Structural, magnetic and electrical transport properties of non-conventionally prepared MAX phases V2AlC and (V/Mn)2AlC†
Christin M.
Hamm‡
a,
Michael
Dürrschnabel
b,
Leopoldo
Molina-Luna
 b,
Ruslan
Salikhov
c,
Detlef
Spoddig
c,
Michael
Farle
cd,
Ulf
Wiedwald
b,
Ruslan
Salikhov
c,
Detlef
Spoddig
c,
Michael
Farle
cd,
Ulf
Wiedwald
 ce and
Christina S.
Birkel
ce and
Christina S.
Birkel
 *a
*a
aEduard-Zintl-Institut für Anorganische und Physikalische Chemie, Technische Universität Darmstadt, 64287 Darmstadt, Germany. E-mail: birkel@ac.chemie.tu-darmstadt.de
bDepartment of Materials and Earth Sciences, Technische Universität Darmstadt, 64287 Darmstadt, Germany
cFaculty of Physics and Center for Nanointegration Duisburg-Essen, University of Duisburg-Essen, 47057 Duisburg, Germany
dCenter for Functionalized Magnetic Materials (FunMagMa), Immanuel Kant Baltic Federal University, Kaliningrad, Russia
eNational University of Science and Technology “MISIS”, Moscow, Russian Federation
First published on 11th January 2018
Abstract
A plethora of magnetic ground states along with intriguing magnetic properties have been reported in thin films of Mn-containing MAX phases. However, fewer results and therefore less knowledge in the area of bulk magnetic MAX phases exist resulting in many open research questions that still remain unanswered. Synthesis of high quality materials is key and is here achieved for bulk V2AlC and its Mn-doped analogs by means of microwave heating and spark plasma sintering. The obtained materials are carefully characterized by structural and microstructural investigations resulting in an average Mn-content of 2% corresponding to the mean chemical composition of (V0.96±0.02Mn0.04±0.02)2AlC in the Mn-doped V2AlC samples. While the parent MAX phase as well as the sample with the nominally lowest Mn-content are obtained essentially single-phase, samples with higher Mn-levels exhibit Mn-rich side phases. These are most likely responsible for the ferromagnetic behavior of the corresponding bulk materials. Besides, we show Pauli paramagnetism of the parent compound V2AlC and a combination of Pauli and Langevin paramagnetism in (V0.96±0.02Mn0.04±0.02)2AlC. For the latter, a magnetic moment of μM = 0.2(2) μB per M atom can be extracted.
Introduction
V2AlC is a member of the group of 70+ transition metal-based ternary carbides that were initially described as H-phases by Jeitschko and Nowotny et al.1,2 and are now better known as MAX phases.3 Structurally, they consist of alternating layers of M6X octahedra and A atoms along the c-axis crystallizing in the hexagonal space group P63/mmc. As a result of this layered structure, MAX phase compounds exhibit interesting properties: they are elastically stiff, yet easily machinable as well as thermal shock resistant and damage tolerant. Since these materials are electrically and thermally conductive they are considered to bridge the gap between ceramics and metals and are therefore a unique class of materials with particularly useful mechanical properties.4 Depending on their chemical composition some MAX phases are also resistant to chemical attack (e.g. Ti3SiC25), and can exhibit self-healing properties (e.g. Ti2AlC,6 Ti2SnC7 and their solid solution Ti2Al(1−x)SnxC8). This makes MAX phases interesting for a plethora of applications where the materials are susceptible to damage cracks (caused by machining, overloading and creep for example) since they can provide/maintain structural integrity.9Long-range magnetic order, as an additional functionality of this class of materials, will significantly broaden the possible fields of application. The earliest discussion about magnetism in MAX phases returns back to 2004, when the electronic structure and related properties of Cr2AlC have been considered.10 The presence of the long-range ordering in these materials and their magnetic structure, however, remains controversial and unsolved.11 The general complexity in this topic arises from the phase purity of MAX phase compounds and presence of different side phases depending on the synthesis method and form (thin films or bulk materials). In 2013, it was shown that Mn-based MAX phases can be stabilized by partial substitution of M elements by Mn.12,13 Furthermore, the Mn2GaC thin films with Mn as the only M element were synthesized extending the M-group members.11,14,15 Further, the first magnetic (V/Mn)3GaC2 MAX phase of a 312 stoichiometry was reported recently.16 The long-range magnetic order with high ordering temperature in Mn-based compounds was predicted theoretically11–13 and confirmed experimentally using magnetometry methods,11–14,16–19 ferromagnetic resonance (FMR),19,20 neutron powder diffraction in bulk (Cr0.96Mn0.04)2GeC21 and neutron reflectometry in Mn2GaC films.22 It was concluded that magnetic moments of highly doped (M1−xMnx)2GaC (x > 0.25) compounds in the ground state (zero magnetic fields) are ferromagnetically (FM) coupled in the basal plane and have canted antiferromagnetic (AFM) spin structure across the Ga-atomic layer.
