Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalysed synthesis of alkylidene 2-pyrrolinone derivatives from the combination of α-keto amides and alkynes†
Qian Wen
Tan
a,
Praful
Chovatia
b and
Michael C.
Willis
 *a
*a
aDepartment of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: michael.willis@chem.ox.ac.uk
bEvotec, 114 Innovation Drive, Milton Park, Abingdon, Oxfordshire OX14 4RZ, UK
First published on 17th October 2018
Abstract
A Cu(I)-catalysed addition and cyclisation sequence has been developed for the synthesis of (E)-alkylidene pyrrolinone derivatives. The reactions incorporate simple α-keto amides and alkynes as substrates, and employ a commercially available Cu(I) catalyst. The process tolerates good variation of both starting materials, and delivers the desired pyrrolinones in good yields, with high levels of stereocontrol.
Nitrogen-based heterocycles feature in almost 60% of FDA-approved drugs.1 In addition, they are common motifs in agrochemicals,2 in new materials,3 and can function as versatile synthetic intermediates.4 These attributes drive efforts to deliver efficient atom-economic methods for the synthesis of N-heterocycles using readily available starting materials.
Towards this goal, we were interested in exploring routes to substituted 2-pyrrolinones. These unsaturated amide-containing heterocycles are present in a diverse range of biologically active natural products and pharmaceutical compounds (Scheme 1),5 and their multiple functional groups make them attractive synthetic building blocks.
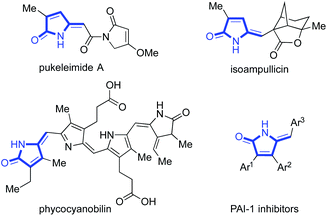 | ||
| Scheme 1 Selected examples of natural products and pharmaceutical compounds containing 2-pyrrolinone derivatives. | ||
Classically, the pyrrolinone core is synthesised using Wittig reactions on maleimides,6 or by treating γ-alkylidenebutenolides with amines,7 while the cyclisation of unsaturated substrates such as dienamides8 and iodoacetamides is also known.9 More recently, methods have been reported employing rhodium catalysts for the addition and cyclisation of α,β-unsaturated oximes to isocyanates,10 or acrylamides to gem-difluoroacrylates.11 Despite the range of approaches available, these protocols often require complex starting materials that require multi-step synthesis, resulting in increased costs, and significant waste generation.
The use of simple addition processes to facilitate bond formation is attractive due to the rapid increase in complexity and the inherent atom economy of these reactions.12 As such, the use of hydroamination or hydroamidation reactions, which combine amines or amides, respectively, with either alkenes or alkynes, presents an attractive and versatile method for the formation of nitrogen-containing organic compounds. Although these reactions can potentially produce a wide range of nitrogen-containing heterocycles, they have yet to be exploited fully in this context. Many hydroamination and hydroamidation reactions display a narrow substrate scope and require non-trivial, or precious metal catalysts.13 The development of alkene and alkyne hydroamination reactions using earth-abundant copper catalysts has been significant in recent years,14 with notable examples being reported from the Buchwald laboratory based on the use of Cu(I)-hydride chemistry.15 However, systems for copper-catalysed hydroamidation are less common.16,17
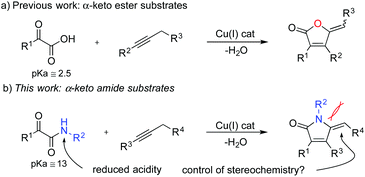
We took on the challenge of developing a system that utilises copper-catalysed hydroamidation to synthesise 2-pyrrolinone motifs using simple and readily accessible starting materials. In earlier work, we reported a copper-catalysed route to ylidenebutenolides from the combination of alkynes and α-oxo acids, which we proposed proceeds via an initial hydrocarboxylation of the alkyne (Scheme 2a).18 We speculated that the use of α-keto amides as substrates in a related reaction would deliver a new route to 2-pyrrolinone derivatives, based on simple addition chemistry, and by analogy to our earlier work, would proceed by a hydroamidation pathway (Scheme 2b). At the outset of this work we noted that a significant challenge would be the use of α-keto amides as substrates, as they are significantly less acidic then the corresponding α-oxo acids.19 Conversely, our earlier route to ylidenebutenolides resulted in mixtures of geometrical isomers, and we speculated that the use of keto amide substrates could lead to more selective reactions due to steric interactions from the N-R2 substituent, thus controlling the alkene geometry.
