 animals. Comparisons were made on the basis of the one-way ANOVA followed by Bonferroni multiple test. All groups were compared to the toxic control group (*p < 0.05, **p < 0.01, ***p < 0.001). All groups were compared to the control group (ap < 0.05, bp < 0.01, cp < 0.001). | ||||||
| Control (normal saline, 3 ml kg−1) | 6.00 ± 0.78 | 1.75 ± 0.46 | 7.75 ± 0.66 | 1.5 ± 0.53 | 9.25 ± 0.71 | 0.20 ± 0.08 |
| MNU (47 mg kg−1) | 6.75 ± 0.89 | 2.5 ± 0.93 | 9.25 ± 1.58c | 2.63 ± 1.06c | 11.88 ± 1.80c | 0.29 ± 0.13 |
| RFX + MNU (25 mg kg−1 + 47 mg kg−1) | 5.25 ± 1.66 | 1.5 ± 0.53 | 6.75 ± 1.49 | 1.25 ± 0.46* | 8.00 ± 1.77** | 0.19 ± 0.05 |
| RFX + MNU (50 mg kg−1 + 47 mg kg−1) | 5.63 ± 1.85 | 1.63 ± 0.52 | 7.25 ± 1.91 | 1.38 ± 0.52* | 8.63 ± 1.59** | 0.21 ± 0.12 |
Non-significant changes were recorded in the TBAR's and GSH level after the MNU (0.77 ± 0.26 nM of MDA per mg of protein and 0.40 ± 0.02 mg%) and RFX treatment afforded non-significant upregulation of the same. However, significant curtailment of the protein carbonyl level was perceived after the low dose RFX (5.45 ± 1.97 nM ml−1) in comparison to MNU treated group (12.24 ± 1.80 nM ml−1). The enzymatic defense of SOD (0.22 ± 0.12 units of SOD per mg of protein) and catalase (1.10 ± 0.02 nM of H2O2 per min per mg of protein) was favorably regulated towards control by high dose RFX (Table 2).
| Groups | TBARs (nM of MDA per μg of protein) | GSH (mg%) | SOD (units of SOD per mg of protein) | Catalase (nM of H2O2 per min per mg of protein) | Protein carbonyl (nM ml−1) | AchE (nM ml−1) | Ach (nM ml−1) |
|---|---|---|---|---|---|---|---|
| a (Values are presented as mean ± SD), each group contains 6 animals. Comparisons were made on the basis of the one-way ANOVA followed by Bonferroni multiple test. All groups were compared to the toxic control group (*p < 0.05, **p < 0.01, ***p < 0.001). All groups were compared to the control group (ap < 0.05, bp < 0.01, cp < 0.001). | |||||||
| Control (normal saline, 3 ml kg−1) | 0.54 ± 0.08 | 0.80 ± 0.07*** | 0.26 ± 0.07*** | 0.59 ± 0.02*** | 9.03 ± 2.55 | 2033.36 ± 114.33*** | 1191.54 ± 67.37*** |
| MNU (47 mg kg−1) | 0.77 ± 0.26 | 0.40 ± 0.02c | 0.31 ± 0.08***c | 1.41 ± 0.02c | 12.24 ± 1.80 | 2363.94 ± 17.64c | 923.97 ± 27.72c |
| RFX + MNU (25 mg kg−1 + 47 mg kg−1) | 0.68 ± 0.19 | 0.48 ± 0.18c | 0.07 ± 0.01***c | 1.34 ± 0.09c | 5.45 ± 1.97***a | 2710.24 ± 103.23***c | 1262.88 ± 19.47***a |
