Recent progress in Zn-based anodes for advanced lithium ion batteries
Lei
Wang
 a,
Guanhua
Zhang
a,
Guanhua
Zhang
 *ab,
Quanhui
Liu
c and
Huigao
Duan
ab
*ab,
Quanhui
Liu
c and
Huigao
Duan
ab
aState Key Laboratory of Advanced Design and Manufacturing for Vehicle Body, School of Physics and Electronics, Hunan University, Changsha, 410082, P. R. China. E-mail: guanhuazhang@hnu.edu.cn
bNational Engineering Research Center for High Efficiency Grinding, College of Mechanical and Vehicle Engineering, Hunan University, Changsha, 410082, P. R. China
cSchool of Physics and Electronics, Hunan University, Changsha, 410082, P. R. China
First published on 8th May 2018
Abstract
Developing high-performance anode materials is essential for advanced next-generation lithium ion batteries. ZnO-based nanomaterials have been considered promising candidates owing to their high theoretical specific capacity, environmental friendliness, and relatively low cost. However, major problems, including inherent poor conductivity and huge volume expansion, have severely impeded ZnO-based nanomaterials from being viable. Here, we first present a brief review of the most effective strategies to improve the lithium ion storage performance of ZnO-based anodes. In addition, recent advances in other novel Zn-based nanomaterials are also summarized and discussed. Finally, we describe the challenges and prospects of future research trends for Zn-based nanomaterials.
1. Introduction
Renewable energy sources such as solar energy, wind and waves have attracted enormous attention to address the shortage of fossil fuels and the increasingly serious environmental issues.1,2 However, without effective energy storage and conversion systems, these green energy sources cannot be widely used owing to their intermittent availability and their instability. In addition, high-performance energy storage and conversion devices are becoming more and more important for the rapid development of smart portable devices, electric vehicles (EVs) and hybrid electric vehicles (HEVs).3–19 As one of the most important energy storage and conversion systems, lithium ion batteries (LIBs) have achieved wide commercial application owing to their high energy density, lack of memory effect, wide operating temperature range, long cycling life, and low self-discharge rate.20,21 The electrodes (including anodes and cathodes) are the key components which will strongly affect the electrochemical performance of LIBs. For the anodes of LIBs, carbon-based materials, especially graphite, have played a crucial role since they were first introduced into commercial LIBs by Sony in 1991. Nevertheless, the low theoretical capacity (372 mA h g−1) and serious safety concerns greatly limit further practical applications of the graphite anode. In particular, an ever-increasing demand for power batteries with high energy density and high safety cannot be satisfied. Therefore, researchers are focusing on developing novel anode materials with high performance for next-generation LIBs.Based on conversion reaction mechanism, nano-sized transition metal oxides (TMOs) deliver much higher theoretical specific capacity than the commercial carbon-based anodes.22,23 Additionally, the higher lithiation potential of the TMOs can avoid the formation of lithium dendrites in the anodes, ensuring a better safety capability for LIBs. Among various TMOs, zinc oxide (ZnO) has been considered a promising anode material candidate for LIBs, benefiting from high theoretical specific capacity, environmental friendliness and low cost.24 In addition, the low lithiation/delithiation potential of ZnO (lithiation potential ca. 0.5 V, delithiation potential 0–0.7 V and ca. 1.4 V) delivers an obvious advantage for high-output voltage and energy density.25,26 Unfortunately, the huge volumetric change of ZnO during the Li ion interaction/extraction process usually causes severe pulverization and electrical disconnection between the active material and the current collector, leading to a dramatic decline of the cycling stability. In addition, the poor intrinsic electrical conductivity of ZnO is unfavorable for fast electron transport, limiting the rate capability of the ZnO anodes. Therefore, optimizing the nanostructure to improve the electrochemical performance of ZnO-based anodes is highly desirable.
Recently, various strategies have been developed to effectively improve the lithium ion storage performance of ZnO-based anodes. Despite a previous review outlining the structural design of ZnO-based anodes, a systematic summary of the different improvement approaches is of great importance. Under these circumstances, in this review we summarize the recently developed key approaches, including structure design, surface modification and component regulation, to enhance structure stability, ionic diffusion and electronic conductivity of ZnO-based anodes. In addition, other novel Zn-based nanomaterials such as ZnS, ZnSe, ZnP, ZnSb, and ZnTe are introduced. Finally, the future challenges and new opportunities of Zn-based anodes are outlined.
2. Synthetic methods for ZnO-based anodes
To date, various methods have been developed to synthesize ZnO nanostructures with controllable morphologies for lithium ion storage. According to the initial state of the reactive materials, the fabrication methods can be classified into solid-phase method, liquid-phase method and gas-phase method, as shown in Table 1.| Synthetic methods | ZnO nanostructures | ||||
|---|---|---|---|---|---|
| 0D (nanoparticle, nanosphere) | 1D (nanorod, nanowire, nanofiber, nanotube, nanobelt) | 2D (nanosheet, nanoplate, nanofilm, nanoflake, nanodisk) | 3D (nanoflower, nanofoam, et al.) | ||
| Solid phase | Ball milling | ✓ | ✓ | ||
| Solid state reaction | ✓ | ✓ | |||
| Liquid phase | Electro depositing | ✓ | ✓ | ✓ | ✓ |
| Sol–gel | ✓ | ✓ | ✓ | ✓ | |
| Hydrothermal | ✓ | ✓ | ✓ | ✓ | |
| Solvothermal | ✓ | ✓ | ✓ | ✓ | |
| Electrospinning | ✓ | ✓ | ✓ | ||
| Precipitation | ✓ | ✓ | ✓ | ✓ | |
| Gas phase | Magnetron sputtering | ✓ | ✓ | ✓ | |
| Pulsed laser deposition | ✓ | ✓ | ✓ | ||
| Atomic layer deposition | ✓ | ✓ | |||
| Plasma chemical vapor deposition | ✓ | ✓ | ✓ | ||
| Thermal chemical vapor deposition | ✓ | ✓ | ✓ | ||
3. Lithium ion storage mechanism of ZnO-based anodes
ZnO, as a typical TMO, delivers a high theoretical specific capacity by redox reaction in a multi-electron process. The electrochemical reaction mechanism of the ZnO anodes can be described as follows:23,24| Conversion reaction: ZnO + 2Li+ + 2e− ⇌ Zn + Li2O | (1) |
| Alloying–dealloying reaction: Zn + xLi+ + xe− ⇌ LixZn (x ≤ 1) | (2) |
As the above equations represent, the Li ion storage mechanism of ZnO anodes includes two stages: conversion reaction to form Zn metal and Li2O, and then alloying reaction to form lithium zinc alloy, accompanied by a multi-electron transfer process.
The theoretical specific capacity of ZnO anodes is related to the number of electrons transferred during the electrochemical reaction process and can be calculated according the following equation:
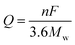 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1), n is the number of electrons transferred in the electrochemical reaction, and Mw is the molecular weight of active material (g mol−1). As we can see from eqn (1) and (2), three electrons are transferred per mole of ZnO during the electrochemical reaction. Therefore, based on eqn (3), we can calculate a theoretical specific capacity of 987 mA h g−1, which is almost twice that of graphite. This high theoretical specific capacity demonstrates that ZnO is a promising anode material for next-generation LIBs. However, the rapid capacity fading during cycling caused by a huge volume expansion (about 228%) is a big challenge of ZnO anodes. Moreover, the low inherent electrical conductivity of ZnO results in a limited rate performance. In order to overcome the above drawbacks and realize the practical application of ZnO-based anodes for next-generation batteries, lots of effective approaches have been developed to improve the battery performance. The recent key progress in designing and fabricating ZnO-based anodes with improved performance will be discussed in detail in this review.
500 C mol−1), n is the number of electrons transferred in the electrochemical reaction, and Mw is the molecular weight of active material (g mol−1). As we can see from eqn (1) and (2), three electrons are transferred per mole of ZnO during the electrochemical reaction. Therefore, based on eqn (3), we can calculate a theoretical specific capacity of 987 mA h g−1, which is almost twice that of graphite. This high theoretical specific capacity demonstrates that ZnO is a promising anode material for next-generation LIBs. However, the rapid capacity fading during cycling caused by a huge volume expansion (about 228%) is a big challenge of ZnO anodes. Moreover, the low inherent electrical conductivity of ZnO results in a limited rate performance. In order to overcome the above drawbacks and realize the practical application of ZnO-based anodes for next-generation batteries, lots of effective approaches have been developed to improve the battery performance. The recent key progress in designing and fabricating ZnO-based anodes with improved performance will be discussed in detail in this review.
4. Strategies for improving lithium ion storage performance
4.1 Nanostructure design
Poizot et al. first discovered that TMOs with nanostructure can be used as high-performance anode materials for LIBs in 2000.27 Since then, numerous efforts have been devoted to this new research field.28–31 Compared with the bulk materials, the novel nanostructured materials possess a series of unique advantages for the improved performance of the batteries. The nanoscale size can not only remarkably relieve the volume expansion to enhance the cycling capability, but also shorten the path distances of electron transfer and Li ion diffusion for better rate performance. Moreover, nanostructured materials with large specific surface areas are beneficial for providing more active sites to participate in the electrochemical energy storage reactions. Benefiting from the structural advantages, various nanostructured ZnO materials ranging from low to high dimensions have been designed and prepared as the anodes for LIBs with obviously enhanced electrochemical performance.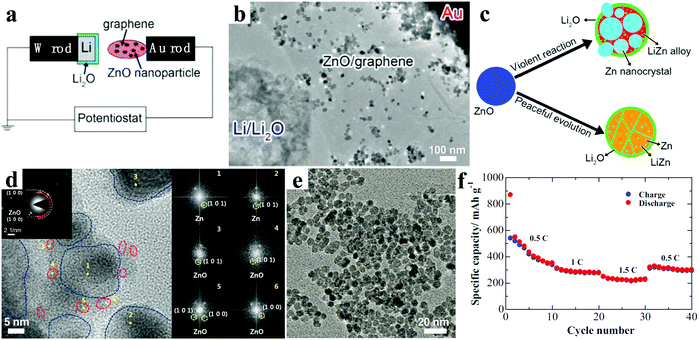 | ||
| Fig. 1 (a) Schematic illustration and (b) TEM image of the in situ electrochemical nano-LIB constructed inside a TEM; (c) schematic illustration of the electrochemical reaction of ZnO with Li+. Reproduced with permission.32 Copyright 2013, IOP Publishing Ltd. (d) High-resolution transmission electron microscopy (HRTEM) image of ZnO electrode at the fully charged state with the corresponding selected area electron diffraction (SAED) and Fourier transform (FT) patterns. Reproduced with permission.33 Copyright 2016, Elsevier. (e) HRTEM image of the ZnO nanoparticles; (f) rate capability of lithium ion cells with the ZnO electrode. Reproduced with permission.34 Copyright 2016, Springer. | ||
Developing a facile and scalable method to prepare ZnO nanoparticles is extremely important to their practical application as LIB anode. Highly dispersed ZnO nanoparticles prepared by a versatile and scalable sol–gel synthetic technique were reported by Li et al.34 According to Fig. 1e, the spherical ZnO nanoparticles obtained had an average size of only about 7 nm. Owing to the nano-size of the highly dispersed ZnO nanoparticles, outstanding electrochemical performance was demonstrated with a good rate capability of 229 mA h g−1 at 1.5C (Fig. 1f).
A series of 1D ZnO nanostructures were designed to further improve the performance of LIBs.35–43 For example, Wang et al. prepared ZnO nanorod arrays with dandelion-like morphology directly grown on copper substrates by a facial hydrothermal method (Fig. 2a).36 Compared with the bulk ZnO, the ZnO nanorod arrays showed superior electrochemical performance with a stable capacity after 40 cycles, which was about four times that of the bulk ZnO. Kundu et al. further reduced the size of ZnO nanorods via a simple chemical route (Fig. 2b).37 The ZnO nanorod electrodes held a capacity of 230 mA h g−1 after 100 cycles at 0.3C. The crystal structural evolution of a single ZnO nanowire electrode during the lithiation reaction was investigated by Kushima and co-workers.38 They observed that the solid state amorphization in single-crystalline ZnO nanowires was mainly driven by nanocracking, as shown in Fig. 2c and d. Initially, in the crystalline nanowire, large tensile stresses near the surface induced by partial lithiation resulted in multiple nanocracking ahead of the main lithiation front, and the nanocracking penetrated the nanowire cross-section. Then, Li+ diffused and solid state amorphization occurred along the open crack surfaces. Finally, a glass–glass interface was left after the crack surfaces merged. In the divided segments, the same reaction also repeated, further pulverizing the nanowire into different nanoglass domains (nano-amorphization). These results provide useful guidance for developing promising ZnO electrode materials with regard to the crystal structure.
 | ||
| Fig. 2 (a) Scanning electron microscopy (SEM) image of the ZnO nanorod arrays. Reproduced with permission.36 Copyright 2008, Elsevier. (b) HRTEM image of the ZnO nanorod. Reproduced with permission.37 Copyright 2015, Elsevier. (c) Schematic illustration and (d) the corresponding TEM images explaining the leapfrog cracking and nanoamorphization lithiation mechanism of ZnO nanowires. Reproduced with permission.38 Copyright 2011, American Chemical Society. SEM images of patterned arrays of ZnO nanorods (e) and nanotubes (f). Reproduced with permission.40 Copyright 2012, American Chemical Society. | ||
Based on the unique properties of the hollow nanostructures, ultrathin 1D ZnO nanotubes were introduced as the anodes for LIBs.40 Pristine solid ZnO nanorods were synthesized by a simple hydrothermal method, as shown in Fig. 2e. Then the solid nanorods were spontaneously converted into ultrathin ZnO nanotubes in ambient NH3via a gas-phase chemical process, according to Fig. 2f. Owing to improved ion diffusion and effective alleviation of volume expansion, the ZnO nanotubes delivered a reversible capacity of 386 mA h g−1 at a current density of 0.5C after 50 cycles, which was about five times higher than that of the original ZnO nanorods (only 83 mA h g−1).