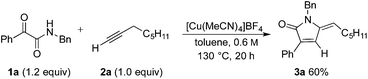
We selected the combination of N-benzyl-2-oxo-2-phenylacetamide (1a) and 1-octyne (2a) as a suitable test reaction (Table 1). Pleasingly, the reaction conditions developed for our butenolide chemistry were effective with the amide substrate, with the use of Cu(MeCN)4BF4 (10 mol%) as the catalyst in toluene at 130 °C, delivering 60% of pyrrolinone 3a. Performing the reaction at a slightly higher concentration (1.0 M) increased the yield to 69%. The use of alternative copper catalysts or solvents, the addition of supporting nitrogen- or phosphorous-based ligands, or the addition of base, failed to improve the yield further. Selected optimization data are shown in Table 1, with full details available in the ESI.† Importantly, in all cases, only the (E)-isomer of pyrrolinone 3a was formed.20
| Entry | Variation from above | Yieldb (%) |
|---|---|---|
| a Reaction conditions: α-Keto amide (0.36 mmol), alkyne (0.30 mmol), [Cu(MeCN)4]BF4 (0.03 mmol), toluene (0.50 mL), 130 °C, 20 h, under N2. b Yields determined using 1H NMR spectroscopy of the crude reaction mixtures with nitromethane as an internal standard. Single isomer of product. c 10 mol% ligand added. d 1 equiv. of base added. | ||
| 1 | 2.0 equiv. 1a | 61% |
| 2 | 1.0 M | 69% |
| 3 | 1.0 equiv. 1a, 2.0 equiv. 2a | 30% |
| 4 | Cu2O as catalyst | 0% |
| 5 | DMF as solvent | 4% |
| 6 | PhCl as solvent | 23% |
| 7c | Bipyridine added | 0% |
| 8c | dppm added | 10% |
| 9d | K2CO3 added | 0% |
| 10d | Et3N added | 0% |
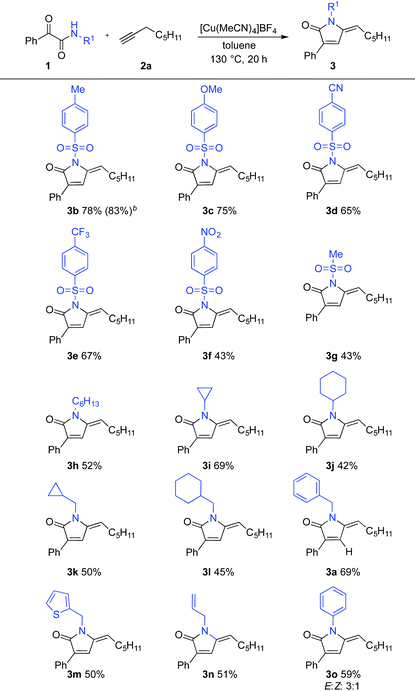
We next explored variation of the N-substituent of the α-keto amides, keeping 1-octyne constant as the second reaction component (Table 2). N-Sulfonyl α-keto amides were effective substrates, although it was apparent that the electronic nature of the sulfonyl group played a role in reaction efficiency. For example, aryl sulfonamides featuring electron-donating substituents (3b,c) delivered higher yielding reactions than those featuring electron-withdrawing groups (3d–f). The N-tosyl example was scaled without issue, delivering 1.2 grams of pyrrolinone 3b in an improved yield of 83%. Importantly, the reaction could be used to produce pyrrolinone cores with various N-alkyl substituents, including linear alkyl (3h), 3- and 6-membered carbocycles (3i–l) as well as a methylthienyl group (3m). The scope was also not limited to N-alkyl substrates as allylic and aryl substituents (3n, 3o) were also tolerated, delivering the expected products in good yields. A mixture of E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z isomers was obtained for the N-phenyl example, with substrates featuring all other N-substituents returning single isomers of product.
Z isomers was obtained for the N-phenyl example, with substrates featuring all other N-substituents returning single isomers of product.
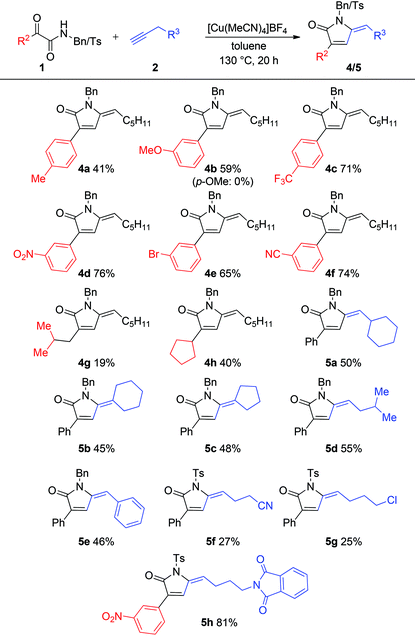
We then focused on evaluating variation in the substituents on the aromatic ring of aryl N-benzyl α-keto amides (Table 3). While both electron-donating (4a,b) and electron-withdrawing (4c–f) substituents, positioned meta and para on the aryl ring were tolerated, the isolated yields suggested that reducing the electron density on the aryl ring provided more efficient reactions. Pyrrolinone 4b illustrates this well; moving the OMe substituent on the arene from the para to the meta position results in a non-productive reaction being converted into one that proceeds in 59% yield. Although they were less reactive, alkyl α-keto amides (4g, 4h) can also be tolerated in the system.
| a Reaction conditions: α-Keto amide (0.36 mmol), alkyne (0.30 mmol), [Cu(MeCN)4]BF4 (0.03 mmol), toluene (0.50 mL), 130 °C, 20 h, under N2. Isolated yields of single isomers. |
|---|
|
Variation of the alkyne reaction component was explored next. Both carbocyclic (5a–c) and linear (5d) aliphatic alkynes were tolerated to give moderate yields of the expected pyrrolinones. However, the presence of nitrile, chloro and phthalimide functional groups in the alkyne did not give any product when combined with N-benzyl α-keto amide 1a. However, when the more reactive N-tosyl α-keto amide 1b was employed in combination with these alkynes it was possible to obtain low to moderate yields of the targeted functionalized pyrrolinones (5f,g). Reactivity could be further increased by employing an α-keto amide featuring an electron-withdrawing aryl substituent, with pyrrolinone 5h, combining a nitro-substituted keto amide with a phthalamide-substituted alkyne, being obtained in an excellent 81% yield.
Conclusions
In conclusion, we have reported a Cu(I)-catalysed synthesis of alkylidene pyrrolinones. The reactions combine simple α-keto amides with alkynes, and deliver E-configured products with high levels of stereocontrol. The scope of the reaction is good, with variation of both reaction components possible. Scale up of the process to >1 gram proceeded without incident. This simple addition process is notable for the use of an earth-abundant metal catalyst and the high-degree of functionality delivered in the products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Sangwon Seo (Oxford) for fruitful discussions, the Agency for Science, Technology and Research (A*STAR), and the Engineering and Physical Sciences Research Council (EPSRC) Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for the studentship, generously supported by AstraZeneca, Diamond Light Source, Defense Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex.Notes and references
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- (a) C. Lamberth, Pest Manage. Sci., 2013, 69, 1106–1114 CrossRef CAS PubMed; (b) C. Lamberth, S. Jeanmart, T. Luksch and A. Plant, Science, 2013, 341, 742–746 CrossRef PubMed.
- (a) G. Mlostoń, Chem. Heterocycl. Compd., 2017, 53, 1; ; (b) P. Yin, Q. Zhang and J. n. M. Shreeve, Acc. Chem. Res., 2016, 49, 4–16 CrossRef CAS PubMed.
- A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611–6637 RSC.
- (a) W. J. Cole, D. J. Chapman and H. W. Siegelman, J. Am. Chem. Soc., 1967, 89, 3643–3645 CrossRef CAS; (b) G. D. James, S. D. Mills and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1993, 2581–2584, 10.1039/P19930002581; (c) H. Miyazaki, T. Miyake, Y. Terakawa, H. Ohmizu, T. Ogiku and A. Ohtani, Bioorg. Med. Chem. Lett., 2010, 20, 546–548 CrossRef CAS PubMed; (d) C. Peschko and W. Steglich, Tetrahedron Lett., 2000, 41, 9477–9481 CrossRef CAS.
- G. B. Gill, G. D. James, K. V. Oates and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1993, 2567–2579, 10.1039/P19930002567.