| RFX + MNU (50 mg kg−1 + 47 mg kg−1) | 0.68 ± 0.04 | 0.43 ± 0.07c | 0.22 ± 0.12c | 1.10 ± 0.02***c | 7.36 ± 1.95** | 2561.65 ± 106.91*c | 1323.96 ± 8.62***c |
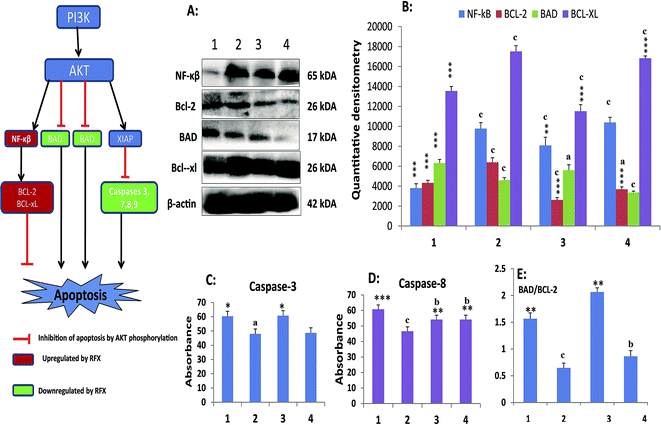
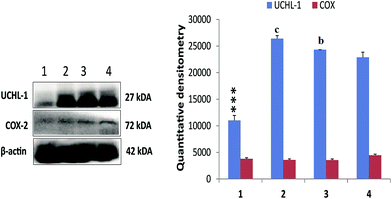
Treatment with MNU up regulated the NFκBp65 expression in the mammary gland tissue and RFX (25 mg kg−1) afforded a significant curtailment of the same. The expression of the antiapoptotic markers (BCL-2 and BCL-xl) was significantly up regulated after the MNU treatment and RFX (25 mg kg−1) afforded significant down regulation of the same. In consonance to the antiapoptotic markers, the expression proapoptotic BAD was favorably up regulated by RFX (25 mg kg−1). The favorable regulation by the RFX towards the apoptotic marker was also evident through BAD/BCL-2 ratio, wherein RFX (25 mg kg−1) observed significant increase. The above sets of observation were also endorsed, once scrutinized through the effector caspase 3 and caspase 8. The RFX (25 mg kg−1) up regulated the levels of caspase 3 and caspase 8 in the MNU treated animals (Fig. 2). The overall tumor progression and prognosis by RFX was further scrutinized through the biological markers like UCHL-1, COX-2 and autonomic dysfunction. RFX treatment non-significantly curtailed the expression of UCHL-1 in comparison to MNU treatment and COX-2 expression remained unaffected after the MNU or low dose of RFX treatment (Fig. 3).
The experimental animals were also scrutinized for the autonomic dysfunction as a prognostic marker for tumor progression. The study endorsed the increase in heart rate (HR) after the MNU treatment (494.95 ± 14.68 BPM) in comparison to control (415.4 ± 53.13 BPM). RFX (25 mg kg−1) imparted a better restoration of HR (454.93 ± 18.21 BPM). The MNU treatment was also evident with the QTc prolongation (0.21 ± 0.03 S), which was restored to normal after RFX (0.16 ± 0.05 S). QRS and QT interval of all the groups was not affected by either of the treatments (Table 3). The autonomic control was further scrutinized through the time and frequency domain HRV parameters. The ratio of lower frequency (LF) to higher frequency (HF) was decreased in MNU treated animals (0.11 ± 0.01) which was increased by the low dose RFX (0.33 ± 0.01) (Table 4) (Fig. 4). The autonomic dysfunction after the MNU treatment was also evident with the decreased levels of Ach (923.97 ± 27.72 nM ml−1). Concomitant treatment with RFX dose dependently increased the Ach concentration with vice versa effect upon the plasma AchE levels.