Pioneering studies, particularly in the field of MAX phase thin films, have already shown highly interesting magnetic phenomena.11 Mn2GaC thin films exhibit magnetically driven anisotropic structural changes interesting for magnetocaloric and magnetoelectric applications.14 (Cr/Mn)2GaC thin films show magnetizations of 0.69 μB per Mn atom and a Curie temperature of TC = 210 K.17,20 Its magnetic behavior is driven by pure spin magnetism yielding a g-factor of 2.00.20 Besides these results obtained from epitaxial thin films, however, there are only a few examples of the preparation and characterization of the corresponding bulk phases. Bulk Mn2GaC has not been synthesized yet, thus a common strategy is needed for incorporation of Mn into known MAX phases. Bulk (Cr1−xMnx)2GeC samples with a maximum x = 0.25 indeed show ferromagnetic behavior with an increasing Curie temperature with increasing x.23 Bulk (Cr1−xMnx)2GaC and (Cr1−xMnx)2AlC (with x = 0.3 and x = 0.06, respectively) have also been prepared. Whereas the Ga-containing MAX phases show magnetic behavior, no magnetic response was found for the Al-containing compound.24
Stabilization of the long-range magnetic order in Al-based MAX phases is of particular interest, since etching of Al atomic layers in these compounds suggests the intriguing opportunity to discover new magnetic MXenes.25,26 It was found, however, that Mn solubility in (Cr/Mn)2AlC phase is low.24,27,28 Exceeding the Mn solubility limit in this compound causes binary carbides and metal-rich phases.27,28 Thus, the search for alternative Al-based MAX phases for Mn incorporation is particularly relevant. Furthermore, partial substitution of Cr or Mn for M elements in ternary MAX phases has influence on the electronic structure and, accordingly, on the physical properties of the host materials.24,27–31
In a previous work, we have prepared bulk Cr2AlC as well as its Mn- and Fe-doped analogs using susceptor-assisted microwave heating as an alternative non-conventional method for MAX phase synthesis.27 The method is used to access intermetallic32 as well as oxide33 phases and is also particularly beneficial for carbide synthesis.34 The main advantage of this time and energy efficient process is the unique heating mechanism, i.e. combination of internal (carbon in precursor mixture) and external (graphite as susceptor) heating. In the present study, we extend the microwave and spark plasma sintering approach to the synthesis of V2AlC and (V1−xMnx)2AlC MAX phases. Subsequent to their non-conventional preparation, we report on comparative analysis of their structure and composition investigated by powder X-ray diffraction and electron microscopy as well as their electrical transport and magnetic properties investigated using a standard four-probe resistivity method and vibrating sample magnetometry.
Experimental section
Bulk samples were prepared starting from vanadium (>99.5%, ∼325 mesh, Sigma Aldrich), manganese (>99.5%, ∼325 mesh, Sigma Aldrich), aluminum (>99.97%, ∼325 mesh, ChemPur) and carbon (>99.9999%, 2–15 micron, Carbone of America) elementan powders that were used as received and stored in an Ar-filled glovebox.In a typical synthesis, the precursors were mixed according to the ratio V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C of 2–2x
C of 2–2x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2x
2x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 with x = 0, 0.05, 0.1 and 0.15, and thoroughly ground inside the glovebox. The resulting mixture was then pressed into a dense pellet with a diameter of 10 mm (5 t, 30 s) and sealed into an evacuated quartz ampoule. For the microwave reaction, the ampoule was surrounded by 7 g of granular graphite (DARCO, Sigma Aldrich), which acts as a susceptor material coupling strongly to the electromagnetic microwave radiation. The reaction setup was placed into an insulation housing to protect the microwave oven (Panasonic) from excessive heat. The heating process was allowed to run for 30 min at a microwave power of 1000 W. After the reaction the ampoule was allowed to cool to room temperature. The sample was then ground to a fine black powder and characterized by powder X-ray diffraction.
0.9 with x = 0, 0.05, 0.1 and 0.15, and thoroughly ground inside the glovebox. The resulting mixture was then pressed into a dense pellet with a diameter of 10 mm (5 t, 30 s) and sealed into an evacuated quartz ampoule. For the microwave reaction, the ampoule was surrounded by 7 g of granular graphite (DARCO, Sigma Aldrich), which acts as a susceptor material coupling strongly to the electromagnetic microwave radiation. The reaction setup was placed into an insulation housing to protect the microwave oven (Panasonic) from excessive heat. The heating process was allowed to run for 30 min at a microwave power of 1000 W. After the reaction the ampoule was allowed to cool to room temperature. The sample was then ground to a fine black powder and characterized by powder X-ray diffraction.