 | ||
| Fig. 3 (a) SEM image of the as-deposited ZnO at 500 °C. Reproduced with permission.44 Copyright 2003, The Electrochemical Society. (b) SEM image of mesoporous ZnO nanosheets; (c) the first two charging/discharging curves of mesoporous ZnO nanosheets. Reproduced with permission.45 Copyright 2014, Elsevier. (d) SEM image of the ZnO nanosheets. Reproduced with permission.46 Copyright 2016, Springer. | ||
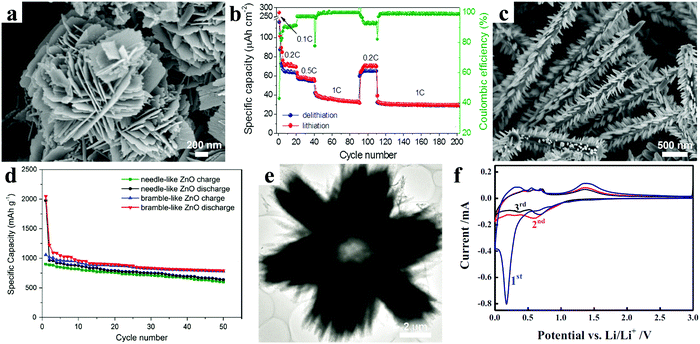 | ||
| Fig. 4 (a) SEM image of the flower-like ZnO nanostructure; (b) rate performance at different current densities. Reproduced with permission.48 Copyright 2013, Elsevier. (c) SEM image of the bramble-like ZnO arrays; (d) cycling performance of needle- and bramble-like ZnO electrodes. Reproduced with permission.50 Copyright 2015, Springer. (e) TEM image of a radial ZnO microstructure; (f) CV curves of a hollow radial ZnO electrode. Reproduced with permission.51 Copyright 2014, Springer. | ||
Other complex 3D structures also demonstrate their distinctive advantages. A bramble-like ZnO array was designed and successfully prepared through a facile two-step hydrothermal process.50 The corresponding scanning electron microscopy image is shown in Fig. 4c. Meanwhile, the electrochemical properties of the bramble-like ZnO electrode were compared with those of the needle-like ZnO electrode. As shown in Fig. 4d, after 50 cycles, the bramble-like ZnO still retained a capacity of 782 mA h g−1 corresponding to 74% capacity retention, which was obviously higher than that of the needle-like ZnO with 67% capacity retention. The enhanced electrochemical performance of the bramble-like ZnO electrode mainly resulted from the nanorod subunits acting as “bridges” to connect the adjacent ZnO needles and providing extra active sites on the ZnO needles. Another typical 3D structure, hollow ZnO with radial morphology, was synthesized based on a polystyrene sphere template via a hydrothermal method; the TEM image of the sample is shown in Fig. 4e.51 Good reproducibility and almost accordance in shape were found in the current–voltage (C–V) curves after the first cycle, indicating high reversibility of the electrode (Fig. 4f). Benefiting from the hollow radial architecture providing enormous void spaces and plenty of active sites, the electrodes revealed good cycling performance and rate capability for reversible lithium storage. For instance, the electrode held a reversible capacity of about 320 mA h g−1 at a current density of 0.2 A g−1 after 100 cycles.
The relationship between lithium ion storage performance and structural dimensions of ZnO-based anodes is systematically exhibited, suggesting the significance of structural design. The 0D ZnO nanostructures have exhibited potential to overcome the challenges existing in the traditional bulk materials owing to their unique nanostructural properties, presenting improved battery performance. ZnO electrodes with high-dimensional nanostructures commonly display better electron transmission rate, larger surface area, more interspaces to alleviate volume change, and more abundant ion diffusion pathways, promoting the reaction kinetics to some extent. Nevertheless, a series of issues accompany high-dimensional ZnO nanostructures, such as low initial coulombic efficiency resulting from extra lithium ion consumption for solid electrolyte interface (SEI) formation, and low packing density because of large numbers of voids within the nanostructures. Hence, rational structure design with different dimensions is necessary to develop high-performance ZnO electrodes.
4.2 Hybridization with carbonaceous materials
Other than structure design, introducing carbonaceous materials (graphene, carbon nanotubes, and derived carbon) has been demonstrated to be a promising way to overcome the inherent poor conductivity of the ZnO material.25,54–64 Additionally, these carbon materials with good mechanical ductility can act as a buffer matrix to relieve the huge volume expansion during the charging/discharging process.Hsieh et al. reported that highly-crystalline ZnO nanocrystals with a size of 80–100 nm were uniformly anchored on both sides of graphene nanosheets (GN) by an efficient method (Fig. 5a).74 The ZnO/GN composites with an optimum ratio showed a superior cycling stability with only 8% capacity decay after 50 cycles (Fig. 5b). Moreover, an excellent rate performance was also achieved, with 60% capacity retention, even with the current density increasing from 0.07 A g−1 to 3.5 A g−1. These excellent electrochemical properties were attributed to rational structure design and composition regulation. The ZnO nanocrystals could efficiently prevent the restacking of graphene nanosheets. Meanwhile, the graphene nanosheets not only provided a high conductive network, but also exhibited a wide buffering effect to accommodate the volume change.
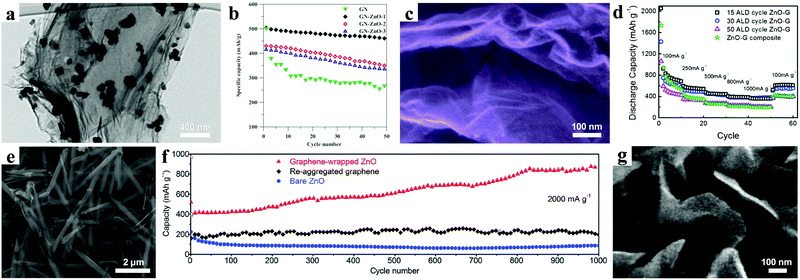 | ||
| Fig. 5 (a) TEM image of ZnO@GN composite; (b) cycling performances at 1C. Reproduced with permission.74 Copyright 2013, Elsevier. (c) SEM image and (d) rate performances of graphene nanosheets coated by ZnO QDs. Reproduced with permission.75 Copyright 2014, Royal Society of Chemistry. (e) SEM image of graphene-wrapped ZnO nanotubes; (f) cycling performance at 2 A g−1. Reproduced with permission.76 Copyright 2016, Royal Society of Chemistry. (g) SEM image of ZnO-VAGNs. Reproduced with permission.77 Copyright 2014, American Chemical Society. | ||
Sun and co-workers prepared uniform ZnO quantum dots (QDs) with size ranging from 2 to 7 nm homogeneously anchored along wrinkled graphene sheets by an atomic layer deposition (ALD) method (Fig. 5c).75 According to the investigation of the relationship between the size and the electrochemical performance, they found that smaller-sized QDs on graphene exhibit better electrochemical performance. For 2 nm ZnO QDs, a high reversible specific capacity of 960 mA h g−1 was achieved by deducting the graphene contribution from the composites, approaching the theoretical specific capacity. Moreover, the comparison of the rate performances showed that the appropriate proportion of ZnO and graphene is critical to obtaining high rate capability (Fig. 5d). A graphene-wrapped ZnO nanotube structure was synthesized by using graphene oxide nanosheets as a “soft” sealing layer confining the growth of ZnO nanotubes, as shown in Fig. 5e.76 An impressive cycling stability with a high reversible specific capacity of 891 mA h g−1 over 1000 cycles at a current of 2 A g−1 was achieved (Fig. 5f).
In order to avoid the use of insulating binder, Li et al. prepared vertically aligned graphene nanosheets (VAGNs) grown in situ on Cu foil.77 Then ultra-small ZnO nanoparticles uniformly grew on both sides of the VAGNs as a result of a hydrothermal process (Fig. 5g). Owing to the improved electrical conductivity and reduced Li ion transport path, the ZnO-VAGNs composites revealed excellent electrochemical performance. After 100 cycles, the reversible capacity still remained at 809 mA h g−1 at a charging/discharging current of 0.08 A g−1, displaying good capacity retention.
CNTs-supported ZnO composites were synthesized via an in situ co-precipitation process, in which ZnO nanohexagonal disks were well dispersed on the surface of the modified CNTs (Fig. 6a).79 As displayed in Fig. 6b, the ZnO/CNT electrodes showed a reversible capacity of 602 mA h g−1 after 50 cycles, which was much higher than that of the bare ZnO electrodes (478 mA h g−1). Köse et al. reported free-standing ZnO and multi-walled carbon nanotube (MWCNT) nanocomposites prepared with two different chelating agent additives, namely, triethanolamine (TEA) and glycerin (GLY) (Fig. 6c).81 It is interesting that the chelating agent additives have an effect on the electrochemical performance of ZnO/MWCNT nanocomposites. With regard to the rate capability, as shown in Fig. 6d, the ZnO/MWCNT/GLY free-standing anode reached a capacity of 375 mA h g−1 at 2C, which is obvious higher than that of ZnO/MWCNT/TEA. In addition, the ZnO/MWCNT/GLY anode also delivered a higher discharge capacity – as high as 460 mA h g−1 – than the capacity of 180 mA h g−1 for the ZnO/MWCNT/TEA anode after 100 cycles at 0.2C. The authors suggested that the better performance of ZnO/MWCNT/GLY composites resulted from their smaller ZnO crystallite size, thinner ZnO film and mesoporous morphology.
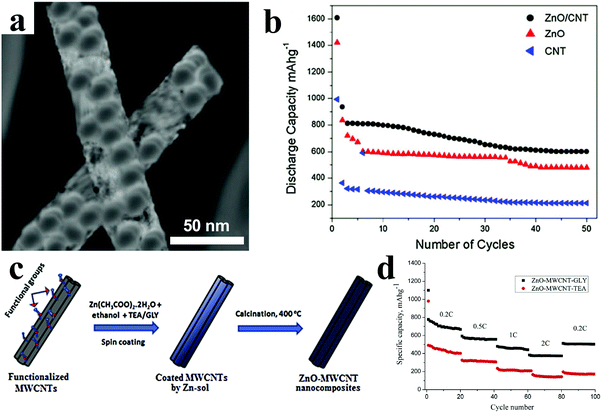 | ||
| Fig. 6 (a) TEM image of ZnO/CNT composites; (b) cycling performances of bare CNTs, ZnO, and ZnO/CNT electrodes. Reproduced with permission.79 Copyright 2013, Springer. (c) Schematic illustration of facile sol–gel synthesis of nanocomposite anodes; (d) the rate capabilities of the ZnO/MWCNT buckypaper nanocomposite anodes. Reproduced with permission.81 Copyright 2015, Elsevier. | ||
Yang et al. converted the metal–organic framework IRMOF-1 into porous carbon-coated ZnO QDs (∼3.5 nm) via pyrolysis at high temperature.93Fig. 7a and b confirm that the highly crystalline ZnO QDs were well dispersed without agglomeration in the amorphous carbon matrix. When evaluated as an anode for lithium ion batteries, the porous carbon-coated ZnO QDs electrode exhibited a remarkable cycling stability with nearly 100% capacity retention after 50 cycles and an outstanding rate performance of 400 mA h g−1 at 3.75 A g−1. In another representative work, Han et al. proposed a strategy by coating ZIF-8 nanoparticles with chitosan, and then the coated ZIF-8 was further pyrolyzed at 800 °C (Fig. 7c).94 This strategy can effectively enhance the electrochemical performance of LIBs. As a result, a reversible capacity of 750 mA h g−1 was obtained at a current density of 0.05 A g−1, and 99.6% capacity was retained after 50 cycles.
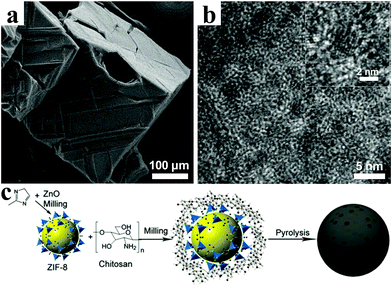 | ||
| Fig. 7 (a) SEM image and (b) TEM image of ZnO QDs@porous carbon. Reproduced with permission.93 Copyright 2013, American Chemical Society. (c) Synthetic route of ZIF-8@chitosan–800N. Reproduced with permission.94 Copyright 2014, Royal Society of Chemistry. | ||
It is worth noting that our group constructed self-supported ZnO@ZnO QDs/C core–shell nanorod arrays (NRAs) via an ionic exchange route (Fig. 8a).95 Ultimately, an integrated, binder-free, and lightweight ZnO@ZnO QDs/C electrode was obtained, as shown in Fig. 8b. As clearly displayed in Fig. 8c, the ZnO QDs were wrapped by ZIF-8-derived porous carbon matrices with a thickness of several nanometers. The carbon matrices protected both the ZnO particles and the residual ZnO core from aggregating and expanding. Meanwhile, abundant mesopores were beneficial for lithium ion access and transport, which could result in high cycling performance and excellent rate capability. When directly used as the anode, the ZnO@ZnO QDs/C core–shell NRAs presented a high first-cycle coulombic efficiency of around 92% and an outstanding rate capability (Fig. 8d and e). It was concluded that the integration of ZnO@ZnO QDs/C and current collector without using binder reduced interface resistance, ensuring fast electron transport; furthermore, the in situ coating of the MOF-derived N-doped porous carbon suppressed the volume expansion of ZnO, guaranteeing superior cycling stability. Similarly, Li et al. provided new insight into the preparation of nanomaterials via the pyrolysis of MOFs.96 ZnO nanosheets grown on MOF-derived porous carbon matrix were synthesized by controlling the intermediate structures of MOF precursors during the pyrolysis process by introducing terephthalic acid and triethylenediamine as ligands (Fig. 8f). These ZnO nanosheets had an average thickness of 18.2 nm based on statistical results. The cycling performance of the composites manifested a rather high specific capacity of 920 mA h g−1 after 150 cycles at a current density of 0.05 A g−1, suggesting a promising prospect in lithium storage application.