| ECG parameters | Control (normal saline, 3 ml kg−1, p.o.) | Toxic control (MNU, 47 mg kg−1, i.v.) | MNU + RFX (47 mg kg−1 i.v.) + (25 mg kg−1, p.o.) | MNU + RFX (47 mg kg−1 i.v.) + (50 mg kg−1, p.o.) |
|---|---|---|---|---|
| a (Values are presented as mean ± SD), each group contains 6 animals. Comparisons were made on the basis of the one-way ANOVA followed by Bonferroni multiple test. All groups were compared to the toxic control group (*p < 0.05, **p < 0.01, ***p < 0.001). All groups were compared to the control group (ap < 0.05, bp < 0.01, cp < 0.001). | ||||
| RR interval (s) | 0.15 ± 0.02 | 0.18 ± 0.02 | 0.17 ± 0.01 | 0.22 ± 0.04 |
| Heart rate (BPM) | 415.4 ± 53.13* | 494.95 ± 14.68a | 454.93 ± 18.21 | 273.64 ± 38.35*** |
| PR interval (s) | 0.04 ± 0.003* | 0.05 ± 0.002a | 0.05 ± 0.005 | 0.05 ± 0.004 |
| P duration (s) | 0.01 ± 0.002 | 0.02 ± 0.002 | 0.02 ± 0.01 | 0.02 ± 0.002 |
| QRS interval (s) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.002 |
| QT interval (s) | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.01 |
| QTc (s) | 0.16 ± 0.04** | 0.21 ± 0.03b | 0.16 ± 0.05** | 0.16 ± 0.02** |
| JT interval (s) | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.06 ± 0.01 |
| T peak tend interval (s) | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| P amplitude (mV) | 0.07 ± 0.03 | 0.10 ± 0.04 | 0.06 ± 0.03 | 0.08 ± 0.03 |
| Q amplitude (mV) | −0.01 ± 0.02* | 0.05 ± 0.09a | 0.01 ± 0.02*** | 0.02 ± 0.01*** |
| R amplitude (mV) | 1.20 ± 0.36 | 1.70 ± 0.39 | 1.20 ± 0.23 | 1.27 ± 0.37 |
| S amplitude (mV) | −0.18 ± 0.22 | −0.09 ± 0.14 | −0.11 ± 0.09 | −0.20 ± 0.26 |
| ST segment (mV) | −0.01 ± 0.09 | −0.02 ± 0.04 | 0.03 ± 0.04 | 0.08 ± 0.05* |
| T amplitude (mV) | 0.25 ± 0.12* | 0.44 ± 0.11a | 0.18 ± 0.12a | 0.34 ± 0.09***a |
| HRV | Control (normal saline, 3 ml kg−1, p.o.) | Toxic control (MNU 47 mg kg−1 i.v.) | RFX + MNU (25 mg kg−1 p.o + 47 mg kg−1 i.v.) | RFX + MNU (50 mg kg−1 p.o + 47 mg kg−1 i.v.) |
|---|---|---|---|---|
| a (Values are presented as mean ± SD), each group contains 6 animals. Comparisons were made on the basis of the one-way ANOVA followed by Bonferroni multiple test. All groups were compared to the toxic control group (*p < 0.05, **p < 0.01, ***p < 0.001). All groups were compared to the control group (ap < 0.05, bp < 0.01, cp < 0.001). | ||||
| Average RR (ms) | 145.98 ± 10.21** | 118.3 ± 17.58b | 169.6 ± 13.50**c | 237.86 ± 16.94***c |
| Median RR (ms) | 146.83 ± 19.88** | 119.33 ± 18.41b | 170.57 ± 14.60**c | 238.66 ± 15.96***c |
| SDRR (ms) | 4.20 ± 02.94** | 2.81 ± 0.10b | 7.27 ± 1.95**b | 9.64 ± 1.09**b |
| CVRR | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.02 |
| SD rate (BPM) | 10.93 ± 1.17 | 6.09 ± 1.23 | 16.05 ± 3.33 | 13.11 ± 5.55 |
| SDSD (ms) | 2.57 ± 0.06 | 1.24 ± 0.17 | 2.84 ± 0.08 | 5.63 ± 1.64 |
| RmSSD (ms) | 2.57 ± 0.02 | 1.24 ± 0.01 | 2.84 ± 0.18 | 5.63 ± 1.64 |
| LF (ms2) | 0.62 ± 0.01 | 0.19 ± 0.08 | 0.91 ± 1.37 | 0.24 ± 0.011 |
| HF (ms2) | 2.21 ± 0.76 | 1.77 ± 0.05 | 2.71 ± 0.02 | 3.24 ± 0.09 |
| LF/HF (%) | 0.28 ± 0.01 | 0.11 ± 0.01 | 0.33 ± 0.01 | 0.19 ± 0.01 |
Discussion