For densification by means of spark plasma sintering (Dr. SinterLab Spark Plasma Sintering System SPS-211Lx, Fuji Electronic Industrial Co., Ltd, Japan), the samples were thoroughly ground and loaded into a graphite die with a diameter of 10 mm using a graphite foil as a separation between the powder and the die. The pressing process was started with a pressure of 30 MPa at room temperature. The pressure was increased to 100 MPa at 572 °C. The final temperature of 1000 °C was held for 15 min. After the holding time the pressure was removed and the sample was allowed to cool to room temperature. The obtained pellet was polished using a diamond plate to remove the graphite foil on the surface. Experimental details on the characterization techniques can be found in the ESI.†
Results and discussion
Structural characterization
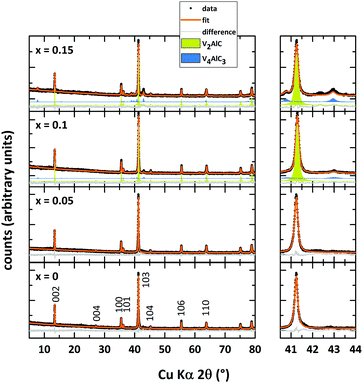
Rietveld refinements of the powder X-ray diffraction data of V2AlC and the Mn-doped analogs after microwave heating and spark plasma sintering are shown in Fig. 1. The structural model with space group P63/mmc (as reported by Jeitschko et al.1) is fitted to the obtained data. Refined unit cell parameters match the literature values well (see Table 1). The parent MAX phase V2AlC is prepared essentially phase pure without any noticeable side phases. According to the XRD data, small doping amounts of Mn (x = 0.05) do not result in the formation of side phases since no extra reflections are detected. Refined unit cell parameters as well as the cell volume of (V0.95Mn0.05)2AlC do not differ significantly from those of the undoped sample. We are not aware of any doping studies of V2AlC with later transition metals thus compare our findings to similar studies of the closely related MAX phase Cr2AlC. We did not find a significant shift in peak positions/change in unit cell parameter for Mn-doped Cr2AlC.27| Nominal composition | x = 0 | x = 0.05 | x = 0.1 | x = 0.15 |
|---|---|---|---|---|
| a Literature data of V2AlC are included for comparison. | ||||
| Unit cell parameter a (Å) | 2.91431(5) 2.91(3)a | 2.91394(6) | 2.91111(5) | 2.91369(7) |
| Unit cell parameter c (Å) | 13.1406(4) 13.1(4)a | 13.1419(5) | 13.1241(4) | 13.1339(6) |
| Cell volume V (Å) | 96.653(5) | 96.639(5) | 96.320(5) | 96.564(6) |
| z (V) | 0.0873(2) | 0.0879(2) | 0.0882(2) | 0.0867(2) |
| GOF | 0.999 | 1.057 | 1.033 | 1.122 |
| Additional phases | — | — | V4AlC3 | V4AlC3 |
Furthermore, Mockute et al. reported that the incorporation of Mn into Cr2AlC does not affect the unit cell parameter c.24 The same is also observed for thin films where the unit cell parameter c of (Cr0.84Mn0.16)2AlC is not changed in comparison to Cr2AlC thin films.35 Increasing amounts of Mn lead to side phase formation of V4AlC3 (blue fit in Fig. 1, maximum of 20% in (V0.85Mn0.15)2AlC). Again, no systematic shift of the peak position of the MAX phase can be observed. However, the lowest cell volume is found for the compound with x = 0.1. In comparison with our results on (Cr1−xMnx)2AlC where the sample with the lowest refined cell volume has incorporated the highest amount of Mn, here this may also provide an indication of V2AlC with the largest Mn doping.
Microstructural characterization
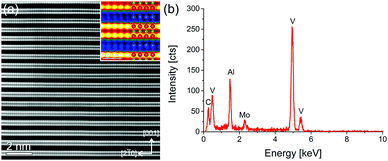
The EDX mapping in Fig. 2 and elemental analysis of EDX point spectra show a homogeneous distribution of Mn in the tested sample area for x = 0.05. Higher Mn dopant levels lead to stronger variations of the local Mn concentration. EDX point spectra analysis at 15 spots with a ratio of V/Al = 2.0 ± 0.1 for each sample reveals Mn concentrations of 1.5 ± 0.8%, 1.2 ± 0.5%, and 2.4 ± 0.6% for x = 0.05, 0.1, and 0.15 incorporated in the V2AlC parent compound, respectively. Note that this amount is small and identical within the error bar and statistics of the EDX measurements.Thus, we conclude that a homogeneous MAX phase can be formed for Mn doping levels up to 3%, while the average doping level is 2% corresponding to (V0.96±0.02Mn0.04±0.02)2AlC (from here on the lowest doped sample will be referred to as (V0.96Mn0.04)2AlC to reflect the actual mean composition). Higher amounts lead to inhomogeneous alloying and Mn-rich precipitates. The presence of different amounts of side phases may dominate the electrical and magnetic properties as discussed below.
Fig. 3(a) shows an atomic resolution STEM-HAADF image of a representative V2AlC grain in [120] zone axis orientation. The layered structure of the material can be identified. The inset on the upper right shows a magnified part of Fig. 3(a) in false colors to enhance the visibility of the Al atomic positions. In addition, an atomic model of the V2AlC unit cell is overlaid for clarity (V: red, Al: blue, and C: green). Fig. 3(b) shows an EDX spectrum of the grain. The Mo peak is due to resputtering during the TEM sample preparation and is not further considered. Quantification of the spectrum by the Cliff–Lorimer k-factor method yielded the following elemental concentration: 25.4 ± 1.9 at% C, 28.0 ± 1.1 at% Al and 46.6 ± 1.4 at% V, which is close to the stoichiometric composition.
