Enantioselective synthesis of (−)-phaseic acid†
Wentong
Tu
a,
Chengqing
Ning
*ab and
Jing
Xu
 *abc
*abc
aDepartment of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong Province, China. E-mail: xuj@sustc.edu.cn; ningcq@sustc.edu.cn
bSUSTech Academy for Advanced Interdisciplinary Studies, Shenzhen, Guangdong Province, China
cGrubbs Institute, Shenzhen, Guangdong Province, China
First published on 18th September 2017
Abstract
Phaseic acid (PA) has long been considered an inactive oxidation catabolite of the essential plant hormone abscisic acid (ABA). However, a recent study disclosed that PA actually acts as an ABA receptor agonist and is capable of prolonging ABA effects in vivo. Here, we report a simple, enantioselective synthesis of (−)-phaseic acid. Key transformations of our strategy include a facile hydrozirconation reaction and a critical, Mn(III)-catalyzed allylic oxidation.
Introduction
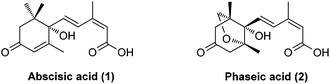
Abscisic acid (ABA, 1, Fig. 1), commonly known as the “stress hormone”, plays an essential role in regulating the adaptive responses of plants to abiotic stress.1,2 For example, under drought stress, ABA levels increase strongly and rapidly, triggering stomatal closure that enables plants to conserve water by preventing transpiration and survive under harsh conditions. Exogenous ABA treatment has also been shown to improve the survival rates and quality of plants subjected to drought stress.3 However, agricultural application of ABA is limited due to its rapid catabolism in vivo.4,5 In plants, natural (+)-ABA is catabolized through oxidation and/or conjugation. (−)-Phaseic acid (PA, 2, Fig. 1) has long been considered to be an inactive, oxidative catabolite of ABA resulting from the catalysis of the CYP707A family of cytochrome P450.6 Its physiological function in plants was not disclosed until recently, when Noel et al.7 demonstrated that PA is an ABA receptor agonist that is capable of prolonging the effects of ABA in vivo. ABA has previously been found to be synthesized to mediate fast responses to water deficiency before being catabolized to PA that can enable long-term responses. Moreover, exogenous PA showed ABA-like hormonal activity and is more stable than ABA in vivo. Therefore, PA may overcome the shortcomings of ABA and emerge as a promising ABA receptor agonist to enhance agricultural water use efficiency, thus addressing global concerns around water scarcity. Furthermore, a recent study found that PA could act as an endogenous neuroprotective agent against strokes by reversibly inhibiting glutamate receptors.8 Given the significant physiological functions of PA in plants and mammals, a simple, enantioselective synthesis of PA is evidently required for further biological investigations and applications.Two pioneering syntheses of PA were reported by Kitahara et al. (asymmetric synthesis by a longest linear sequence (LLS) of 17 steps from a known chiral synthon)9 and Abrams et al. (racemic synthesis by an LLS of 14 steps from a known starting material), respectively.10 The three continuous quaternary/fully-substituted carbon centers and a unique 1,3-diene motif of (E,Z)-configuration present a significant challenge for synthesis. Here, we describe a short, enantioselective synthesis of (−)-phaseic acid by an LLS of seven steps.11
Results and discussion
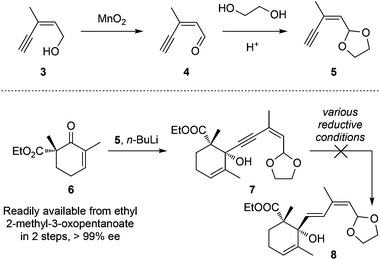
Our synthetic trial started from commercially available (Z)-3-methylpent-2-en-4-yn-1-ol (3, Scheme 1) that was first oxidized with MnO2 to aldehyde 4 followed by acetal transformation to achieve 5. The lithiated alkyne 5 was then reacted with the known chiral β-ketoester 6 (readily available from ethyl 2-methyl-3-oxopentanoate in 2 steps, >99% ee) to generate propagyl alcohol 7 (dr = ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).11l,12,13 However, all attempts to chemo/stereo-selectively reduce the alkyne motif to the desired E-alkene were unsuccessful. The red-Al11l or other attempted conditions only led to decomposition, probably due to the over-reduction of the carboxylic ethyl ester motif.
1).11l,12,13 However, all attempts to chemo/stereo-selectively reduce the alkyne motif to the desired E-alkene were unsuccessful. The red-Al11l or other attempted conditions only led to decomposition, probably due to the over-reduction of the carboxylic ethyl ester motif.
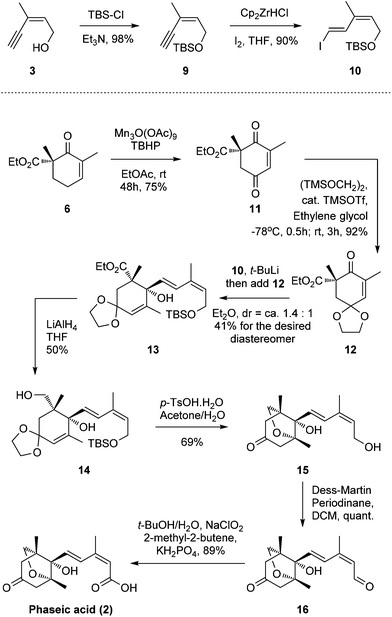
To address this problem, we introduced the 1,3-diene motif of (E,Z)-configuration in a more direct way. The alcohol 3 was protected as tert-butyl dimethylsilyl ether followed by hydrozirconation/iodination under Schwartz's reagent/I2 conditions to produce the vinyl iodide 10 of the desired (E,Z)-configuration (88% yield in two steps, Scheme 2).14
Concurrently, β-ketoester 6 was oxidized to ketone 11 under Shing's mild, Mn(III) acetate-catalyzed allylic oxidative conditions (75% yield).15,16 The newly generated carbonyl motif was protected as a ketal to afford compound 12. Subsequently, we are very pleased to find that 12 can be attacked by the vinyl lithium formed in situ through an iodide-lithium exchange of vinyl iodide 10 with t-BuLi to smoothly furnish allylic alcohol 13.17,18 Various solvents, temperatures, and lithium sources, such as n-Buli, s-BuLi and t-BuLi, were screened for this optimal result (dr = ca. 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 41% for the desired diastereomer, no 1,4-adduct was observed). The yield and chemo-/stereo-selectivity are quite acceptable considering the severe steric hindrance of the ketone motif. Reduction of the carboxylic ester group afforded moderate yields of alcohol 14 (50%) with the corresponding aldehyde by-product (38%), which can be isolated and further reduced to 14 (LiAlH4, 80%, see the ESI†) to improve the overall yield.19,20 Inspired by Takahashi's work, a one-pot global deprotection/oxa-Michael addition then successfully produced the bicyclic motif 15.11l,21 Finally, a two-step oxidation sequence (Dess–Martin oxidation/Pinnick oxidation) produced the desired (−)-phaseic acid at a yield of 89%. The synthetic material, thus obtained, possessed identical spectroscopic and analytical properties to those reported for the natural product and the synthetic enantiomer.6,9,10,22
1, 41% for the desired diastereomer, no 1,4-adduct was observed). The yield and chemo-/stereo-selectivity are quite acceptable considering the severe steric hindrance of the ketone motif. Reduction of the carboxylic ester group afforded moderate yields of alcohol 14 (50%) with the corresponding aldehyde by-product (38%), which can be isolated and further reduced to 14 (LiAlH4, 80%, see the ESI†) to improve the overall yield.19,20 Inspired by Takahashi's work, a one-pot global deprotection/oxa-Michael addition then successfully produced the bicyclic motif 15.11l,21 Finally, a two-step oxidation sequence (Dess–Martin oxidation/Pinnick oxidation) produced the desired (−)-phaseic acid at a yield of 89%. The synthetic material, thus obtained, possessed identical spectroscopic and analytical properties to those reported for the natural product and the synthetic enantiomer.6,9,10,22
Conclusions
In summary, we have completed a short, enantioselective synthesis of a newly identified ABA receptor agonist (−)-phaseic acid by an LLS of seven steps. A convenient PA preparation method is certainly to be of great assistance in probing its comprehensive effects as an exogenous agent in plants and mammals.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21402082), the Shenzhen Tech-Innovation Program from Shenzhen Science, Technology and Innovation Commission (no. Pu20150267), and SUSTC Neural & Cognitive Sciences Research Center (no. Y01221001) is greatly appreciated.Notes and references
- S. K. Sah, K. R. Reddy and J. Li, Front. Plant Sci., 2016, 7, 571 CrossRef PubMed.
- K. Vishwakarma, N. Upadhyay, N. Kumar, G. Yadav, J. Singh, R. K. Mishra, V. Kumar, R. Verma, R. G. Upadhyay, M. Pandey and S. Sharma, Front. Plant Sci., 2017, 8, 161 Search PubMed.
- Z. Wang, B. Huang and Q. Xu, J. Am. Soc. Hortic. Sci., 2003, 128, 36 CAS.
- Y. Todoroki and N. Hirai, in Studies in Natural Products Chemistry, ed. Atta-ur-Rahman, 2002, vol. 27, pp. 321–360 Search PubMed.
- X. Han, L. Jiang, C. Che, C. Wan, H. Lu, Y. Xiao, Y. Xu, Z. Chen and Z. Qin, Sci. Rep., 2017, 7, 43863 CrossRef PubMed.
- (a) J. MacMillan, J. C. Seaton and P. J. Suter, Tetrahedron, 1960, 11, 60 CrossRef CAS; (b) J. MacMillan and R. J. Pryce, Chem. Commun., 1968, 124 RSC; (c) B. V. Milborrow, Chem. Comm., 1969, 966 RSC; (d) B. V. Milborrow, Phytochemistry, 1975, 14, 1045 CrossRef CAS; (e) S. Saito, N. Hirai, C. Matsumoto, H. Ohigashi, D. Ohta, K. Sakata and M. Mizutani, Plant Physiol., 2004, 134, 1439 CrossRef CAS PubMed.
- J. K. Weng, M. Ye, B. Li and J. P. Noel, Cell, 2016, 166, 881 CrossRef CAS PubMed.
- (a) S. T. Hou, S. X. Jiang, L. I. Zaharia, X. Han, C. L. Benson, J. Slinn and S. R. Abrams, J. Biol. Chem., 2016, 291, 27007 CrossRef CAS PubMed; (b) J. Xu, M. H. Lacoske and E. A. Theodorakis, Angew. Chem., Int. Ed., 2014, 53, 956 CrossRef CAS PubMed.
- T. Kitahara, K. Touhara, H. Watanabe and K. Mori, Tetrahedron, 1989, 45, 6387 CrossRef CAS.
- G. D. Abrams, S. R. Abrams, L. A. K. Nelson and L. V. Gusta, Tetrahedron, 1990, 46, 5543 CrossRef CAS.
- For the synthesis of ABA see: (a) M. G. Constantino, P. M. Donate and N. Petragnani, J. Org. Chem., 1986, 51, 253 CrossRef CAS; (b) M. Acemoglu, P. Uebelhart, M. Rey and C. H. Eugster, Helv. Chim. Acta, 1988, 71, 931 CrossRef CAS; (c) M. G. Constantino and P. Losco, J. Org. Chem., 1989, 54, 681 CrossRef CAS; (d) M. Soukup, T. Lukai, B. Lohri and E. Widmer, Helv. Chim. Acta, 1989, 72, 361 CrossRef CAS; (e) S. J. Cornforth, J. E. Hawes and R. Mallaby, Aust. J. Chem., 1992, 45, 179 CrossRef; (f) B. Lei, S. R. Abrams, B. Ewan and L. V. Gusta, Phytochemistry, 1994, 37, 289 CrossRef CAS; (g) J. R. Hanson and C. Uyanik, J. Chem. Res., Synop., 2003, 7, 426 CrossRef; (h) T. R. Smith, A. J. Clark, G. J. Clarkson, P. C. Taylor and A. Marsh, Org. Biomol. Chem., 2006, 4, 4186 RSC; (i) C. Liu, R. Wen, W. Xiao, H. Gu and B. Yang, Chin. J. Appl. Chem., 2009, 26, 1297 CAS. For the synthesis of methyl phaseate see: (j) S. Takahashi, T. Oritani and K. Yamashita, Agric. Biol. Chem., 1986, 50, 1589 CAS; (k) S. Takahashi, T. Oritani and K. Yamashita, Agric. Biol. Chem., 1988, 52, 1633 CAS; (l) S. Takahashi, T. Oritani and K. Yamashita, Agric. Biol. Chem., 1989, 53, 2711 CAS; (m) T. Inoue and T. Oritani, Biosci., Biotechnol., Biochem., 2000, 64, 1071 CrossRef CAS PubMed.
- S. Maiti, B. Achari and A. K. Banerjee, Synlett, 1998, 129 CrossRef CAS.
- X. Han, C. Wan, X. Li, H. Li, D. Yang, S. Du, Y. Xiao and Z. Qin, Bioorg. Med. Chem. Lett., 2015, 25, 2438 CrossRef CAS PubMed.
- N. Yin, G. W. Wang, M. X. Qian and E. Negishi, Angew. Chem., Int. Ed., 2006, 45, 2916 CrossRef CAS PubMed . The desired (E,Z)-configuration was determined by the J-coupling constants.
- T. K. M. Shing, Y. Y. Yeung and P. L. Su, Org. Lett., 2006, 8, 3149 CrossRef CAS PubMed.
- L. Trzoss, J. Xu, M. H. Lacoske, W. C. Mobley and E. A. Theodorakis, Org. Lett., 2011, 13, 4554 CrossRef CAS PubMed.
- G. Pandey and V. Janakiram, Chem. – Eur. J., 2015, 21, 13120 CrossRef CAS PubMed.
- C. Spino, M. C. Tremblay and C. Godbout, Org. Lett., 2004, 6, 2801 CrossRef CAS PubMed.
- M. E. Jung and N. Nishimura, Org. Lett., 2001, 3, 2113 CrossRef CAS PubMed.
- D. Demeke and C. J. Forsyth, Org. Lett., 2000, 2, 3177 CrossRef CAS PubMed.
- K. Ishigami, R. Katsuta and H. Watanabe, Tetrahedron, 2006, 62, 2224 CrossRef CAS.
- (a) Y. Todoroki, N. Hirai and H. Ohigashi, Tetrahedron, 2000, 56, 1649 CrossRef CAS; (b) N. Hirai, S. Kondo and H. Ohigashi, Biosci., Biotechnol., Biochem., 2003, 67, 2408 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qo00653e |
| This journal is © the Partner Organisations 2018 |