The present study demonstrated the effect of RFX against MNU induced mammary gland carcinogenesis in albino wistar rats. The mammary gland tissue was examined through whole mount preparation to study the small proliferative lesions represented by the increase in number of AB and lobules. AB and lobules represent the structure from which premalignant pathologic changes to ductal carcinoma and corresponds to the terminal ductal lobular units in human breast.33 Increase in AB number is directly correlated with the increased chances of developing malignancy and numerically represented through DF.34 The MNU administration was evident with the increase in AB and DF score. Treatment with RFX afforded significant curtailment of the same, suggesting demarcating effect of RFX upon vascularization and subsequently angiogenesis in mammary gland tissue.14 The architecture of the mammary gland tissue was further scrutinized through the H&E staining and SEM. The MNU administration was evident for loss to duct cuboidal epithelial, myoepithelial cells along with LCT, which are in corroboration with the previous reports.35 The corresponding SEM analysis revealed the loss of capillary network along with collagen fiber and development of nodules representing tumor lesions.36 The H&E staining and SEM analysis confirmed the vascularization of mammary gland tissue by MNU as perceived through the whole mount preparation and is in line with the previous reports. Concomitant treatment with RFX afforded restoration of the duct, LCT, DCT and cuboidal epithelial cells when perceived through H&E staining. On the similar lines, SEM analysis of the mammary gland tissue treated with RFX helped to restore the capillary network and collagen covering of the tissue. Studies have perceived a significant demarcating effect of RFX towards culminating the deleterious effects of MNU against mammary gland carcinoma in albino wistar rats.Reactive oxygen species (ROS) generation and oxidative stress are a well-established testimony for the MNU induced carcinogenesis.37 Oxidative stress is defined as an imbalance between the generation and metabolism of ROS and reactive nitrogen species (RNS), surmounting to impaired cellular metabolism and changes in the intra and extracellular environmental conditions.38 Excessive ROS and RNS production could lead to DNA damage including mutations/deletions/amplifications/rearrangements which can lead to defects in apoptosis.39 A delicate balance between the ROS/RNS production is mediated through exogenous or endogenous antioxidant defense regulated through enzymatic and non-enzymatic mechanisms. MNU treatment was evident with increase in lipid and protein peroxidation as perceived through increased TBAR's and protein carbonyl levels and thereby endorsing the ROS generation. The enzymatic antioxidant defense of SOD and catalase were significantly up-regulated after MNU treatment and the same could be attributed to the feedback or activated enzymatic defense to counteract high circulating levels of ROS. It would be appropriate to notify that SOD, performs the dismutation of the H2O2 to OH, which is further catalyzed to H2O and molecular O2 by catalase. Both the enzymes work in tandem to neutralize the ROS and a similar pattern of fold change in the SOD/catalase is expected and was perceived in the present experiment. The concomitant RFX treatment afforded significant curtailment in the products of lipid and protein peroxidation products along with up-regulation in the enzymatic levels of SOD and catalase. All in all, from the above one could derive significant negating effects of RFX against MNU induced cancer. However, the possible mechanisms beneath the same remain a stand still question and therefore authors considered it worth to study the effect of RFX on apoptotic markers.
