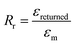Recoverable hydrogel with high stretchability and toughness achieved by low-temperature hydration of Portland cement†
Rui
Liang
ab,
Zongjin
Li
b,
Lu-Tao
Weng
c,
Lina
Zhang
d and
Guoxing
Sun
 *b
*b
aDepartment of Civil and Environmental Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China
bJoint Key Laboratory of the Ministry of Education, Institute of Applied Physics and Materials Engineering, University of Macau, Avenida da Universidade, Taipa, Macau, China. E-mail: gxsun@umac.mo
cDepartment of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China
dSchool of Material Science and Engineering, University of Jinan, No. 336 Nanxinzhuang West Road, Shandong 250022, China
First published on 14th September 2018
Abstract
A novel calcium hydroxide nanospherulite (CNS) strengthened super elastic hydrogel with excellent mechanical properties has been successfully invented and investigated. The CNSs are one major hydration product of cement hydrated at low temperature. They are incorporated into the hydrogel polymer network through the Ca2+ ions diffusing from the cement particles into the hydrogel matrix first and then forming calcium hydroxide nanospherulites with diameters <4 nm uniformly in the matrix. This develops an innovative method to form such nanometer-sized calcium hydroxide. The uniformly distributed 4 nm-sized spherulites formed by such a method can act as a crosslinker and hence enhance the properties of the hydrogel remarkably. By incorporating about 40 ppm of 4 nm-sized calcium hydroxide spherulites, the modified hydrogel sample can be stretched to 112 times of its initial length without breaking and withstand a maximum stress of 400 kPa. The strain recovery rate Rr, which is defined as the ratio of recovered strain to the maximum strain, increases from 18.0% for the original hydrogel to 96.7% for the hydrogel with incorporation of around 200 ppm 4 nm-sized calcium hydroxide spherulites. This research contributes to the field with a unique formulation method of uniformly distributed 4 nm or smaller nanoparticles acting as crosslinkers of a hydrogel, achieving enhancement of the excellent overall mechanical properties for the hydrogel. It opens a new direction for nanoparticle strengthened material development by controlling the nanoparticle size from hydrolyzing the matrix.
Hydrogels are crosslinked polymer networks that contain a large amount of water inside.1 Although hydrogels have great potential applications in biomedical and tissue engineering, the current use of hydrogels has been severely limited by several shortcomings such as small stretchability, low mechanical strength and unsatisfactory recoverability.2–4 The demand for robust hydrogels in tissue engineering,5 cell supports,6 drug delivery,7 and industrial applications8 has motivated intense research on the mechanical enhancement of these wet polymeric materials. Consequently, various polymeric hydrogels with satisfactory mechanical strength, such as macromolecular microsphere composite gels,9 double-network gels (DN gels),10–12 and sliding-ring gels (SR gels),13,14 have been developed. Meanwhile, nanocomposite hydrogels (NC gels) were also investigated for their facile synthesis, superior mechanical strength, sensitive stimuli and outstanding reversible properties.3,4,15–18 Haraguchi and Takehisa first prepared an NC gel by an in situ free radical polymerization method of monomers.3 Later, reinforced polymeric hydrogels were developed with the combination of a polymeric network and various nanoparticles, such as inorganic/ceramic nanoparticles (hectorite, montmorillonite, layered double hydroxide),4,19–23 metal–oxide nanoparticles (titanate(IV) nanosheet),16 and carbon-based nanomaterials (graphene oxide).18,24–27 However, in these works, as the nanoparticles were added as an inner filler without strong chemical bonding, the enhancing effects on the hydrogel's properties were rather limited. Moreover, nanoscale particles usually aggregate, and the aggregation becomes more serious with the decrease of particle size, which negates any benefits associated with the nanoscopic dimension.28 There is a critical need to invent a novel processing method that can effectively generate nanoscale particles with uniform distribution in a polymer matrix and develop new hydrogels with both excellent mechanical properties and potential for large-scale production.
