Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anion transport by ortho-phenylene bis-ureas across cell and vesicle membranes†
Christopher M.
Dias
 a,
Hongyu
Li
a,
Hongyu
Li
 b,
Hennie
Valkenier
b,
Hennie
Valkenier
 c,
Louise E.
Karagiannidis
d,
Philip A.
Gale
c,
Louise E.
Karagiannidis
d,
Philip A.
Gale
 *e,
David N.
Sheppard
*e,
David N.
Sheppard
 b and
Anthony P.
Davis
b and
Anthony P.
Davis
 *a
*a
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: anthony.davis@bristol.ac.uk
bSchool of Physiology, Pharmacology and Neuroscience, University of Bristol, Biomedical Sciences Building, University Walk, Bristol, BS8 1TD, UK
cEngineering of Molecular NanoSystems, Ecole Polytechnique de Bruxelles, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, CP165/64, B-1050 Brussels, Belgium
dChemistry, University of Southampton, Southampton, SO17 1BJ, UK
eSchool of Chemistry, The University of Sydney, NSW 2006, Australia. E-mail: philip.gale@sydney.edu.au
First published on 29th January 2018
Abstract
Ortho-Phenylene bis-ureas serve as anionophores in cells expressing halide-sensitive yellow fluorescent protein, as well as in synthetic vesicles. Activities can reach high levels, and are strongly dependent on the deliverability of the transporters.
The development of synthetic anion transporters (anionophores) has become a major theme of supramolecular chemistry.1 An important motivation is the potential for anionophores to be applied therapeutically, for example in the treatment of cancer and channelopathies such as cystic fibrosis (CF).2 Much of the work focuses on using small molecule anion carriers, which can bind an anion on one side of the membrane, traverse the membrane and then release the anion on the other side.2f,3,4
If anionophores are to be exploited in medicine, it is necessary to show that they can be effective in living cells, and not just in synthetic bilayer systems such as vesicles. We recently developed an assay for biological anion transport which employs Fischer rat thyroid cells expressing the iodide-sensing yellow fluorescent protein (YFP-FRT cells).5,6 The assay was applied to anionophores of the “1,5-diaxial” family3 with steroidal, trans-decalin or cyclohexane scaffolds (e.g.1–3, see Fig. 1).6a All tested positive for chloride transport, with trans-decalin 2 showing especially high activity. Transporter 2 showed negligible toxicity to several cell lines, suggesting that anionophore activity is not necessarily damaging to cells and raising the hope of “channel replacement therapy” as a treatment for CF.
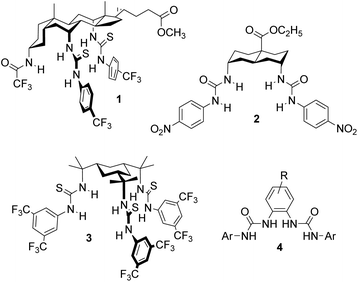 | ||
| Fig. 1 1,5-Diaxial anionophores 1–3, previously shown to be active in YFP-FRT cells and the general structure 4 for the OPBU carriers studied in the present work. | ||
While the 1,5-diaxial anionophores are known to be exceptionally powerful,7 other families have also shown good activity in synthetic vesicle assays. In particular, ortho-phenylene bis-ureas (OPBUs) of general structure 4 have proven effective for Cl−/NO3−, Cl−/HCO3−, and Cl−/carboxylate antiport, in some cases at very low transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid ratios.8,9 These carriers are also especially easy to prepare, being accessible from commercially available starting materials in one or two steps. They thus provide a valuable opportunity for the study of structure–activity relationships. Here, we report that OPBUs are active in cells, at levels comparable to the 1,5-diaxial anionophores. We also explore the effects of substituents which modify lipophilicity and electronic characteristics, and the correlation between results in synthetic vesicles and data from the biological assay. The results highlight the importance of deliverability in the development of anionophores for biological applications.
lipid ratios.8,9 These carriers are also especially easy to prepare, being accessible from commercially available starting materials in one or two steps. They thus provide a valuable opportunity for the study of structure–activity relationships. Here, we report that OPBUs are active in cells, at levels comparable to the 1,5-diaxial anionophores. We also explore the effects of substituents which modify lipophilicity and electronic characteristics, and the correlation between results in synthetic vesicles and data from the biological assay. The results highlight the importance of deliverability in the development of anionophores for biological applications.