Phosphoinositide-3 kinase/AKT (PI3K/AKT) signaling plays a vital role in cell survival and it has been implicated in various types of malignancies.40 PI3K/AKT performs as a prosurvival factor by inhibiting apoptotic signal including inhibition of proapoptotic family members (e.g. BAX, BAD); activation of NF-κβ transcription factor (promoting transcription of anti-apoptotic genes, in particular and BCL-xl). Additionally AKT also activates prosurvival proteins XIAP (X-linked inhibitor of apoptosis protein) resulting in inhibition of caspases.41–44 All in all, PI3K/AKT phosphorylation inhibits the apoptotic signaling through multidimensional pathways. It would be appropriate to mention that PI3K/AKT pathway is one among the most important signaling cascade in human breast cancer and a recent study has accorded that activation of PI3K/AKT pathway in the progression of MNU induced cancer. In accordance to the previous report, we recorded the activation PI3K/AKT signaling cascade endorsed by increased expression of NF-κβp65, BCL-XL and down regulation of the levels of BAD, caspase 3 and caspase 8; subsequent to MNU treatment. Concomitant RFX treatment helped to down regulate the anti-apoptotic markers (NF-κβp65 and BCL-XL) and upregulated the pro-apoptotic signals (BAD, caspase 3 and caspase 8). It would be appropriate to remark that the low dose of RFX demonstrated more commendable regulation in comparison to high dose. The group of evidences derived through the above line of experiments gives a clear testimony for the efficacy of RFX against experimental carcinogenesis in albino wistar rats. The present observation is in line with the recent report, in fact adds to the small set of existing data that PXR activators can impart proliferative inhibition mediated through upregulation of pro-apoptotic markers. Authors would also like to pen down that the present report is the first in vivo report affirming the efficacy of PXR activators against an experimental model for breast cancer as warranted/suggested by the previous researchers.45
All in all, low dose of RFX was more effective to regulate the deleterious effects of MNU in comparison to high dose. It would be appropriate to pen down that despite being less effective, no adverse effects could be recorded even after the high dose of RFX. The less effectiveness of high dose could be attributed to the counterproductive effects of RFX. The present findings are in consonance to the previous report suggesting aggressive/high dose treatment produces the best short-term results but may counterproductive as observed in the present study.46
Integrated multidimensional prognostic biomarkers approach is always preferred/utilized to define the effectiveness of the proposed therapy. Henceforth, authors considered it worth to evaluate the COX-2, UCHL-1 and markers of autonomic dysfunction in the present study to further validate the efficacy of PXR activation through RFX. UCHL-1 is a tumor suppressor gene and has gained importance for its diagnostic and antimetastatic potential, particularly in case of breast cancer due to its deubiquitinating effect upon hypoxia-inducible factor-1α (HIF-1α).47 Previously, we have reported the up-regulated levels of UCHL-1 in case of MNU induced carcinogenesis and similar observation was taken on record in the present study as well. RFX at the high dose was able to nudge down the UCHL-1 levels. The association between inflammation, cancer and COX-2 is well established. Plethora of scientific literature, including from our own laboratory has endorsed significant up-regulation of COX-2 in MNU induced mammary gland carcinoma. However we could not observe any change in the COX-2 expression in either of the groups. Authors would like to submit that appropriate control was incorporated during the experiment to avoid misidentification of COX-2. However, the present observation is beyond the scope of present manuscript.
Autonomic dysfunction represents of autonomic control usually associated with sympathetic over activity and parasympathetic down regulation. The autonomic dysfunction associated cardiovascular risk among the early breast cancer and breast cancer survivors is a well reported phenomenon.48 The interrelationship between the sympathetic and parasympathetic nervous system can be read using HRV. Therefore to have a broader prospect of the present study authors evaluated the HRV of the animals treated with MNU and subsequently RFX. Most of the antineoplastic therapy has been reported to be associated with autonomic dysfunction in breast cancer patients.49 The poor autonomic control has been reported to be associated with increased HR and decreased HRV in the early breast cancer and survivors of breast cancer patients and MNU treatment was evident for the similar pattern of increased HR and decreased HRV.50 The increased HR and decreased HRV, endorses the autonomic dysfunction in the animals treated with MNU and is in corroboration with the previous clinical reports of autonomic dysfunction in early breast cancer cases.51 The autonomic dysfunction in the animals treated with MNU was also supported by the fact that we observed significant decline in the Ach levels after MNU treatment. The decrease in Ach represents parasympathetic down regulation and could be associated with sympathetic overactivity.17 However, concomitant RFX treatment afforded a significant increase in the Ach level. The said observation is a clear indicative of the restoration of the autonomic function and therefore suggests a good prognosis by RFX.
To the best of our knowledge the present report is first to elaborate the prognostic relationship between carcinogenesis and autonomic dysfunction in experimental model. All in all, RFX demonstrated good prognostic valve in the management of experimental carcinogenesis through restoration of UCHL-1 and regulating the autonomic dysfunction.