- C. Haase and P. Langer, Synlett, 2005, 453–456 CAS.
- (a) K. Cherry, A. Duchêne, J. Thibonnet, J.-L. Parrain, E. Anselmi and M. Abarbri, Synthesis, 2009, 257–270 CAS; (b) K. Cherry, J. Thibonnet, A. Duchêne, J.-L. Parrain and M. Abarbri, Tetrahedron Lett., 2004, 45, 2063–2066 CrossRef CAS.
- Y. Tang and C. Li, Org. Lett., 2004, 6, 3229–3231 CrossRef CAS PubMed.
- W. Hou, B. Zhou, Y. Yang, H. Feng and Y. Li, Org. Lett., 2013, 15, 1814–1817 CrossRef CAS PubMed.
- H. Liu, S. Song, C. Q. Wang, C. Feng and T. P. Loh, ChemSusChem, 2017, 10, 58–61 CrossRef CAS PubMed.
- X. Zeng, Chem. Rev., 2013, 113, 6864–6900 CrossRef CAS PubMed.
- (a) C. Brinkmann, A. G. Barrett, M. S. Hill and P. A. Procopiou, J. Am. Chem. Soc., 2012, 134, 2193–2207 CrossRef CAS PubMed; (b) M. R. Crimmin, I. J. Casely and M. S. Hill, J. Am. Chem. Soc., 2005, 127, 2042–2043 CrossRef CAS PubMed; (c) M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2000, 122, 9546–9547 CrossRef CAS; (d) F. E. Michael and B. M. Cochran, J. Am. Chem. Soc., 2006, 128, 4246–4247 CrossRef CAS PubMed; (e) J.-S. Ryu, G. Y. Li and T. J. Marks, J. Am. Chem. Soc., 2003, 125, 12584–12605 CrossRef CAS PubMed; (f) R. Sarma and D. Prajapati, Chem. Commun., 2011, 47, 9525–9527 RSC; (g) C. S. Sevov, J. S. Zhou and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 3200–3207 CrossRef CAS PubMed; (h) X. Zhang, T. J. Emge and K. C. Hultzsch, Angew. Chem., Int. Ed., 2012, 51, 394–398 CrossRef CAS; (i) L. B. Huang, M. Arndt, K. Goossen, H. Heydt and L. J. Goossen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed.
- (a) J. Bahri, B. Jamoussi, A. van Der Lee, M. Taillefer and F. Monnier, Org. Lett., 2015, 17, 1224–1227 CrossRef CAS PubMed; (b) R. Blieck, J. Bahri, M. Taillefer and F. Monnier, Org. Lett., 2016, 18, 1482–1485 CrossRef CAS PubMed; (c) T. Ishikawa, T. Sonehara, M. Minakawa and M. Kawatsura, Org. Lett., 2016, 18, 1422–1425 CrossRef CAS PubMed.
- (a) M. T. Pirnot, Y. M. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 48–57 CrossRef CAS PubMed; (b) S. L. Shi and S. L. Buchwald, Nat. Chem., 2015, 7, 38–44 CrossRef CAS PubMed; (c) H. Wang, J. C. Yang and S. L. Buchwald, J. Am. Chem. Soc., 2017, 139, 8428–8431 CrossRef CAS PubMed; (d) S. Zhu, N. Niljianskul and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 15746–15749 CrossRef CAS PubMed.
- (a) R. Martin, M. Rodriguez Rivero and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 7079–7082 CrossRef CAS PubMed; (b) Y. Zhou, O. D. Engl, J. S. Bandar, E. D. Chant and S. L. Buchwald, Angew. Chem., Int. Ed., 2018, 57, 6672–6675 CrossRef CAS PubMed.
- For an example of the Pd-catalysed synthesis of alkyne hydroamination products from alkenyl tosylates, see: M. C. Willis, G. N. Brace and I. P. Holmes, Synthesis, 2005, 3229–3234 CrossRef CAS.
- S. Seo and M. C. Willis, Org. Lett., 2017, 19, 4556–4559 CrossRef CAS PubMed.
- W. M. Haynes, in CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data, ed. D. R. Lide and W. M. Haynes, CRC Press, Boca Raton, 2009 Search PubMed.
- Established by NOESY data; see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ob02205d |
| This journal is © The Royal Society of Chemistry 2018 |