Asymmetric ring-opening reactions of azabenzonorbornadienes through transfer hydrogenation using secondary amines as hydrogen sources: tuning of absolute configuration by acids†
Abstract
Reductive transfer hydrogenation of bicyclic alkenes has been developed under the co-catalytic system of palladium and silver by using secondary amines as reductants. The reaction results in the asymmetric ring-opening product. A wide range of azabenzonorbornadienes reacted well giving 1,2-dihydronaphthalen-1-amine derivatives in high yields with good enantioselectivities. The control of the absolute configuration of the product by addition of carboxylic acids has been demonstrated.
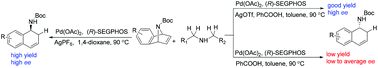


 Please wait while we load your content...
Please wait while we load your content...