Authors contributions
SG and PS performed the experimental work, performed the calculations, organised the data; JKR performed the electrocardiographic studies; SR and MS performed the western blotting; RKY, and UD performed the SEM, histopathological studies; PSG and SAS performed the statistical analysis; GK perceived the idea, designed and supervised the overall study, prepared and proof read the final manuscript.Conflicts of interest
Authors declare no competing interest.Acknowledgements
The author would like to thanks University Grants Commission, Government of India for granting senior research fellowship to MS, JKR and SG. Authors are also thankful to Department of Science and Technology, Government of India for granting senior research fellowship SR.References
- G. Laustsen and L. Wimmett, Drug Approval Highlights FDA Update, Nurse Pract., 2005, 30(2), 14–29 CrossRef PubMed.
- H. DuPont, Rifaximin: an antibiotic with important biologic effects, Mini-Rev. Med. Chem., 2016, 16(3), 200–205 CrossRef CAS.
- G. Esposito, S. Gigli, L. Seguella, N. Nobile, A. D'Alessandro and M. Pesce, Rifaximin, a non-absorbable antibiotic, inhibits the release of pro-angiogenic mediators in colon cancer cells through a pregnane X receptor-dependent pathway, Internet J. Oncol., 2016, 49(2), 639–645 CrossRef CAS PubMed.
- Y. Jia, N. Viswakarma and J. K. Reddy, Med1 subunit of the mediator complex in nuclear receptor-regulated energy metabolism, liver regeneration, and hepatocarcinogenesis, Gene Expression, 2016, 16(2), 63–75 CrossRef PubMed.
- I. Koutsounas, S. Theocharis, E. Patsouris and C. Giaginis, Pregnane X receptor (PXR) at the crossroads of human metabolism and disease, Curr. Drug Metab., 2013, 14(3), 341–350 CrossRef CAS PubMed.
- P. Pavek, Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions, Front. Pharmacol., 2016, 25(7), 456 Search PubMed.
- D. Robbins and T. Chen, Tissue-specific regulation of pregnane X receptor in cancer development and therapy, Cell Biosci., 2014, 4(1), 17 CrossRef PubMed.
- C. T. Brewer and T. Chen, PXR variants: the impact on drug metabolism and therapeutic responses, Acta Pharm. Sin. B, 2016, 6(5), 441–449 CrossRef PubMed.
- L. Mo and J. He, Nuclear hormone receptors PXR and CAR and metabolic diseases, Horm. Mol. Biol. Clin. Invest., 2014, 19(2), 129–140 CAS.
- J. Yan and W. Xie, A brief history of the discovery of PXR and CAR as xenobiotic receptors, Acta Pharm. Sin. B, 2016, 6(5), 450–452 CrossRef PubMed.
- D. Kotiya, B. Jaiswal, S. Ghose, R. Kaul, K. Datta and R. K. Tyagi, Role of PXR in Hepatic Cancer: Its Influences on Liver Detoxification Capacity and Cancer Progression, PLoS One, 2016, 11(10), e0164087 Search PubMed.
- H. Masuyama, K. Nakamura, E. Nobumoto and Y. Hiramatsu, Inhibition of pregnane X receptor pathway contributes to the cell growth inhibition and apoptosis of anticancer agents in ovarian cancer cells, Internet J. Oncol., 2016, 49(3), 1211–1220 CrossRef CAS PubMed.
- S. R. Pondugula, P. Pavek and S. Mani, Pregnane X Receptor and Cancer: Context-Specificity is Key, Nucl. Receptor Res., 2016, 12, 3 Search PubMed.
- N. Zucchini, G. de Sousa, B. Bailly-Maitre, J. Gugenheim, R. Bars and G. Lemaire, et al., Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes, BBA, Biochim. Biophys. Acta, Mol. Cell Res., 2005, 1745(1), 48–58 CrossRef CAS PubMed.
- J. Zhou, M. Liu, Y. Zhai and W. Xie, The antiapoptotic role of pregnane X receptor in human colon cancer cells, Mol. Endocrinol., 2008, 22(4), 868–880 CrossRef CAS PubMed.
- H. Masuyama, H. Nakatsukasa, N. Takamoto and Y. Hiramatsu, Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells, Mol. Pharmacol., 2007, 72(4), 1045–1053 CrossRef CAS PubMed.
