Reversible colour changes of mixed films composed of 4,4′-bis[bis(4-methylphenyl)amino]azobenzene and organic acids in response to exhaled breath†
Yuya
Kitamura
,
Ryoji
Ichikawa
and
Hideyuki
Nakano
 *
*
Department of Applied Chemistry, Muroran Institute of Technology, 27-1, Mizumoto-cho, Muroran, Hokkaido 050-8585, Japan. E-mail: nakano@mmm.muroran-it.ac.jp
First published on 1st November 2017
Abstract
We found that mixed films made of the title aminoazobenzene-based material with various organic acids exhibited reversible colour changes in response to exhaled breath. Such reversible colour changes were shown to be general phenomena for films composed of aminoazobenzene-based amorphous molecular materials mixed together with organic acids. The ability of this type of film to reversibly change colour was suggested to be due to protonation of the aminoazobenzene caused by the organic acid and deprotonation by moisture, and the acidity levels and concentrations of the organic acids were suggested to be important factors affecting the colour of the film and its response to moisture.
Introduction
The phenomena of reversible colour changes in response to external stimuli are attracting current interest both from the viewpoints of fundamental science and practical applications. Azobenzene derivatives are materials that exhibit such phenomena and have been widely used not only as colouring agents such as food dyes and pigments but also for a variety of stimuli-responsive materials such as pH indicators,1 photochromic and photo-responsive materials,2etc. Very recently, photomechanical effects observed for azobenzene-based materials have been attracting a great deal of attention.3–5We have been studying the creation of low-molecular-mass materials that readily form amorphous glasses above room temperature, namely amorphous molecular materials.6,7 Amorphous molecular materials form smooth amorphous films easily obtained not only by the spin-coating method but also by vacuum deposition. Photo- and electro-active amorphous molecular materials have been used to provide a variety of functional films having a quite high concentration of functional moieties and having no grain boundaries between their moieties. Amorphous molecular materials also function as matrices that dissolve a variety of materials to readily provide mixed amorphous films.
Of the various types of stimuli-induced reversible colour changes, vapochromism is one of the most attractive such phenomena in that it involves responses to the surrounding vapours.8 Vapochromic materials are expected to provide visually recognizable environmental sensors. For example, nanoporous TiO2 and Al2O3 films adsorbed with methylene blue were recently reported to exhibit a rapid colour change in response to water vapour.9 As a related phenomenon, vapochromic luminescence is also a subject of interest.8,10 We recently reported that light-emitting amorphous molecular materials based on diarylaminobenzaldehydes function as luminescent vapochromic materials; that is, the colour of light emitted by their amorphous films has been shown to drastically change upon exposure of the films to a variety of organic solvent vapours.11
In addition to single amorphous molecular material systems, the hybridization of such materials with other materials is also of interest for the purpose of providing unique amorphous systems exhibiting reversible colour changes. We have reported preliminary results about mixed amorphous films, specifically those consisting of aminoazobenzene-based amorphous molecular materials such as 4-[bis(4-methylphenyl)amino]azobenzene (BMAB) and 4-[phenyl(biphenyl-4-yl)amino]azobenzene (PBAB) mixed with p-toluene sulfonic acid (TsOH), exhibiting drastic and reversible colour change in response to exhaled breath.12 In addition, reversible changes in fluorescence emission in response to exhaled breath were achieved using the mixed film composed of 4-[bis(4-methylphenyl)amino]benzaldehyde and TsOH.13 In these systems, moisture in the exhaled breath was suggested to play an important role in producing the reversible changes.
In the present study, in order to clarify the generality of the phenomena of reversible colour changes in response to exhaled breath observed for such mixed systems and to gain information about the mechanism or mechanisms of the phenomena, we fabricated new types of mixed films, specifically films including an aminoazobenzene-based amorphous molecular material, 4,4′-bis[bis(4-methylphenyl)amino]azobenzene (BBMAB), mixed together with various organic acids, specifically p-toluene sulfonic acid (TsOH), 2-naphthalenesulfonic acid (NSA), pentafluorobenzoic acid (PFBA), phenylphosphonic acid (PPA) or benzoic acid (BA), and investigated the relationship between the kind of acid used and the behaviour of the reversible colour change in response to exhaled breath.