The OPBU anionophores employed in this work are shown in Fig. 2. To ensure high activities (and thus ease of measurement), the urea units were uniformly terminated with 3,5-bis(trifluoromethyl)phenyl groups. Studies conducted by ourselves and others have shown that anionophores bearing this substituent are often the most powerful derivatives in their respective series.7,9,10 Bis-urea 4a was known, and had previously been shown to possess good transport activity in large unilamellar vesicles (LUVs).9 The new compounds 4b–g were synthesised by refluxing the appropriate diaminobenzene with 3,5-bis(trifluoromethyl)phenyl isocyanate in DCM. The preparation of esters 4f and 4g required prior synthesis of the corresponding alkyl diaminobenzoates from 3,4-diaminobenzoic acid. Full experimental details are given in the ESI.†
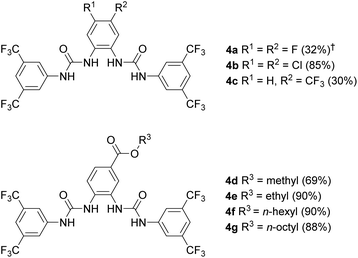 | ||
| Fig. 2 Structures of ortho-phenylene bis-ureas investigated in this work. Isolated yields for the final step of the synthesis are given in brackets. †Yield reported in previous work (see ref. 9). | ||
To characterise 4a–g, we first investigated their ability to function as anion receptors by performing NMR titrations in DMSO-d6/0.5% H2O with tetrabutylammonium chloride as guest. In all experiments the most significant movements were observed for the urea NH signals, indicating that they are all involved in chloride binding.11 Residual distribution analysis indicated that all receptors bind the anion in both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (host
2 (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) stoichiometries (see ESI† for further discussion) and titration data were subsequently fitted to this model. The second binding event was found to be very weak in all cases and 1
guest) stoichiometries (see ESI† for further discussion) and titration data were subsequently fitted to this model. The second binding event was found to be very weak in all cases and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding is the predominant process. The results are given in Table 1. In line with previous work, K1:1 values are rather moderate and not especially sensitive to the type of substituent present on the central aromatic ring.8,9 For comparison, diureidodecalin 2 bound Bu4N+Cl− with Ka = 680 M−1 under the same conditions.6a
1 binding is the predominant process. The results are given in Table 1. In line with previous work, K1:1 values are rather moderate and not especially sensitive to the type of substituent present on the central aromatic ring.8,9 For comparison, diureidodecalin 2 bound Bu4N+Cl− with Ka = 680 M−1 under the same conditions.6a
| Compound | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Binding to Bu4N+Cl−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
Transport in LUVs (initial slope I of F0/F)c (s−1) | Deliverability, D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
Transport in YFP-FRT cellse | ||
|---|---|---|---|---|---|---|---|
| K a, 1:1 (M−1) | K a, 1:2 (M−1) | Preincorporated | External addition | |dF/dt| (s−1) | |||
a Calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, an estimate of lipophilicity where P is the partition coefficient between n-octanol and water. Values were calculated using TorchLite (available as freeware from http://www.cresset-group.com).
b Determined by 1H NMR titration in DMSO-d6/0.5% H2O at 298 K. Data were analysed using a 1 P, an estimate of lipophilicity where P is the partition coefficient between n-octanol and water. Values were calculated using TorchLite (available as freeware from http://www.cresset-group.com).
b Determined by 1H NMR titration in DMSO-d6/0.5% H2O at 298 K. Data were analysed using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 1 1 + 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (host 2 (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) binding model.
c Values are proportional to rates of Cl−/NO3− exchange. Experiments were conducted in 200 nm POPC/cholesterol (7 guest) binding model.
c Values are proportional to rates of Cl−/NO3− exchange. Experiments were conducted in 200 nm POPC/cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) LUVs with transporter 3) LUVs with transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid = 1 lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. I was obtained by fitting F0/F versus time (0–500 s) to a double exponential decay function, for further details see ref. 7 and ESI.
d Ratio of I from experiments employing external addition/preincorporation of transporter (see discussion in text).
e I−/Cl− exchange mediated by the transporter (50 μM) in YFP-FRT cells. Obtained by fitting the decay in YFP fluorescence to a first order exponential function and subtracting fluorescence quenching of the vehicle (0.5–1% v/v DMSO). 000. I was obtained by fitting F0/F versus time (0–500 s) to a double exponential decay function, for further details see ref. 7 and ESI.
d Ratio of I from experiments employing external addition/preincorporation of transporter (see discussion in text).