- S. Revathidevi, R. Sudesh, V. Vaishnavi, M. Kaliyanasundaram, K. G. MaryHelen, G. Sukanya and A. K. Munirajan, Screening for the 3′UTR Polymorphism of the PXR Gene in South Indian Breast Cancer Patients and its Potential role in Pharmacogenomics, Asian Pac. J. Cancer Prev., 2016, 17(8), 3971–3977 Search PubMed.
- S. Verma, M. M. Tabb and B. Blumberg, Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells, BMC Cancer, 2009, 9(1), 1 CrossRef PubMed.
- T. Shintaku, T. Ohba, H. Niwa, T. Kushikata, K. Hirota, K. Ono, Y. Matsuzaki, T. Imaizumi, K. Kuwasako, D. Sawamura and M. Murakami, Effects of propofol on electrocardiogram measures in mice, J. Pharmacol. Sci., 2014, 126(4), 351–358 CrossRef CAS PubMed.
- J. Russo, B. A. Gusterson, A. E. Rogers, I. H. Russo, S. R. Wellings and M. J. VanZwieten, Comparative study of human and rat mammary tumorigenesis, Pathol Rev, Springer, 1990, pp. 217–251 Search PubMed.
- C. Manral, S. Roy, M. Singh, S. Gautam, R. K. Yadav, J. K. Rawat, U. Devi, M. N. Ansari, S. A. Saeedan and G. Kaithwas, Effect of β-sitosterol against methyl nitrosourea-induced mammary gland carcinoma in albino rats, BMC Complementary Altern. Med., 2016, 16(1), 260 CrossRef PubMed.
- T. Nagato, H. Yoshida, A. Yoshida and Y. Uehara, A scanning electron microscope study of myoepithelial cells in exocrine glands, Cell Tissue Res., 1980, 209(1), 1–10 CrossRef CAS PubMed.
- D. S. Murray, L. A. Ruble, H. Willis and C. A. Molloy, Parent and teacher report of social skills in children with autism spectrum disorders, Lang. Speech Hear Serv. Sch., 2009, 40(2), 109–115 CrossRef PubMed.
- G. Kaithwas and D. K. Majumdar, In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats, Eur. J. Lipid Sci. Technol., 2012, 114(11), 1237–1245 CrossRef CAS.
- G. Kaithwas, A. Mukherjee, A. Chaurasia and D. K. Majumdar, Antiinflammatory, analgesic and antipyretic activities of Linum usitatissimum L. (flaxseed/linseed) fixed oil, Indian J. Exp. Biol., 2011, 49, 932–938 CAS.
- G. Kaithwas Dubey, D. Bhatia, A. D. Sharma and K. Pillai, Reversal of sodium nitrite induced impairment of spontaneous alteration by Aloe vera gel: Involvement of cholinergic system, Pharmacologyonline, 2007, 3, 428–437 Search PubMed.
- A. Z. Reznick and L. Packer, [38] Oxidative damage to proteins: Spectrophotometric method for carbonyl assay, Methods Enzymol., 1994, 233, 357–363 CAS.
- J. Liphardt, S. Dumont, S. B. Smith, I. Tinoco and C. Bustamante, Equilibrium information from nonequilibrium measurements in an experimental test of Jarzynski's equality, Science, 2002, 296(5574), 1832–1835 CrossRef CAS PubMed.
- B. R. Celli, W. MacNee, A. Agusti, A. Anzueto, B. Berg and A. S. Buist, et al., Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper, Eur. Respir. J., 2004, 23(6), 932–946 CrossRef CAS PubMed.
- N. Sharma and Y. Ahmad, An effective method for the analysis of human plasma proteome using two-dimensional gel electrophoresis, J. Proteomics Bioinf., 2011, 2009 Search PubMed.
- U. K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, 227, 680–685 CrossRef CAS PubMed.
- H. Towbin, T. Staehelin and J. Gordon, Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications, Proc. Natl. Acad. Sci. U. S. A., 1979, 76(9), 4350–4354 CrossRef CAS.
- T. L. Mastracci, F. I. Boulos, I. L. Andrulis and W. L. Lam, Genomics and premalignant breast lesions: clues to the development and progression of lobular breast cancer, Breast Cancer Res., 2007, 9(6), 1 CrossRef PubMed.