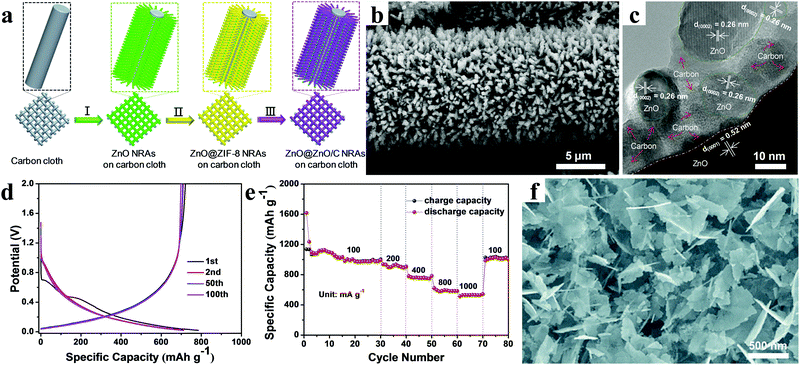 | ||
| Fig. 8 (a) Schematic illustrating the synthesis procedure, (b) SEM image, (c) TEM image, (d) galvanostatic charging/discharging profiles, and (e) rate performance of core–shell ZnO@ZnO QDs/C NRAs grown on flexible carbon cloth. Reproduced with permission.95 Copyright 2015, Wiley-VCH. (f) SEM image of ZnO nanosheets grown on porous carbon matrix. Reproduced with permission.96 Copyright 2017, Royal Society of Chemistry. | ||
Recently, we proposed a solid-solution-like (SSL) concept to prepare ZnO/C composite nanofibers as anode materials for LIBs by a simple electrospinning technology, using polyacrylonitrile (PAN) as carbon source (Fig. 9a).98 In this SSL nanostructure, amorphous ZnO was atomically “dissolved” in a carbon matrix to maximize the synergistic effect by creating the most metal oxide/carbon interfaces and defects possible, as shown in Fig. 9b. The composite nanofibers showed a high reversible capacity of 813.3 mA h g−1 after 100 cycles at a current density of 0.1 A g−1 (Fig. 9c). Furthermore, an outstanding rate performance was also demonstrated: even when the current density was increased by 40 times, the discharge capacity still remained at 53.5% of the initial value. Fan et al. used polyvinyl pyrrolidone (PVP) as the carbon source to synthesize hierarchical yolk–shell carbon-coated ZnO microspheres (YC-ZnO), as shown in Fig. 9d and e.100 An impressive performance delivering a discharge capacity of 659 mA h g−1 was retained after 300 cycles at a current density of 0.5 A g−1. Even with high current density of up to 10 A g−1, the YC-ZnO electrodes also manifested superior cycling stability, with 96.9% capacity retention after 5000 cycles.
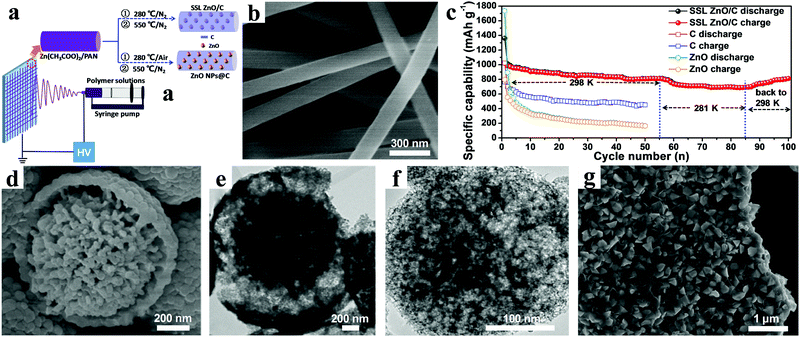 | ||
| Fig. 9 (a) Schematic illustration of preparation process of SSL ZnO/C and ZnO NPs@C composites; (b) SEM image of SSL ZnO/C NFs; (c) cycling performance of SSL ZnO/C NFs at 0.1 A g−1 under the various ambient temperatures. Reproduced with permission.98 Copyright 2015, Elsevier. (d) SEM image and (e) TEM image of yolk–shell structured YC–ZnO. Reproduced with permission.100 Copyright 2017, Elsevier. (f) TEM image of a single ZnO–NMPCS particle. Reproduced with permission.103 Copyright 2016, Elsevier. (g) SEM image of ZnO@C-5. Reproduced with permission.105 Copyright 2014, Elsevier. | ||
Sun et al. prepared a mesoporous N-doping spherical carbon matrix from melamine and phenol precursors.103 Then a novel ZnO/carbon mesoporous composite (ZnO–NMPCS) was formed by the confined growth of ZnO nanoparticles in the carbon sphere (Fig. 9f). As a result, the developed ZnO–NMPCS nanocomposite exhibited an excellent cycling stability, with a capacity of 1058.9 mA h g−1 after 100 cycles at a current density of 0.2 A g−1. Moreover, a capacity of 425 mA h g−1 after 1800 cycles was obtained even at a current density of 5 A g−1. A composite material consisting of tetrahedral ZnO nanocrystals embedded in a three-dimension (3D) carbon framework derived from oleic acid and oleylamine was designed and fabricated via a simple internal-reflux method by Ren and co-workers (Fig. 9g).105 When treated by subsequent calcination at 500 °C, the ZnO@C composite demonstrated the best electrochemical performance. For instance, a high capacity of 518 mA h g−1 was achieved after 300 cycles under a current density of 0.11 A g−1. Chae et al. synthesized nano-sized ZnO/C composites to investigate the role of carbon in the electrochemical properties, citric acid being chosen as carbon precursor.107 Compared with the pure ZnO electrodes, the ZnO/C composite electrodes showed obviously enhanced rate capability and cycling performance. After 200 cycles, the ZnO/C composite electrodes held a reversible capacity of 700 mA h g−1 at a current density of 0.1 A g−1.
As a typical organometallic complex, C4H4ZnO6, as the precursor for both ZnO and carbon, was pyrolyzed in an inert atmosphere to prepare zinc oxide nanoparticles embedded in a carbon framework (ZnO@C).108 Benefiting from the in situ introduction of carbon, the ZnO@C composite displayed an initial reversible capacity of 680 mA h g−1 at 1 A g−1, and 89.7% capacity retention even after 300 cycles.
4.3 Doping with metal/metal ion
Heteroatom doping is another important way to improve the electrochemical properties of electrode materials, which can obviously enhance the electrical conductivity. A variety of metal/metal ions, including Au, Ag, Co, Cu, Sn, Ni, Mn, Al, and Fe, have been doped into ZnO-based materials.112–128 In general, for the metal doping, the heteroatoms and ZnO are hybridized at the nanoscale level. However, for metal ion doping, the doping occurs at the atomic level by substituting Zn in the lattice by other metals. Large numbers of results have certified that this modified strategy highlights a promising approach to develop high-performance ZnO-based electrode materials.For instance, Peng and coworkers combined a facile electrostatic attraction approach and subsequent annealing treatment in argon to produce sandwich-like Ag–C@ZnO–C@Ag–C hybrid hollow microspheres (Fig. 10a and b).129 As the anode materials for LIBs, the composites revealed a very high reversible capacity of 1670 mA h g−1 after 200 cycles at a current density of 0.2 A g−1 (Fig. 10c). Meanwhile, at current densities of 1.6 and 10 A g−1, specific capacities of about 1063 and 526 mA h g−1, respectively, could be retained. These superior electrochemical performances resulted from the modification of silver and 3D carbon framework, as well as hollow nanostructure. As we know, Cu not only possesses high electrical conductivity, but also has a considerably lower cost than noble metals. C/Cu/ZnO porous hybrids were prepared by Wang and co-workers, in which the sizes of ZnO and Cu nanoparticles were distributed in the range 5–10 nm, as shown in Fig. 10d.130 The current–voltage (C–V) curves after the first cycle are almost coincident, indicating excellent reversibility of the C/Cu/ZnO electrode (Fig. 10e). The cycling performance showed high and stable specific capacities of 818 mA h g−1 and 689 mA h g−1 at current densities of 0.05 A g−1 and 0.2 A g−1, respectively, after 100 cycles. Yue et al. synthesized Co-doped MOF-5s by a secondary growth method, and then obtained Co-doped ZnO coated with carbon (CZO@C) by heat treatment under a nitrogen atmosphere (Fig. 10f).131 Interestingly, they found that the doping with Co had a dual function, including improving the conductivity of the material and enhancing the graphitization degree of the carbon in the material, which was beneficial for fast electron transfer. Based on the synergetic effect of Co doping and carbon coating, the CZO@C showed a high reversible capacity of 725 mA h g−1 at a current density of 0.1 A g−1 after 50 cycles, which was much higher than that of ZnO@C composites without Co doping (335 mA h g−1).
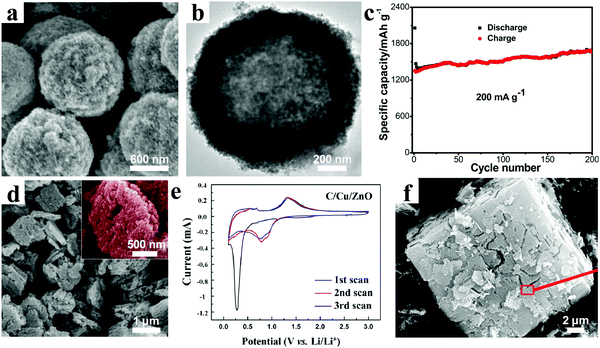 | ||
| Fig. 10 (a) SEM and (b) TEM images of sandwich-like Ag–C@ZnO–C@Ag–C hybrid hollow microspheres; (c) cycling performances at 0.2 A g−1. Reproduced with permission.129 Copyright 2015, American Chemical Society. (d) SEM image and (e) CV curves of the C/Cu/ZnO hybrids. Reproduced with permission.130 Copyright 2014, American Chemical Society. (f) SEM image of the CZO@C. Reproduced with permission.131 Copyright 2014, American Chemical Society. | ||
Bresser et al. presented n-type transition-metal (TM)-doped zinc oxide nanoparticles with the general formula Zn0.9TM0.1O (TM = Fe or Co), in which the transition metals mixed with zinc on the atomic level rather than on the microscale or nanoscale, Zn atoms being replaced by transition metal atoms within the wurtzite lattice (Fig. 11a and b).132 The results of in situ X-ray diffraction revealed that the alloying of lithium and zinc and the reduction of iron and zinc occurred simultaneously to a large extent. The Fe-doped ZnO nanoparticles displayed obviously enhanced specific capacities and rate performance compared with pristine zinc oxide when used as lithium ion anode. Inspired by natural fibrous roots, Yu et al. designed ZnxCo3−xO4@Zn1−yCoyO binary TMO nanoarrays on Cu substrates as an integrated LIB anode, in which the Zn1−yCoyO nanowires acted as the root part and the ZnxCo3−xO4 nanorods were the fibrous part (Fig. 11c).133 Owing to the synergistic effect, the binary TMO nanoarray electrodes could hold a high reversible capacity of 804 mA h g−1 after 100 cycles at 0.5 A g−1, which was much higher than that of the unitary cobalt-based and zinc-based nanoarrays.
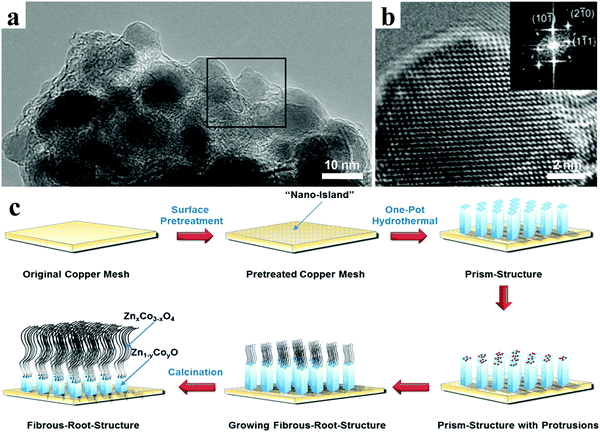 | ||
| Fig. 11 (a) TEM and (b) HRTEM images of Fe-doped ZnO nanoparticles, inset in panel (b) showing the corresponding FT. Reproduced with permission.132 Copyright 2013, American Chemical Society. (c) Preparation procedure of ZnxCo3−xO4@Zn1−yCoyO binary nanoarrays. Reproduced with permission.133 Copyright 2016, American Chemical Society. | ||
4.4 Compositing with metal oxides
ZnO-based composites synthesized by addition of other metal oxides, such as CoOx, CuO, NiO, Fe2O3, SnOx, TiO2, MnOx, Al2O3, and ZnFe2O4, have also demonstrated unique characteristics for high-performance LIBs.134–163 They have an obvious effect in relieving the volume expansion and improving the specific capacity, owing to the synergistic effect between the components.Fang et al. prepared 2D porous hybrid bimetallic Co3O4/ZnO nanosheets via a facile thermal treatment, using the synergistic effects between the two components for outstanding electrochemical capability (Fig. 12a).164 A series of materials with different Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratios (1
Zn molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 0
2, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were designed and their corresponding electrochemical properties were compared in detail. The results suggested that the Co3O4/ZnO composite with the molar ratio of 1
1) were designed and their corresponding electrochemical properties were compared in detail. The results suggested that the Co3O4/ZnO composite with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the best electrochemical performance for LIBs, which benefited from an appropriate ratio of Co3O4 and ZnO and the electrochemical synergistic effects. Flexible, freestanding, and binder-free electrodes consisting of amorphous SnOx–ZnO particles uniformly dispersed in carbon nanofibers (CNFs) were developed via a simple electrospinning method by Joshi et al. (Fig. 12b).165 Compared with pure CNFs, ZnO CNFs, and SnOx CNFs electrodes, SnOx–ZnO CNFs with a Sn
1 showed the best electrochemical performance for LIBs, which benefited from an appropriate ratio of Co3O4 and ZnO and the electrochemical synergistic effects. Flexible, freestanding, and binder-free electrodes consisting of amorphous SnOx–ZnO particles uniformly dispersed in carbon nanofibers (CNFs) were developed via a simple electrospinning method by Joshi et al. (Fig. 12b).165 Compared with pure CNFs, ZnO CNFs, and SnOx CNFs electrodes, SnOx–ZnO CNFs with a Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio of 3
Zn ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 displayed the highest first discharge and charge capacities (1910 and 1400 mA h g−1, respectively) and a high reversible capacity of 963 mA h g−1 at a current density of 0.1 A g−1 after 55 cycles. Lu et al. developed ZnO–NiO–Co3O4 hybrid nanoflakes based on the idea of introducing a small amount of Co3O4 as a catalyzing and performance-enhancing agent (Fig. 12c and d).166 Although only a small amount was present, Co3O4 played a vital role as it can further enhance the conductivity and catalytic activity of the hybrid materials. When applied as anode materials, the hybrid nanoflakes exhibited an excellent reversible capacity of 1060 mA h g−1 after 300 cycles at a current density of 0.5 A g−1.
