A tailored nanosheet decorated with a metallized dendrimer for angiography and magnetic resonance imaging-guided combined chemotherapy†
Guilong
Zhang‡
a,
Ruohong
Du‡
b,
Junchao
Qian
c,
Xiaojia
Zheng
b,
Xiaohe
Tian
d,
Dongqing
Cai
a,
Jiacai
He
b,
Yiqun
Wu
e,
Wei
Huang
e,
Yuanyin
Wang
b,
Xin
Zhang
f,
Kai
Zhong
c,
Duohong
Zou
*be and
Zhengyan
Wu
 *a
*a
aKey Laboratory of High Magnetic Field and Ion Beam Physical Biology, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, People's Republic of China. E-mail: zywu@ipp.ac.cn
bDepartment of Dental Implant Center, Stomatologic Hospital & College, Key Laboratory of Oral Diseases Research of Anhui Province, Anhui Medical University, Hefei 230032, People's Republic of China. E-mail: zdhyy@ahmu.edu.cn
cHigh Magnetic Field Laboratory, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei, Anhui 230031, People's Republic of China
dSchool of Life Sciences, Anhui University, Hefei 230601, People's Republic of China
eThe Department of Oral Surgery, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Key Laboratory of Stomatology, National Clinical Research Center of Stomatology, Shanghai 200001, People's Republic of China
fSchool of Life Sciences, Anhui Agricultural University, Hefei 230036, People's Republic of China
First published on 27th November 2017
Abstract
Considering the chemical exchange between gadolinium centers and water protons, nanosystems comprising gadolinium conjugated with high specific area nanocarriers might serve as more robust clinical tools for diagnosis and imaging-guided therapy. Herein, a pH-responsive nanosystem containing graphene oxide conjugated with a folic acid- and gadolinium-labeled dendrimer (FA-GCGLD) to boost its T1 contrast ability was developed, and doxorubicin (DOX) and colchicine (COLC) were efficiently loaded onto this nanosystem (FA-GCGLD-DOX/COLC). This nanosystem showed a prominent T1 contrast with an ultrahigh relaxivity of up to 11.6 mM−1 s−1 and pH-responsive drug release behavior. HepG2 cells treated with FA-GCGLD-DOX/COLC were efficiently inhibited, and the cell contrast was enhanced. In vivo, the tumor accumulation of FA-GCGLD-DOX/COLC significantly increased, thereby facilitating the systemic delivery of particles and exerting tumor growth inhibition and an enhanced tumor contrast effect. Moreover, compared to free drugs, FA-GCGLD-DOX/COLC effectively decreased the drug resistance of the tumor, thereby improving the cancer chemotherapeutic efficacy. In addition, injecting rats with FA-GCGLD afforded excellent magnetic resonance angiography (MRA) images with high-resolution vascular structures because of the long blood circulation time of FA-GCGLD. Thus, this study provides a powerful tool for diverse applications in the biomedical field, including accurate diagnosis and chemotherapy of tumors and the detection of cardiovascular diseases.