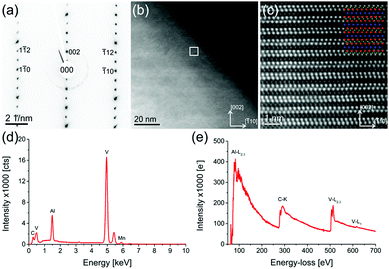
Beside the parent compound, a Mn-doped V2AlC grain is also investigated (sample with nominally x = 0.15). Fig. 4 shows a combined TEM/STEM measurement of a single, representative Mn-doped V2AlC grain. Fig. 4(a) is a selected area diffraction pattern in [110] zone axis orientation, which was acquired using a selected area aperture size of 120 nm. Fig. 4(b) shows a STEM-HAADF image of a thin representative area of the (V1−xMnx)2AlC grain. The white square, indicated in the image, shows the region from which an atomic resolution image (Fig. 4(c)) was acquired. An atomic model of V2AlC with red V atoms, green C atoms and blue Al atoms was overlaid to confirm the layered structure in this orientation. The intensity of the single atomic columns suggests that Mn replaces V. Fig. 4(d) is an EDX spectrum of the grain with a visible Mn peak appearing below 6 keV. Quantification of the spectrum yielded values shown in Table 2. Fig. 4(e) shows a wide-range EELS spectrum of the (V1−xMnx)2AlC grain including all relevant edges, i.e. Al-L2,3, C-K, and V-L2,3. The Mn-L2,3 edge was not observed due to the low Mn concentration and an additional overlap with the V-L1 edge. The fine structure of the C-K and V-L2,3 is similar to those observed in in-plane geometry by Bugnet et al.36 in Cr2AlC except the V-L2,3 white line ratio, which is for V of course different from that of Cr. This also confirms that there is no detectable influence of Mn on the structure of V2AlC. Table 2 contains the quantification of eight EDX spectra acquired in eight different arbitrarily selected grains. The C content is underestimated due to reduced sensitivity of the detector in that particular energy range and X-ray absorption. The TEM-EDX analysis confirms the presence of side phases also observed in the SEM measurements. We find Mn-, V- and C-rich grains as well as aluminium/nitrogen-containing grains that probably formed due to traces of air present during the synthesis. Furthermore, successful incorporation of Mn into MAX phase grains is demonstrated.
| V/at% | Al/at% | C/at% | Mn/at% | Phase |
|---|---|---|---|---|
| a Note that Al, N-rich grains contain roughly 22 at% N. | ||||
| 60.0 ± 0.3 | 28.1 ± 0.2 | 10.9 ± 0.2 | 0.9 ± 0.1 | V2AlC |
| 62.7 ± 0.3 | 25.0 ± 0.2 | 11.4 ± 0.3 | 1.0 ± 0.1 | V2AlC |
| 55.9 ± 0.2 | 30.6 ± 0.2 | 12.6 ± 0.2 | 1.0 ± 0.1 | V2AlC |
| 12.3 ± 0.1 | 52.6 ± 0.2 | 0.0 | 33.2 ± 0.2 | Mn-rich |
| 10.8 ± 0.2 | 1.9 ± 0.1 | 86.6 ± 0.9 | 0.8 ± 0.1 | Carbide |
| 3.5 ± 0.1 | 74.1 ± 0.2 | 0.0 | 0.0 | Al, N-richa |
| 3.1 ± 0.1 | 0.7 ± 0.1 | 96.1 ± 0.7 | 0.0 | C inclusion |
| 91.2 ± 0.5 | 7.3 ± 0.2 | 0.0 | 1.3 ± 0.1 | V-rich |
Transport properties
The temperature dependence of the electrical resistivity ρ in polished (V1−xMnx)2AlC pellets is shown in Fig. 5. Starting from ambient temperature, the resistivity decreases with decreasing temperature in all samples showing an overall standard metallic behavior. The electrical resistivity ρ = 0.8 μΩ m in the ternary parent compound V2AlC at room temperature is a factor of two larger than previously reported values for this MAX phase,37 presumably due to different pellet preparation. The residual resistivity ratio (RRR = ρ(300 K)/ρ(40 K) = 3.2), however, is only two times smaller than reported in Hettinger et al.37 indicating good sample quality and the mismatch in resistivity is the matter of sample homogeneity (grain size, impurity level, structural imperfections etc.). The (V0.96Mn0.04)2AlC MAX phase sample has similar RRR, but smaller residual resistivity at low temperatures as evident in Fig. 5.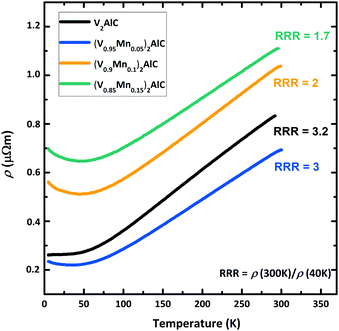 | ||
| Fig. 5 Temperature dependence of the electrical resistivity of polished (V1−xMnx)2AlC pellets measured in zero magnetic field. Note that nominal compositions are given here. | ||
This can be ascribed to an enhanced charge concentration at the Fermi level by substitution of Mn for V in the MAX phase. Electronic density of states (DOS) calculations for different MAX phase compounds suggested that 3d states of M elements dominate the DOS at the Fermi level (D(EF)) and contribute mainly to the electrical conductivity.31,38 This explains the decrease of the residual resistivity when substituting Mn for V. In the magnetic properties section below we show that D(EF) in (V0.96Mn0.04)2AlC is 17% larger than in the parent V2AlC compound. Further Mn doping, however, leads to noticeable enhancement of the resistivity as seen in Fig. 5. This is attributed to the decrease of the carrier mobility due to reduced sample quality as a result of side phases formation revealed by XRD measurements. More reliably, the decrease of the RRR with Mn doping in nominally (V0.9Mn0.1)2AlC and (V0.85Mn0.15)2AlC samples (Fig. 5) evidences sizable scattering from side phase precipitates.