Experimentals
Materials
BBMAB was prepared according to the method described in our previous paper.7c TsOH, NSA, PFBA, PPA, BA and poly(methyl methacrylate) (PMMA) were purchased commercially.Preparation of sample films
BBMAB (1 mg) and an appropriate amount of the tested organic acid were dissolved in ca. 0.5 mL of THF together with 1 mg of PMMA in order to stabilize the amorphous state of the film. For each acid tested, the amorphous film was obtained by spin-coating (1500 rpm) the mixed solution onto a transparent glass substrate. Each of the sample films was dried in vacuum conditions for more than 1 h before being used.Measurements and apparatus
Electronic absorption spectra of the films were acquired by using BLUE-Wave miniature spectrometers (StellarNet, Inc.) in a handmade Styrofoam box. For acquiring the spectra at high humidity, the humidity around the sample films was increased by placing a wet paper in the box and monitored by using a Palma-II hygrometer (Sato Keiryoki MFG Co., Ltd).DFT calculations
Optimized molecular structures of BBMAB and its protonated species were calculated based on density-function theory at the B3LYP/6-31G(d,p) level. The results are described in ESI.† In order to calculate their electronic absorption spectra, we performed time-dependent DFT calculations involving single point energy calculations using the last optimized structures. These calculations were performed using the Gaussian 09 software package.14Results and discussion
Because of the difficulty in obtaining stable amorphous films including PFBA due to the crystallization of PFBA, PMMA was used as a stabilizer for all films in the present study although other films could be obtained as smooth films without the stabilizer. Fig. 1 shows photographs of the film containing BBMAB and PFBA with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 together with PMMA as a stabilizer (hereafter, in the present paper, we abbreviate the film as “BBMAB–PFBA(6) film” indicating the kind of organic acid with the molar ratio). In the ambient atmosphere (at ca. 21 °C and less than 20% RH), the film exhibited a bluish green colour (Fig. 1a). When we breathed onto the film, the colour was found to change instantly and drastically to yellow as shown in Fig. 1b. When pausing the exposure of the film to exhaled breath, the colour returned to the original bluish green within a few seconds. The response time of these colour changes was apparently too short to be measured by our equipment. Such colour changes were observed repeatedly, being clearly visible after more than a few dozen cycles. As described below, the BBMAB–PFBA(3), BBMAB–TsOH(6), BBMAB–NSA(6) and BBMAB–PPA(6) films also exhibited similar colour changes in response to exhaled breath. Thus, such reversible colour change seemed to be a general phenomenon observed for the films containing aminoazobenzene-based materials and organic acids.
6 together with PMMA as a stabilizer (hereafter, in the present paper, we abbreviate the film as “BBMAB–PFBA(6) film” indicating the kind of organic acid with the molar ratio). In the ambient atmosphere (at ca. 21 °C and less than 20% RH), the film exhibited a bluish green colour (Fig. 1a). When we breathed onto the film, the colour was found to change instantly and drastically to yellow as shown in Fig. 1b. When pausing the exposure of the film to exhaled breath, the colour returned to the original bluish green within a few seconds. The response time of these colour changes was apparently too short to be measured by our equipment. Such colour changes were observed repeatedly, being clearly visible after more than a few dozen cycles. As described below, the BBMAB–PFBA(3), BBMAB–TsOH(6), BBMAB–NSA(6) and BBMAB–PPA(6) films also exhibited similar colour changes in response to exhaled breath. Thus, such reversible colour change seemed to be a general phenomenon observed for the films containing aminoazobenzene-based materials and organic acids.
 | ||
| Fig. 1 Photographs of the BBMAB–PFBA(6) film (a) in the ambient atmosphere and (b) when exposed to exhaled breath. | ||
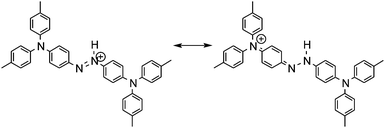
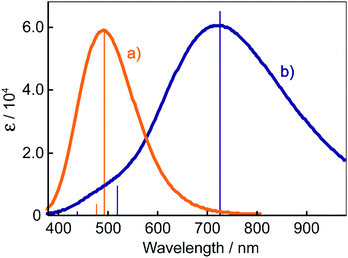
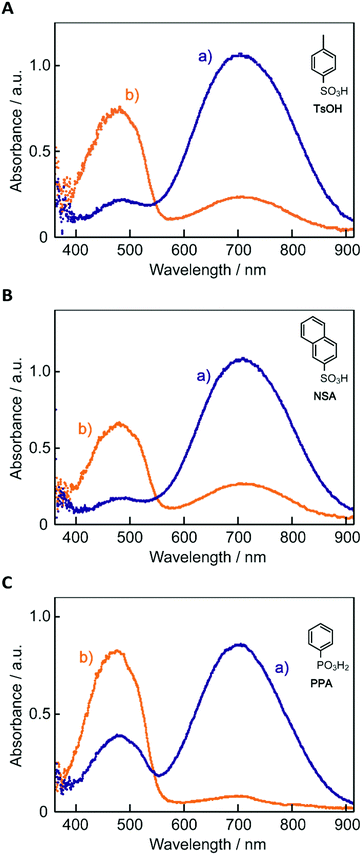
The electronic absorption spectra of BBMAB–PFBA(3) and BBMAB–PFBA(6) films are shown in Fig. 2. Each spectrum was found to have two bands, at wavelengths of about 480 nm and 700 nm. When we breathed onto these films, the band at about 480 nm was enhanced and simultaneously the absorbance at 700 nm decreased. The original spectra were recovered immediately upon pausing the exposure of the films to the exhaled breath. With regard to the spectra in the ambient atmosphere, the ratio of the absorbance at 700 nm relative to that at 480 nm was greater for the BBMAB–PFBA(6) film for the BBMAB–PFBA(3) film. The band at about 480 nm was almost identical in shape and position to that of the electronic absorption spectrum of the amorphous film of BBMAB without organic acid,5 and was hence attributed to neutral BBMAB. On the other hand, the band at about 700 nm was enhanced by increasing the concentration of the acid PFBA, and was hence attributed to the protonated species of BBMAB (BBMAB–H+). Since resonance structures of BBMAB–H+ can be drawn as shown in Scheme 1, a considerable protonation-induced red shift of the electronic absorption may be caused by extension of the conjugation as well as a change in electronic structure caused by protonation. The absorption bands at about 480 nm and 700 nm were also suggested, based on the results of ab initio calculations, to be due to the neutral BBMAB and protonated BBMAB–H+ species, respectively. These electronic absorption spectra obtained by performing DFT calculations are shown in Fig. 3. Both the BBMAB and BBMAB–H+ spectra were observed to agree well with experimental results, with these absorption bands being attributed to their HOMO–LUMO transitions.
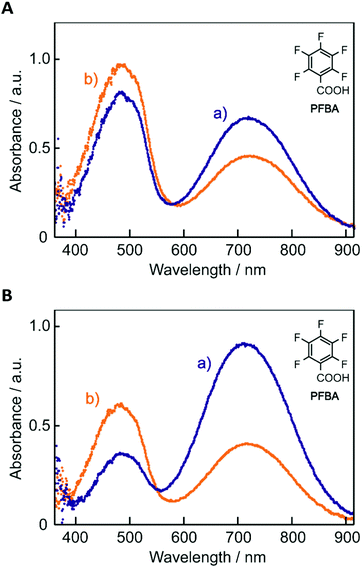 | ||
| Fig. 2 Electronic absorption spectra of (A) BBMAB–PFBA(3) and (B) BBMAB–PFBA(6) films (a) in the ambient atmosphere and (b) exposed to exhaled breath. | ||
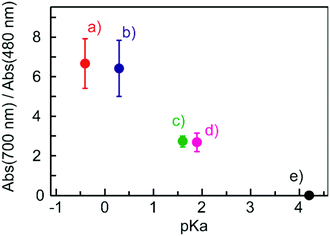
Fig. 4 shows electronic absorption spectra of the films using the other organic acids. These films were found to exhibit reversible changes in the spectrum similar to those for the BBMAB–PFBA(3) and BBMAB–PFBA(6) films. With regard to the spectra at ambient atmosphere, the ratio of the absorbance at 700 nm relative to that at 480 nm seemed to depend on the kind of organic acid used. In addition, no band at about 700 nm was observed for the BBMAB–BA(6) film, the spectrum being identical to that for the single BBMAB film. Thus, the acidity of the organic acid was assumed to be an important factor. Fig. 5 shows the ratio of absorbance at 700 nm relative to that at 480 nm vs. pKa value of the organic acid.‡ The results indicated this ratio increased with decreasing pKa; that is, the concentration of BBMAB–H+ increased with increasing acidity of the organic acid. In addition to the acidity levels of the organic acids, their concentrations also affected the colour of the film as described above. In fact, the ratios of absorbance at 700 nm relative to that at 480 nm were 0.81 and 2.71 for the BBMAB–PFBA(3) and BBMAB–PFBA(6), respectively, suggesting that the concentration of BBMAB–H+ increased with increasing concentration of the organic acid. With regard to TsOH, the ratios of the absorbance at 700 nm relative to that at 480 nm were 4.91 and 6.67 for BBMAB–TsOH(3) and BBMAB–TsOH(6), respectively. Although the ratio also depended on the concentration of TsOH, the concentration dependence of the ratio was lower than that for PFBA system, the concentration of BBMAB–H+ being high enough even for BBMAB–TsOH(3). This relatively low concentration dependence was caused by the strong acidity of TsOH.