Introduction
In clinical settings, magnetic resonance imaging (MRI) remains the benchmark diagnostic tool, as it can acquire 3D tomographic images with excellent soft tissue contrast and anatomical detail, both in a non-invasive manner and without the ionization hazards that plague other imaging modalities.1–3 To further enhance its diagnostic capabilities, approximately 50% of all MR examinations involve the use of contrast agents (CAs) that shorten the spin–lattice (T1) or spin–spin (T2) relaxation time of water protons.4–6 Within the past few decades, Gd chelates (i.e., Gd-DTPA and Gd-DOTA) serving as T1-weighted CAs have been successfully utilized in the clinic, but most of them are small and non-targeted compounds that are passively distributed in the interstitial space of tissues and organs, which decreases their blood circulation time and results in low contrast enhancement.7–9To compensate for these shortcomings, nanocarriers that can load Gd chelates have been developed.10,11 These nanocarriers could significantly enhance the Gd payload and increase the T1 relaxivity per nanoparticle. A wide range of nanocarrier-labeled Gd chelates have already been tested and have shown high contrast ability, including dendrimers,12–15 polymers,16–18 silica nanoparticles,19 and carbonaceous materials.20–22 Arguably, any significant future improvements in the r1 per particle will be achieved by developing a nanocarrier that supports larger Gd payloads. Graphene oxide (GO) possesses a high specific area and diverse functional groups and therefore could be used to enhance the Gd payload by conjugating gadolinium chelates in the intraparticle space. In addition, based on the Solomon–Bloembergen–Morgen (SBM) theory, the strategies for enhancing the relaxivity per Gd mainly have optimized the water residence time, increased the number of bound water molecules, and prolonged the rotational correlation time. Therefore, GO could also affix plenty of water molecules on its surface and optimize the contact efficiency between water protons and gadolinium chelates, thereby achieving an enhanced MRI contrast ability. However, according to the reported studies,23–25 the Gd payload was still low because of the direct grafting between gadolinium chelates and GO. Moreover, these nanosystems were comparatively larger and thus were hardly applied in vivo. Therefore, there is an urgent need to develop GO-based CAs with a high Gd payload and suitable size.
Conversely, GO-based drug delivery systems (DDSs) have attracted widespread attention and displayed an excellent cancer therapeutic effect in animal models.26–29 However, such systems suffer from several issues, such as low permeability,30 retention effect,31 and ligand recognition,32 which have seriously limited their further clinical use. One approach to resolve these problems is to develop DDSs with active targeting ligands and controlled-release ability. Mimicking the responsiveness of a living organism has yielded GO-based DDSs that sensitively respond to the microenvironment of the cancer cells of organisms; currently, these DDSs appear to be feasible strategies for enhancing the drug utilization efficiency and reducing the side effects. However, this approach might contribute to the problem of drug resistance, causing medicines to lose their potency against tumor tissue. Therefore, more efficient delivery strategies are needed.
In this study, we demonstrate a highly efficient theranostic system that was obtained by tailoring GO (TGO) conjugated with a folic acid- and gadolinium-labeled dendrimer (FA-GCGLD). Two types of anticancer drugs (doxorubicin, DOX and colchicine, COLC) were effectively loaded onto FA-GCGLD via π–π interactions with a loading capacity of up to 154%. Meanwhile, the release of these anticancer drugs could be triggered under acidic conditions, and the release rate increased as the pH decreased. Compared with Gd-DTPA, the T1 relaxivity of FA-GCGLD was greatly enhanced, which could be attributed to the high Gd payload and contact efficiency between the Gd centers and water protons. Cell assays indicated that this nanosystem enables not only the intracellular drug release but also the detection of the targeted accumulation of nanodrugs by MRI. The systemic delivery of drugs loaded in FA-GCGLD significantly enhanced the drug efficacy and inhibited the tumor growth. Moreover, the tumor contrast was dramatically enhanced, thereby improving the accuracy of liver cancer diagnosis. In addition, FA-GCGLD nanosheets with ultrahigh T1 relaxivity also showed a clear vascular structure by magnetic resonance angiography (MRA), indicating this nanosystem as a potential CA candidate in the future for the diagnosis of cardiovascular diseases.