At high temperatures (150–300 K), all samples show a linear temperature dependence due to the phonon contribution as expected for metals and non-magnetic MAX phase compounds.37 The monotonic temperature dependence of the electrical resistivity suggests the absence of phase transitions. For example, a magnetic phase transition has led to a slope change in the ρ(T) curve at the critical temperature as seen in the (Mo0.5Mn0.5)2GaC quaternary19 or to a hump in the Cr2GaN nitride MAX phases.39
While the V2AlC parent compound shows a constant electrical resistivity at low temperatures, the rise of ρ in Mn-doped systems at temperatures below 40 K (Fig. 5) is obvious. Although such increase is not observed in phase pure Mn-based MAX phases,19,39 the increased resistivity at low temperatures can be understood as a result of enhanced electrical charge scattering from paramagnetic Mn atoms in the MAX phase compound as well as from paramagnetic or ferromagnetic Mn-based side phase precipitates. We note that the rise of the resistivity below 40 K becomes larger at larger nominal concentrations of Mn and the deflection temperature coincides with the onset of the Langevin paramagnetism discussed in the next section. Fig. SI-3 (ESI†) presents the identical data on a logarithmic temperature scale. The observation of the logarithmic decrease of the resistivity of Mn-doped samples as indicated by the linear fits can be ascribed to either Kondo effect or weak localization,40 both scaling logarithmically. Based on the present data, however, we cannot decide to one or the other. To separate these two possible contributions, the study of V2AlC doped with other elements would be helpful, but is, however, out of focus of the present study.
Magnetic properties
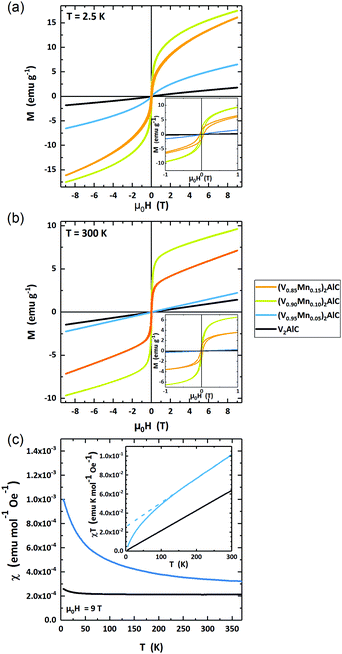
The magnetic response of the four samples is measured in variable fields μ0H = ±9 T and temperatures T = 2.5–370 K. Fig. 6(a) and (b) present a set of selected data of field-dependent magnetization at 2.5 K and 300 K. Additional curves at intermediate temperatures are shown in the ESI† (Fig. SI-1).The parent compound V2AlC and low-doped, (V0.96Mn0.04)2AlC MAX phases are paramagnetic at T = 2.5 K and 300 K. The highly doped samples with nominal composition (V0.90Mn0.10)2AlC and (V0.85Mn0.15)2AlC exhibit magnetic hysteresis with finite remanent magnetization at all temperatures as can be seen in the magnified low field region in the insets of Fig. 6(a) and (b). The observation of hysteresis loops is likely due to a significant amount of ferromagnetic side phases governing the overall magnetic response of the samples. However, the formation of a ferromagnetic MAX phase cannot be excluded. The paramagnetic high field slopes are likely the response of the paramagnetic, Mn-doped MAX phase (V0.96Mn0.04)2AlC as suggested by the microstructural analysis above. Their deconvolution in a strong ferromagnetic and weak paramagnetic component, however, is not unambiguously possible from the magnetometry data. Thus, we restrict the analysis to the phase-pure paramagnetic V2AlC and the lowest doped (V0.96Mn0.04)2AlC samples.
The temperature-dependent magnetic susceptibility χ(T) is presented in Fig. 6(c). The data have been recorded in an external field μ0H = 9 T. The parent compound V2AlC has a constant susceptibility χ = 2.19 × 10−4 emu mol−1 Oe−1 for T > 25 K and a slightly rising χ at lower temperatures. This behavior suggests almost temperature-independent Pauli paramagnetism. Earlier studies reported χ = 2.75–3.50 × 10−4 emu mol−1 Oe−1 for Cr2AlC and χ = 4.1 × 10−4 emu mol−1 Oe−1 for Cr2GaC MAX phases which are similar to the experimental value for V2AlC.23,39,41
The susceptibility of (V0.96Mn0.04)2AlC is strongly temperature dependent and decreases with rising temperature from χ(3 K) = 9.91 × 10−4 emu mol−1 Oe−1 to χ(300 K) = 3.23 × 10−4 emu mol−1 Oe−1. Such behavior can be classified as a Langevin type of paramagnetism. However, the hyperbolic χ(T) of the Langevin paramagnetism is shifted by a constant value (Fig. 6(c)). We ascribe this to the superposition of Langevin and Pauli paramagnetic contributions.