e I−/Cl− exchange mediated by the transporter (50 μM) in YFP-FRT cells. Obtained by fitting the decay in YFP fluorescence to a first order exponential function and subtracting fluorescence quenching of the vehicle (0.5–1% v/v DMSO).
|
|||||||
| 4a | 9.9 | 136 | 1 | 0.073 | 0.041 | 0.57 | 0.02 |
| 4b | 10.4 | 146 | 1 | 0.073 | 0.027 | 0.37 | 0.0023 |
| 4c | 10.1 | 157 | 1 | 0.042 | 0.018 | 0.43 | 0.0093 |
| 4d | 8.8 | 166 | 3 | 0.0073 | 0.012 | 1.61 | 0.0071 |
| 4e | 9.2 | 146 | 2 | 0.011 | 0.013 | 1.17 | 0.00099 |
| 4f | 10.8 | 138 | 3 | 0.017 | 0.011 | 0.66 | 0.0017 |
| 4g | 11.6 | 134 | 1 | 0.019 | 0.0029 | 0.15 | 0.0018 |
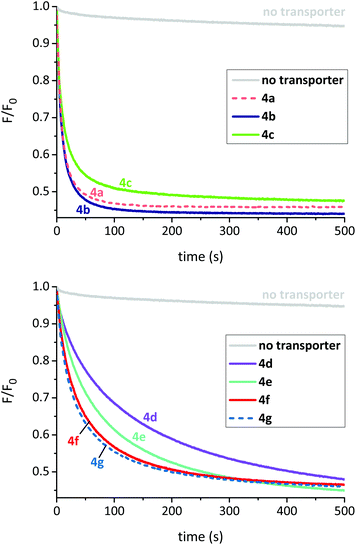
To assess the transport properties of these molecules in synthetic membranes, we preferred a method which could distinguish between intrinsic activity (the ability to transport anions once in the membrane) and overall practical effectiveness (which also depends on the ability to find and enter the membrane). In the previous studies on OPBUs, transport activity in LUVs was assayed using an ion selective electrode (ISE) to detect chloride anions leaving the vesicles.8,9 This method requires that the test anionophore be externally added to the vesicles after they have been prepared; this is because preincorporation leaves the vesicles permeable to anions at all stages of the experiment, so that chloride efflux can occur before the measurement is initiated. The test thus gives a measure of practical effectiveness, but cannot be adapted to show intrinsic transport ability.13 For the present work, we employed an alternative assay which relies on a halide-sensitive fluorophore (lucigenin) which can be trapped inside the vesicles. This detects chloride influx, allowing the experiment to be started by addition of chloride to the external solution. Anionophores can therefore be preincorporated in the vesicles (to measure intrinsic activity) or added externally (to assess practical effectiveness).6a
To measure intrinsic activity, we prepared LUVs, (∼200 nm diameter) from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) with the putative transporter preincorporated in the membrane. The interior of the LUVs contained aqueous NaNO3 (225 mM) and lucigenin. The vesicles were suspended in aqueous NaNO3 (225 mM) and the experiment commenced by the addition of a pulse of NaCl (25 mM). The decay in lucigenin fluorescence emission corresponds to an increase in intravesicular chloride concentration. To obtain values for comparison, the data were expressed as F0/F (which is proportional to intravesicular chloride concentrations) then initial slopes I were obtained using a curve fitting procedure.7 Decay curves (F/F0) for a series of experiments with transporter
3 ratio) with the putative transporter preincorporated in the membrane. The interior of the LUVs contained aqueous NaNO3 (225 mM) and lucigenin. The vesicles were suspended in aqueous NaNO3 (225 mM) and the experiment commenced by the addition of a pulse of NaCl (25 mM). The decay in lucigenin fluorescence emission corresponds to an increase in intravesicular chloride concentration. To obtain values for comparison, the data were expressed as F0/F (which is proportional to intravesicular chloride concentrations) then initial slopes I were obtained using a curve fitting procedure.7 Decay curves (F/F0) for a series of experiments with transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid = 1
lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 are shown in Fig. 3, and corresponding values for I are listed in Table 1.
000 are shown in Fig. 3, and corresponding values for I are listed in Table 1.
The experiments revealed that all compounds in this series possess high intrinsic anionophore activity, clearly measurable at a very low transporter loading (0.004 mol%). The most powerful were bis-ureas 4a and 4b, based on halogenated central scaffolds. In the case of 4a, a dose–response study was performed and showed activity down to transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid = 1
lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000k (Fig. S38†).
1000k (Fig. S38†).
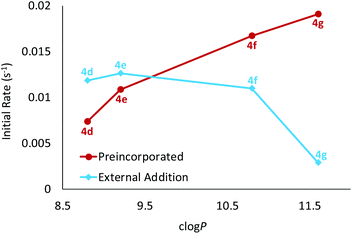
When comparing the activities of esters 4d–g, there is clearly a positive correlation between initial rate and the length of the ester alkyl chain/lipophilicity (Table 1 and Fig. 4, red circles). In principle this effect could arise due to different amounts of transporter in the membrane; less lipophilic esters (shorter chains) might be present in lower concentrations due to leaching from the membrane into the aqueous phase. To examine this possibility, we conducted a leaching study10c,12 on methyl ester 4d, the least lipophilic member in this series and thus the most likely to partition into water. Briefly, LUVs with 4d preincorporated in the membrane were prepared as previously described. The vesicles were then successively diluted with aqueous NaNO3 which should result in a loss of transporter from the lipid bilayers if it were capable of leaching. However, these studies indicated that 4d resides exclusively within the membrane and is not lost to the aqueous phase (see ESI† for further details). It thus seems that the longer alkyl chains promote intrinsic transport activity, possibly by positioning the transporter or complex more favourably within the membrane.10c,13,14
After measuring the intrinsic activities of OPBUs 4a–g we next sought to establish how readily they could be delivered by external addition to membranes (the second component of practical effectiveness). LUVs were prepared in a manner analogous to that previously described, except that the anionophore was not added to the initial lipid mixture. Instead a solution of the anionophore in acetone was externally added to rapidly stirred preformed vesicles to give a notional transporter to lipid loading of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. After 5 minutes, NaCl (25 mM) was added and the decay in fluorescence analysed as before (Table 1 and ESI†). Deliverability (D) was calculated by dividing the initial rate of transport from this experiment to that observed for preincorporated anionophore. If a transporter is perfectly deliverable, values for D are found to be ≥1 and this was observed for esters 4d and 4e.15 However, all other OPBUs tested displayed inferior activity when externally added, indicating that they are not perfectly deliverable (D < 1). Within the ester series it is evident that deliverability decreases as a function of lipophilicity (Table 1 and Fig. 4). This has a substantial effect on the activity of 4g; whereas it was the most powerful transporter in the ester series when preincorporated inside vesicles, it is the least active when added externally. Octyl ester 4g would thus be an ideal candidate for further investigation in coiled-coil driven membrane fusion studies. As demonstrated previously,6b this is a highly efficient method of delivering lipophilic anionophores to membranes.
000. After 5 minutes, NaCl (25 mM) was added and the decay in fluorescence analysed as before (Table 1 and ESI†). Deliverability (D) was calculated by dividing the initial rate of transport from this experiment to that observed for preincorporated anionophore. If a transporter is perfectly deliverable, values for D are found to be ≥1 and this was observed for esters 4d and 4e.15 However, all other OPBUs tested displayed inferior activity when externally added, indicating that they are not perfectly deliverable (D < 1). Within the ester series it is evident that deliverability decreases as a function of lipophilicity (Table 1 and Fig. 4). This has a substantial effect on the activity of 4g; whereas it was the most powerful transporter in the ester series when preincorporated inside vesicles, it is the least active when added externally. Octyl ester 4g would thus be an ideal candidate for further investigation in coiled-coil driven membrane fusion studies. As demonstrated previously,6b this is a highly efficient method of delivering lipophilic anionophores to membranes.
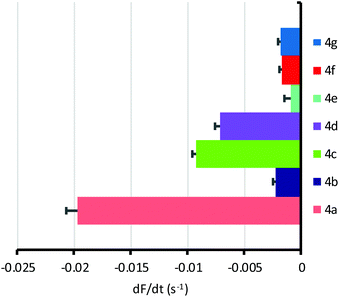
Following characterisation of their transport properties in synthetic membranes, OPBUs 4a–g were tested in cells. For these studies, we used Fischer rat thyroid cells expressing the halide sensor YFP-H148Q/I152L (YFP-FRT cells). YFP-FRT cells were seeded in a 96-well plate and cultured for 3 to 4 days until 90% confluence. The cells were washed twice with phosphate buffered saline (PBS) solution before incubating with a PBS solution containing the test anionophore (50 μM) for 10 min. The experiment was commenced by the addition of NaI (100 mM) and the rate of chloride/iodide exchange measured by fitting the decay in YFP fluorescence to a first order exponential function (see ESI† for full experimental details).