1 displayed the highest first discharge and charge capacities (1910 and 1400 mA h g−1, respectively) and a high reversible capacity of 963 mA h g−1 at a current density of 0.1 A g−1 after 55 cycles. Lu et al. developed ZnO–NiO–Co3O4 hybrid nanoflakes based on the idea of introducing a small amount of Co3O4 as a catalyzing and performance-enhancing agent (Fig. 12c and d).166 Although only a small amount was present, Co3O4 played a vital role as it can further enhance the conductivity and catalytic activity of the hybrid materials. When applied as anode materials, the hybrid nanoflakes exhibited an excellent reversible capacity of 1060 mA h g−1 after 300 cycles at a current density of 0.5 A g−1.
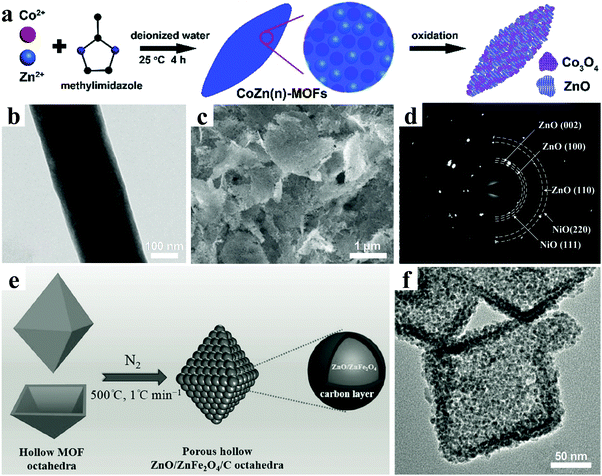 | ||
| Fig. 12 (a) Schematic of the synthesis of 2D porous hybrid Co3O4/ZnO nanosheets. Reproduced with permission.164 Copyright 2017, Royal Society of Chemistry. (b) TEM image of SnOx–ZnO CNFs. Reproduced with permission.165 Copyright 2016, American Chemical Society. (c) SEM image and (d) SAED pattern of ZnO–NiO–Co3O4 hybrid nanoflakes. Reproduced with permission.166 Copyright 2017, Royal Society of Chemistry. (e) Schematic illustration for the preparation process and (f) TEM image of ZnO/ZnFe2O4/C nanoparticles. Reproduced with permission.173 Copyright 2014, Wiley-VCH. | ||
Recently, nanostructured ZnO and ternary ZnM2O4 (M = Ge, Fe, Co etc.) composites have generated increasing interest as promising lithium-storage materials for LIBs.167–172 Zou et al. reported porous hollow ZnO/ZnFe2O4/C octahedra using a MOF as both the precursor and the self-sacrificing template by an ordinary refluxing reaction followed by thermal annealing in N2, as shown in Fig. 12e.173 In the nanostructure, ultrafine ZnO and ZnFe2O4 nanocrystals of less than 5 nm were uniformly embedded in a 3D interconnected, porous carbon framework (Fig. 12f). An outstanding electrochemical lithium-storage performance was demonstrated, in which superior specific capacities were as high as 1390 mA h g−1 and 988 mA h g−1 after 100 cycles at the current densities of 0.5 A g−1 and 2 A g−1, respectively. The rate performance exhibited a specific capacity of 762 mA h g−1 even at a high current density of 10 A g−1.
An intriguing phenomenon, that nano-sized Zn-based composite anodes always deliver higher specific capacities than the theoretical capacity, has been reported in the literature.68,76,77,113 Similar to other nano-sized transition-metal oxides, the extra capacity may originate from the formation of polymer/gel-like film, the surface/interface lithium ion storage and synergistic effects of composites.93,123,126,133,174–177 For example, the hybrid ZnO QDs@porous carbon anode exhibited a reversible capacity of 1150 mA h g−1, which was obviously beyond the theoretical capacity of 817 mA h g−1 considering the relative amounts of ZnO and amorphous carbon.93 The extra capacity came from interfacial charge storage via weak bonding between Li+ and the surface of ZnO QDs, reactions with the electrolyte, charge separation at the SEI layer, and synergistic effects of the composites of ZnO and amorphous carbon. The mechanism of this phenomenon needs to be further studied in order to better optimize the performance of the Zn-based negative electrodes from the aspects of structure design and component control.
5. Other Zn-based nanomaterials
In addition to ZnO, lots of other Zn-based nanomaterials, such as ZnS, ZnSe, ZnP, ZnSb, and ZnTe, have also been reported as attractive anode candidates for Li ion batteries. Generally, these Zn-based nanomaterials show different advantages for electrochemical features owing to their different properties.As a kind of typical transition metal sulfide, ZnS has been considered a promising electrode material for next-generation LIBs, owing to its high theoretical specific capacity of 962.3 mA h g−1. Unfortunately, similar to ZnO electrodes, the huge volume and structure changes in the lithiation/delithiation process and the poor conductivity greatly hinder the practical application of ZnS-based electrodes. Rational structure design, by reducing the size to the nanoscale and hybridizing with conductive carbonaceous materials, has been demonstrated an effective strategy for improving the lithium storage performance. Up to now, various carbonaceous materials, such as citric acid, graphene, porous carbon, reduced graphene oxide, C2H2, and glucose, have been adopted to alleviate the volume variations and enhance the electrical conductivity of ZnS electrodes, which has usually resulted in obviously improved electrochemical performances.178–185 Especially, Li et al. prepared ZnS nanoparticles decorated on nitrogen-doped porous carbon (NPC) polyhedra by carbonizing and vulcanizing zeolitic imidazolate framework-8 (ZIF-8) with polyhedral morphology (Fig. 13a and b).186 Thus a ZnS/NPC hybrid showed a high reversible specific capacity of 1067.4 mA h g−1 at a current density of 0.1 A g−1 after 200 cycles. As shown in Fig. 13c, it displayed an excellent rate capability with a specific capacity of 364.6 mA h g−1 even at 4 A g−1. It exhibited superior long cycling performance, with a reversible capacity of 856.8 mA h g−1 after 1000 cycles at a high current density of 1 A g−1.
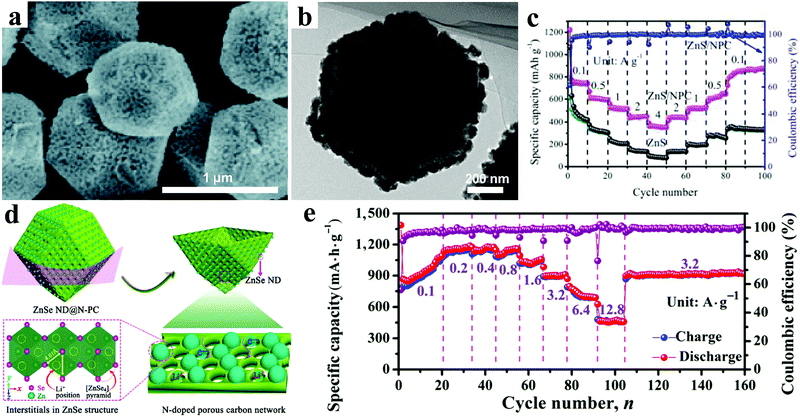 | ||
| Fig. 13 (a) SEM image, and (b) TEM image of ZnS/NPC hybrid; (c) rate performances of ZnS/NPC and ZnS. Reproduced with permission.186 Copyright 2017, Royal Society of Chemistry. (d) Schematic illustration for the construction of ZnSe ND@N-PC architecture; (e) rate capability at various current densities of ZnSe ND@N-PC composite electrodes. Reproduced with permission.191 Copyright 2017, Tsinghua University Press and Springer. | ||
As another kind of typical metal chalcogenide, ZnSe possesses a high theoretical capacity of 557 mA h g−1 based on the alloying and conversion reaction mechanism.187 Thus, numerous efforts have been made to realize its much better performance as anode for LIBs.188,189 In order to address the drawbacks of huge volume change up cycling and the intrinsic low electrical conductivity, composites of ZnSe and carbon are considered promising electrode materials with an obvious enhanced electrochemical performance.190–192 Chen et al. designed three-dimensional composites consisting of ZnSe nanodots uniformly confined within an N-doped porous carbon network, showing a high reversible capacity of 1134 mA h g−1 after 500 cycles at a current density of 0.6 A g−1 and excellent rate capability of 474 mA h g−1 at a current density of 12.8 A g−1 (Fig. 13d and e).191 In general, ZnSe electrodes displayed rising and additional reversible capacities beyond their theoretical capacities during cycling. Xu et al. investigated the origin of the additional capacities in ZnSe@C composites by employing ex situ X-ray photoelectron spectroscopy and controlling the electrochemical charging/discharging process.192 Their results showed that the generated and activated Se made the major contribution to the extra rising capacity in this system during the electrochemical process.
As promising anode materials for LIBs, ZnxP2 (x = 1, 3) have also been reported by many research groups.193,194 Bichat et al. obtained Zn3P2 powders with various morphologies by three different solid state preparation routes and revealed the Li insertion mechanism into the Zn3P2 electrode.195 The Li ion storage process included two distinct but parallel reversible pathways: one pathway employed exclusively phosphide phases (Zn3P2, LiZnP, Li4ZnP2, and Li3P) and the other pathway involved only Li–Zn alloys (Zn, LiZn4, and LiZn). Self-supported Zn3P2 nanowire arrays grafted on different conductive substrates have been designed and directly used for electrodes.196,197 For instance, Zn3P2 nanowire arrays on carbon fabric cloth as an integrated binder-free anode via a facile chemical vapor deposition (CVD) were reported by Li et al. (Fig. 14a).197 The results showed an impressive electrochemical performance with a coulombic efficiency up to 88%, excellent cycling stability without obvious decay after 200 cycles, and high rate capability of 400 mA h g−1 even at 15 A g−1. Compared with zinc-rich Zn3P2 (1241 mA h g−1), phosphorus-rich ZnP2 possesses a higher theoretical specific capacity (1581 mA h g−1). Monoclinic ZnP2, tetragonal ZnP2, and orthorhombic ZnP2 electrodes have been investigated. Recently, Chen et al. synthesized orthorhombic ZnP2 nanowires with diameters ranging from 10 to 50 nm by a solvothermal method (Fig. 14b).198 The electrochemical measurement results showed a high first discharge specific capacity of 1415 mA h g−1 at 0.3C with a high initial coulombic efficiency of 89% and an excellent cycling performance, which demonstrated that ZnP2 nanowires are a promising anode material for lithium ion batteries.
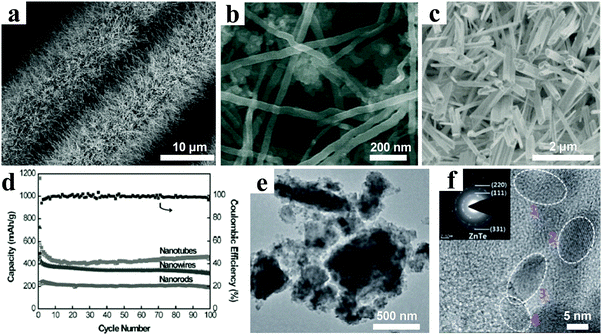 | ||
| Fig. 14 (a) SEM image of Zn3P2 nanowire arrays grown on carbon cloth. Reproduced with permission.197 Copyright 2016, Royal Society of Chemistry. (b) SEM image of ZnP2 nanowires. Reproduced with permission.198 Copyright 2017, Royal Society of Chemistry. (c) SEM image of Zn4Sb3 nanotubes deposited on copper foil; (d) cycling performances of Zn4Sb3 nanotubes, nanowires, and nanorods at 0.1 A g−1. Reproduced with permission.204 Copyright 2013, Wiley-VCH. (e) TEM image and (f) HRTEM image of ZnTe/C nanocomposite, insert showing the SAED pattern. Reproduced with permission.205 Copyright 2014, Royal Society of Chemistry. | ||
Intermetallic compounds ZnxSby, such as ZnSb and Zn4Sb3, have been investigated as anodes for LIBs.199–202 Orthorhombic ZnSb possesses a puckered layer structure, in which Zn and Sb atoms are connected by screw-type layered chains along the b-axis. Park & Sohn reported the quasi-intercalation behavior of ZnSb reacting with Li ions.203 During the charging process, the insertion of Li ions transformed the puckered hexagonal channels into regular hexagonal channels. Then, the layered Zn–Sb planes were rearranged, periodic Li arrays passed through the cleavage, and puckered Zn–Sb chains were recombined. The electrochemical testing results showed an excellent cycling stability with the ZnSb electrode demonstrating 80% capacity retention compared with the first discharge capacity after 1000 cycles at 0.1 A g−1. Xu et al. directly grew one-dimensional Zn4Sb3 structures such as nanotubes, nanowires and nanorods on Cu foil by a simple CVD method (Fig. 14c).204 The obtained Zn4Sb3 nanotubes exhibited better electrochemical performance than the other Zn4Sb3 structures, because the one-dimensional transport nature and the tubular structures are beneficial for high rate performance and excellent cycling durability. In Fig. 14d, a reversible capacity of 450 mA h g−1 after 100 cycles at a current of 0.1 A g−1 is demonstrated.
