Aromaticity control via modifications of a macrocyclic frame: 5,6-dimethoxyphenanthriporphyrin and 5,6-dioxophenanthriporphyrin†
Abstract
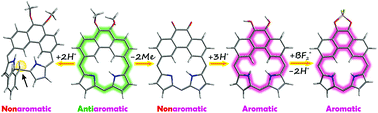
The incorporation of a 9,10-dimethoxyphenanthrene moiety into a porphyrin framework results in the formation of a hybrid macrocycle – 5,6-dimethoxyphenanthriporphyrin 1, fusing the structural features of polycyclic aromatic hydrocarbons and porphyrins. Simple transformations of antiaromatic 1 led to two macrocycles incorporating phenanthrene and phenanthrenequinone units: isophenanthriporphyrin and 5,6-dioxophenanthriporphyrin. The reversible protonation of 1 at the central meso-carbon atom stabilizes its constitutional isomer, i.e. the Cs-symmetric isophenanthriporphyrin in its dicationic form 1-A-H22+. The addition of an acid to nonaromatic 5,6-dioxophenanthriporphyrin 2 yielded the aromatic tricationic form protonated at the carbonyl oxygen atoms. In the presence of tetrafluoroboric acid, etherate, 2 underwent borylation at carbonyl oxygen atoms forming the aromatic BF2-derivative.


 Please wait while we load your content...
Please wait while we load your content...