Results and discussion
Synthesis and characterization
Fig. 1a shows the preparation process of drug-loaded FA-GCGLD. We first demonstrated that Gd-labeled dendrimers could be further functionalized with the targeting ligand folic acid (FA-GLD) and subsequently loaded the dendrimer onto TGO nanosheets via amide bond formation. Next, FA-GCGLD was further modified using PEG to obtain good biocompatibility and prolong the blood circulation time in vivo. Finally, the anticancer drugs (DOX and COLC) were effectively loaded onto FA-GCGLD (FA-GCGLD-DOX/COLC) via π–π interactions, and the release of drugs could be triggered by adjusting the pH of the solution. Therefore, this nanosystem was endowed with the ability to diagnose and treat disease (Fig. 1b).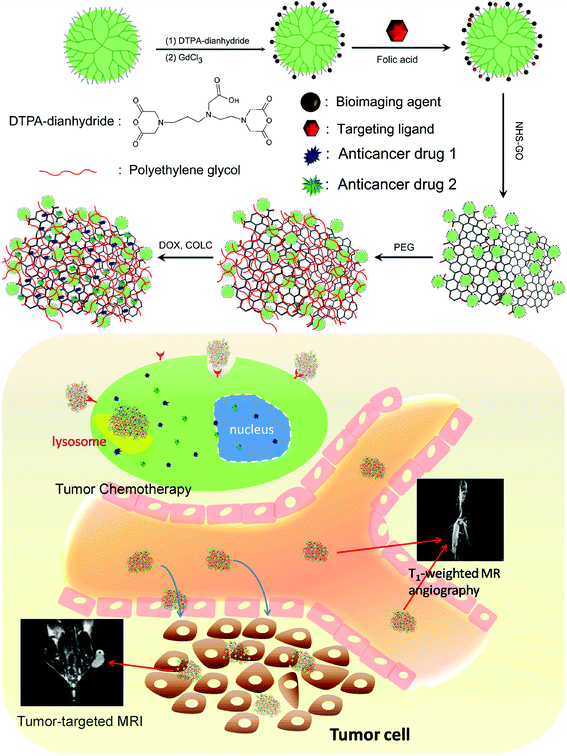 | ||
| Fig. 1 Schematic diagram of (a) the preparation of FA-GCGLD-DOX/COLC and (b) its subsequent use for the accurate diagnosis and therapy of cancer in vivo. | ||
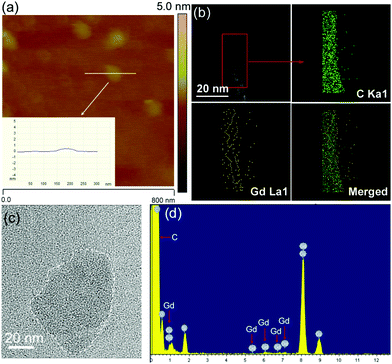
GO was fabricated from graphite powder via the classical Hummer's method.33 The original GO displayed a 2D sheet-like morphology (Fig. S1a†) and a lateral width of approximately 1.5 μm (Fig. S1c†), which was too large for in vivo applications. Subsequently, the original GO nanosheets were further tailored to afford smaller nanosheets of different shapes (Fig. S1b,†Fig. 2c). The analysis of the particle size distribution showed that the size of GO was reduced from 692 nm before tailoring to 108 nm after tailoring (Fig. S1d†). In addition, the atomic force microscopy (AFM) images show that the thickness of TGO was still less than 1 nm (Fig. 2a), indicating that TGO was a single layer and could be utilized in vivo. After FA-GLD was conjugated to the TGO nanosheets, energy-dispersive X-ray (EDX) analysis showed an obvious peak for the Gd element, implying that considerable FA-GLD was conjugated to the surface of TGO (Fig. 2d). Elemental mapping and surface scanning analysis demonstrated the uniform distribution of FA-GLD within the TGO nanosheets (Fig. 2b).
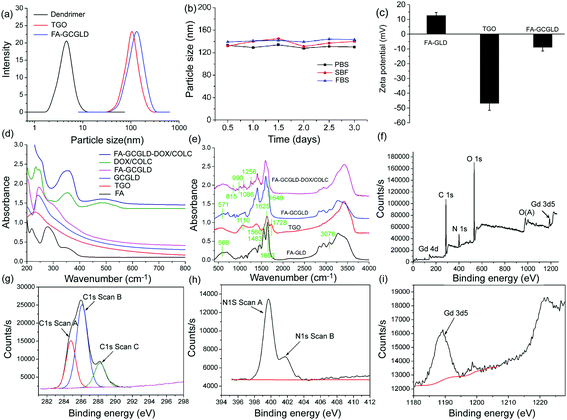
The hydrodynamic diameter of the particles was measured via dynamic light scattering (DLS). As shown in Fig. 3a, FA-GLD particles displayed a very small size of approximately 5.7 nm. TGO was 108 nm, and the size of the FA-GCGLD composite further increased to 186 nm, further indicating that abundant FA-GLD particles were successfully loaded onto the TGO nanosheets. In addition, Fig. 3b reveals that the size of FA-GCGLD did not increase over time and that no precipitation occurred, indicating that FA-GCGLD possessed good stability. Furthermore, we investigated the process of conjugating FA-GLD onto the GO nanosheets via zeta potential analysis (Fig. 3c). The FA-GLD particles had a mean charge of 12.5 mV, and TGO possessed a mean charge of −46.5 mV. After FA-GLD was conjugated to TGO, the charge of the particles became −8.7 mV.
In this context, the modification process of FA-GCGLD was investigated via ultraviolet visible (UV-Vis) and Fourier transform infrared (FT-IR) spectroscopy. As shown in Fig. 3d, GO possessed a typical absorption peak at 227 nm. Moreover, the absorption peak after the loading of GLD onto TGO shifted to 276 nm, indicating that the oxide groups in TGO were reduced or removed during the binding of the GLD particles. These findings suggested that the GLD particles were successfully loaded onto the TGO lattice. In addition, the new peak at 295 nm exhibited by FA-GCGLD suggested that FA-GCGLD featured many folic acid targeting ligands. For FA-GCGLD-DOX/COLC, the strong peaks at 355 and 496 nm were assigned to the absorption of COLC and DOX, respectively, which suggested that a considerable amount of drugs were loaded onto the FA-GCGLD nanosheet. In the FT-IR spectra (Fig. 3e), the FA-GLD peaks at 568, 1483, 1652, and 3078 cm−1 were ascribed to the Gd–O stretching vibration, benzene skeleton vibration, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and
O stretching vibration, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching vibration, respectively, which indicated that GLD and folic acid were attached to the dendrimers. The TGO peak at 1728 cm−1 corresponded to the –COOH stretching vibration; this functionality was used to conjugate FA-GLD via an amide bond. For FA-GCGLD, the –COOH peak shifted from 1728 to 1649 cm−1, indicating the successful formation of the amide bond between FA-GLD and the TGO nanosheet. For FA-GCGLD-DOX/COLC, the new peaks appearing at 1256, 1086, 990, and 815 cm−1 suggested that many drug molecules were loaded onto the nanosystem. These data were consistent with the results from the UV-Vis analysis.
C–H stretching vibration, respectively, which indicated that GLD and folic acid were attached to the dendrimers. The TGO peak at 1728 cm−1 corresponded to the –COOH stretching vibration; this functionality was used to conjugate FA-GLD via an amide bond. For FA-GCGLD, the –COOH peak shifted from 1728 to 1649 cm−1, indicating the successful formation of the amide bond between FA-GLD and the TGO nanosheet. For FA-GCGLD-DOX/COLC, the new peaks appearing at 1256, 1086, 990, and 815 cm−1 suggested that many drug molecules were loaded onto the nanosystem. These data were consistent with the results from the UV-Vis analysis.