As discussed in our previous communication,12 reversible colour changes of these films in response to exhaled breath were suggested to be due to moisture. Then, changes in the electronic absorption spectra of these films resulting from changing the surrounding humidity were investigated. Fig. 6 shows the electronic absorption spectra of BBMAB–PPA(6) films exposed to various relative humidity (RH) levels. The band at about 700 nm decreased with increasing humidity, and simultaneously, the band at about 480 nm increased, with an isosbestic point at 543 nm. Thus, the moisture played an important role in the reversible colour changes.
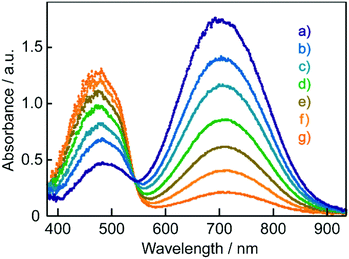 | ||
| Fig. 6 Electronic absorption spectra of BBMAB–PPA(6) films at (a) 20% RH, (b) 30% RH, (c) 40% RH, (d) 50% RH, (e) 60% RH, (f) 70% RH, and (g) 80% xRH. | ||
Fig. 7 shows the plots of humidity dependence of the normalized absorbance of the films at a wavelength of 700 nm. The response of the absorbance to humidity was found to depend on the kind of organic acid used. With organic acids with relatively low pKa, that is, the stronger acids such as TsOH and NSA, the colour change took place at only high humidity levels, specifically greater than 70% RH. On the other hand, the BBMAB–PPA(6) film exhibited colour changes for a relatively wide range of humidity, with the absorbance changing even at quite low humidity levels of 20% RH or less. Thus, the behaviours of the colour changes in response to humidity were also suggested to depend on the acidity levels of the organic acids.
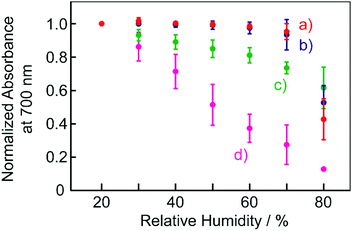 | ||
| Fig. 7 Humidity dependence of the normalized absorbance of the films at 700 nm. (a) BBMAB–TsOH(6), (b) BBMAB–NSA(6), (c) BBMAB–PFBA(6), (d) BBMAB–PPA(6). | ||
It was suggested that the two equilibriums described by eqn (1) and (2) were operating in the present system.
| BBMAB + RH ⇌ BBMAB–H+⋯R− | (1) |
| H2O + RH ⇌ H3O+⋯R− | (2) |
In these equations, RH and R− denote the organic acid and its conjugated base anion, respectively. Due to the quite high concentrations of BBMAB and organic acids in the film, a large amount of the protonated species BBMAB–H+ was produced at ambient atmosphere, caused by the forward reaction of eqn (1). With an increase in the concentration of the organic acid, the amount of BBMAB–H+ increased, resulting in an increase in the ratio of the absorbance at 700 nm to that at 480 nm. Similarly, when we used a stronger organic acid, i.e., with a lower pKa value, the forward reaction of eqn (1) became more dominant, and increased the amount of BBMAB–H+. Thus, the ratio of absorbance at 700 nm to that at 480 nm increased when we used stronger acids. When we breathed onto these films, the moisture in the exhaled breath was absorbed into their films and the forward reaction of eqn (2) was induced. Then, the concentration of RH decreased, resulting in inducing the reverse reaction of eqn (1), which decreased the amount of BBMAB–H+. When pausing the exposure of the film to exhaled breath, H2O evaporated immediately from their films, resulting in the recovery of the colour to the original according to the reverse reaction of eqn (2) followed by the forward reaction of eqn (1). When we used a relatively strong acid such as TsOH or NSA, the equilibrium of eqn (1) proceeded sufficiently to the right even when the concentration of the acid was relatively low as described above; so that the concentration of BBMAB–H+ was not susceptible to a change in humidity in the relatively low humidity range, i.e., did much follow eqn (2). Therefore, the colour change only took place at relatively high humidity. When we used a weaker acid such as PPA, the equilibrium of eqn (1) was more sensitive to the reaction of eqn (2), and therefore the colour change was observed for a wide range of humidity levels. Thus, the acidity of the organic acid was suggested to control the colour and the response to moisture for the present mixed systems.