In our previous work, we used tricalcium silicate to fabricate calcium hydroxide (Ca(OH)2) nano-spherulites (CNS) with diameters <5 nm, which acted as the crosslinker in polyacrylamide (PAM) hydrogel.29 Due to the elimination of aggregation by downsized CNS nanoparticles, a super stretchable and high-toughness hydrogel network at very low inorganic content was achieved. As an extension of previous work, we were seeking novel approaches that would allow NC gels to be engineered with desirable properties and mass produced at low cost. Here we used a common construction material, Portland cement, as the source to generate CNSs. And ‘ce-CNSs’ is used to represent the cement-generated-CNSs. We focused on the dispersion of cement particles, the diffusion of Ca2+ ions from cement particles and formation of calcium hydroxide through chemical reaction between Ca2+ and H2O in a hydrogel matrix to ensure the size and uniform dispersion of ce-CNSs. The stretchability, mechanical strength and recoverability of polymeric hydrogels could be greatly enhanced by the nanometer-sized uniformly distributed ce-CNSs acting as the crosslinks in the polymer matrix. In the following description, we use ‘PAM/cement hydrogel’ to represent the PAM hydrogel enhanced by cement-generated-CNSs. We believe that it could offer innovative insights into the design of robust polymeric hydrogels for industrial applications.
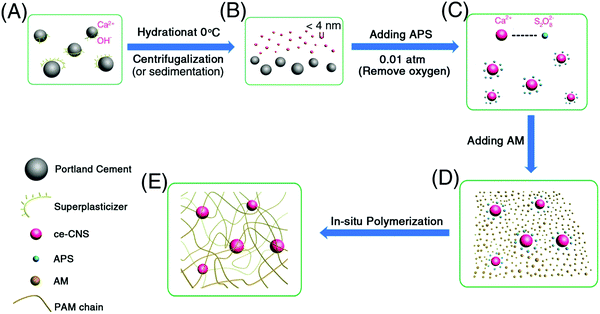
In our experiment, we prepared PAM/cement hydrogel via in situ Ca2+ diffusion, calcium hydroxide formation and polymerization of acrylamide (AM) in a diluted calcium hydroxide suspension (Fig. 1). In the first step, cement particles were dispersed in a polycarboxylate–ether based superplasticizer (PCE) solution (Fig. 1A). The environmental temperature of the solution was strictly kept at 0 °C to control the Ca2+ ion diffusion rate. Consequently, the size of calcium hydroxide could be controlled. After sufficient Ca2+ ions diffused, the remaining cement particles were removed by sedimentation or filtration (Fig. 1B). Next, PAM/cement hydrogels were synthesized by in situ radical polymerization of AM initiated by ammonium persulfate (APS) on the surface of ce-CNSs at 0.01 atm (Fig. 1C–E), following the mechanism discussed in our previous work.29 After polymerization, none of the hydrogel specimens were dissolved after being kept in water for a long time or sonicated, implying the successful formation of a network structure by PAM and ce-CNSs.
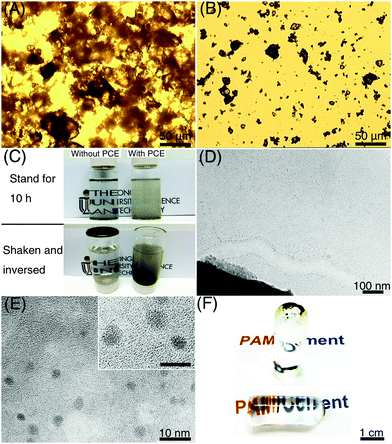
Compared with tricalcium silicate (Ca3SiO5) particles with size <500 nm (Fig. S1, ESI†), which were used to produce CNSs in our previous work, the dispersibility of large Portland cement particles (size 10–20 μm) is much worse. Cement particles could easily aggregate in water, and form clusters with size larger than 100 μm, which greatly decreases the activated surface area for the generation of ce-CNSs (Fig. 2A). The aggregation becomes more serious with the increase of hydration time. As shown in Fig. 2C, cement particles were totally precipitated after 10 h in diluted suspension with a cement concentration of 0.1 wt%. The sediment is a solid hydrated cement block, which stably adheres to the container even after shaking and inverting. In this case, sufficient ce-CNSs are difficult to achieve due to the fast sedimentation and limited surface area of cement particles exposed to water. To ensure the uniform distribution of the diffused Ca2+ ions and formation of ce-CNSs, we used Polycarboxylate–ether (PCE) as a dispersant in the cement suspension. PCE is a comb-like copolymer with a sodium polymethacrylate (PMA) backbone partially esterified with polyethyleneglycol (PEG) chains. It has been proven that the adsorption of PCE on cement particles imposes a strong static electrical field to enforce the separation of cement particles.30 With the adsorption of PCE, cement particles can be separated and dispersed uniformly (Fig. 2B). The cement suspension containing PCE remains stable after standing for 10 h, and the solid hydrated cement block does not form when the container is shaken and inverted (Fig. 2C).
Due to the improved dispersibility of Portland cement achieved by PCE, ce-CNSs can be formed intensively surrounding the edge of a cement particle (Fig. 2D). The dispersion of individual ce-CNSs is very homogeneous in the entire suspension, disregarding the distance from the cement particles (Fig. S2, ESI†). The aqueous medium with a concentration of 0.1 wt% cement suspension has counterions (determined by dynamic light scattering testing), which ensure efficient dispersion of ce-CNSs in cement suspensions with concentrations of 0.02–0.1 wt% to avoid aggregation. Inductively coupled plasma optical emission spectrometry (ICP-OES) test shows that the weight of ce-CNSs is about 20 wt% of the Portland cement matrix, which means 40–200 ppm ce-CNSs can be generated from a 0.02–0.1 wt% cement suspension. Because of the upper limit of calcium cation (Ca2+) concentration in the cement suspension, the maximum concentration of ce-CNSs is 200 ppm. An enlarged high resolution transmission electron microscopy (TEM) image shows that the diameter of ce-CNSs is 2–4 nm, even smaller than the CNSs generated from tricalcium silicate suspension (Fig. 2E) as reported in our previous work.29 The significant difference in size between the ce-CNSs (2–4 nm) and cement matrix (10–20 μm) proves that the concept of using ce-CNSs to strengthen hydrogels is feasible. As described in Fig. 1B, the remaining cement precipitates are removed by centrifugation or sedimentation, while the homogeneous ce-CNS suspension stays in the hydrogel matrix. In the case that the remaining cement precipitates are not removed, the transparency of the main body of the PAM/cement hydrogel can remain the same as that of the original PAM hydrogel (Fig. 2F), due to such a small amount ce-CNSs being incorporated.
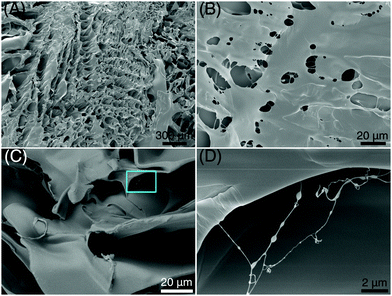
Homogeneously dispersed ce-CNSs with size <4 nm form cross links with PAM chains via electrostatic interaction, as proved in our previous work.29 A regular honeycomb structure is observed from a freeze-dried xerogel of a thoroughly swelled PAM/cement specimen using scanning electron microscopy (SEM) (Fig. 3A). A lot of filamentous structures are also spotted with diameters around 50 nm, crosslinked inside the pores of flat-on layers (Fig. 3B) and gaps among edge-on layers (Fig. 3C) of the honeycomb. Unique bumps with diameters of 100–500 nm locate linearly on the filaments, with a distance of 500 nm to 2 μm from each other (Fig. 3D). It seems that the internal structure of the xerogel is very uniform at different scales. Therefore, we can infer that when being connected with polymer chains in a PAM matrix, ce-CNSs retain a similar dispersibility as in aqueous media. The homogeneous distribution of crosslinked polymer chains helps make the overall network structure strong and well-regulated, which improves the mechanical strength, deformability, and recoverability of the PAM/cement hydrogel.
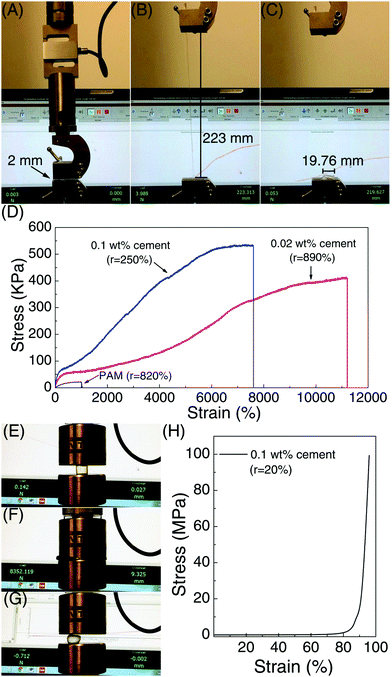
The stretchability and shape recoverability of PAM/cement hydrogel have been evaluated by a uniaxial tension test (Fig. 4A–D). As shown in Fig. 4A, the transparent part of the hydrogel with an initial length of 2 mm is clamped in a MTS machine for uniaxial tension. As expected, the specimen containing 40 ppm ce-CNS can be easily stretched to 223 mm, which is about 112 times the initial length (Fig. 4B). The elongation at break is much higher than that for the most highly reported stretchable hydrogels, accompanied by a noteworthy tensile strength of 400 kPa. Compared with the original PAM hydrogel with an elongation toughness (which is defined as the area under the stress-deformation curve) of 0.17 MJ m−3, the elongation toughness of the PAM/cement hydrogel strengthened by 40 and 200 ppm of ce-CNSs are increased to 25.2 and 25.8 MJ m−3, respectively (Fig. 4D). One important index to be used for describing the shape recovery properties of the hydrogel at the maximum strain εm is the strain recovery rate Rr, which is the ratio of recovered strain to the maximum strain and quantifies the ability of the material to recover to its permanent shape.31Rr can be calculated using the following equation:
The shape recovery of the PAM/cement hydrogel is also impressive in a compressive test, as shown in Fig. 4E–H. Because of the strong elastic network crosslinked by ce-CNSs, the PAM/cement hydrogel containing 200 ppm of ce-CNSs did not fail under a compressive strain of 95% with 100 MPa pressure (Fig. 4E, F and H). After the force was released, it was surprising that the specimen almost reverted to its original shape in 1 second (Fig. 4G). Relevant Rr calculated was 78.9%, indicating a strong shape recovery efficiency during compressing. Compared with the original PAM hydrogel with Rr less than 15% (under 95% compressive strain), the PAM/cement hydrogel shows much stronger recoverability under large compressive deformation.
In summary, we have developed a unique method to produce a uniformly distributed nanoscale hydration product of Portland cement, ce-CNSs, and used them as a crosslinker to fabricate a highly stretchable, tough, transparent and shape-memorable PAM/cement hydrogel. The formation of ce-CNSs from the cement suspension is achieved by using PCE as the dispersant. Also, controlling the temperature and pressure of the environment is essential in order to ensure that calcium ions diffuse from the cement particles and form calcium hydroxide. Compared with the CNSs formed from tricalcium silicate suspension,29 the ce-CNSs have similar concentration, size, and enhancement effect on the polymer network, even though the cement particles for ce-CNS formation are 2 manganite bigger than tricalcium silicate. This demonstrates that the CNS generation method is effective regardless of the particle size of the CNS generator. Comparison of the elongation properties between PAM/cement hydrogel and some reported NC gels with various crosslinker nanoparticles is shown in Fig. 5. Due to the much smaller size and uniform distribution of ce-CNSs, the PAM/cement hydrogel shows superior properties to other nanoparticle enhanced hydrogels. The additive amount is the lowest among the reported NC gels, while the stretchability is almost the highest with a satisfactory elongation stress. Normally, an overall mechanical enhancement is required to expand the application of hydrogels. However, the elongation strain of the reported tough hydrogels is below 2000%, while highly stretchable hydrogels are usually accompanied by low stress and recoverability.29 This newly developed PAM/cement hydrogel can achieve high deformability, stress, and shape recovery at the same time. Moreover, the low-content additive, Portland cement, is a commonly used construction material with very low cost. Therefore, this work may help to create new application fields of hydrogels, and possibly transfer the expensive and brittle bio-materials to cheap engineering materials with excellent mechanical properties.
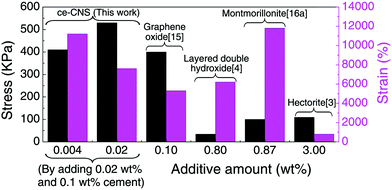 | ||
| Fig. 5 Comparison of elongation stress and strain for the synthetic elastic NC gels with different additives. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the China Ministry of Science and Technology under grant (2015CB655104) managed by HKUST Shenzhen Research Institute Science and Technology; Science and Technology Development Fund from Macau (FDCT-078/2017/A2); Research & Development Grant for Chair Professor (CPG2017-00029-FST), and a Start-up Research Grant (SRG2017-00093-IAPME) from University of Macau.References
- H. Kamata, Y. Akagi, Y. Kayasuga-Kariya, U. Chung and T. Sakai, Science, 2014, 343, 873 CrossRef PubMed.
- J. Y. Sun, et al. , Nature, 2012, 489, 133 CrossRef PubMed.
- K. Haraguchi and T. Takehisa, Adv. Mater., 2002, 14, 1120 CrossRef.
- Z. Q. Hu and G. M. Chen, Adv. Mater., 2014, 26, 5950 CrossRef PubMed.
- E. Alsberg, K. W. Anderson, A. Albeiruti, R. T. Franceschi and D. J. Mooney, J. Dent. Res., 2001, 80, 2025 CrossRef PubMed.
- G. D. Nicodemus and S. J. Bryant, Tissue Eng., Part B, 2008, 14, 149 CrossRef PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2002, 54, 3 CrossRef PubMed.
- N. Dehbari and Y. H. Tang, J. Appl. Polym. Sci., 2015, 132, 46 CrossRef.
- T. Huang, et al. , Adv. Mater., 2007, 19, 1622 CrossRef.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155 CrossRef.
- W. Yang, H. Furukawa and J. P. Gong, Adv. Mater., 2008, 20, 4499 CrossRef.
- J. P. Gong, Soft Matter, 2010, 6, 2583 RSC.
- Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485 CrossRef.
- K. Ito, Curr. Opin. Solid State Mater. Sci., 2010, 14, 28 CrossRef.
- Q. Wang, et al. , Nature, 2010, 463, 339 CrossRef PubMed.
- M. J. Liu, et al. , Nature, 2015, 517, 68 CrossRef PubMed.
- L.-W. Xia, et al. , Nat. Commun., 2013, 4, 2226 CrossRef PubMed.
- J. Q. Liu, et al. , ACS Nano, 2012, 6, 8194 CrossRef PubMed.
- G. Gao, G. Du, Y. Sun and J. Fu, ACS Appl. Mater. Interfaces, 2015, 7, 5029 CrossRef PubMed.
- K. Haraguchi and H. J. Li, Angew. Chem., Int. Ed., 2005, 44, 6500 CrossRef PubMed.
- K. Haraguchi, M. Ebato and T. Takehisa, Adv. Mater., 2006, 18, 2250 CrossRef.
- K. Haraguchi, H. J. Li, K. Matsuda, T. Takehisa and E. Elliott, Macromolecules, 2005, 38, 3482 CrossRef.
- K. Haraguchi and H. J. Li, Macromolecules, 2006, 39, 1898 CrossRef.
- R. Q. Liu, et al. , J. Mater. Chem., 2012, 22, 14160 RSC.
- N. N. Zhang, et al. , Soft Matter, 2011, 7, 7231 RSC.
- J. C. Fan, Z. X. Shi, M. Lian, H. Li and J. Yin, J. Mater. Chem. A, 2013, 1, 7433 RSC.
- J. Q. Liu, G. S. Song, C. C. He and H. L. Wang, Macromol. Rapid Commun., 2013, 34, 1002 CrossRef PubMed.
- A. C. Balazs, T. Emrick and T. P. Russell, Science, 2006, 314, 1107 CrossRef PubMed.
- G. Sun, Z. Li, R. Liang, L.-T. Weng and L. Zhang, Nat. Commun., 2016, 7, 12095 CrossRef PubMed.
- G. X. Sun, et al. , RSC Adv., 2014, 4, 25479 RSC.
- A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods. See DOI: 10.1039/c8qm00391b |
| This journal is © the Partner Organisations 2018 |