Recently, ZnTe has been proposed as a potential electrode material for rechargeable LIBs. ZnTe possesses a much higher density of 6.34 g cm−3 compared with those of ZnO (5.61 g cm−3), ZnS (4.09 g cm−3), and ZnSe (5.27 g cm−3), which will result in high volumetric capacity when it is applied in the Li ion battery. Seo & Park prepared a ZnTe/C nanocomposite, composed of 5–10 nm ZnTe nanocrystallites uniformly distributed within a carbon matrix (Fig. 14e and f).205 They revealed the reaction process during the first charging/discharging cycle was as follows:
| ZnTe → Li2Te + Zn → Li2Te + LixZn → Li2Te + LiZn (discharge process) | (4) |
| Li2Te + LiZn → Li2Te + LixZn → Li2Te + Zn → ZnTe (charge process) | (5) |
When applied as anode, the ZnTe/C nanocomposite showed an outstanding electrochemical performance: an initial charge capacity of 530 mA h g−1, long cycling stability holding a specific capacity of 623 mA h g−1 over 200 cycles, and fast rate capability with 92% and 84% retention of the initial charge capacity at rates of 1C and 3C, respectively.
The above novel Zn-based nanomaterials demonstrate superior electrochemical performance including high specific capacity, excellent rate capability, and long cycling life. Compared with ZnO-based materials, these Zn-based electrodes exhibit a series of advantages, such as improved conductivity for ZnS, higher theoretical specific capacity for ZnP2, and higher density for ZnTe. However, continuous efforts are still required to overcome challenges to the practical application of the novel Zn-based electrodes, such as low yields, high cost, complicated procedures, poor conductivity and structural stability.
In general, an SEI film will be formed during the first cycle, which results from the interface reaction of the active materials and the electrolyte. Owing to the irreversibility of Li+ consumption in forming the SEI film, electrodes suffer a relatively large irreversible capacity loss, leading to a low initial coulombic efficiency. But the stable SEI film is favorable to avoid the further reaction of the active materials and the electrolyte and improve the electrochemical performance. During the charging/discharging process, traditional bulk Zn-based anodes undergo huge volumetric change and structural breaking, causing the continuous forming of the SEI film and low coulombic efficiency. Furthermore, the thus continuously thickened SEI film also reduces electrical contact between the active materials and the current collector. Nanostructural Zn-based anodes can effectively overcome the pulverization of electrode materials and form a stable SEI film. Unfortunately, larger irreversible capacity loss and lower initial coulombic efficiency are observed for nanostructural Zn-based anodes, because the high surface area of nanostructural materials provides more contact area between the electrodes and the electrolyte for interface reaction. Therefore, the irreversible capacity loss and the initial coulombic efficiency hold a close relationship with the size, structure and morphology of Zn-based materials.
Enormous efforts have been taken to ameliorate the initial coulombic efficiency of Zn-based anodes. Electrolyte optimization is another effective strategy to improve the lithium storage performance of Zn-based anodes through modifying the surface chemistry.206–208 For example, the addition of fluoroethylene carbonate (FEC) in the electrolyte could promote the formation of a more uniform and stable SEI film and improve the electrochemical performance of the ZnCo2O4 electrode.208 Additionally, coating a protective layer on the Zn-based materials, supplying additional Li resources for pre-lithiation, and modifying binder also have been deployed to reduce the irreversible capacity loss and improve the initial coulombic efficiency.67,209–212
6. Lithium ion full cells with Zn-based anodes
It is worth noting that the aforementioned progress of Zn-based anodes is based on half cells (Table 2). However, in order to further evaluate the performance and practical applicability of Zn-based composites, the study of Zn-based anodes assembled in full cells is desperately needed.213 Zn-based anode full cells formed by coupling with commercial cathode materials (such as LiCoO2, LiFePO4, and LiNi1/3Co1/3Mn1/3O2) have been investigated by a few research groups (Table 3).214–223| Material | Synthesis method | 1st discharge–charge capacity (mA h g−1) | Cycling stability | Rate capability (mA h g−1) | Ref. |
|---|---|---|---|---|---|
| ZnO nanoparticles | Ball milling | 1100/683 at 0.1 A g−1 | 570 mA h g−1 (200 cycles) | 371 at 2C | 33 |
| ZnO nanosheets | Solvothermal | 1523/∼780 at 0.2 A g−1 | 421 mA h g−1 (100 cycles) | 325 at 1 A g−1 | 46 |
| ZnO/carbon black | Atomic layer deposition | ∼1572/1081 at 0.1 A g−1 | 769.5 mA h g−1 (500 cycles) | ∼1014 at 0.5 A g−1 | 60 |
| ∼810 at 2 A g−1 | |||||
| ZnO QD/carbon bubble | CVD & annealing | 1914/1054 at 0.1 A g−1 | 510 mA h g−1 (400 cycles) at 1 A g−1 | 530 at 1 A g−1 | 61 |
| 210 at 8 A g−1 | |||||
| ZnO/graphene oxide | Precipitation | ∼1540/780 at 0.1 A g−1 | 752.8 mA h g−1 (65 cycles) | 404.4 at 1 A g−1 | 68 |
| 259.7 at 2 A g−1 | |||||
| Graphene-wrapped ZnO nanotubes | Hydrothermal & annealing | 1511/1011 at 0.1 A g−1 | 891 mA h g−1 (1000 cycles) at 2 A g−1 | 514 at 0.5 A g−1 | 76 |
| 389 at 2 A g−1 | |||||
| ZnO/multiwalled CNT | Precipitation & annealing | 1477/845 at 0.1 A g−1 | 419.8 mA h g−1 (100 cycles) at 0.2 A g−1 | 326.8 at 1 A g−1 | 82 |
| ZnO@ZnO QDs/C core–shell NRAs | Hydrothermal & annealing | ∼1610/1160 at 0.1 A g−1 | 699 mA h g−1 (100 cycles) at 0.5 A g−1 | 530 at 1 A g−1 | 95 |
| ZnO nanosheet/porous carbon | Hydrothermal & annealing | ∼1920/880 at 0.05 A g−1 | 920 mA h g−1 (150 cycles) | 363 at 1 A g−1 | 96 |
| 50 at 10 A g−1 | |||||
| ZnO/C nanofibers | Electrospinning & annealing | 1750/1319 at 0.1 A g−1 | 452 mA h g−1 (500 cycles) at 2 A g−1 | 591 at 2 A g−1 | 97 |
| YC-ZnO | Precipitation & annealing | 1501/1168 at 0.1 A g−1 | ∼254 mA h g−1 (5000 cycles) at 10 A g−1 | 485.7 at 2 A g−1 | 100 |
| 289.6 at 10 A g−1 | |||||
| ZnO-NMPCS | Sol–gel & hydrothermal | 2627/1667 at 0.2 A g−1 | 425 mA h g−1 (1800 cycles) at 5 A g−1 | 840 at 0.2 A g−1 | 103 |
| 471 at 4 A g−1 | |||||
| Ge-Doped ZnO | CVD | 1496.5/1014 at 0.1 A g−1 | ∼690 mA h g−1 (100 cycles) | ∼515 at 0.4 A g−1 | 112 |
| ∼430 at 0.8 A g−1 | |||||
| Co-Doped ZnO | Sol–gel & annealing | 1338.7/1134.7 at 0.1 A g−1 | 412.5 mA h g−1 (1000 cycles) at 1 A g−1 | 370.4 at 3 A g−1 | 123 |
| Cu-Doped ZnO/carbon | Precipitation & annealing | 1472/876 at 0.2 A g−1 | 769 mA h g−1 (500 cycles) | 350 at 1 A g−1 | 124 |
| Ag-Doped ZnO/carbon | Sol–gel & annealing | 2396/1569 at 0.1 A g−1 | 1670 mA h g−1 (200 cycles) at 0.2 A g−1 | 1063 at 1.6 A g−1 | 129 |
| 526 at 10 A g−1 | |||||
| Co-Doped ZnO | Hydrothermal & annealing | 1692/1241 at 0.5 A g−1 | 804 mA h g−1 (100 cycles) | 738 at 1 A g−1 | 133 |
| ZnO/ZnFe2O4 submicrocubes | Precipitation & annealing | 1892/1371 at 0.1 A g−1 | 837 mA h g−1 (200 cycles) at 1 A g−1 | 728 at 1 A g−1 | 163 |
| 667 at 2 A g−1 | |||||
| Co3O4/ZnO nanosheets | Precipitation & annealing | ∼690/420 at 0.1 A g−1 | 442 mA h g−1 (1000 cycles) at 3 A g−1 | 404 at 4 A g−1 | 164 |
| SnOx/ZnO/C nanofibers | Electrospinning & annealing | 1910/1400 at 0.1 A g−1 | 963 mA h g−1 (55 cycles) | 600 at 0.5 A g−1 | 165 |
| 400 at 1 A g−1 | |||||
| Co3O4/NiO/ZnO nanoflakes | Hydrothermal & annealing | 1454/993 at 0.1 A g−1 | 1060 mA h g−1 (300 cycles) at 0.5 A g−1 | 667 at 2 A g−1 | 166 |
| ZnO/ZnFe2O4/C | Precipitation & annealing | 1385/1047 at 0.5 A g−1 | 1390 mA h g−1 (100 cycles) | 762 at 10 A g−1 | 173 |
| ZnS/C nanoparticles | Hydrothermal & CVD | 1157/694 at 0.1 A g−1 | 506 mA h g−1 (600 cycles) at 0.5 A g−1 | 363 at 5 A g−1 | 184 |
| ZnS/porous carbon | Precipitation & annealing | 1445.3/839.3 at 0.1 A g−1 | 1067.4 mA h g−1 (200 cycles) | 364.6 at 4 A g−1 | 186 |
| ZnSe nanodots/porous carbon | Precipitation & annealing | 871/494 at 0.6 A g−1 | 1134 mA h g−1 (500 cycles) | 696 at 6.4 A g−1 | 191 |
| 474 at 12.8 A g−1 | |||||
| ZnP2 nanowires | Solvothermal | 1575/1415 at 0.3C | 1066 mA h g−1 (500 cycles) | 309 at 4C | 198 |
| Zn4Sb3 nanotubes | CVD | 1160/∼590 at 0.1 A g−1 | 450 mA h g−1 (100 cycles) | 204 | |
| ZnTe/C | Ball milling | 690/530 at 0.1 A g−1 | 623 mA h g−1 (200 cycles) | 550 at 1C | 205 |
| 504 at 3C |
| Anode material | Cathode material | Capacity (mA h g−1) | Cycling stability | Rate capability (mA h g−1) | Ref. |
|---|---|---|---|---|---|
| ZnO/C | LiNi0.6Co0.2Mn0.2O2 | 180 at 0.1C | 126.1 mA h g−1 (30 cycles) at 1C | 214 | |
| ZnCo2O4 nanowires | LiCoO2 | 1314 at 0.2 A g−1 | 1300 mA h g−1 (40 cycles) | 215 | |
| ZnCo2O4 urchins | LiCoO2 | 1175 at 0.2C | 1172 mA h g−1 (50 cycles) | ∼810 at 5C | 216 |
| ZnCo2O4 spheres | LiNi1/3Co1/3Mn1/3O2 | ∼70 at 0.2C | ∼70 mA h g−1 (10 cycles) | 217 | |
| ZnMn2O4/graphene | LiFePO4 | 132 at 0.2C | 124 mA h g−1 (100 cycles) | 122 at 0.5C | 218 |
| 90 at 2C | |||||
| ZnFe2O4/graphene | LiFePO4 | 805 at 0.1 A g−1 | ∼440 mA h g−1 (10 cycles) | 219 | |
| ZnFe2O4/C | LiFePO4/CNT | 41 mA h g−1 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) at 3 mA cm−2 000 cycles) at 3 mA cm−2 |
220 |
Liu et al. directly grew 3D ZnCo2O4 nanowire arrays on flexible carbon cloth as a binder-free anode and coupled this anode with a conventional LiCoO2 cathode to construct a flexible full cell (Fig. 15a).215 The initial discharge capacity of 1314 mA h g−1 and the capacity retention of approximately 1300 mA h g−1 indicated a high reversibility (Fig. 15b). They also synthesized ZnCo2O4 urchins on carbon fiber cloth to fabricate high-performance flexible cells.216 A full cell combining the ZnCo2O4 anode and a commercial LiCoO2 cathode demonstrated a lithium storage capacity of 1172 mA h g−1 even after 50 cycles at 0.2C. Xiong et al. reported flexible full cells with 2D ZnMn2O4/graphene nanosheet anodes and LiFePO4 nanosheet cathodes, considering the advantages of the unique 2D nanosheets, such as excellent electronic conductivity, short Li ion diffusion path, and more active surfaces (Fig. 15c).218 The as-prepared full cells exhibited superior cycling performance and rate capability. Furthermore, the stable cycling behavior could still be obtained under bending, folding and rolling (Fig. 15d). An impressive cycling performance with 85% capacity retention after 10000 cycles based on carbon-coated ZnFe2O4 anode and LiFePO4–CNT composite cathode coupled full cells was demonstrated by Varzi et al. (Fig. 15e).220 In addition, the authors claimed that the observed maximum specific energy and power densities were 202 W h kg−1 and 3.72 kW kg−1, respectively (Fig. 15f). However, the related research on Zn-based full cells is still limited, and more efforts should be made in this field.