Here, the present results were compared with the previously reported BMAB–TsOH system.12 Regarding the electronic absorption spectra, the absorption maxima of the film at ambient conditions and when breathing on to the film for the present BBMAB systems (ca. 700 nm and 480 nm, respectively) were somewhat shifted to longer wavelengths than those for the BMAB system (ca. 560 nm and 450 nm, respectively). These results were suggested to be due to extension of π-conjugation by substitution of another diarylamino moiety for BBMAB. It is of interest to note that the sensitivity levels of the films to humidity were found to depend on the type of aminoazobenzene used as well as the kind and concentration of organic acid as described above. Fig. 8 shows plots of the humidity dependence of normalized absorbance at λmax of the films of BBMAB and BMAB (700 nm and 560 nm, respectively) together with TsOH at a couple of different molar ratios. The BMAB–TsOH(6) film was found to be somewhat more sensitive to change in humidity than was the BBMAB–TsOH(6) (Fig. 8a and b). This tendency became more marked when the concentration of TsOH was decreased (Fig. 8c and d). The absorbance of the BMAB–TsOH(3) film was found to change for a wide range of humidity levels while that of BBMAB–TsOH(3) changed for only an RH level greater than 60%. The basicity of BBMAB was assumed to be stronger than that of BMAB due to BBMAB having another electron-donating diarylamino moiety substituent. This property resulted in the forward reaction of eqn (1) having been more dominant for the BBMAB system than for the BMAB system and, therefore, the BBMAB system having become insusceptible to a change in humidity, in contrast to the case for the BMAB system. Thus, the basicity of aminoazobenzene was also suggested to control the colour and response to moisture for the mixed systems of the current work.
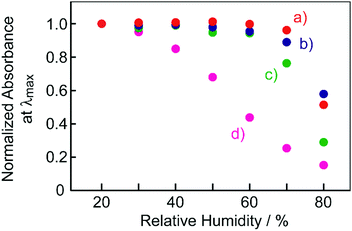 | ||
| Fig. 8 Humidity dependence of the normalized absorbance of the films at λmax. (a) BBMAB–TsOH(6), (b) BMAB–TsOH(6), (c) BBMAB–TsOH(3), (d) BMAB–TsOH(3). | ||
Conclusions
The mixed films containing BBMAB and organic acids were demonstrated to exhibit reversible colour changes in response to exhaled breath in the present study. Such phenomena of reversible colour changes seems to generally occur for amorphous films containing aminoazobenzene-based materials together with organic acids with appropriate acidity levels. In addition, protonation of the aminoazobenzene caused by organic acid and deprotonation by moisture were suggested to be key issues, and the acidity levels of the organic acids and the basicity levels of the aminoazobenzenes as well as their concentrations were also suggested to be the important factors affecting the colour of the film and the response to moisture. The systems developed in the current work are new examples of taking advantage of amorphous molecular materials as excellent matrices and of azobenzene-based dyes as materials whose colours change in response to acid. Using appropriate amorphous molecular materials and organic acids is expected to provide further unique hybrid films that respond to external stimuli such as exhaled breath.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI Grant Number JP26107006 in Scientific Research on Innovative Areas “Photosynergetics”.Notes and references
- I. M. Kolthoff, Acid-base indicators, ed. C. Rosenblum, The Macmillan Co., 1937 Search PubMed; R. W. Sabnis, Handbook of Acid-Base Indicators, CRC Press, Boca Raton, USA, 2007 Search PubMed.
- Photochromism, ed. G. H. Brown, Wiley & Sons, Inc., 1973 CrossRef CAS; Photochromism, Molecules and Systems, ed. H. Dürr and H. Bouas-Laurant, Elsevier, 1990 CrossRef CAS; Applied Photochromic Polymer Systems, ed. C. B. McArdle, Blackie & Son Ltd., 1992 CrossRef CAS; K. Ichimura, Y. Suzuki, T. Seki, A. Hosoki and K. Aoki, Langmuir, 1988, 4, 1214 CrossRef CAS; T. Seki, H. Sekizawa, S. Morino and K. Ichimura, J. Phys. Chem. B, 1998, 102, 5313 CrossRef; K. Ichimura, Chem. Rev., 2000, 100, 1847 CrossRef PubMed.
- Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef CAS PubMed; M. Kondo, Y. Yu and T. Ikeda, Angew. Chem., Int. Ed., 2006, 45, 1378 CrossRef PubMed; T. Ikeda, J. Mamiya and Y. Yu, Angew. Chem., Int. Ed., 2007, 46, 506 CrossRef PubMed.
- P. Rochon, E. Batalla and A. Natansohn, Appl. Phys. Lett., 1995, 66, 136 CrossRef CAS; D. Y. Kim, S. K. Tripathy, L. Li and J. Kumar, Appl. Phys. Lett., 1995, 66, 116 Search PubMed; P. Lefin, C. Fiorini and J.-M. Nunzi, Pure Appl. Opt., 1998, 7, 71 CrossRef; N. K. Viswanathan, D. Y. Kim, S. Bian, J. Williams, W. Liu, L. Li, L. Samuelson, J. Kumar and S. K. Tripathy, J. Mater. Chem., 1999, 9, 1941 RSC; A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139 CrossRef PubMed.
- H. Nakano, T. Takahashi, T. Kadota and Y. Shirota, Adv. Mater., 2002, 14, 1157 CrossRef CAS; H. Nakano, T. Tanino, T. Takahashi, H. Ando and Y. Shirota, J. Mater. Chem., 2008, 18, 242 RSC; H. Nakano, T. Takahashi, T. Tanino and Y. Shirota, Dyes Pigm., 2009, 84, 102 CrossRef; H. Nakano, Chem. Lett., 2011, 40, 473 CrossRef; H. Nakano, J. Mater. Chem., 2010, 20, 2071 RSC; H. Nakano and M. Suzuki, J. Mater. Chem., 2012, 22, 3702 RSC; R. Ichikawa and H. Nakano, Appl. Phys. Express, 2013, 6, 035602 CrossRef; H. Nakano, R. Ichikawa and R. Matsui, Micromachines, 2013, 4, 128 CrossRef; R. Ichikawa and H. Nakano, RSC Adv., 2016, 6, 36761 RSC.
- Y. Shirota, J. Mater. Chem., 2000, 10, 1 RSC; Y. Shirota, J. Mater. Chem., 2005, 15, 75 RSC , and references cited therein.
- (a) Y. Shirota, K. Moriwaki, S. Yoshikawa, T. Ujike and H. Nakano, J. Mater. Chem., 1998, 8, 2579 RSC; (b) H. Utsumi, D. Nagahama, H. Nakano and Y. Shirota, J. Mater. Chem., 2000, 10, 2436 RSC; (c) T. Tanino, S. Yoshikawa, T. Ujike, D. Nagahama, K. Moriwaki, T. Takahashi, Y. Kotani, H. Nakano and Y. Shirota, J. Mater. Chem., 2007, 17, 4953 RSC.
- O. S. Wegner, Chem. Rev., 2013, 113, 3686 CrossRef PubMed.
- R. Ishizaki and R. Katoh, Chem. Phys. Lett., 2016, 652, 36 CrossRef CAS.
- H. Iida, S. Iwahana, T. Mizoguchi and E. Yashima, J. Am. Chem. Soc., 2012, 134, 15103 CrossRef CAS PubMed; T. Hayashi, A. Kobayashi, H. Ohara, M. Yoshida, T. Matsumto, H.-C. Chang and M. Kato, Inorg. Chem., 2015, 54, 8905 CrossRef PubMed.
- K. Ogura and H. Nakano, Kobunshi Ronbunshu, 2017, 72, 199 CrossRef.
- R. Ichikawa, E. Nagata and H. Nakano, RSC Adv., 2015, 5, 2934 RSC.
- H. Nakano, T. Nishimura, E. Nagata and R. Ichikawa, ChemistrySelect, 2016, 1, 1737 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: DFT calculations of BBMAB and BBMAB-H+. See DOI: 10.1039/c7qm00385d |
| ‡ The pKa values of TsOH, NSA, PFBA, PPA and BA were −0.43, 0.27, 1.60, 1.88 and 4.20, respectively. These values were addressed under the database (SciFinder®), being calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02. |
| This journal is © the Partner Organisations 2018 |