The Pauli and Langevin paramagnetism can be decomposed from the susceptibility curves when plotting χ·T as function of T as shown in the inset of Fig. 6(c). In this diagram, the slope is a measure of the Pauli paramagnetism while the extrapolated intercept gives the Langevin contribution. Here, it is obvious that the parent compound V2AlC is a pure Pauli paramagnet since the line crosses the origin. The extrapolation of the high temperature susceptibility of (V0.96Mn0.04)2AlC yields a Langevin contribution of χL·T = 2.57 × 10−2 emu K mol−1 Oe−1 while the Pauli contribution is χP = 2.56 × 10−4 emu mol−1 Oe−1. The latter is 17% larger than in the V2AlC MAX phase. Assuming the free electron model, the Pauli susceptibility χP is connected to the density of states at the Fermi level D(EF) via χP = μ0μB2D(EF) with μ0 the vacuum permeability and μB Bohr's magneton. Thus, we can calculate D(EF) = 6.78 and 7.91 states per eV and formula unit for V2AlC and (V0.96Mn0.04)2AlC, respectively. This suggests that Mn doping increases D(EF) supporting the reduced residual resistivity of the (V0.95Mn0.05)2AlC MAX phase as compared to V2AlC (Fig. 5). It seems that the experimental D(EF) value using the free electron model is overestimated, since the DFT calculation of V2AlC in Fig. SI-2 (ESI†) delivers 2.7 states per eV and formula unit which is in agreement with earlier theoretical results.42,43 However, the χP of V2AlC is comparable to Cr2AlC which has D(EF) = 8.51 states per eV and formula unit being only about 10% larger (7.3 states per eV and formula unit) than calculated from specific heat measurements.41 This means that the free electron model holds for Cr2AlC, and presumably also for V2AlC. We therefore assume that the discrepancy of D(EF) derived from susceptibility measurements and DFT calculations for V2AlC are the result of an additional temperature-independent contribution to the Pauli paramagnetism.
The Langevin paramagnetism can be attributed to the Mn-doping of the V2AlC MAX phase. Using Curie's law χL = μ0nμ2·(3kBT)−1 with n the number of M atoms per formula unit in the MAX phase, and kB the Boltzmann constant, the magnetic moment μ of the Langevin paramagnetic atoms can be estimated. Here, χL·T = 2.57 × 10−2 emu K mol−1 Oe−1. We deduce the magnetic moment μ = 0.453 μB per formula unit. Since the parent compound shows negligibly small Langevin paramagnetism, it is natural ascribing this contribution to the incorporation of Mn atoms in the MAX phase. If the Langevin part solely belongs to the Mn atoms for (V0.96Mn0.04)2AlC, it follows a Mn magnetization of μeff = 4.53 μB per Mn atom, which is close to the high spin Mn2+ configuration of 5 μB per Mn atom according Hund's rules. This value, however, appears too high as compared to many intermetallic Mn compounds of 1.2–3.5 μB per Mn atom.44–46 Thus, a polarization of V should be considered in future studies.
Overall, the magnetic characterization is in line with the structural data suggesting phase pure MAX phase samples with up to 4% Mn doping. Higher doping results in ferromagnetic response that is likely due to the formation of side phases, since the above limit of Mn incorporation is reached and the remaining Mn forms Mn-rich regions, probably in different phases. (V0.96Mn0.04)2AlC is paramagnetic as the parent compound V2AlC. In addition to the Pauli paramagnetism, the Mn-doped crystal exhibits Langevin paramagnetism that is about four times larger than the Pauli contribution at T = 5 K.