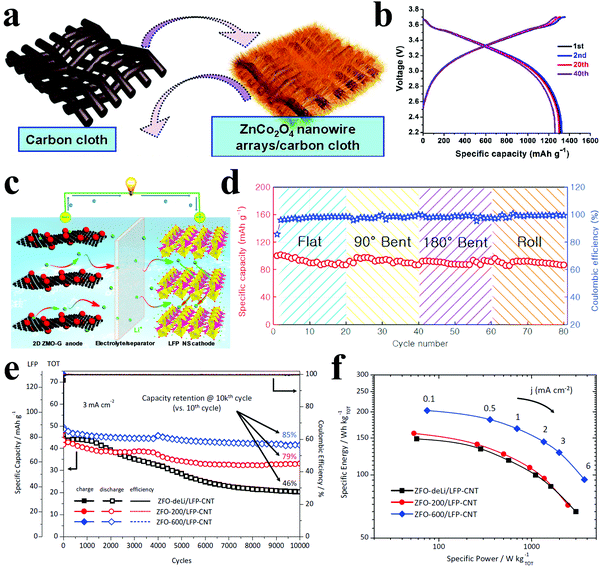 | ||
| Fig. 15 (a) Schematic illustration of the synthesis of flexible 3D ZnCo2O4 nanowire arrays/carbon cloth; (b) charging/discharging curves of the full flexible battery. Reproduced with permission.215 Copyright 2012, American Chemical Society. (c) Schematic of Li-ion full cell using 2D ZnMn2O4/grapheme (ZMO–G) nanosheet anode and LiFePO4 (LFP) nanosheet cathode; (d) cycling performance of the pouch cell under flat, 90° bent, 180° bent, and roll states for 20 cycles, respectively. Reproduced with permission.218 Copyright 2015, Elsevier. (e) Cycling stability and the coulombic efficiency of LFP and TOT (TOT = LFP+ZFO). (f) Ragone-like plot of ZnFe2O4/LiFePO4-CNT (ZFO/LFP-CNT) full cells employing anodes with different degrees of lithiation. Reproduced with permission.220 Copyright 2014, Wiley-VCH. | ||
7. Summary and outlook
In this review, we have summarized the most recent progress of zinc-based nanomaterials as LIB anodes. So far, the main modification methods used to improve the capability for lithium ion storage of ZnO-based anodes fall into four categories. First, various ZnO nanostructures with tunable dimensions have been rationally designed. Benefiting from rationally designed structures with more active sites and superior mechanical stability, these ZnO electrodes display obviously enhanced electrochemical performance. Second, combining ZnO with carbonaceous materials can not only effectively improve electronic conductivity of the electrodes, but also relieve the huge volume expansion during the charging/discharging process. Third, doping ZnO with highly conductive metal heteroatoms is an effective method to enhance the electronic conductivity of ZnO-based anodes, resulting in improved electrochemical properties. Fourth, compositing ZnO with other metal oxides produces outstanding electrochemical performance with the aid of the synergistic effect between the different components. In addition, other newly developed Zn-based nitrogen and oxygen group compounds with unique physical and chemical properties demonstrate fascinating features as anode candidates for LIBs.In view of the unclear mechanism of the synergistic effect in increasing the capacity of ZnO and other metal oxide composites, more attention needs to be paid to in-depth mechanism analysis. At present, ZnO electrode performance still can be further enhanced by heteroatom doping and carbon coating, but the major drawbacks are difficulty in determining the optimized additive proportion of heteroatoms or carbon in the ZnO material and realizing uniform doping or coating. A single modification method can only address a partial issue to a certain degree. Combining two or more approaches simultaneously is worth exploring for Zn-based electrodes with satisfactory reversible capacity and cycling stability. The currently developed modification strategies concentrate on promoting the electrochemical performance of the electrodes. Unfortunately, the issues of cost and mass production are commonly ignored. Development of a cost-effective method to produce high-performance Zn-based electrode materials on a large scale for commercial applications is urgently needed. The solid-phase method may be a potential strategy for the commercialization of ZnO-based anodes because of the facile reaction conditions, low cost, and excellent compatibility with industrial equipment.
Over the past few years, intense research has proved Zn-based nanomaterials to be a promising anode material for LIBs, but there is still a long way to go before real-life application. A series of problems such as cost, initial coulombic efficiency, capacity decay, volumetric energy density, electrical conductivity of materials, and safety should be solved to meet the actual market requirements. In addition, advanced equipment and techniques should also be developed for the nanomaterial systems. Further studies are necessary to determine how nanostructure and morphology can be manipulated to achieve optimal electrochemical performance. And more continuous efforts are required to achieve the final success of Zn-based nanomaterials for next-generation LIBs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the Fundamental Research Funds for the Central Universities (531107040992), National Natural Science Foundation of China (Grant no. 11574078, 51702095), the National Excellent Doctoral Dissertation of China (201318), and the Natural Science Foundation of Hunan Province (2015JJ1008, 2015RS4024).References
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef PubMed.
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef.
- K. Kang, Y. S. Meng, J. Breger, C. P. Grey and G. Ceder, Science, 2006, 311, 977–980 CrossRef PubMed.
- V. Aravindan, Y. S. Lee and S. Madhavi, Adv. Energy Mater., 2015, 5, 1402225 CrossRef.
- Q.-C. Liu, T. Liu, D.-P. Liu, Z.-J. Li, X.-B. Zhang and Y. Zhang, Adv. Mater., 2016, 28, 8413–8418 CrossRef PubMed.
- G. H. Zhang, Y. Song, H. Zhang, J. Xu, H. G. Duan and J. Y. Liu, Adv. Funct. Mater., 2016, 26, 3012–3020 CrossRef.
- J.-J. Xu, Z.-W. Chang, Y.-B. Yin and X.-B. Zhang, ACS Cent. Sci., 2017, 3, 598–604 CrossRef PubMed.
- K. X. Wang, X. H. Li and J. S. Chen, Adv. Mater., 2015, 27, 527–545 CrossRef PubMed.
- S. Yuan, Y.-H. Zhu, W. Li, S. Wang, D. Xu, L. Li, Y. Zhang and X.-B. Zhang, Adv. Mater., 2017, 29, 1602469 CrossRef PubMed.
- L. Wang, G. H. Zhang, X. J. Zhang, H. M. Shi, W. Zeng, H. Zhang, Q. Liu, C. C. Li, Q. H. Liu and H. G. Duan, J. Mater. Chem. A, 2017, 5, 14801–14810 Search PubMed.
- G. M. Zhou, F. Li and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1307–1338 Search PubMed.
- S. Wang, T. Sun, S. Yuan, Y.-h. Zhu, X.-b. Zhang, J.-m. Yan and Q. Jiang, Mater. Horiz., 2017, 4, 1122–1127 RSC.
- J. Zhang, H. Ren, J. Wang, J. Qi, R. Yu, D. Wang and Y. Liu, J. Mater. Chem. A, 2016, 4, 17673–17677 Search PubMed.
- W. Liu, M. S. Song, B. Kong and Y. Cui, Adv. Mater., 2017, 29, 1603436 CrossRef PubMed.
- H. Li, H. Ma, M. Yang, B. Wang, H. Shao, L. Wang, R. Yu and D. Wang, Mater. Res. Bull., 2017, 87, 224–229 CrossRef.
- Q. Liu, Z. Chang, Z. Li and X. Zhang, Small Methods, 2018, 2, 1700231 CrossRef.
- Z. Tang, G. Zhang, H. Zhang, L. Wang, H. Shi, D. Wei and H. Duan, Energy Storage Mater., 2018, 10, 75–84 CrossRef.
- J.-J. Xu and X.-B. Zhang, Nat. Energy, 2017, 2, 17133 CrossRef.
- J. Xu, J. Ma, Q. Fan, S. Guo and S. Dou, Adv. Mater., 2017, 29, 1606454 CrossRef PubMed.
- L.-P. Wang, N.-W. Li, T.-S. Wang, Y.-X. Yin, Y.-G. Guo and C.-R. Wang, Electrochim. Acta, 2017, 244, 172–177 CrossRef.
- Z.-D. Huang, Z. Gong, Q. Kang, Y. Fang, X.-S. Yang, R. Liu, X. Lin, X. Feng, Y. Ma and D. Wang, Mater. Chem. Front., 2017, 1, 1975–1981 RSC.
- M. V. Reddy, G. V. Subba Rao and B. V. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef PubMed.
- K. Cao, T. Jin, L. Yang and L. Jiao, Mater. Chem. Front., 2017, 1, 2213–2242 RSC.
- J. Zhang, P. Gu, J. Xu, H. Xue and H. Pang, Nanoscale, 2016, 8, 18578–18595 RSC.
- Q. Xie, X. Zhang, X. Wu, H. Wu, X. Liu, G. Yue, Y. Yang and D.-L. Peng, Electrochim. Acta, 2014, 125, 659–665 CrossRef.
- Z. Zhou, K. Zhang, J. Liu, H. Peng and G. Li, J. Power Sources, 2015, 285, 406–412 CrossRef.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. Tarascon, Nature, 2000, 407, 496–499 CrossRef PubMed.
- J. Zhang, T. He, W. Zhang, J. Sheng, I. S. Amiinu, Z. Kou, J. Yang, L. Mai and S. Mu, Adv. Energy Mater., 2017, 7, 1602092 CrossRef.
- L. Yu, H. Hu, H. B. Wu and X. W. Lou, Adv. Mater., 2017, 29, 1604563 CrossRef PubMed.
- J.-Y. Li, Q. Xu, G. Li, Y.-X. Yin, L.-J. Wan and Y.-G. Guo, Mater. Chem. Front., 2017, 1, 1691–1708 RSC.
- H. Sun, L. Mei, J. Liang, Z. Zhao, C. Lee, H. Fei, M. Ding, J. Lau, M. Li, C. Wang, X. Xu, G. Hao, B. Papandrea, I. Shakir, B. Dunn, Y. Huang and X. Duan, Science, 2017, 356, 599–604 CrossRef PubMed.
- Q. Su, Z. Dong, J. Zhang, G. Du and B. Xu, Nanotechnology, 2013, 24, 255705 CrossRef PubMed.
- M.-G. Park, G.-K. Sung, N.-E. Sung, J.-H. Kim and C.-M. Park, J. Power Sources, 2016, 328, 607–614 CrossRef.
- H. Li, Y. Wei, Y. Zhang, F. Yin, C. Zhang, G. Wang and Z. Bakenov, Ionics, 2016, 22, 1387–1393 CrossRef.
- W. H. Shin, T. H. Hwang, Y. S. Huh and J. W. Choi, J. Electrochem. Soc., 2012, 159, 2143–2147 CrossRef.
- H. Wang, Q. Pan, Y. Cheng, J. Zhao and G. Yin, Electrochim. Acta, 2009, 54, 2851–2855 CrossRef.
- S. Kundu, S. Sain, M. Yoshio, T. Kar, N. Gunawardhana and S. K. Pradhan, Appl. Surf. Sci., 2015, 329, 206–211 CrossRef.
- A. Kushima, X. H. Liu, G. Zhu, Z. L. Wang, J. Y. Huang and J. Li, Nano Lett., 2011, 11, 4535–4541 CrossRef PubMed.
- Y. Zhang, Z. Wang, Y. Li and K. Zhao, Mech. Mater., 2015, 91, 313–322 CrossRef.
- K. T. Park, F. Xia, S. W. Kim, S. B. Kim, T. Song, U. Paik and W. I. Park, J. Phys. Chem. C, 2013, 117, 1037–1043 Search PubMed.
- H. J. Yang, S. C. Lim, S. Y. He and H. Y. Tuan, RSC Adv., 2015, 5, 33392–33399 RSC.
- H. Asayesh-Ardakani, W. Yao, Y. Yuan, A. Nie, K. Amine, J. Lu and R. Shahbazian-Yassar, Small Methods, 2017, 1, 1700202 CrossRef.
- M. Laurenti, N. Garino, S. Porro, M. Fontana and C. Gerbaldi, J. Alloys Compd., 2015, 640, 321–326 CrossRef.
- Z.-W. Fu, F. Huang, Y. Zhang, Y. Chu and Q.-Z. Qin, J. Electrochem. Soc., 2003, 150, 714–720 CrossRef.
- X. H. Huang, R. Q. Guo, J. B. Wu and P. Zhang, Mater. Lett., 2014, 122, 82–85 CrossRef.
- X. Wang, L. Huang, Y. Zhao, Y. Zhang and G. Zhou, Nanoscale Res. Lett., 2016, 11, 37 CrossRef PubMed.
- F. Li, L. Yang, G. Xu, H. Xiaoqiang, X. Yang, X. Wei, Z. Ren, G. Shen and G. Han, J. Alloys Compd., 2013, 577, 663–668 CrossRef.
- V. Cauda, D. Pugliese, N. Garino, A. Sacco, S. Bianco, F. Bella, A. Lamberti and C. Gerbaldi, Energy, 2014, 65, 639–646 CrossRef.
- N. Garino, A. Lamberti, R. Gazia, A. Chiodoni and C. Gerbaldi, J. Alloys Compd., 2014, 615, 454–458 CrossRef.
- J. Yan, G. Wang, H. Wang, Z. Zhang, X. Ruan, W. Zhao, J. Yun and M. Xu, J. Nanopart. Res., 2015, 17, 52 CrossRef.
- G. Yuan, G. Wang, H. Wang and J. Bai, Ionics, 2014, 21, 365–371 CrossRef.
- L. Xiao, D. Mei, M. Cao, D. Qu and B. Deng, J. Alloys Compd., 2015, 627, 455–462 CrossRef.
- J. Wang, H. Tang, H. Wang, R. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- C. Xiao, S. Zhang, S. Wang, Y. Xing, R. Lin, X. Wei and W. Wang, Electrochim. Acta, 2016, 189, 245–251 CrossRef.
- X. Shen, D. Mu, S. Chen, B. Wu and F. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 3118–3125 Search PubMed.
- C. Zhang, Z. Zhang, F. Yin, Y. Zhang, A. Mentbayeva, M.-R. Babaa, A. Molkenova and Z. Bakenov, ChemElectroChem, 2017, 4, 2359–2365 CrossRef.
- E. Quartarone, V. Dall'Asta, A. Resmini, C. Tealdi, I. G. Tredici, U. A. Tamburini and P. Mustarelli, J. Power Sources, 2016, 320, 314–321 CrossRef.
- C. Chen, H. Zhang, Y. Xu, M. Ji, H. Dong and C. Zhao, RSC Adv., 2015, 5, 40219–40226 RSC.
- J. Liu, Y. Li, R. Ding, J. Jiang, Y. Hu, X. Ji, Q. Chi, Z. Zhu and X. Huang, J. Phys. Chem. C, 2009, 113, 5336–5339 Search PubMed.
- S. Lu, H. Wang, J. Zhou, X. Wu and W. Qin, Nanoscale, 2017, 9, 1184–1192 RSC.
- Z. Tu, G. Yang, H. Song and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 439–446 Search PubMed.
- Z. Bai, Y. Zhang, N. Fan, C. Guo and B. Tang, Mater. Lett., 2014, 119, 16–19 CrossRef.
- G. Z. Yang, H. W. Song, H. Cui, Y. C. Liu and C. X. Wang, Nano Energy, 2013, 2, 579–585 CrossRef.
- Y. Mao, Q. Wang, X. Wu, H. Xie, X. Ma and M. Chen, Energy Technol., 2018, 6, 188–195 CrossRef.
- J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng, Y. Li, H. Liu and S. Dou, Adv. Sci., 2017, 4, 1700146 CrossRef PubMed.
- R. Guo, W. Yue, Y. An, Y. Ren and X. Yan, Electrochim. Acta, 2014, 135, 161–167 CrossRef.
- M. Yu, A. Wang, Y. Wang, C. Li and G. Shi, Nanoscale, 2014, 6, 11419–11424 RSC.
- C. Kim, J. W. Kim, H. Kim, D. H. Kim, C. Choi, Y. S. Jung and J. Park, Chem. Mater., 2016, 28, 8498–8503 CrossRef.
- J. Zheng, J. Lin, R. Chu, C. Wu, J. Zhang, Y. Chen, Y. Zhang and H. Guo, J. Appl. Electrochem., 2017, 47, 969–978 CrossRef.
- M. Yu, D. Shao, F. Lu, X. Sun, H. Sun, T. Hu, G. Wang, S. Sawyer, H. Qiu and J. Lian, Electrochem. Commun., 2013, 34, 312–315 CrossRef.
- Y. Feng, Y. Zhang, X. Song, Y. Wei and V. S. Battaglia, Sustainable Energy Fuels, 2017, 1, 767–779 Search PubMed.
- S. Li, Y. Xiao, X. Wang and M. Cao, Phys. Chem. Chem. Phys., 2014, 16, 25846–25853 RSC.
- H. Fang, L. Zhao, W. Yue, Y. Wang, Y. Jiang and Y. Zhang, Electrochim. Acta, 2015, 186, 397–403 CrossRef.
- C.-T. Hsieh, C.-Y. Lin, Y.-F. Chen and J.-S. Lin, Electrochim. Acta, 2013, 111, 359–365 CrossRef.
- X. Sun, C. Zhou, M. Xie, H. Sun, T. Hu, F. Lu, S. M. Scott, S. M. George and J. Lian, J. Mater. Chem. A, 2014, 2, 7319–7326 Search PubMed.
- Z. H. Li, X. Yu, Y. Liu, W. X. Zhao, H. Zhang, R. M. Xu, D. H. Wang and H. Shen, J. Mater. Chem. A, 2016, 4, 19123–19131 Search PubMed.
- N. Li, S. X. Jin, Q. Y. Liao and C. X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 20590–20596 Search PubMed.
- Y. Zhang, Y. Wei, H. Li, Y. Zhao, F. Yin and X. Wang, Mater. Lett., 2016, 184, 235–238 CrossRef.
- S. M. Abbas, S. T. Hussain, S. Ali, N. Ahmad, N. Ali and S. Abbas, J. Mater. Sci., 2013, 48, 5429–5436 CrossRef.
- Y. Zou, Z. Qi, W. Jiang, J. Duan and Z. Ma, Mater. Lett., 2017, 199, 57–60 CrossRef.
- H. Köse, Ş. Karaal, A. O. Aydın and H. Akbulut, J. Power Sources, 2015, 295, 235–245 CrossRef.
- Y. Zou, Z. Qi, Z. Ma, W. Jiang, R. Hu and J. Duan, J. Electroanal. Chem., 2017, 788, 184–191 CrossRef.
- D. Wang, J. Guo, C. Cui, J. Ma and A. Cao, Mater. Res. Bull., 2018, 101, 305–310 CrossRef.
- H. Zhang, Y. Wang, W. Zhao, M. Zou, Y. Chen, L. Yang, L. Xu, H. Wu and A. Cao, ACS Appl. Mater. Interfaces, 2017, 9, 37813–37822 Search PubMed.
- L. Shen and C. Wang, RSC Adv., 2015, 5, 88989–88995 RSC.
- Q. Gan, K. Zhao, S. Liu and Z. He, Electrochim. Acta, 2017, 250, 292–301 CrossRef.
- Y. Song, Y. Chen, J. Wu, Y. Fu, R. Zhou, S. Chen and L. Wang, J. Alloys Compd., 2017, 694, 1246–1253 CrossRef.
- S. Gao, R. Fan, B. Li, L. Qiang and Y. Yang, Electrochim. Acta, 2016, 215, 171–178 CrossRef.
- Q. Li, H. Zhang, S. Lou, Y. Qu, P. Zuo, Y. Ma, X. Cheng, C. Du, Y. Gao and G. Yin, Ceram. Int., 2017, 43, 11998–12004 CrossRef.
- Y. Han, D. Yu, J. Zhou, P. Xu, P. Qi, Q. Wang, S. Li, X. Fu, X. Gao, C. Jiang, X. Feng and B. Wang, Chemistry, 2017, 23, 11513–11518 CrossRef PubMed.
- C. Shi, Y. Gao, L. Liu, Y. Song, X. Wang, H.-J. Liu and Q. Liu, J. Nanopart. Res., 2016, 18, 371 CrossRef.
- Y. Zhang, Y. Lu, S. Feng, D. Liu, Z. Ma and S. Wang, J. Mater. Chem. A, 2017, 5, 22512–22518 Search PubMed.
- S. J. Yang, S. Nam, T. Kim, J. H. Im, H. Jung, J. H. Kang, S. Wi, B. Park and C. R. Park, J. Am. Chem. Soc., 2013, 135, 7394–7397 CrossRef PubMed.
- Y. Han, P. Qi, S. Li, X. Feng, J. Zhou, H. Li, S. Su, X. Li and B. Wang, Chem. Commun., 2014, 50, 8057–8060 RSC.
- G. Zhang, S. Hou, H. Zhang, W. Zeng, F. Yan, C. C. Li and H. Duan, Adv. Mater., 2015, 27, 2400–2405 CrossRef PubMed.
- A. Li, H. H. Song, Z. Bian, L. L. Shi, X. H. Chen and J. S. Zhou, J. Mater. Chem. A, 2017, 5, 5934–5942 Search PubMed.
- G. H. An, D. Y. Lee and H. J. Ahn, ACS Appl. Mater. Interfaces, 2017, 9, 12478–12485 Search PubMed.
- G. Zhang, H. Zhang, X. Zhang, W. Zeng, Q. Su, G. Du and H. Duan, Electrochim. Acta, 2015, 186, 165–173 CrossRef.
- P. Li, Y. Liu, J. Liu, Z. Li, G. Wu and M. Wu, Chem. Eng. J., 2015, 271, 173–179 CrossRef.
- H. Fan, H. Yu, Y. Zhang, J. Guo, Z. Wang, H. Wang, X. Hao, N. Zhao, H. Geng, Z. Dai, Q. Yan and J. Xu, Nano Energy, 2017, 33, 168–176 CrossRef.
- H. Ning, H. Xie, Q. Zhao, J. Liu, W. Tian, Y. Wang and M. Wu, J. Alloys Compd., 2017, 722, 716–720 CrossRef.
- Y. Liu, Y. Li, M. Zhong, Y. Hu, P. Hu, M. Zhu, W. Li and Y. Li, Mater. Lett., 2016, 171, 244–247 CrossRef.
- F. Sun, J. Gao, H. Wu, X. Liu, L. Wang, X. Pi and Y. Lu, Carbon, 2017, 113, 46–54 CrossRef.
- S. Y. Kim and B.-H. Kim, Synth. Met., 2015, 210, 386–391 CrossRef.
- Z. Ren, Z. Wang, C. Chen, J. Wang, X. Fu, C. Fan and G. Qian, Electrochim. Acta, 2014, 146, 52–59 CrossRef.
- G. Xia, L. Zhang, F. Fang, D. Sun, Z. Guo, H. Liu and X. Yu, Adv. Funct. Mater., 2016, 26, 6188–6196 CrossRef.
- O. B. Chae, S. Park, J. H. Ryu and S. M. Oh, J. Electrochem. Soc., 2012, 160, 11–14 CrossRef.
- D. Wei, Z. Xu, J. Wang, Y. Sun, S. Zeng, W. Li and X. Li, J. Alloys Compd., 2017, 714, 13–19 CrossRef.
- Y. Li, Y. Zhao, G. Huang, B. Xu, B. Wang, R. Pan, C. Men and Y. Mei, ACS Appl. Mater. Interfaces, 2017, 9, 38522–38529 Search PubMed.
- T. Kim, H. Kim, J.-M. Han and J. Kim, Electrochim. Acta, 2017, 253, 190–199 CrossRef.
- Y. Hao, S. Wang, J. Zeng, H. Li, P. Yang, B. Liu, S. Zhang and Y. Xing, Ceram. Int., 2018, 44, 1321–1327 CrossRef.
- Y. Sun, G. Z. Yang, H. Cui, J. Wang and C. X. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 15230–15239 Search PubMed.
- X. Shen, D. Mu, S. Chen, R. Huang and F. Wu, J. Mater. Chem. A, 2014, 2, 4309–4315 Search PubMed.
- L. Fan, J. Zhang, Y. Zhu and Y. Qian, Chin. Sci. Bull., 2014, 59, 2006–2011 CrossRef.
- F. Mueller, D. Geiger, U. Kaiser, S. Passerini and D. Bresser, ChemElectroChem, 2016, 3, 1311–1319 CrossRef.
- L. Zhang, J. Zhang, Y. Liu, P. Zheng, X. Yuan and S. Guo, Mater. Lett., 2016, 165, 165–168 CrossRef.
- J. Duan, S. Yuan, C. Zhu, Z. Chen, G. Zhang, H. Duan, L. Li and Z. Zhu, Synth. Met., 2017, 226, 39–45 CrossRef.
- H. Yang, W. Cui, Y. Han and B. Wang, Chin. Chem. Lett., 2017 DOI:10.1016/j.cclet.2017.09.024.
- Q. Xie, Y. Ma, X. Zhang, L. Wang, G. Yue and D.-L. Peng, J. Alloys Compd., 2015, 619, 235–239 CrossRef.
- M. Ahmad, S. Yingying, A. Nisar, H. Sun, W. Shen, M. Wei and J. Zhu, J. Mater. Chem., 2011, 21, 7723–7729 RSC.
- H. Yue, Z. Shi, Q. Wang, T. du, Y. Ding, J. Zhang, N. Huo and S. Yang, RSC Adv., 2015, 5, 75653–75658 RSC.
- Q. Xie, Y. Ma, D. Zeng, X. Zhang, L. Wang, G. Yue and D. L. Peng, ACS Appl. Mater. Interfaces, 2014, 6, 19895–19904 Search PubMed.
- Y. Xiao and M. Cao, J. Power Sources, 2016, 305, 1–9 CrossRef.
- Q. Xie, L. Lin, Y. Ma, D. Zeng, J. Yang, J. Huang, L. Wang and D.-L. Peng, Electrochim. Acta, 2017, 226, 79–88 CrossRef.
- L. Wang, K. Tang, M. Zhang and J. Xu, Nanoscale Res. Lett., 2015, 10, 280 CrossRef PubMed.
- A. K. Giri, P. Pal, R. Ananthakumar, M. Jayachandran, S. Mahanty and A. B. Panda, Cryst. Growth Des., 2014, 14, 3352–3359 Search PubMed.
- J. Zhao, J. Su, S. Liu, X. Chen, T. Huang and A. Yu, RSC Adv., 2017, 7, 5459–5465 RSC.
- X. Lu, A. Xie, C. Jiang, M. Lu, Y. Zhang, H. Zhong and S. Zhuang, RSC Adv., 2017, 7, 4269–4277 RSC.
- Q. Xie, Y. Ma, X. Wang, D. Zeng, L. Wang, L. Mai and D. L. Peng, ACS Nano, 2016, 10, 1283–1291 CrossRef PubMed.
- Y. Wang, X. Jiang, L. Yang, N. Jia and Y. Ding, ACS Appl. Mater. Interfaces, 2014, 6, 1525–1532 Search PubMed.
- H. Yue, Z. Shi, Q. Wang, Z. Cao, H. Dong, Y. Qiao, Y. Yin and S. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 17067–17074 Search PubMed.
- D. Bresser, F. Mueller, M. Fiedler, S. Krueger, R. Kloepsch, D. Baither, M. Winter, E. Paillard and S. Passerini, Chem. Mater., 2013, 25, 4977–4985 CrossRef.
- J. Yu, S. Chen, W. Hao and S. Zhang, ACS Nano, 2016, 10, 2500–2508 CrossRef PubMed.
- Y. Feng, R. Zou, D. Xia, L. Liu and X. Wang, J. Mater. Chem. A, 2013, 1, 9654–9658 Search PubMed.
- H. Yan, Y. Luo, X. Xu, L. He, J. Tan, Z. Li, X. Hong, P. He and L. Mai, ACS Appl. Mater. Interfaces, 2017, 9, 27707–27714 Search PubMed.
- L. Liu, C. Zhao, H. Zhao, Q. Zhang and Y. Li, Electrochim. Acta, 2014, 135, 224–231 CrossRef.
- X. Chen, Y. Huang, X. Zhang, C. Li, J. Chen and K. Wang, Mater. Lett., 2015, 152, 181–184 CrossRef.
- Z. Yin, J. Qin, W. Wang and M. Cao, Nano Energy, 2017, 31, 367–376 CrossRef.
- J. Li, D. Yan, S. Hou, T. Lu, Y. Yao, D. H. C. Chua and L. Pan, Chem. Eng. J., 2018, 335, 579–589 CrossRef.
- J. Deng, X. Yu, X. Qin, B. Liu, Y.-B. He, B. Li and F. Kang, Energy Storage Mater., 2018, 11, 184–190 CrossRef.
- Q. Pan, L. Qin, J. Liu and H. Wang, Electrochim. Acta, 2010, 55, 5780–5785 CrossRef.
- Y. Huang, X. Chen, K. Zhang and X. Feng, Ceram. Int., 2015, 41, 13532–13540 CrossRef.
- L. Qiao, X. Wang, L. Qiao, X. Sun, X. Li, Y. Zheng and D. He, Nanoscale, 2013, 5, 3037–3042 RSC.
- Q. Xie, Y. Ma, D. Zeng, L. Wang, G. Yue and D. L. Peng, Sci. Rep., 2015, 5, 8351 CrossRef PubMed.
- M.-S. Wu and H.-W. Chang, J. Phys. Chem. C, 2013, 117, 2590–2599 Search PubMed.
- L. Qin, Q. Zhu, G. Li, F. Liu and Q. Pan, J. Mater. Chem., 2012, 22, 7544–7550 RSC.
- C. Zhang, J. Dai, P. Zhang, S. Zhang, H. Zhang, Y. Shen and A. Xie, Ceram. Int., 2016, 42, 1044–1049 CrossRef.
- L. Luo, W. Xu, Z. Xia, Y. Fei, J. Zhu, C. Chen, Y. Lu, Q. Wei, H. Qiao and X. Zhang, Ceram. Int., 2016, 42, 10826–10832 CrossRef.
- H. Köse, Ş. Dombaycıoğlu, A. O. Aydın and H. Akbulut, Int. J. Hydrogen Energy, 2016, 41, 9924–9932 CrossRef.
- N. Feng, L. Qiao, D. Hu, X. Sun, P. Wang and D. He, RSC Adv., 2013, 3, 7758–7764 RSC.
- Q. Guo, S. Chen and X. Qin, Mater. Lett., 2014, 128, 50–53 CrossRef.
- L. Gao, S. Li, D. Huang, Y. Shen and M. Wang, Electrochim. Acta, 2015, 182, 529–536 CrossRef.
- S. H. Choi and Y. C. Kang, Chemistry, 2014, 20, 3014–3018 CrossRef PubMed.
- M. S. Song, S. Nahm, W. I. Cho and C. Lee, Phys. Chem. Chem. Phys., 2015, 17, 23496–23502 RSC.
- J. Deng, X. Yu, Y. He, B. Li, Q.-H. Yang and F. Kang, Energy Storage Mater., 2017, 6, 61–69 CrossRef.
- H.-Q. Dai, H. Xu, Y.-N. Zhou, F. Lu and Z.-W. Fu, J. Phys. Chem. C, 2011, 116, 1519–1525 Search PubMed.
- L. Zhang, T. Wei, J. Yue, L. Sheng, Z. Jiang, D. Yang, L. Yuan and Z. Fan, J. Mater. Chem. A, 2017, 5, 11188–11196 Search PubMed.
- C. Yuan, H. Cao, S. Zhu, H. Hua and L. Hou, J. Mater. Chem. A, 2015, 3, 20389–20398 Search PubMed.
- X. Yang, H. Xue, Q. Yang, R. Yuan, W. Kang and C.-S. Lee, Chem. Eng. J., 2017, 308, 340–346 CrossRef.
- J. Li, K. Du, Y. Lai, Y. Chen and Z. Zhang, J. Mater. Chem. A, 2017, 5, 10843–10848 Search PubMed.
- X. Lu, A. Xie, Y. Zhang, H. Zhong, X. Xu, H. Liu and Q. Xie, Electrochim. Acta, 2017, 249, 79–88 CrossRef.
- D. Cai, H. Zhan and T. Wang, Mater. Lett., 2017, 197, 241–244 CrossRef.
- L. Hou, L. Lian, L. Zhang, G. Pang, C. Yuan and X. Zhang, Adv. Funct. Mater., 2015, 25, 238–246 CrossRef.
- G. Fang, J. Zhou, Y. Cai, S. Liu, X. Tan, A. Pan and S. Liang, J. Mater. Chem. A, 2017, 5, 13983–13993 Search PubMed.
- B. N. Joshi, S. An, H. S. Jo, K. Y. Song, H. G. Park, S. Hwang, S. S. Al-Deyab, W. Y. Yoon and S. S. Yoon, ACS Appl. Mater. Interfaces, 2016, 8, 9446–9453 Search PubMed.
- L. Lu, H. Y. Wang, J. G. Wang, C. Wang and Q. C. Jiang, J. Mater. Chem. A, 2017, 5, 2530–2538 Search PubMed.
- C. W. Lee, S.-D. Seo, D. W. Kim, S. Park, K. Jin, D.-W. Kim and K. S. Hong, Nano Res., 2013, 6, 348–355 CrossRef.
- X. Ge, Z. Li, C. Wang and L. Yin, ACS Appl. Mater. Interfaces, 2015, 7, 26633–26642 Search PubMed.
- Z. Li and L. Yin, J. Mater. Chem. A, 2015, 3, 21569–21577 Search PubMed.
- X. H. Xu, K. Z. Cao, Y. J. Wang and L. F. Jiao, J. Mater. Chem. A, 2016, 4, 6042–6047 Search PubMed.
- M. Jiang, T. Zhou, W. Liu, C. Feng, J. Liu and Z. Guo, RSC Adv., 2017, 7, 17769–17772 RSC.
- J.-L. Niu, C.-H. Zeng, H.-J. Peng, X.-M. Lin, P. Sathishkumar and Y.-P. Cai, Small, 2017, 13, 1702150 CrossRef PubMed.
- F. Zou, X. Hu, Z. Li, L. Qie, C. Hu, R. Zeng, Y. Jiang and Y. Huang, Adv. Mater., 2014, 26, 6622–6628 CrossRef PubMed.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dolle, L. Dupont and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, 627–634 CrossRef.
- Y.-Y. Hu, Z. Liu, K.-W. Nam, O. J. Borkiewicz, J. Cheng, X. Hua, M. T. Dunstan, X. Yu, K. M. Wiaderek, L.-S. Du, K. W. Chapman, P. J. Chupas, X.-Q. Yang and C. P. Grey, Nat. Mater., 2013, 12, 1130–1136 CrossRef PubMed.
- J. Wang, H. Tang, L. Zhang, H. Ren, R. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. Liu, Y. Zhang, Y. Wen, L. Gu, G. Ma, Z. Su, Z. Tang, H. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef.
- M. Keppeler and M. Srinivasan, ChemElectroChem, 2017, 4, 2727–2754 CrossRef.
- L. He, X.-Z. Liao, K. Yang, Y.-S. He, W. Wen and Z.-F. Ma, Electrochim. Acta, 2011, 56, 1213–1218 CrossRef.
- M. L. Mao, L. Jiang, L. C. Wu, M. Zhang and T. H. Wang, J. Mater. Chem. A, 2015, 3, 13384–13389 Search PubMed.
- Z. Chen, R. Wu, H. Wang, Y. Jiang, L. Jin, Y. Guo, Y. Song, F. Fang and D. Sun, Chem. Eng. J., 2017, 326, 680–690 CrossRef.
- J. Li, Y. Fu, X. Shi, Z. Xu and Z. Zhang, Chem. – Eur. J, 2017, 23, 157–166 CrossRef PubMed.
- Y. Fu, Z. A. Zhang, X. Yang, Y. Q. Gan and W. Chen, RSC Adv., 2015, 5, 86941–86944 RSC.
- Y. Feng, Y. Zhang, Y. Wei, X. Song, Y. Fu and V. S. Battaglia, Phys. Chem. Chem. Phys., 2016, 18, 30630–30642 RSC.
- X. Du, H. Zhao, Z. Zhang, Y. Lu, C. Gao, Z. Li, Y. Teng, L. Zhao and K. Świerczek, Electrochim. Acta, 2017, 225, 129–136 CrossRef.
- X. Du, H. Zhao, Y. Lu, Z. Zhang, A. Kulka and K. Świerczek, Electrochim. Acta, 2017, 228, 100–106 CrossRef.
- J. B. Li, D. Yan, X. J. Zhang, S. J. Hou, T. Lu, Y. F. Yao and L. K. Pan, J. Mater. Chem. A, 2017, 5, 20428–20438 Search PubMed.
- Z. H. Wang, X. Y. Cao, P. Ge, L. M. Zhu, L. L. Xie, H. S. Hou, X. Q. Qiu and X. B. Ji, New J. Chem., 2017, 41, 6693–6699 RSC.
- H.-T. Kwon and C.-M. Park, J. Power Sources, 2014, 251, 319–324 CrossRef.
- Y. Fu, Z. Zhang, K. Du, Y. Qu, Q. Li and X. Yang, Mater. Lett., 2015, 146, 96–98 CrossRef.
- Z. Zhang, Y. Fu, X. Yang, Y. Qu and Q. Li, Electrochim. Acta, 2015, 168, 285–291 CrossRef.
- Z. Chen, R. Wu, H. Wang, K. H. L. Zhang, Y. Song, F. Wu, F. Fang and D. Sun, Nano Res., 2018, 11, 966–978 CrossRef.
- Y. Xu, J. Liang, K. Zhang, Y. Zhu, D. Wei and Y. Qian, Electrochem. Commun., 2016, 65, 44–47 CrossRef.
- C. M. Park and H. J. Sohn, Chem. Mater., 2008, 20, 6319–6324 CrossRef.
- X. Wang, H.-M. Kim, Y. Xiao and Y.-K. Sun, J. Mater. Chem. A, 2016, 4, 14915–14931 Search PubMed.
- M.-P. Bichat, J.-L. Pascal, F. Gillot and F. Favier, Chem. Mater., 2005, 17, 6761–6771 CrossRef.
- X. Li, W. Li, J. Yu, H. Zhang, Z. Shi and Z. Guo, J. Alloys Compd., 2017, 724, 932–939 CrossRef.
- W. Li, L. Gan, K. Guo, L. Ke, Y. Wei, H. Li, G. Shen and T. Zhai, Nanoscale, 2016, 8, 8666–8672 RSC.
- J.-Y. Chen, L.-C. Chin, G.-A. Li and H.-Y. Tuan, CrystEngComm, 2017, 19, 975–981 RSC.
- S. Saadat, Y. Y. Tay, J. Zhu, P. F. Teh, S. Maleksaeedi, M. M. Shahjamali, M. Shakerzadeh, M. Srinivasan, B. Y. Tay, H. H. Hng, J. Ma and Q. Yan, Chem. Mater., 2011, 23, 1032–1038 CrossRef.
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC.
- L. Fan, Y. Liu, A. G. Tamirat, Y. Wang and Y. Xia, New J. Chem., 2017, 41, 13060–13066 RSC.
- M.-G. Park, C. K. Lee and C.-M. Park, RSC Adv., 2014, 4, 5830–5833 RSC.
- C. M. Park and H. J. Sohn, Adv. Mater., 2010, 22, 47–52 CrossRef PubMed.
- J. Xu, H. Wu, F. Wang, Y. Xia and G. Zheng, Adv. Energy Mater., 2013, 3, 286–289 CrossRef.
- J.-U. Seo and C.-M. Park, J. Mater. Chem. A, 2014, 2, 20075–20082 Search PubMed.
- F. M. Courtel, H. Duncan, Y. Abu-Lebdeh and I. J. Davidson, J. Mater. Chem., 2011, 21, 10206–10218 RSC.
- H. Duncan, F. M. Courtel and Y. Abu-Lebdeh, J. Electrochem. Soc., 2015, 162, 7110–7117 CrossRef.
- H. Rong, Z. Jiang, S. Cheng, B. Chen, Z. Zhen, B. Deng, Y. Qin, G. Xie, Z.-J. Jiang and M. Liu, RSC Adv., 2017, 7, 18491–18499 RSC.
- J.-H. Lee, M.-H. Hon, Y.-W. Chung and I.-C. Leu, Appl. Phys. A: Mater. Sci. Process., 2010, 102, 545–550 CrossRef.
- J. Li, J. Wang, D. Wexler, D. Shi, J. Liang, H. Liu, S. Xiong and Y. Qian, J. Mater. Chem. A, 2013, 1, 15292–15299 Search PubMed.
- R. Zhang, X. Yang, D. Zhang, H. Qiu, Q. Fu, H. Na, Z. Guo, F. Du, G. Chen and Y. Wei, J. Power Sources, 2015, 285, 227–234 CrossRef.
- J. Fei, Q. Sun, Y. Cui, J. Li and J. Huang, J. Electroanal. Chem., 2017, 804, 158–164 CrossRef.
- M.-S. Balogun, W. Qiu, Y. Luo, H. Meng, W. Mai, A. Onasanya, T. K. Olaniyi and Y. Tong, Nano Res., 2016, 9, 2823–2851 CrossRef.
- G.-L. Xu, Y. Li, T. Ma, Y. Ren, H.-H. Wang, L. Wang, J. Wen, D. Miller, K. Amine and Z. Chen, Nano Energy, 2015, 18, 253–264 CrossRef.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef PubMed.
- B. Liu, X. Wang, B. Liu, Q. Wang, D. Tan, W. Song, X. Hou, D. Chen and G. Shen, Nano Res., 2013, 6, 525–534 CrossRef.
- D. Chen, G. Chen, J. Pei, C. Yan, Y. Hu and Z. Qin, New J. Chem., 2017, 41, 6973–6976 RSC.
- P. Xiong, L. Peng, D. Chen, Y. Zhao, X. Wang and G. Yu, Nano Energy, 2015, 12, 816–823 CrossRef.
- J. Xie, W. Song, G. Cao, T. Zhu, X. Zhao and S. Zhang, RSC Adv., 2014, 4, 7703–7709 RSC.
- A. Varzi, D. Bresser, J. von Zamory, F. Müller and S. Passerini, Adv. Energy Mater., 2014, 4, 1400054 CrossRef PubMed.
- S. Zhong, H. Zhang, J. Fu, H. Shi, L. Wang, W. Zeng, Q. Liu, G. Zhang and H. Duan, ChemElectroChem, 2018 DOI:10.1002/celc.201800287.
- D. Wang, W. Zhou, R. Zhang, X. Huang, J. Zeng, Y. Mao, C. Ding, J. Zhang, J. Liu and G. Wen, J. Mater. Chem. A, 2018, 6, 2974–2983 Search PubMed.
- L. Li, S. Liu, H. Zhou, Q. Lei and K. Qian, Mater. Lett., 2018, 216, 135–138 CrossRef.
| This journal is © the Partner Organisations 2018 |