- I. H. Russo and J. Russo, Mammary gland neoplasia in long-term rodent studies, Environ. Health Perspect., 1996, 104(9), 938 CrossRef CAS PubMed.
- R. D. Cardiff and S. R. Wellings, The comparative pathology of human and mouse mammary glands, J. Mammary Gland Biol. Neoplasia, 1999, 4(1), 105–122 CrossRef CAS PubMed.
- T. Yasugi, T. Kaido and Y. Uehara, Changes in density and architecture of microvessels of the rat mammary gland during pregnancy and lactation, Arch. Histol. Cytol., 1989, 52(2), 115–122 CrossRef CAS PubMed.
- A. Rani, S. Roy, M. Singh, U. Devi, R. K. Yadav, S. Gautam, J. K. Rawat, M. N. Ansari, S. A. Saeedan, A. Prakash and G. Kaithwas, α-Chymotrypsin regulates free fatty acids and UCHL-1 to ameliorate N-methyl nitrosourea induced mammary gland carcinoma in albino wistar rats, Inflammopharmacology, 2016, 24(5), 277–286 CrossRef CAS PubMed.
- B. Halliwell, and J. M. Gutteridge, Free radicals in biology and medicine: Oxford University Press, USA, 2015 Search PubMed.
- H. Wiseman and B. Halliwell, Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer, Biochem. J., 1996, 313(Pt 1), 17 CrossRef CAS PubMed.
- J. A. F. Vara, E. Casado, J. de Castro, P. Cejas, C. Belda-Iniesta and M. González-Barón, PI3K/Akt signalling pathway and cancer, Cancer Treat. Rev., 2004, 30(2), 193–204 CrossRef CAS PubMed.
- Y. Chen, Y. Tang, S. Chen and D. Nie, Regulation of drug resistance by human pregnane X receptor in breast cancer, Cancer Biol. Ther., 2009, 8(13), 1265–1272 CrossRef CAS PubMed.
- R. C. Richie and J. O. Swanson, Breast cancer: a review of the literature, J. Insur. Med., 2003, 35(2), 85–101 Search PubMed.
- H. E. M. zu Schwabedissen, R. G. Tirona, C. S. Yip, R. H. Ho and R. B. Kim, Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer, Cancer Res., 2008, 68(22), 9338–9347 CrossRef PubMed.
- Y. M. Shah, X. Ma, K. Morimura, I. Kim and F. J. Gonzalez, Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression, Am. J. Physiol.: Gastrointest. Liver Physiol., 2007, 292(4), G1114–G1122 CrossRef CAS PubMed.
- N. A. Venier, Capsaicin as a Novel Chemopreventive and Therapeutic Option for Prostate Cancer, University of Toronto, 2015 Search PubMed.
- C. A. Dinarello, Why not treat human cancer with interleukin-1 blockade?, Cancer Metastasis Rev., 2010, 29(2), 317–329 CrossRef CAS PubMed.
- Y. Goto, L. Zeng, C. J. Yeom, Y. Zhu, A. Morinibu and K. Shinomiya, et al., UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α, Nat. Commun., 2015, 6, 6153 CrossRef CAS PubMed.
- S. Julius and S. Nesbitt, Sympathetic overactivity in hypertension a moving target, Am. J. Hypertens., 1996, 9(S4), 113S–120S CrossRef CAS PubMed.
- D. C. Doll, Q. S. Ringenberg and J. Yarbro, Vascular toxicity associated with antineoplastic agents, J. Clin. Oncol., 1986, 4(9), 1405–1417 CrossRef CAS PubMed.
- E. L. Worthington Jr, C. V. O. Witvliet, P. Pietrini and A. J. Miller, Forgiveness, health, and well-being: A review of evidence for emotional versus decisional forgiveness, dispositional forgivingness, and reduced unforgiveness, J. Behav. Med., 2007, 30(4), 291–302 CrossRef PubMed.
- E. Caro-Morán, C. Fernández-Lao, N. Galiano-Castillo, I. Cantarero-Villanueva, M. Arroyo-Morales and L. Díaz-Rodríguez, Heart Rate Variability in Breast Cancer Survivors After the First Year of Treatments A Case-Controlled Study, Biol. Res. Nurs., 2016, 18(1), 43–49 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |