Open Access Article
Open Access ArticleHighly selective non-enzymatic electrochemical sensor based on a titanium dioxide nanowire–poly(3-aminophenyl boronic acid)–gold nanoparticle ternary nanocomposite
N. Muthuchamy†
a,
A. Gopalan†ab and
Kwang-Pill Lee *ab
*ab
aResearch Institute of Advanced Energy Technology, Kyungpook National University, Daegu, South Korea. E-mail: kplee@knu.ac.kr
bDepartment of Nanoscience and Nanotechnology, Kyungpook National University, Daegu, South Korea
First published on 9th January 2018
Abstract
A novel three component (titanium dioxide nanowire (TiO2 NW), poly(3-aminophenyl boronic acid) (PAPBA) and gold nanoparticles (Au NPs)) based ternary nanocomposite (TNC) (designated as TiO2 NW/PAPBA–Au TNC) was prepared by a simple two-stage synthetic approach and utilized for the fabrication of a non-enzymatic (enzyme-free) glucose (NEG) sensor. In stage 2, the PAPBA–Au NC was formed by oxidative polymerization of 3-APBA using HAuCl4 as oxidant on the surface of pre-synthesized TiO2 NW via electrospinning (stage 1). The formation of PAPBA–Au NC as the shell on the surface of the TiO2 NW (core) was confirmed by field emission scanning electron microscopy (FE-SEM). Notably, we obtained a good peak to peak separation, and a high peak current for the redox Fe(CN)63−/4− process indicating excellent electron transfer capability at the glassy carbon electrode (GCE)/TiO2 NW/PAPBA–Au TNC interface. Also, the fabricated TiO2 NW/PAPBA–Au TNC provides excellent electrocatalytic activity towards glucose detection in neutral (pH = 7.0) phosphate buffer solution. The detection of glucose was monitored using differential pulse voltammetry. The obtained sensitivity and detection limits are superior to many of the TiO2 based enzymatic and non-enzymatic glucose sensors reported in the literature. Furthermore, the TiO2 NW/PAPBA–Au TNC sensor is preferred because of its high selectivity to glucose in the presence of co-existing interfering substances and practical application for monitoring glucose in human blood serum samples.
1. Introduction
Diabetes, a global health problem affecting over 200 million people, can cause disorders of the kidney, heart, neural system and retina.1 Hence, the monitoring of blood glucose levels is essential to prevent diabetic complications such as diabetes from becoming more prevalent in modern society. Conventional glucose sensors use glucose oxidase immobilized on a solid electrode, to catalyze the oxidation of glucose in the presence of oxygen to produce hydrogen peroxide and selectively monitor the glucose levels.2 Although such enzymatic glucose sensors are available commercially, they suffer from some limitations such as complex enzyme immobilization procedures, instability due to the intrinsic nature of enzymes, sensitivity to the chemical environment (e.g. pH), and temperature.3 Besides, long-term monitoring of the blood glucose levels using an enzyme-based electrode is typically hampered by surface fouling by the absorption/passivation of the products. The surface fouling inevitably limits the selectivity and sensitivity towards glucose oxidation over time.4,5 As a result, development of non-enzymatic glucose (NEG) sensors is becoming essential.6Various nanostructured metals (e.g., Au,7 Pt,8 Pd,9 metal oxides (e.g., NiO,10 Co3O4,11 CuO12), their composites (e.g., CuO–SWCNT)),13 and nanocomposites (Cu based multicomponent nanobead),14 BiOCl–G nanohybrid sheets,15 CuO–MWCNT16 had been used to fabricate NEG sensors. A comprehensive review has been published on the fabrication of NEG sensors using various nanomaterials.17 Among the metal oxide nanostructures based sensors, TiO2 nanostructures have attracted much interest due to their combinational properties such as high surface area, electrocatalysis, non-toxicity, biocompatibility, and oxygen storage capacity. However, pristine TiO2 electrodes usually have poor electrochemical activity because of the low conductivity of TiO2.18,19 Nanocomposites of TiO2, such as TiO2 modified with metal nanoparticles, are thus promising in NEG sensors.20 Several binary composites based on TiO2 such as TiO2–G,21 NiO–TiO2,22 TiO2/CuO23 and TiO2/Pd,24 TiO2/Co3O4 (ref. 25 and 26) were used for the fabrication of NEG sensors. One dimensional (1D) nanostructures of TiO2, specifically nanowires (NW), possess essentially the common physical characteristics as that of 2D and 3D nanostructures of TiO2, but geometrically offer the additional advantages like good transport of electrons and mechanical stability.27 1D TiO2 NWs, could also be integrated with other materials or nanomaterials to improve the surface area and electron transport properties. Our recent research studies demonstrated that TiO2 based nanocomposite can serve as the highly sensitive electrode for the determination of cholesterol and nitrate ions.28,29 However, studies on the use of TiO2 NW in combination with a glucose selective polymer towards NEG sensor has not been reported so far.
Boronic acid is well known to interact with 1,2- or 1,3-diols, at physiological pHs or above, and form reversible covalent bonds to generate five or six-membered cyclic complexes.30 The chemical interactions between boronic acid and diols have been utilized for the detection of diol-containing biomolecules, such as sugars, dopamine, glycoproteins, and bacteria.31–33 Boronic acid groups containing polymers such as poly(3-aminophenyl boronic acid) (PAPBA) have exhibited good operational stability and selectivity towards detection of glucose levels as compared to glucose oxidase and hence utilized for the fabrication of NEG sensors.34–37 Due to their fascinating electrical and catalytic properties, gold nanoparticles (Au NPs) have been utilized widely in electrochemical applications.38–40 Au NPs in combination with G (carbon 98, 90 (2016)); calix41 arene/boronic acids42 were used to improve the performance of NEG sensors.
In this background, we have constructed the NEG sensor based on a new ternary nanocomposite (TNC) comprised of TiO2 NW, Au NPs and PAPBA (designated as TiO2 NW/PAPBA–Au TNC) (Scheme 1). Firstly, 1D TiO2 NWs were synthesized by electrospinning and hydrothermal processes.43 Followed by the synthesis of TiO2 NWs, Au NPs distributed PAPBA NC was formed on the surface of TiO2 NW by a one-step polymerization of 3-APBA using auric chloride (HAuCl4) as the oxidizing agent. The morphology and electrochemical properties of TiO2 NW/PAPBA–Au TNC were investigated by field emission scanning electron microscopy (FE-SEM) electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). The fabricated NEG sensor based on GCE/TiO2 NW/PAPBA–Au TNC electrode exhibited excellent performances towards electrochemical glucose detection (Scheme 1), including high sensitivity and good selectivity in the presence of common interfering species as compared to the pristine components, PAPBA and TiO2 NW, as well over the glucose sensing performances based on other TiO2 based materials reported in literature (Table 1). Moreover, the capability of utilizing GCE/TiO2 NW/PAPBA–Au TNC electrode for determination of glucose levels in human serum is demonstrated, suggesting the potential of the fabricated NEG sensor for practical diabetes diagnosis.
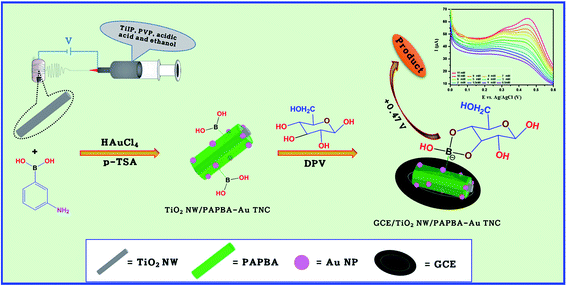 | ||
| Scheme 1 Schematic diagram of the preparation of TiO2 NW/PAPBA–Au TNC and electrochemical performance towards glucose detection. | ||
| Glucose sensor | Sensitivity [μA cm−2 mM−1] | Linear range [mM] | Reference |
|---|---|---|---|
| GOx/PANI-NF/GCE | — | 0.01–1.0 | 44 |
| Cyt c/AuNPs/PANI-NS | 63.1 | 0.01–3.2 | 45 |
| PANI-NT/GOx | 4.62 | 0.01–5.5 | 46 |
| GOx/n-TiO2/PANI/GCE | 6.31 | 0.02–6.0 | 47 |
| Pt/CNTs/TiO2 NTAs | 0.24 | 0.006–1.5 | 48 |
| GOx/TiO2/CNTs | 11.3 ± 1.3 | Up to 3.0 | 49 |
| TiO2–SWCNT NWS | 5.32 | 0.010–1.42 | 50 |
| Cu2O/TiO2 | 14.56 | 3.0–9.0 | 51 |
| GOx/Ag/TiO2 NTAs | 0.39 | 0.1–4.0 | 52 |
| GOx/Pt/Gr/TiO2 NTAs | 0.94 | 0.1–8.0 | 53 |
| AuNPs–TiO2 NT | — | 0.40–8.0 | 54 |
| TiO2–GR | 6.20 | 0–8.0 | 21 |
| Chitosan–AuNPs | — | 0.006–0.14 | 55 |
| GOD/1DH S–TiO2 | 9.9 | 0.2–1.0 | 56 |
| GOD/HNF–TiO2/GC | 32.6 | 0.002–3.17 | 57 |
| Nafion-silica/MWCNTs-g-PANI/GOx | 5.01 | 1–10 | 58 |
| GCE/PAPBA–Au NC | 34.6 | 0.5–11.0 | This work |
| GCE/TiO2 NW/PAPBA–Au TNC | 66.8 | 0.5–11.0 | This work |
2. Experimental details
2.1. Chemicals
HAuCl4, p-toluenesulfonic acid (p-TSA), 3-APBA, Ti(IV) isopropoxide, polyvinylpyrrolidone (PVP, molecular weight < 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Fluka) acetic acid, glucose and human serum (from human male AB plasma, USA origin, sterile-filtered) were purchased from Sigma-Aldrich, South Korea and used as received. Ammonium peroxydisulfate (APS) was obtained from Samchum Chemicals, South Korea. Phosphate buffer solution (PBS, pH = 7) and ethanol were obtained from OCI Company Ltd., South Korea.
000, Fluka) acetic acid, glucose and human serum (from human male AB plasma, USA origin, sterile-filtered) were purchased from Sigma-Aldrich, South Korea and used as received. Ammonium peroxydisulfate (APS) was obtained from Samchum Chemicals, South Korea. Phosphate buffer solution (PBS, pH = 7) and ethanol were obtained from OCI Company Ltd., South Korea.
2.2. Formation of TiO2 NW/PAPBA–Au TNC
The formation stages of TiO2 NW/PAPBA–Au TNC include: (i) preparation of TiO2-NWs and (ii) simultaneous formation of PAPBA and Au NPs on TiO2 NW.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (3
ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) 18 mL solution and stirred for 2 h. The resulting electrospinning dope was transferred into a plastic syringe equipped with a needle (0.25 mm diameter) and subjected to electrospinning by applying a DC voltage of 25 kV using a programmable syringe pump at a flow rate of 0.7 mL h−1. The composite titanate nanofibers were collected on a grounded rotating drum covered with an aluminum foil placed at a distance of 15 cm from the spinneret. The composite titanate nanofibers were sintered by calcination in air at 500 °C for 3 h. The calcined materials, TiO2-NWs, was collected and washed with ethanol to remove impurities.
7 v/v) 18 mL solution and stirred for 2 h. The resulting electrospinning dope was transferred into a plastic syringe equipped with a needle (0.25 mm diameter) and subjected to electrospinning by applying a DC voltage of 25 kV using a programmable syringe pump at a flow rate of 0.7 mL h−1. The composite titanate nanofibers were collected on a grounded rotating drum covered with an aluminum foil placed at a distance of 15 cm from the spinneret. The composite titanate nanofibers were sintered by calcination in air at 500 °C for 3 h. The calcined materials, TiO2-NWs, was collected and washed with ethanol to remove impurities.2.3. Fabrication of modified electrodes for NEG sensing
TiO2 NW/PAPBA–Au TNC, PAPBA–Au NC, TiO2 NW/PAPBA NC and pristine PAPBA modified electrodes were prepared by dispersing 2 mg of the respective electrode modifying the material in 0.1 mL of isopropyl alcohol and Nafion (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) solution. The mixture was sonicated for 10 min to obtain an ink. About, 6 μL of the ink was dropped on the surface of polished glassy carbon electrode (GCE, area = 0.07065 cm2) and dried at room temperature.
2 v/v) solution. The mixture was sonicated for 10 min to obtain an ink. About, 6 μL of the ink was dropped on the surface of polished glassy carbon electrode (GCE, area = 0.07065 cm2) and dried at room temperature.
2.4. Morphological characterization
Field emission scanning electron microscopy (FE-SEM) coupled with energy dispersive X-ray (EDX) (Hitachi S-4300, Japan) analysis was used to study morphology and elemental composition. The crystal structure of the prepared materials was investigated by X-ray diffraction (XRD, Quantera SXM, ULVAC-PHI, Japan) using Cu Kα radiation (λ = 1.5406 Å) in the 2θ range of 20–85°.2.5. Electrochemical properties and NEG sensing
Electrochemical measurements were performed using Ivium-Stat (Netherland) electrochemical interface with PC-controlled analyzer workstation. A conventional three-electrode cell assembly was used. The modified electrode was used as the working electrode. Ag/AgCl and platinum wire were used as reference and counter electrodes, respectively. The electroactivity of the modified electrodes was evaluated using cyclic voltammetry in 0.1 M PBS (pH 7). The glucose detection experiments were carried out using differential pulse voltammetry measurements at a scan rate of 0.05 V s−1 with a pulse amplitude of 0.05 V, pulse rate of 0.5 s and pulse width of 50 ms for various glucose concentrations in an electrochemical cell containing a magnetically stirred PBS electrolyte.3. Result and discussion
3.1. Morphology and structural characterization
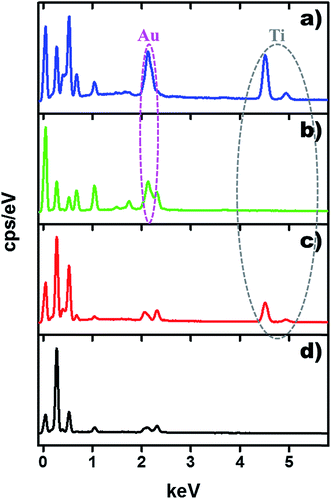
The morphologies of the as-prepared TiO2 NW/PAPBA–Au TNC, PAPBA–Au NC, TiO2 NW/PAPBA NC and pristine PAPBA were characterized by FE-SEM analysis. As shown in the FE-SEM image of TiO2 NW/PAPBA–Au TNC (Fig. 1a), the smooth surface of TiO2 NW (Fig. 1a, inset) was transformed into an uneven and bumpy surface, suggested that the surface of TiO2 NW was completely covered with PAPBA–Au NC. On close perusal of the surface of TiO2 NW/PAPBA–Au TNC (Fig. 1a), one can identify two kinds of materials, spherical bright dots (Au NPs) and the dark puffy mass (PAPBA). It is clear that Au NPs were distributed within PAPBA matrix and existed on the surface of TiO2 NW. EDAX analysis of the TiO2 NW/PAPBA–Au TNC informed that nearly 17.63 wt% of elemental Au is presented in (Fig. 2). Thus, the treatment of HAuCl4 with 3-APBA in the presence of TiO2 NW modified the TiO2 NW surface with a PAPBA–Au shell layer. FESEM image of TiO2 NW/PAPBA NC (Fig. 1b) also reveals the modification of the surface of TiO2 NW. In this case, a much increased (about 150 nm thick) shell layer of PAPBA was formed on TiO2 NW surface. Fig. 1c displays the image of PAPBA–Au NC with the distribution of larger sized spherical Au NPs (diameters in the range 130–200 nm) on the amorphous host matrix of PAPBA with minimum aggregations. The pristine PAPBA had a granular morphology (Fig. 1d).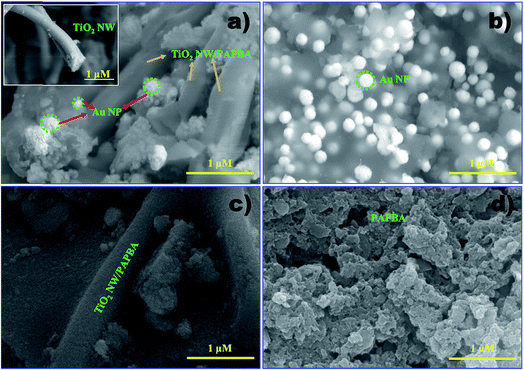 | ||
| Fig. 1 FE-SEM images of (a) TiO2 NW/PAPBA–Au TNC, (inset: image of TiO2 NW) (b) PAPBA–Au NC, (c) TiO2 NW/PAPBA NC, (d) pristine PAPBA. | ||
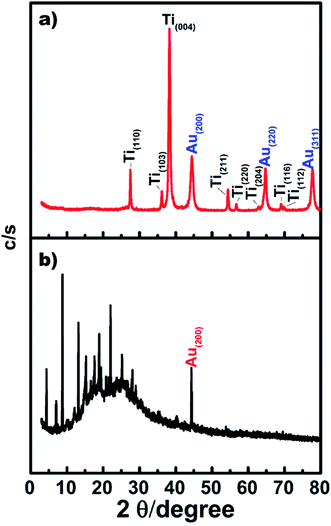
Fig. 3 shows the XRD patterns of the TiO2 NW/PAPBA–Au TNC (Fig. 3a), PAPBA–Au NC (Fig. 3b). The XRD pattern of TiO2 NW/PAPBA–Au TNC (Fig. 3a) exhibits peaks centered at 2θ = 27.48°, 36.18°, 38.24°, 54.46°, 56.68°, 62.96°, 69.08° and 69.86° that correspond to (110), (103), (004), (211), (220), (204), (116) and (112) reflections of anatase TiO2, peaks corresponding to the atomic planes of Au [2θ = 44.56° (200), 64.82° (220), and 77.68° (311)] and a broad band around 10–25°. The XRD pattern of PAPBA–Au NC (Fig. 3b) comprises of an intense broadband around 2θ = 10–25° (PAPBA) that is attributed to the periodically parallel chain of amorphous polymers of PAPBA and a sharp peak Bragg reflection peak at 2θ = 44.56° (Au (200)). The Au reflection peaks at 38.24° (100) and 64.8° (220) are less intense as compared to XRD pattern of the TiO2 NW/PAPBA–Au TNC (Fig. 3a). Thus, XRD pattern of TiO2 NW/PAPBA–Au TNC (Fig. 3a) corroborates with the coexistence of crystalline TiO2, Au atomic planes, and amorphous PAPBA.
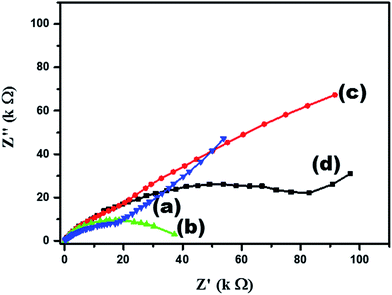
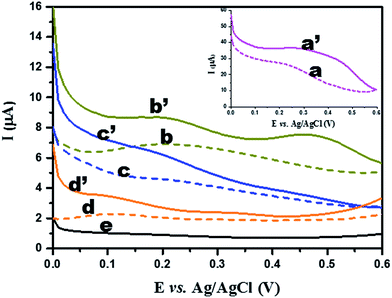
3.2. Electrochemical properties
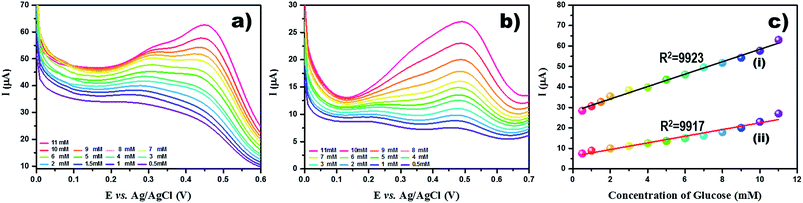
3.3. NEG sensor performances
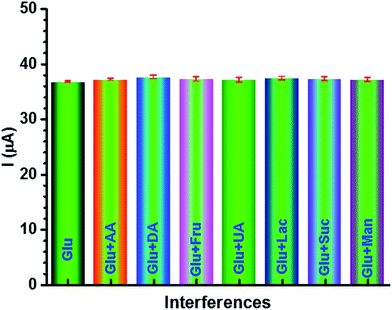
Our results reveal that TiO2 NW/PAPBA–Au TNC exhibits superior electroactivity, higher electrochemical surface area, faster electron transfer kinetics, and interfacial characteristics as compared to the pristine components. Also, it exhibits the highest glucose sensing performances as compared to the TiO2 based materials reported in the literature (Table 1). We attribute a multisource synergistic electrocatalytic effect for TiO2 NW/PAPBA–Au TNC arising from the catalytic activity of TiO2 NW in axial and radial directions and deliberate dispersion of electrocatalytic nanosized Au particles onto the electron mediating conducting polymer (PAPBA) having boronic acid binding groups to selectively bind glucose.
| Sample | Added glucose [mM] | Concentration [mM] | Recovery [%] | RSDa [%] | |
|---|---|---|---|---|---|
| Determined by glucometer | Determined at the fabricated electrode | ||||
| a Number of measurements = 3. | |||||
| Human serum samples | 0 | 4.44 ± 0.01 | 4.43 ± 0.04 | 99.8 | |
| 1 | 5.415 ± 0.06 | 5.412 ± 0.01 | 99.94 | ||
| 1 | 5.44 ± 0.02 | 5.43 ± 0.05 | 99.81 | ||
| 1 | 5.43 ± 0.07 | 5.413 ± 0.04 | 99.68 | ||
| 2 | 6.43 ± 0.03 | 6.425 ± 0.07 | 99.92 | ||
| 2 | 6.36 ± 0.01 | 6.358 ± 0.02 | 99.97 | ||
| 2 | 6.383 ± 0.05 | 6.38 ± 0.03 | 99.95 | ||
| 3 | 7.388 ± 0.3 | 7.37 ± 0.01 | 99.75 | 2.84 | |
| 3 | 7.34 ± 0.03 | 7.35 ± 0.07 | 100.13 | ||
| 3 | 7.442 ± 0.04 | 7.31 ± 0.06 | 98.22 | ||
| 4 | 8.41 ± 0.01 | 8.43 ± 0.02 | 100.23 | ||
| 4 | 8.45 ± 0.06 | 8.442 ± 0.05 | 99.90 | ||
| 4 | 8.378 ± 0.02 | 8.4 ± 0.04 | 100.26 | ||
| 5 | 9.42 ± 0.07 | 9.431 ± 0.03 | 100.11 | ||
| 5 | 9.39 ± 0.03 | 9.388 ± 0.04 | 99.97 | ||
| 5 | 9.41 ± 0.05 | 9.414 ± 0.03 | 100.04 | ||
4. Conclusion
The ternary nanocomposite (TNC), comprising of the surface area enriched 1D TiO2-NWs, a glucose binding conducting polymer (PAPBA) and electrocatalytic Au NPs materials was utilized for the construction of a sensitive non-enzymatic glucose sensor. The TiO2 NW/PAPBA–Au TNC exhibited good sensor characteristics towards electrochemical detection of glucose. The TiO2 NW/PAPBA–Au TNC sensor outperforms the reported TiO2 based glucose sensors. The peak current for glucose oxidation showed a linear dependence with a concentration of glucose in a wider range, 50 μM to 11 mM with a high sensitivity (66.8 μA cm−2 mM−1). The excellent sensor performances (high sensitivity and wider linear range) at TiO2 NW/PAPBA–Au TNC are due to synergistic roles of the components (TiO2 NW, PAPBA, and Au NPs) in the newly synthesized TNC. Importantly, the peak current of glucose oxidation current was not influenced at TiO2 NW/PAPBA–Au TNC in the presence of electrochemical interfering substances, such as ascorbic acid (AA), uric acid (UA) dopamine (DA), fructose (Fru), lactose (Lac), sucrose (Suc) and mannose (Mon). To note, TiO2 NW/PAPBA–Au TNC sensor exhibited a remarkable performance in the human serum sample and signifies its utility for practical blood glucose detection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Priority Research Center Program through a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science, and Technology (2009-0093819) and National Research Foundation of Korea (2014-R1A1A4A03004026).References
- A. Heller and B. Feldman, Chem. Rev., 2008, 108, 2482–2505 CrossRef CAS PubMed.
- C. Chen, Q. Xie, D. Yang, H. Xiao, Y. Fu, Y. Tan and S. Yao, RSC Adv., 2013, 3, 4473–4491 RSC.
- A. S. Kumar, P. Y. Chen, S. H. Chien and J. M. Zen, Electroanalysis, 2005, 17, 210–222 CrossRef CAS.
- D. P. Manica, Y. Mitsumori and A. G. Ewing, Anal. Chem., 2003, 75, 4572–4577 CrossRef CAS PubMed.
- S. Komathi, A. I. Gopalan, N. Muthuchamy and K. P. Lee, RSC Adv., 2017, 7, 15342–15351 RSC.
- B. K. Jena and C. R. Raj, Chem.–Eur. J., 2006, 12, 2702–2708 CrossRef CAS PubMed.
- W. Liu, X. Wu and X. Li, RSC Adv., 2017, 7, 36744–36749 RSC.
- X. Bo, J. C. Ndamanisha, J. Bai and L. Guo, Talanta, 2010, 82, 85–91 CrossRef CAS PubMed.
- L. M. Lu, H. B. Li, F. Qu, X. B. Zhang, G. L. Shen and R. Q. Yu, Biosens. Bioelectron., 2011, 26, 3500–3504 CrossRef CAS PubMed.
- F. Cao, S. Guo, H. Ma, D. Shan, S. Yang and J. Gong, Biosens. Bioelectron., 2011, 26, 2756–2760 CrossRef CAS PubMed.
- C. Karuppiah, S. Palanisamy, S. M. Chen, V. Veeramani and P. Periakaruppan, Sens. Actuators, B, 2014, 196, 450–456 CrossRef CAS.
- L. Xu, Q. Yang, X. Liu, J. Liu and X. Sun, RSC Adv., 2014, 4, 1449–1455 RSC.
- N. Q. Dung, D. Patil, H. Jung and D. Kim, Biosens. Bioelectron., 2013, 42, 280–286 CrossRef PubMed.
- A. I. Gopalan, N. Muthuchamy, S. Komathi and K. P. Lee, Biosens. Bioelectron., 2016, 84, 53–63 CrossRef CAS PubMed.
- A. I. Gopalan, N. Muthuchamy and K. P. Lee, Biosens. Bioelectron., 2017, 89, 352–360 CrossRef CAS PubMed.
- J. Yang, L. C. Jiang, W. D. Zhang and S. Gunasekaran, Talanta, 2010, 82, 25–33 CrossRef CAS PubMed.
- G. Gnana Kumar, G. Amala and S. M. Gowtham, RSC Adv., 2017, 7, 36949–36976 RSC.
- X. Lu, G. Wang, T. Zhai, M. Yu, J. Gan, Y. Tong and Y. Li, Nano Lett., 2012, 12, 1690–1696 CrossRef CAS PubMed.
- L. Hu, K. Huo, R. Chen, B. Gao, J. Fu and P. K. Chu, Anal. Chem., 2011, 83, 8138–8144 CrossRef CAS PubMed.
- P. V. Suneesh, V. S. Vargis, T. Ramachandran, B. G. Nair and T. S. Babu, Sens. Actuators, B, 2015, 215, 337–344 CrossRef CAS.
- H. D. Jang, S. K. Kim, H. Chang, K. M. Roh, J. W. Choi and J. Huang, Biosens. Bioelectron., 2012, 38, 184–188 CrossRef CAS PubMed.
- Z. D. Gao, Y. Han, Y. Wang, J. Xu and Y. Y. Song, Sci. Rep., 2013, 3, 3323 CrossRef PubMed.
- L. Tian, W. Hu, X. Zhong and B. Liu, Mater. Res. Innovations, 2015, 3, 160–165 CrossRef.
- X. Chen, G. Li, G. Zhang, K. Hou, H. Pan and M. Du, Mater. Sci. Eng., C, 2016, 62, 323–328 CrossRef CAS PubMed.
- X. Y. Lang, H. Y. Fu, C. Hou, G. F. Han, P. Yang, Y. B. Liu and Q. Jiang, Nat. Commun., 2013, 4, 2169 Search PubMed.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- J. Z. Chen, W. Y. Ko, Y. C. Yen, P. H. Chen and K. J. Lin, ACS Nano, 2012, 6, 6633–6639 CrossRef CAS PubMed.
- S. Komathi, N. Muthuchamy, K. P. Lee and A. I. Gopalan, Biosens. Bioelectron., 2016, 84, 64–71 CrossRef CAS PubMed.
- N. Muthuchamy, K. P. Lee and A. I. Gopalan, Biosens. Bioelectron., 2017, 89, 390–399 CrossRef CAS PubMed.
- B. Deore and M. S. Freund, Analyst, 2003, 128, 803–806 RSC.
- X. Wang, Y. Liu, L. Ren, H. Li and Z. Liu, Anal. Methods, 2013, 5, 5444–5449 RSC.
- S. Badhulika, C. Tlili and A. Mulchandani, Analyst, 2014, 139, 3077–3082 RSC.
- H. Torul, H. Çiftçi, F. C. Dudak, Y. Adıgüzel, H. Kulah, İ. H. Boyacı and U. Tamer, Anal. Methods, 2014, 6, 5097–5104 RSC.
- Q. Wu, L. Wang, H. Yu, J. Wang and Z. Chen, Chem. Rev., 2011, 111, 7855–7875 CrossRef CAS PubMed.
- D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine, and Materials, Wiley-VCH, Weinheim, Germany, 2011 Search PubMed.
- R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47, 1106 RSC.
- K. M. Manesh, P. Santhosh, A. Gopalan and K. P. Lee, Anal. Biochem., 2007, 360, 189–195 CrossRef CAS PubMed.
- K. L. Adams, B. K. Jena, S. J. Percival and B. Zhang, Anal. Chem., 2011, 83, 920–927 CrossRef CAS PubMed.
- X. Zhang, T. Zeng, C. Hu and S. Hu, Anal. Methods, 2016, 8, 1162–1169 RSC.
- P. Daggumati, Z. Matharu and E. Seker, Anal. Chem., 2015, 87, 8149–8156 CrossRef CAS PubMed.
- S. D. Bull, M. G. Davidson, J. M. Van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y. B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2012, 46, 312–326 CrossRef PubMed.
- A. Pandya, P. G. Sutariya and S. K. Menon, Analyst, 2013, 138, 2483–2490 RSC.
- H. G. Lee, G. Sai-Anand, S. Komathi, A. I. Gopalan, S. W. Kang and K. P. Lee, J. Hazard. Mater., 2015, 283, 400–409 CrossRef CAS PubMed.
- M. Zhao, X. Wu and C. Cai, J. Phys. Chem. C, 2009, 113, 4987–4996 CAS.
- C. Xiang, Y. Zou, S. Qiu, L. Sun, F. Xu and H. Zhou, Talanta, 2013, 110, 96–100 CrossRef CAS PubMed.
- Z. Wang, S. Liu, P. Wu and C. Cai, Anal. Chem., 2009, 81, 1638–1645 CrossRef CAS PubMed.
- W. Tang, L. Li and X. Zeng, Talanta, 2015, 131, 417–423 CrossRef CAS PubMed.
- X. Y. Pang, D. M. He, S. L. Luo and Q. Y. Cai, Sens. Actuators, B, 2009, 137, 134–138 CrossRef.
- J. H. Lopes, F. X. Colson, J. E. Barralet and G. Merle, Mater. Sci. Eng. C, 2017, 76, 991–996 CrossRef CAS PubMed.
- N. Q. Dung, D. Patil, T. T. Duong, H. Jung, D. Kim and S.-G. Yoon, Sens. Actuators, B, 2012, 166–167, 103–109 CrossRef CAS.
- M. Long, L. Tan, H. Liu, Z. He and A. Tang, Biosens. Bioelectron., 2014, 59, 243–250 CrossRef CAS PubMed.
- C. Feng, G. Xu, H. Liu, J. Lv, Z. Zheng and Y. Wu, J. Solid State Electrochem., 2014, 18, 163–171 CrossRef CAS.
- C. Feng, G. Xu, H. Liu, J. Lv, Z. Zheng and Y. Wu, J. Electrochem. Soc., 2014, 161, B1–B8 CrossRef CAS.
- Z. Zhang, Y. Xie, Z. Liu, F. Rong, Y. Wang and D. Fu, J. Electroanal. Chem., 2011, 650, 241–247 CrossRef CAS.
- C. Jiang, J. Zhu, Z. Li, J. Luo, J. Wang and Y. Sun, RSC Adv., 2017, 7, 44463–44469 RSC.
- P. Si, S. J. Ding, J. Yuan, X. W. Lou and D. H. Kim, ACS Nano, 2011, 5, 7617–7626 CrossRef CAS PubMed.
- Q. Guo, L. Liu, M. Zhang, H. Hou, Y. Song, H. Wang, B. Zhong and L. Wang, Biosens. Bioelectron., 2017, 92, 654–660 CrossRef CAS PubMed.
- A. I. Gopalan, K.-P. Lee, D. Ragupathy, S.-H. Lee and J.-W. Lee, Biomaterials, 2009, 30, 5999–6005 CrossRef CAS PubMed.
Footnote |
| † Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |