Bio-based oil gelling agent for effective removal of oil spills from the surface of water†
Peng
Lv
ab,
Sudong
Yang
a and
Peng-Cheng
Ma
 *a
*a
aLaboratory of Environmental Science and Technology, The Xinjiang Technical Institute of Physics and Chemistry, Key Laboratory of Functional Materials and Devices for Special Environments, Chinese Academy of Sciences, Urumqi, 830011, China. E-mail: mapc@ms.xjb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 22nd May 2018
Abstract
Developing novel materials for oil–water separation is highly desirable to solve the problems caused by oil spills. Herein, a wheat-bran-based hybrid material was prepared using a hydrothermal process, and the feasibility of utilizing the hybrid as an oil gelling agent (OGA) was studied. The results showed that the obtained material exhibited a mild hydrophobicity with a water contact angle above 130°, and an oleophilicity with high adsorption capacity for various oils and organic solvents. Furthermore, the material continuously adsorbed and gelled the oil floating on the water surface, forming an oil–OGA aggregate when in contact with an oil–water mixture. The aggregation-induced gelation observed in the material makes it possible to conveniently remove oil spills from the surface of water. The low cost and environmentally benign process distinguishes the developed material as a candidate that is suitable for the clean-up of oil spills.
Introduction
Petroleum, which is known as an “industrial blood”, plays an important role for the continuous development of the economy. However, oil leakage accidents occur during the exploitation and transportation of petroleum every year, and the world has witnessed increasing environmental pollution and ecological problems caused by oil spills and chemical leakage.1–3 Catastrophes caused by these accidents, such as the oil spill in the Gulf of Mexico, have highlighted the importance and urgency of developing new methods and materials for quickly cleaning up oil spills.3 The most utilized approaches for oil spill remediation include in situ burning,4 mechanical recovery by oil skimming,5 dispersant-aided diffusion,6 and physical separation.7–11 Among the existing methods, physical adsorption of oil by porous materials is a simple and efficient technique. Different adsorbents, such as polymer sponge,9 carbon-based aerogel,10 and bio-based sponge,11 have been developed over the years for the clean-up of oil spills with varying degrees of success. Easy applicability and excellent selectivity for the oil–water mixture are two challenges when designing a suitable porous material for such purposes. In addition, there is a concern that the adsorption method is ineffective for oily liquids with a high viscosity, such as crude oil whose viscosity is generally above 100 mPa s at room temperature.As an emerging idea, phase-selective gelation has been adopted in recent years for the recovery of spilled oil.12–14 The principle of this technique is that by mixing with an oil gelling agent (OGA), oil spills can be selectively congealed into floating gels in the presence of water, thus preventing an oil film from spreading and providing convenience for easy collection and reclamation of gelled oil. Based on the physical state and chemical composition of material, there are two categories of OGAs that have been reported in the literature: liquid materials and solid materials.12 For a liquid OGA, an oil-soluble solvent was used to dissolve the OGA to achieve its uniform distribution in an oil–water mixture for oil gelation.15 This type of OGA necessitates the use of toxic or hot carrier solvents or a large amount of carrier solvents, causing problems for large-scale logistical and technical deployment.16 Solid OGAs originated from natural compounds can be used in a powdered form to directly gel oil.17–19 However, because there is poor diffusion of these OGAs through the liquid, the speed at which these materials act to gel oil is slow. To solve this problem, Raju and co-workers prepared a series of bio-based gelators (amino acid and xylitol derivatives) and applied a conventional heating process to speed the gelation for practical applications.20,21 Cholesterol compounds developed by Fang had the capability to spontaneously gel a variety of organic solvents without heating or addition of a co-solvent.22,23 The multiple steps required for the synthesis and rarity of the starting materials were two major obstacles for the large-scale production of this material. Therefore, the development of new OGAs with characteristics such as excellent gelling capability, fast diffusion/adsorption of oil, and easy process for production is highly desirable.
The current study is a part of a larger project in developing materials with hierarchical structures for environmental and engineering applications. In this work, a powdered OGA was prepared using biodegradable wheat bran as a raw material. The morphology and surface properties of materials were investigated, and the ability of this OGA to gel with and adsorb various organic liquids and oils was evaluated.
Experimental
Materials
Raw wheat bran (Raw-WB) was used as a starting material to prepare the OGA and was obtained from a local flourmill. Vinyltriethoxysilane (VTES) was employed as a modifying agent and was purchased from Guangzhou Kailvwei Chemical Co., Ltd (China). Silicon dioxide (SiO2) particles were obtained from Aladdin Reagent Co., Ltd (China). Sodium hydroxide (NaOH), hydrogen peroxide (H2O2), ethanol, and hydrochloric acid were purchased from Tianjin Baishi Chemical Co., Ltd (China). Commercially available solvents/oil products (diesel, gasoline, motor oil, octane, petroleum ether, toluene) and crude oil (Karamay Oilfield, China) were used as probing liquids to study the oil gelling performance of materials. All chemicals were analytical grade and used as received without further purification.Preparation of the OGA
Raw-WB was initially purified to remove starch and soluble compounds. During the experiment, a powdered sample (40.0 g) was put into a beaker containing NaOH solution (2.0 g L−1, 1.0 L). Then, H2O2 (2.0 mL, 30%) was added dropwise into the beaker. This was followed by increasing the temperature of the mixture to 95 °C and magnetically stirring for 1 h. After cooling to ambient temperature, the pH of the mixture was adjusted to 7.0 by adding hydrochloric acid. The purified sample (Purified-WB) was separated from the solution using vacuum filtration and then dried in an oven at 80 °C for 1 h.For the preparation of the OGA, Purified-WB (3.0 g), SiO2 particles (0.10 g), VTES (3.0 mL), and NaOH solution (0.1 M, 3.0 mL) were added to ethanol (40.0 mL). The resultant mixture was ultrasonically dispersed for 0.5 h at room temperature, and then added to a Teflon-lined stainless steel autoclave and processed in an oven for 1.5 h at 105 °C. The modified sample (Modified-WB), i.e., the OGA, was obtained by filtering the yield and subsequently drying at 80 °C for 2 h. The OGA was in a powder state with particle size in millimetres.
Characterization
Various techniques were employed to study the morphology and properties of bran samples before and after modification. Scanning electron microscopy (SEM, Phenom XL) and optical microscopy (Nikon, LV100D) were used to observe the morphology and structure of materials. Functional groups on the surface of the material were determined using a Fourier transform-infrared spectrometer (FT-IR, J28180-6/7F, Thermo Electron). The thermal stability of the sample was studied using thermogravimetric analysis (TGA, STA 449F3, Netzsch) with a heating rate of 10 °C min−1 from 20 to 1000 °C in air flow. The static contact angle of the material against water was measured on a goniometer (XG-CAMA, Shanghai Xuanyichuangxi) at ambient temperature.The adsorption capacity and oil gelling performance of the raw and OGA materials were studied and compared as well. For the adsorption experiment, the prepared sample was added to a specific oily liquid and immersed for 10 min to reach adsorption equilibrium. The weight gain of the sample was calculated using the weight change of the material before and after immersion. The oil gelling performance of the OGA was evaluated by measuring the viscosity of the oil–OGA mixture. During the measurement, the OGA with different weight ratios to oil was placed into the oil liquid and vigorously mixed. The viscosity of the mixture was then measured on a rotating viscometer (NDJ-8S, Shanghai Changji, China). The organic solvent adsorbed in the oil–OGA mixture was separated using a rotary evaporator under a negative pressure of 0.05 MPa at 60 °C, yielding a recycled OGA. Its recyclability was evaluated by measuring the capacity of the material to adsorb octane under the cyclic runs. All experiments were conducted three times, and an average value with a standard deviation is reported.
Results and discussion
Morphology and property of bran samples
When wheat is grown, it is composed of three distinct parts, which are known as the bran, endosperm, and germ.24 The bran is a coating layer for a wheat grain, providing the seed with protection from parasites and weather conditions. Fig. 1 shows the varying morphologies of bran samples. It is clear to see that there are two distinct sides for Raw-WB (A–C in Fig. 1), i.e., the yellow side and the white side, representing the outer pericarp layer and the inner layer that is in contact with the endosperm in the grain, respectively. The fine microstructures of these two sides at different processing steps were characterized by SEM. The outer layer of the Raw-WB was smooth, and many flour particles were observed (Fig. 1D). These particles were completely removed during the purification of WB as verified by a clean surface in the Purified-WB sample (Fig. 1E), and a close examination confirmed the existence of river-like markings on the sample surface. Similar results have also been found in chemically modified cotton fibers, where the non-cellulosic compounds such as pectin, protein, and wax on natural material were removed upon hot alkali treatment.25 In the Modified-WB (Fig. 1F), the outer layer of bran became rough and was covered by a thin layer, which originated from the polysiloxane produced during the hydrolyzation of silane.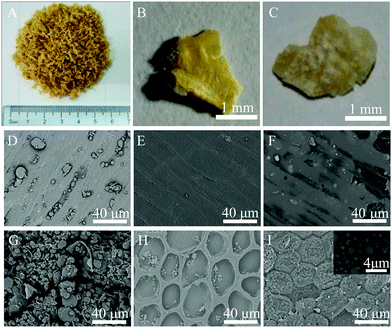 | ||
| Fig. 1 Morphologies of bran at the macro- and micro-scale ((A–C) digital images of Raw-WB; (D–F) outer layer of bran samples; (G–I) inner layer of bran with and without treatment). | ||
In sharp contrast, the inner layer of the corresponding bran showed significant differences in the morphologies. For example, this layer for Raw-WB had many spherical and granular particles with different sizes and irregular shapes (Fig. 1G). The particles may be protein and starch26 and were mostly removed from the surface of the bran during the purification process, leaving a honeycomb-like structure in the Purified-WB sample (Fig. 1H). This structure provides additional benefit for the material, as it can provide space for anchoring flour particles in the material, as less particles were found in the outer layer of bran (Fig. 1D). This assumption was also confirmed by observing the same layer of the Modified-WB, as the honeycomb structures were filled with spherical SiO2 particles, and the surface of the sample became rough due to the deposition of these insoluble particles (Fig. 1I). The diameter of the particles ranged from a few hundred nanometers to up to 2 μm (inset in Fig. 1I), which was expected to increase the contact area and interfacial interactions with oil, and further discussion will be made regarding this in the following section.
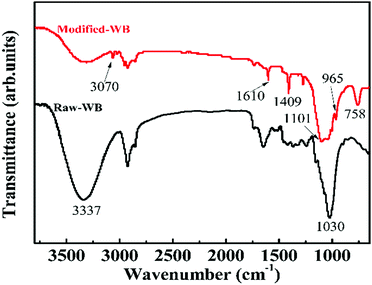
Infrared spectrometry was conducted to evaluate the surface information of the sample. The FT-IR spectra of Raw-WB and Modified-WB over the range of 600–3800 cm−1 are presented in Fig. 2. In the high frequency region, the band located at 3337 cm−1 in both spectra can be assigned to the hydroxyl (–OH) groups, and the intensity of this group was obviously decreased in the Modified-WB, suggesting a reaction between the –OH moieties in WB and the ethoxy (–OCH2CH3) groups in silane.27 The new peaks at 3070 cm−1 and 1409 cm−1 were matched well with stretching vibrations and in-plane deformations of vinyl (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) groups,28 and a characteristic peak at 758 cm−1 can be attributed to the Si–C vibration.29 All these data confirmed the existence of silane in the Modified-WB. Moreover, a peak at 1101 cm−1 was also observed in the modified sample, which can be attributed to the Si–O–Si stretching vibration.30 This bond came either from SiO2 particles or the condensation of silane. Based on these observations, a possible mechanism on the modification process was proposed: as a natural polymer, WB contains numerous –OH groups on its surface, and these groups are available and immediately react with –O–C2H5 groups in silane via hydrolyzation, resulting in the formation of –Si–O–Si– bonds terminated with vinyl groups. Consequently, a thin layer of polysiloxane was formed and attached onto the bran sample. The –OH groups in the SiO2 particles can also react with silane to form short oligomer chains, leading to the linkage of these particles and deposition on the surface of bran, which was in good agreement with the SEM observations in Fig. 1. It should be mentioned that the layer structure of the bran remained during these chemical reactions, as a peak at 1030 cm−1 ascribed to the stretching vibration of C–O–C in cellulose31 was observed in both Raw-WB and Modified-WB samples. In addition, a peak at 1610 cm−1 can be seen in both samples. For Raw-WB, the peak may result from asymmetric stretching vibrations of –C
CH2) groups,28 and a characteristic peak at 758 cm−1 can be attributed to the Si–C vibration.29 All these data confirmed the existence of silane in the Modified-WB. Moreover, a peak at 1101 cm−1 was also observed in the modified sample, which can be attributed to the Si–O–Si stretching vibration.30 This bond came either from SiO2 particles or the condensation of silane. Based on these observations, a possible mechanism on the modification process was proposed: as a natural polymer, WB contains numerous –OH groups on its surface, and these groups are available and immediately react with –O–C2H5 groups in silane via hydrolyzation, resulting in the formation of –Si–O–Si– bonds terminated with vinyl groups. Consequently, a thin layer of polysiloxane was formed and attached onto the bran sample. The –OH groups in the SiO2 particles can also react with silane to form short oligomer chains, leading to the linkage of these particles and deposition on the surface of bran, which was in good agreement with the SEM observations in Fig. 1. It should be mentioned that the layer structure of the bran remained during these chemical reactions, as a peak at 1030 cm−1 ascribed to the stretching vibration of C–O–C in cellulose31 was observed in both Raw-WB and Modified-WB samples. In addition, a peak at 1610 cm−1 can be seen in both samples. For Raw-WB, the peak may result from asymmetric stretching vibrations of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O that existed in protein, and for the Modified-WB, this band originated from the –C
O that existed in protein, and for the Modified-WB, this band originated from the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– vibration in vinyl groups32 because the protein was removed during the pre-treatment.
C– vibration in vinyl groups32 because the protein was removed during the pre-treatment.
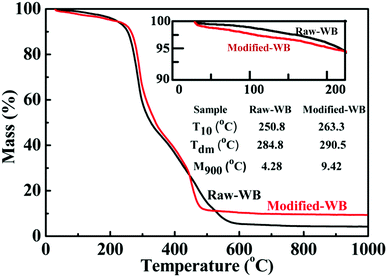
The introduction of silane onto the bran was expected to rectify the thermal properties of the material. Fig. 3 shows the TGA results of Raw-WB and Modified-WB. The mass loss below 200 °C was attributed to the removal of volatile materials (water and hydrocarbon compounds) and hydroxyl groups in the WB. From the enlarged view of the TGA curves below 200 °C (inset in Fig. 3), it can be seen that the mass losses for Raw-WB and Modified-WB at 200 °C were 3.9% and 4.8%, respectively. The slightly higher weight loss in the Modified-WB was possibly due to the removal of the organic backbones in silane. The temperature at 10% loss (T10) and the maximum decomposition rate obtained from derivative TG (Tdm) are listed in the inset table. The T10 of the sample increased from 250.8 to 263.3 °C with a simultaneous increase in Tdm from 284.8 to 290.5 °C, indicating that silane modification is an effective method that can be used to improve the thermal stability of samples in air. These results were attributed to the physical barrier effect, where the hydrolyzed silane impeded the propagation of decomposition reactions in the bran sample. Additionally, the residual yields of Raw-WB and Modified-WB at 900 °C (M900) were 4.28% and 9.42%, respectively. The former yield resulted from the oxides of minerals (Fe, Zn, Mn, Mg, etc.) in WB,33 and the latter, in addition to the minerals, originated from the presence of polysiloxane and SiO2 particles in the Modified-WB. Because the TGA was performed in an air flow, it can be estimated that the increase (5.14%) existed in the form of SiO2. Silicon accounts for 46.7% weight in SiO2, and therefore, it can be deduced that the weight percentage of elemental Si (arising from silane and SiO2) in the Modified-WB is approximately 2.40%.
Oil gelling performance of bran samples
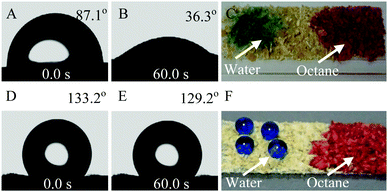
Phase-selective gelling ability can be obtained by adjusting the balance between the hydrophilic and oleophilic groups in an amphipathic material,34 and the wettability of material with water is a parameter that can be used to evaluate the capability of a material to cause oil gelation. Therefore, the contact angle of bran samples against water was measured. As shown in Fig. 4, a water droplet can stand on the surface of Raw-WB with a contact angle of 87.1° (Fig. 4A), which decreased to 36.3° within a minute (Fig. 4B), suggesting the penetration of water inside the material. Comparative results showed that Raw-WB can adsorb water and oil (octane) without any selectivity (Fig. 4C). In contrast, Modified-WB displayed a hydrophobic surface with a stable water contact angle of approximately 130° (D and E in Fig. 4). This property was attributed to the synergistic effect arising from the vinyl functional groups as well as enhanced roughness due to the presence of SiO2 particles in the material. As a result, droplets of octane (dyed with Oil Red O) were instantly adsorbed into the Modified-WB, while water droplets (dyed with brilliant blue) presented spherical shapes on the sample surface (Fig. 4F).To evaluate the oil-gelling capability of Modified-WB as an OGA (Fig. 5A), a demonstration experiment was conducted using octane as a probing liquid. In the experiment, water (480 mL) and octane (4 mL, dyed with Oil Red O for easy observation) were poured into a beaker and then slightly agitated to obtain an oil film on a water surface (Fig. 5B). When the OGA (1.4 g) was manually sprinkled on the liquid mixture, the red oil floating on the surface of the water was gathered within 30 seconds, and was continuously adsorbed and quickly aggregated by the OGA (Fig. 5C, Supporting Movie 1, ESI†), suggesting the excellent gelling performance of the developed material. The obtained gel possesses high viscosity, and can be easily separated from water by using a net spoon (Fig. 5D).
The OGA also exhibited a reasonable recyclability for practical applications. Fig. 6 shows the oil gelling performance of the OGA under cyclic operations. The material exhibited a stable adsorption capacity for octane during the cyclic adsorption/evaporation process (Fig. 6A), and retained more than 90% of its original gelling capacity after 10 runs (Fig. 6B).
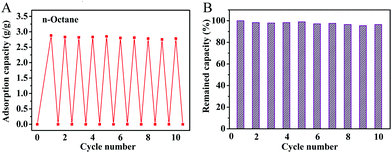 | ||
| Fig. 6 Recyclability of OGA for oil gelation ((A) oil gelling recyclability of OGA over ten cycles; (B) remaining oil gelling capacity of the sample). | ||
The mechanism behind such an interesting observation is schematically described in Fig. 7. During the pretreatment of raw materials, the starch and soluble compounds were removed (Fig. 7A), and thus, the hydroxyl groups (–OH) on the surface of the WB were exposed. These moieties had the capability to readily react with ethoxy groups (–O–C2H5) of VTES via the silanization reaction (reaction III in Fig. 7A). This process along with the self-polymerization of silane (reaction I in Fig. 7A) generated polysiloxane on the WB surface, resulting in the formation of a hydrophobic surface for the material. In addition, there were –OH groups on the surface of the SiO2 particles, and this offered an opportunity for these particles to be covalently bonded onto the WB with the assistance of VTES (reaction II in Fig. 7A). Consequently, the developed OGA contained lipophilic parts, such as hydrophobic vinyl groups from organic silane, resulting in the adsorption of oil droplets inside the material. However, the hydrophilic component in the material, such as the unreacted –OH on the surface of the WB and SiO2, provided a possibility for the formation of hydrogen bonds among the OGA particles as well as with water molecules (Fig. 7B), thus preventing the scattering of OGA particles with adsorbed oil on the surface of the water. The synergy of the above two aspects resulted in excellent oil-gelling performance of the material, i.e., aggregation-induced gelation (AIG), in macro-scale.
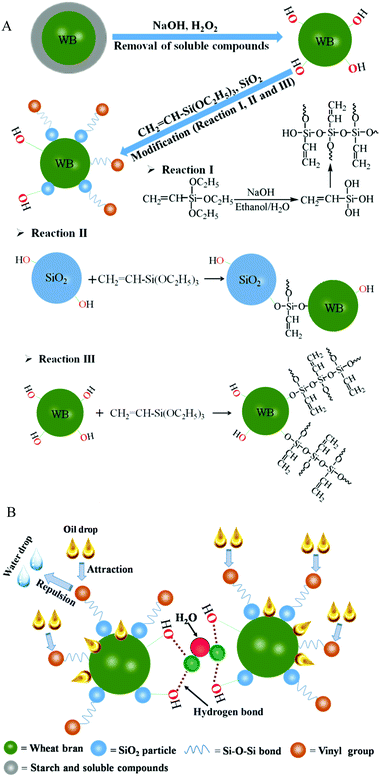 | ||
| Fig. 7 Schematic showing (A) the chemical reactions taking place on the WB surface and (B) the gelling mechanism of the OGA for oil–water separation. | ||
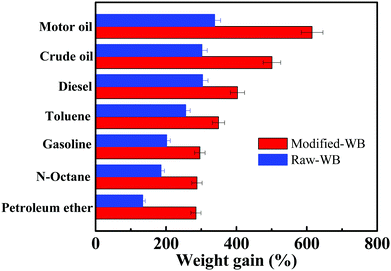
It should be mentioned here that the affinity of the OGA to oil is a key factor in determining the gelling performance of the material. The adsorption capacity of the bran samples before and after modification was measured and compared (Fig. 8). The results showed that for a specific oil, the weight gain of Modified-WB was always higher than its counterpart. For example, Modified-WB can adsorb motor oil with a capacity of 6.15 ± 0.12 g g−1, nearly 80% higher than that of Raw-WB (3.38 ± 0.08 g g−1). The reasons for this were three-fold: (i) Modified-WB possesses not only a lower apparent density (0.10 g cm−3) than that of R-WB (0.24 g cm−3, the density of the sample was simply calculated by measuring the weight of the sample filled up in a graduated cylinder with a maximum capacity of 50 mL), but also a higher degree of pores in the structure, and therefore, a certain quantity of the former provides more unoccupied volume for the adsorption of oily liquid; (ii) the SiO2 nanoparticles modified by the silane on the surface of the bran enhanced the hydrophobicity and roughness of the material, thus offering increased affinity for the organic molecules, and (iii) the capillary effect is considered to be the driving force for the penetration of oil into a porous material, and this process was strengthened because of the increased oleophilicity of the interconnected pores in the Modified-WB.
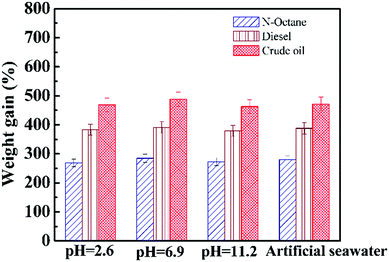
The versatility of the OGA for removing oil on the surface of water was further demonstrated by studying the adsorption capacity of material under different conditions. The oil uptake was measured in solutions with various pH values and in artificial seawater (prepared according to ref. 35). Fig. 9 summarizes the weight gain of the OGA for three organic liquids in different solutions, and the content of OGA was fixed at 8.0 wt% of oil. As can be seen, for a specific oil liquid, the value of the weight gain marginally varied, suggesting the excellent stability of the OGA against acid, alkali, and salty conditions. The material gained weight more than 4.7 times higher than its original weight for crude oil in simulated seawater, and this exciting result shows the potential application of the OGA for removing crude oil spills in marine systems. The oil content in the water after gelation was quantitatively measured and is summarized in Table 1. Remarkably, the efficiency for all samples was higher than 96%, indicating the excellent stability and capability of the OGA to separate oil from the water surface under different conditions.
| Oil type | Oil removal ratio (%) | |||
|---|---|---|---|---|
| Diesel | Octane | Crude oil | ||
| Oil/water mixture | pH = 2.6 | 97.4 ± 0.2 | 97.6 ± 0.2 | 97.1 ± 0.2 |
| pH = 6.9 | 97.6 ± 0.2 | 97.9 ± 0.2 | 97.3 ± 0.2 | |
| pH = 11.2 | 97.1 ± 0.2 | 97.3 ± 0.2 | 96.8 ± 0.2 | |
| Oil/artificial seawater mixture | 96.8 ± 0.2 | 97.2 ± 0.2 | 96.7 ± 0.2 | |
The viscosity is an important indicator for characterizing the oil-gelling ability of material. Three oily liquids (octane, crude oil, and motor oil) were selected as probing liquids to study the effect of the OGA content on the gelling performance of materials. In a typical procedure, an excessive amount of oily liquid (100.0 g) was poured into a container, and then a certain amount of OGA was added to the oil and vigorously stirred to achieve a uniform distribution of OGA in the mixture. The viscosity of the mixture as a function of the OGA content is summarized in Table 2. As can be seen, the viscosity of oil without the OGA was relatively low (ηoctane = 0.65 mPa s, ηcrude oil = 251 mPa s, ηmotor oil = 990 mPa s), and the oils became thicker after adding a small quantity of OGA. Specifically, with 8.0 wt% of OGA, the viscosities of the mixture increased to 0.28, 1.81, and 9.5 Pa s for octane, crude oil, and motor oil, respectively. With further increase in the OGA content, the viscosity of the liquid exhibited non-linearity. When the additive amount of OGA reached 16.0 wt%, the flowable oils were completely gelled by the material, and the viscosities of mixtures increased by several orders of magnitude. The gel with crude oil exhibited a high viscosity of 152 Pa s, and such transformation from oily liquid to solid-like material resulted in easy removal of the oil–OGA combination from the surface of the water.
| η (Pa s) | OGA quantity (wt% of oil) | ||
|---|---|---|---|
| 0.0 | 8.0 | 16.0 | |
| n-Octane | (0.65 ± 0.15) × 10−3 | 0.28 ± 0.02 | 94.20 ± 0.80 |
| Crude oil | 0.25 ± 0.02 | 1.81 ± 0.09 | 152.26 ± 1.30 |
| Motor oil | 0.99 ± 0.05 | 9.50 ± 0.22 | 200.13 ± 1.60 |
Conclusions
In summary, we developed a new type of oil gelator through a simple hydrothermal method using biodegradable wheat bran as a starting material. The obtained gelator showed controlled hydrophilicity to water and oleophilicity to organic compounds, which was due to the synergistic effect arising from the organic silane coating and enhanced surface roughness in the material. The prepared oil gelator had the capability to adsorb various organic liquids with a maximum adsorption capacity of 6.15 g g−1 for motor oil. In addition, the material could selectively solidify the oil phase from an oil–water mixture at room temperature. With advantages such as easy applicability, excellent phase selectivity, short gelling time, and green preparation process, the modified wheat bran demonstrated its remarkable potential as an effective oil solidifier for the cleanup of oil spills.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was supported by the Major Science and Technology Program of Xinjiang Uygur Autonomous Region (Project No: 2018A02002-3), Director Foundation of XTIPC-CAS (Grant No: 2016PY005), and the National 1000-Talent Program (Recruitment Program of Global Expert, In Chinese: Qian-Ren-Ji-Hua).Notes and references
- Z. L. Chu, Y. J. Feng and S. Seeger, Angew. Chem., Int. Ed., 2015, 54, 2328 CrossRef PubMed.
- B. Wang, W. X. Liang, Z. G. Guo and W. M. Liu, Chem. Soc. Rev., 2015, 44, 336 RSC.
- A. K. Kato, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed.
- I. Buist, J. Mccourt, S. Potter, S. Ross and K. Trudel, Pure Appl. Chem., 1999, 71, 43 CrossRef.
- V. Broje and A. Keller, Environ. Sci. Technol., 2006, 40, 7914 CrossRef PubMed.
- R. C. Prince, Environ. Sci. Technol., 2015, 49, 6376 CrossRef PubMed.
- J. P. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699 CrossRef.
- M. M. Tao, L. X. Xue, F. Liu and L. Jiang, Adv. Mater., 2014, 26, 2943 CrossRef PubMed.
- L. Mu, S. D. Yang, B. Hao and P. C. Ma, Polym. Chem., 2015, 6, 5869 RSC.
- C. C. Wang, S. D. Yang, Q. Ma, X. Jia and P. C. Ma, Carbon, 2017, 118, 765 CrossRef.
- G. Wang, Y. He, L. Zhang, Q. Y. Yu, S. S. Peng, X. D. Wu, T. H. Ren, Z. X. Zeng and Q. J. Xue, Green Chem., 2015, 17, 3093 RSC.
- S. Bhattacharya and Y. K. Ghosh, Chem. Commun., 2001, 185 RSC.
- C. L. Ren, J. Shen, F. Chen and H. Q. Zeng, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef.
- A. Prathap and K. M. Sureshan, Chem. Commun., 2012, 48, 5250 RSC.
- S. Basak, J. Nanda and A. Banerjee, J. Mater. Chem., 2012, 22, 11658 RSC.
- M. Samateh, A. Vidyasagar, S. R. Jadhav and G. John, RSC Adv., 2016, 6, 107598 RSC.
- A. M. Vibhute, V. Muvvala and K. M. Surashan, Angew. Chem., Int. Ed., 2016, 55, 7782 CrossRef PubMed.
- C. L. Ren, G. H. Boon, H. Wu, K. H. Chan, J. Shen, C. Teh, J. Y. Ying and H. Q. Zeng, Chem. Mater., 2016, 28, 4001 CrossRef.
- R. Gallego, J. F. Arteaga, C. Valencia and J. M. Franco, Molecules, 2013, 18, 6532 CrossRef PubMed.
- C. S. K. Raju, B. Pramanik, T. Kar, P. V. C. Rao, N. V. Choudary and R. Ravishankar, RSC Adv., 2016, 6, 53415 RSC.
- C. S. K. Raju, B. Pramanik, R. Ravishankar, P. V. C. Rao and G. Sriganesh, RSC Adv., 2017, 7, 37175 RSC.
- M. Xue, D. Gao, K. Q. Liu, J. X. Peng and Y. Fang, Tetrahedron, 2009, 65, 3369 CrossRef.
- J. X. Peng, K. Q. Liu, X. F. Liu, H. Y. Xia, J. Liu and Y. Fang, New J. Chem., 2008, 32, 2218 RSC.
- C. Antoine, S. Peyron, F. Mabille, C. Lapierre, B. Bouchet, J. Abecassie and X. Rouau, J. Agric. Food Chem., 2003, 51, 2026 CrossRef PubMed.
- M. Rana, J. T. Chen, S. D. Yang and P. C. Ma, Adv. Mater. Interfaces, 2016, 3, 1600128 CrossRef.
- J. Guo, Y. Y. Bian, K. X. Zhu, X. N. Guo, W. Peng and H. M. Zhou, Food Bioprocess Technol., 2015, 8, 1552 CrossRef.
- P. C. Ma, J. K. Kim and B. Z. Tang, Carbon, 2006, 44, 3232 CrossRef.
- A. Kuznetsova, E. A. Wovchko and J. T. Yates, Langmuir, 1997, 13, 5322 CrossRef.
- Y. Q. Wu, S. S. Jia, Y. Qing, S. Luo and M. Liu, J. Mater. Chem. A, 2016, 4, 14111 Search PubMed.
- Y. Li, Z. Z. Zhang, B. Ge, X. H. Men and Q. J. Xue, Green Chem., 2016, 18, 5266 RSC.
- K. Kaya, E. Pehlivan, C. Schmidt and M. Bahadir, Food Chem., 2014, 158, 11 CrossRef PubMed.
- X. Yang, Z. P. You and J. Mills-Beale, J. Mater. Civ. Eng., 2015, 27, 04014157 CrossRef.
- O. O. Onipe, A. I. O. Jideani and D. Beswa, Int. J. Food Sci. Tech., 2015, 50, 2509 CrossRef.
- Y. Z. Wang, Y. S. Wang, X. R. Yan, S. Q. Wu, L. Shao, Y. Y. Liu and Z. H. Guo, Chemosphere, 2016, 153, 485 CrossRef PubMed.
- K. Yu, X. Tan, Y. N. Hu, F. W. Chen and S. J. Li, Corros. Sci., 2011, 53, 2035 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00127h |
| This journal is © the Partner Organisations 2018 |