Conclusions
We have successfully used the non-conventional solid-state synthesis method based on microwave heating and spark plasma sintering to prepare the MAX phase V2AlC as well as its Mn-doped analogs (V1−xMnx)2AlC (with x = 0.05, 0.1, 0.15). All compounds are obtained in high quality with perfectly crystalline single grains as supported by TEM analysis. Powder X-ray diffraction data after densification show that V2AlC and (V1−xMnx)2AlC with a nominal x = 0.05 are obtained single-phase while small amounts of V4AlC3 are identified in the Mn-doped samples with higher doping levels (x = 0.1 and 0.15). No clear shift in the reflection position upon incorporation of Mn is observed which has also been reported for other Mn-doped MAX phases.24,27 In order to determine the level of Mn-doping, electron microscopy analyses on different length scales were conducted. EDX mapping as well as EDX point spectra show a homogeneous Mn distribution in the MAX phase with the nominal x = 0.05. Samples with higher nominal x-values exhibit areas with higher Mn concentrations due to Mn-rich segregations. The average amount of Mn incorporation in all doped MAX phases is 2% corresponding to a mean chemical composition of (V0.96±0.02Mn0.04±0.02)2AlC. Successful Mn incorporation in V2AlC is also supported by TEM analyses showing grains with roughly 1 at% Mn that match the MAX phase structure. Electronic transport measurements reveal metallic behavior for all samples studied here, in line with the reported metallic character of MAX phases in general. These data also show the good sample quality of the parent compound as well as the sample with the nominally smallest Mn concentration. The monotonous decrease of the resistivity with decreasing temperature suggests the absence of magnetic phase transitions. The parent compound V2AlC is a Pauli paramagnet with a nearly temperature-independent susceptibility. While the Mn-doped sample, (V0.96Mn0.04)2AlC, also exhibits paramagnetic behavior, the nominally higher doped samples clearly show ferromagnetic behavior with magnetic hysteresis. At this point, we cannot confirm the presence of a ferromagnetic MAX phase and assign the ferromagnetic nature of the materials to the presence of Mn-rich side phases as shown in the microstructural analysis. The magnetic behavior of the essentially single phase Mn-doped MAX phase with a composition of (V0.96Mn0.04)2AlC is the combination of Pauli paramagnetism (as found in the parent compound) and Langevin paramagnetism. Our results clearly demonstrate the necessary combination of detailed structural and microstructural analyses with the measurement of materials properties in order to understand the materials of interest. Here we provide important data and discussions in order to drive the research area of bulk magnetic MAX phases further.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the DFG (BI 1775/2-1) and the German federal state of Hessen through its excellence program LOEWE “RESPONSE” is gratefully acknowledged. M. D. and L. M.-L. also acknowledge financial support from the Hessen State Ministry of Higher Education, Research and the Arts via LOEWE RESPONSE. L. M.-L. acknowledges financial support from DFG Grant MO 3010/3-1. The transmission electron microscope used in this work was partially funded by the German Research Foundation (DFG/INST163/2951). The authors thank S. Kausch for TEM sample preparation. We thank Marina Spasova (Univ. Duisburg-Essen) for fruitful discussions. R. S. acknowledges funding by the DFG under grant number SA 3095/2-1.References
- W. Jeitschko, Monatsh. Chem. Verw. Teile Anderer Wiss., 1963, 94, 672–676 CrossRef CAS.
- W. Jeitschko, PhD thesis, Universität Wien, 1964.
- M. W. Barsoum, MAX phases: Properties of Machinable Carbides and Nitrides, 2013 Search PubMed.
- M. Radovic and M. W. Barsoum, Am. Ceram. Soc. Bull., 2013, 92, 20–27 CAS.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201–281 CrossRef CAS.
- W. G. Sloof, R. Pei, S. A. McDonald, J. L. Fife, L. Shen, L. Boatemaa, A.-S. Farle, K. Yan, X. Zhang, S. van der Zwaag, P. D. Lee and P. J. Withers, Sci. Rep., 2016, 6, 23040 CrossRef CAS PubMed.
- S. Li, G. Bei, X. Chen, L. Zhang, Y. Zhou, M. Mačković, E. Spiecker and P. Greil, J. Eur. Ceram. Soc., 2016, 36, 25–32 CrossRef CAS.
- G. Bei, B.-J. Pedimonte, T. Fey and P. Greil, J. Am. Ceram. Soc., 2013, 96, 1359–1362 CrossRef CAS.
- P. Greil, J. Adv. Ceram., 2013, 1, 249–267 CrossRef.
- J. M. Schneider, Z. Sun, R. Mertens, F. Uestel and R. Ahuja, Solid State Commun., 2004, 130, 445–449 CrossRef CAS.
- A. S. Ingason, M. Dahlqvist and J. Rosén, J. Phys.: Condens. Matter, 2016, 28, 433003 CrossRef CAS PubMed.
- A. S. Ingason, A. Mockute, M. Dahlqvist, F. Magnus, S. Olafsson, U. B. Arnalds, B. Alling, I. A. Abrikosov, B. Hjörvarsson, P. O. Å. Persson and J. Rosén, Phys. Rev. Lett., 2013, 110, 195502 CrossRef CAS PubMed.
- A. Mockute, P. O. Å. Persson, F. Magnus, A. S. Ingason, S. Olafsson, L. Hultman and J. Rosén, Phys. Status Solidi RRL, 2014, 8, 420–423 CrossRef CAS.
- M. Dahlqvist, A. S. Ingason, B. Alling, F. Magnus, A. Thore, A. Petruhins, A. Mockute, U. B. Arnalds, M. Sahlberg, B. Hjörvarsson, I. A. Abrikosov and J. Rosén, Phys. Rev. B, 2016, 93, 014410 CrossRef.
- A. S. Ingason, A. Petruhins, M. Dahlqvist, F. Magnus, A. Mockute, B. Alling, L. Hultman, I. A. Abrikosov, P. O. Å. Persson and J. Rosén, Mater. Res. Lett., 2013, 2, 89–93 CrossRef.
- Q. Tao, R. Salikhov, A. Mockute, J. Lu, M. Farle, U. Wiedwald, J. Rosen, Q. Tao, R. Salikhov, A. Mockute, J. Lu, M. Farle and U. Wiedwald, APL Mater., 2016, 4, 086109 CrossRef.
- A. Petruhins, A. S. Ingason, J. Lu, F. Magnus, S. Olafsson and J. Rosen, J. Mater. Sci., 2015, 50, 4495–4502 CrossRef CAS.
- Z. Liu, T. Waki, Y. Tabata and H. Nakamura, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 054435 CrossRef.
- R. Salikhov, R. Meshkian, D. Weller, B. Zingsem, D. Spoddig, J. Lu, A. S. Ingason, H. Zhang, J. Rosen, U. Wiedwald and M. Farle, J. Appl. Phys., 2017, 121, 0–7 CrossRef CAS.
- R. Salikhov, A. S. Semisalova, A. Petruhins, A. S. Ingason, J. Rosen, U. Wiedwald and M. Farle, Mater. Res. Lett., 2015, 3, 156–160 CrossRef CAS.
- O. Rivin, E. N. Caspi, A. Pesach, H. Shaked, A. Hoser, R. Georgii, Q. Tao, J. Rosen and M. W. Barsoum, Mater. Res. Innovations, 2017, 5, 465–471 Search PubMed.
- A. S. Ingason, G. K. Pálsson, M. Dahlqvist and J. Rosen, Phys. Rev. B, 2016, 94, 024416 CrossRef.
- Z. Liu, T. Waki, Y. Tabata and H. Nakamura, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 054435 CrossRef.
- A. Mockute, J. Lu, E. J. Moon, M. Yan, B. Anasori, S. J. May, M. W. Barsoum and J. Rosén, Mater. Res. Lett., 2014, 3, 16–22 CrossRef.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- C. M. Hamm, J. D. Bocarsly, G. Seward, U. I. Kramm and C. S. Birkel, J. Mater. Chem. C, 2017, 5, 5700–5708 RSC.
- H. Li, S. Li, H. Mao and Y. Zhou, Adv. Appl. Ceram., 2017, 116, 165–172 CrossRef CAS.
- V. Saltas, D. Horlait, E. Sgourou, F. Vallianatos and A. Chroneos, Appl. Phys. Rev., 2017, 4, 041301 Search PubMed.
- P. A. Burr, D. Horlait and W. E. Lee, Mater. Res. Lett., 2017, 5, 144–157 CAS.
- M. Magnuson and M. Mattesini, Thin Solid Films, 2017, 621, 108–130 CrossRef CAS.
- C. S. Birkel, W. G. Zeier, J. E. Douglas, B. R. Lettiere, C. E. Mills, G. Seward, A. Birkel, M. L. Snedaker, Y. Zhang, G. J. Snyder, T. M. Pollock, R. Seshadri and G. D. Stucky, Chem. Mater., 2012, 24, 2558–2565 CrossRef CAS.
- A. Birkel, K. A. Denault, N. C. George, C. E. Doll, B. Hery, A. A. Mikhailovsky, C. S. Birkel, B. Hong and R. Seshadri, Chem. Mater., 2012, 24, 1198–1204 CrossRef CAS.
- C. M. Hamm, T. Schäfer, H. Zhang and C. S. Birkel, Z. Anorg. Allg. Chem., 2016, 642, 1397–1401 CrossRef CAS.
- A. Mockute, M. Dahlqvist, J. Emmerlich, L. Hultman, J. Schneider, P. O. Å. Persson and J. Rosén, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 094113 CrossRef.
- M. Bugnet, M. Jaouen, V. Mauchamp, T. Cabioc’H and G. Hug, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 195116 CrossRef.
- J. D. Hettinger, S. E. Lofland, P. Finkel, T. Meehan, J. Palma, K. Harrell, S. Gupta, A. Ganguly, T. El-Raghy and M. W. Barsoum, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 2–7 CrossRef.
- T. Ouisse and M. W. Barsoum, Mater. Res. Lett., 2017, 5, 365–378 CrossRef.
- Z. Liu, T. Waki, Y. Tabata, K. Yuge, H. Nakamura and I. Watanabe, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 134401 CrossRef.
- G. Bergmann, Phys. Rep., 1984, 107, 1–58 CrossRef CAS.
- Z. Liu, K. Takao, T. Waki, Y. Tabata and H. Nakamura, J. Phys.: Conf. Ser., 2017, 868, 012016 CrossRef.
- J. M. Schneider, R. Mertens and D. Music, J. Appl. Phys., 2006, 99, 013501 CrossRef.
- S. E. Lofland, J. D. Hettinger, T. Meehan, A. Bryan, P. Finkel, S. Gupta, M. W. Barsoum and G. Hug, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 174501 CrossRef.
- K. Kamishima, M. Bartashevich, T. Goto, M. Kikuchi and T. Kanomata, J. Phys. Soc. Jpn., 1998, 67, 1748–1754 CrossRef CAS.
- P. J. Brown, V. Nunez, F. Tasset, J. B. Forsyth and P. Radhakrishna, J. Phys.: Condens. Matter, 1990, 2, 9409–9422 CrossRef CAS.
- S. C. Ma, K. Liu, C. C. Ma, Q. Ge, J. T. Zhang, Y. F. Hu, E. K. Liu and Z. C. Zhong, Appl. Phys. Lett., 2017, 111, 232404 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, magnetization curves, density of states of V2AlC. See DOI: 10.1039/c7qm00488e |
| ‡ Current address: DECHEMA-Forschungsinstitut, Electrochemistry, Theodor-Heuss-Allee 25, 60486 Frankfurt a. Main, Germany. |
| This journal is © the Partner Organisations 2018 |