All compounds were found to mediate chloride/iodide exchange in cells leading to a decay in YFP fluorescence (Fig. 5, Fig. S45† and Table 1). The most active by some margin was bis-urea 4a, based on the difluoroaromatic scaffold. Reassuringly, transporter 4a was also the most active when externally delivered to LUVs, and joint first when preincorporated. This adds to our confidence that the YFP-FRT assay does indeed measure anion transport, rather than some other phenomenon. Anionophore 4a is somewhat more active than 2, which gave |dF/dt| = 0.013 s−1 in the present assay. The overall relationship between activity in cells and vesicles is shown in Fig. 6. Provided the vesicle measurements involve external addition of anionophore, a rough correlation emerges (Fig. 6a). There is, however, considerable scatter; the poor performance of dichloride 4b in the cells, despite its good activity in LUVs, is especially noticeable. This is difficult to explain, but may result from a specific interaction between 4b and the natural membrane. The results imply that, while experiments in LUVs can give some guidance, these synthetic assemblies are not ideal surrogates for cells and cannot substitute for biological testing. When results in cells are compared to those in vesicles with preincorporated OPBU, the correlation is very poor (Fig. 6b). This highlights the importance of considering deliverability as well as intrinsic activity when developing biologically active anionophores.6
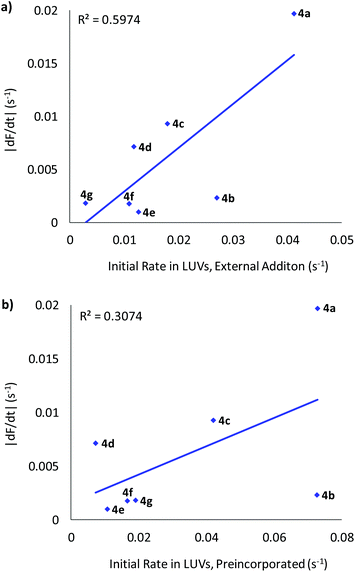 | ||
| Fig. 6 Plot of the rate of chloride transport mediated by OPBUs 4a–g in YFP-FRT cells versus LUVs when (a) externally added or (b) preincorporated. | ||
In conclusion, we have shown that a series of ortho-phenylene bis-urea (OPBU) anionophores are active in cells as well as synthetic vesicles. The most effective in all assays is bis-urea 4a, based on a difluorinated central scaffold. Activities depend both on electronic factors and lipophilicity. In particular, the sequence 4d–g illustrates the role of lipophilicity in promoting intrinsic activity, but the contrary effect on deliverability and thus on effectiveness in cells. These bis-ureas are readily prepared, so that many other variations are potentially accessible. They therefore hold promise as tools for obtaining further insight into anionophore biological activity, and perhaps for the development of medical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank A. S. Verkman for the gift of FRT cells expressing YFP-H148Q/I152L. This work was supported by the EPSRC through the Bristol Chemical Synthesis Centre for Doctoral Training (EP/G036764/1) and research grant number EP/J00961X/1 and by the F. R. S.-FNRS through a Chargée de Recherche grant to HV. PAG thanks the EPSRC for a studentship to LEK and the ARC for funding (DP170100118).Notes and references
- (a) A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348–357 RSC; (b) P. A. Gale, E. N. W. Howe and X. Wu, Chem, 2016, 1, 351–422 CrossRef CAS; (c) N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed; (d) A. Vargas Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791–2800 CrossRef CAS PubMed; (e) S. Matile, A. Vargas Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC.
- (a) P. A. Gale, J. T. Davis and R. Quesada, Chem. Soc. Rev., 2017, 46, 2497–2519 RSC; (b) I. Alfonso and R. Quesada, Chem. Sci., 2013, 4, 3009–3019 RSC; (c) N. Busschaert, S. H. Park, K. H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Felix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017, 9, 667–675 CrossRef CAS PubMed; (d) S. K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed; (e) V. Soto-Cerrato, P. Manuel-Manresa, E. Hernando, S. Calabuig-Farinas, A. Martinez-Romero, V. Fernandez-Duenas, K. Sahlholm, T. Knopfel, M. Garcia-Valverde, A. M. Rodilla, E. Jantus-Lewintre, R. Farras, F. Ciruela, R. Perez-Tomas and R. Quesada, J. Am. Chem. Soc., 2015, 137, 15892–15898 CrossRef CAS PubMed; (f) J. L. Sessler and W. E. Allen, CHEMTECH, 1999, 29, 16–24 CAS.
- H. Valkenier and A. P. Davis, Acc. Chem. Res., 2013, 46, 2898–2909 CrossRef CAS PubMed.
- P. A. Gale, R. Perez-Tomas and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed.
- (a) L. V. J. Galietta, S. Jayaraman and A. S. Verkman, Am. J. Physiol.: Cell Physiol., 2001, 281, C1734–C1742 CrossRef CAS PubMed; (b) A. S. Verkman and L. J. V. Galietta, Nat. Rev. Drug Discovery, 2009, 8, 153–171 CrossRef CAS PubMed; (c) D. N. Sheppard, M. R. Carson, L. S. Ostedgaard, G. M. Denning and M. J. Welsh, Am. J. Physiol., 1994, 266, L405–L413 CAS.
- (a) H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed; (b) N. López Mora, A. Bahreman, H. Valkenier, H. Li, T. H. Sharp, D. N. Sheppard, A. P. Davis and A. Kros, Chem. Sci., 2016, 7, 1768–1772 RSC; (c) X. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Pérez-Tomás, D. N. Sheppard, Y.-B. Jiang, A. P. Davis and P. A. Gale, Chem, 2016, 1, 127–146 CrossRef CAS.
- H. Valkenier, L. W. Judd, H. Li, S. Hussain, D. N. Sheppard and A. P. Davis, J. Am. Chem. Soc., 2014, 136, 12507–12512 CrossRef CAS PubMed.
- S. J. Moore, C. J. E. Haynes, J. Gonzalez, J. L. Sutton, S. J. Brooks, M. E. Light, J. Herniman, G. J. Langley, V. Soto-Cerrato, R. Perez-Tomas, I. Marques, P. J. Costa, V. Felix and P. A. Gale, Chem. Sci., 2013, 4, 103–117 RSC.
- L. E. Karagiannidis, C. J. E. Haynes, K. J. Holder, I. L. Kirby, S. J. Moore, N. J. Wells and P. A. Gale, Chem. Commun., 2014, 50, 12050–12053 RSC.
- (a) H. Valkenier, C. M. Dias, K. L. Porter Goff, O. Jurček, R. Puttreddy, K. Rissanen and A. P. Davis, Chem. Commun., 2015, 51, 14235–14238 RSC; (b) J. A. Cooper, S. T. G. Street and A. P. Davis, Angew. Chem., Int. Ed., 2014, 53, 5609–5613 CrossRef CAS PubMed; (c) S. Hussain, P. R. Brotherhood, L. W. Judd and A. P. Davis, J. Am. Chem. Soc., 2011, 133, 1614–1617 CrossRef CAS PubMed; (d) S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 4592–4596 CrossRef CAS PubMed; (e) N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed; (f) N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed; (g) C. Lang, X. Zhang, Q. Luo, Z. Dong, J. Xu and J. Liu, Eur. J. Org. Chem., 2015, 6458–6465 CrossRef CAS.
- For modelling and crystallography of OPBU·Cl− complexes, see ref. 8 and 9 respectively. Both methods support the participation of all 4 NH groups as H-bond donors.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Sci., 2014, 5, 1128–1134 RSC.
- S. J. Edwards, I. Marques, C. M. Dias, R. A. Tromans, N. R. Lees, V. Félix, H. Valkenier and A. P. Davis, Chem. – Eur. J., 2016, 22, 2004–2011 CrossRef CAS PubMed.
- M. Lisbjerg, H. Valkenier, B. M. Jessen, H. Al-Kerdi, A. P. Davis and M. Pittelkow, J. Am. Chem. Soc., 2015, 137, 4948–4951 CrossRef CAS PubMed.
- With some anionophores, external addition to LUVs gives higher rates than preincorporation, resulting in values of D > 1. We presume that this observation is due to the actual concentration of lipid being lower than the notional 0.4 mM. Vesicle preparation involves some loss of lipid, so that subsequent addition of anionophore (external addition) results in a transporter to lipid loading that is higher than intended. In contrast, when the transporter is preincorporated in the membrane, the lipid and transporter are lost together during vesicle preparation so that the intended transporter to lipid ratio is maintained.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the synthesis and binding and transport studies. See DOI: 10.1039/c7ob02787g. All the data supporting this study are included in the ESI. |
| This journal is © The Royal Society of Chemistry 2018 |