The surface composition, structure, and functional groups of the FA-GCGLD nanosheet were also investigated via X-ray photoelectron spectroscopy (XPS). A full spectral analysis confirmed the presence of C, N, Gd, and O elements in FA-GCGLD (Fig. 3f). The C 1s spectrum showed three peaks at 284.8, 286.7, and 288.2 eV (Fig. 3g), which were ascribed to graphite C (sp2 –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), C–O–C, and the amide bond, respectively. In addition, the N 1s spectrum possessed two peaks at 399.8 and 402.1 eV (Fig. 3h), which were assigned to the –C–N– bond in the dendrimer and the amide bond (HN–C
C–), C–O–C, and the amide bond, respectively. In addition, the N 1s spectrum possessed two peaks at 399.8 and 402.1 eV (Fig. 3h), which were assigned to the –C–N– bond in the dendrimer and the amide bond (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. The strong peak that appeared at 1188.6 eV suggested that many Gd ions had been attached to the FA-GCGLD nanosheet (Fig. 3i). Therefore, these data suggested that the FA-GLD particles had been successfully grafted onto GO nanosheets via amide bond formation.
O), respectively. The strong peak that appeared at 1188.6 eV suggested that many Gd ions had been attached to the FA-GCGLD nanosheet (Fig. 3i). Therefore, these data suggested that the FA-GLD particles had been successfully grafted onto GO nanosheets via amide bond formation.
MRI investigation in vitro
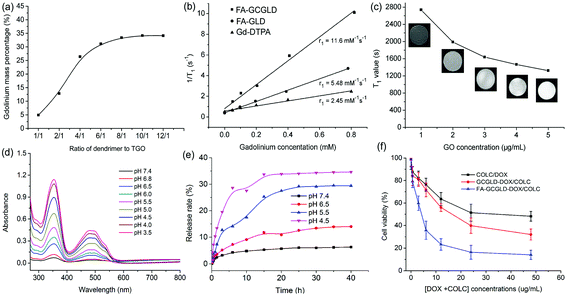
The United States Food and Drug Administration recently cautioned against the use of clinical commercial CAs, particularly in patients with kidney disease, because of the unexpected release of Gd ions in the physiological environment and the resultant kidney damage.34–36 To address these concerns, we demonstrated the stability of the Gd ions in FA-GCGLD under different pH conditions. Fig. S2† shows that the amount of Gd ions released from FA-GCGLD was low and could be deemed negligible at different pH values, indicating that FA-GCGLD was stable under complex physiological conditions.To achieve high MR contrast enhancement, assessing the gadolinium content in the FA-GCGLD nanosystem was necessary. The gadolinium content of FA-GCGLD was found to depend on the ratios of the dendrimer to TGO, with the highest gadolinium payload of approximately 34.2 wt% being achieved when the mass ratio of the dendrimer to TGO was 10/1 (Fig. 4a). In addition, based on the FT-IR spectrum (Fig. S3†),37 each TGO nanosheet was calculated to have 102 carboxyl groups, indicating that each nanosheet could load 102 FA-GLD particles. Thus, approximately 3264 Gd ions were theoretically attached to each TGO nanosheet. In actuality, the number of Gd ions on a TGO nanosheet was 2302, as determined by inductively coupled plasma optical emission spectrometry (ICP-OES).
We then studied the contrast enhancement ability of the nanosheets using MR tubes with different concentrations of gadolinium, which provided intuitional views of the contrast ability by distinguishing the brightness (T1) of the images. Three samples (Gd-DTPA, FA-GLD, and FA-GCGLD) with different concentrations were prepared and used in a T1-weighted MRI study (Fig. S7b†). In the T1-weighted imaging, both Gd-DTPA and FA-GLD showed a weak contrast (slight brightness), whereas FA-GCGLD at the same concentration exhibited a clear T1 contrast (strong brightness), indicating that FA-GCGLD had the best T1 contrast ability. The corresponding longitudinal relaxivity (r1) result of FA-GCGLD was 11.6 mM−1 s−1, which was 5-fold and 2-fold higher than that of Gd-DTPA (2.45 mM−1 s−1) and FA-GLD (5.48 mM−1 s−1, Fig. 4b). The high r1 of FA-GCGLD might be because of the fact that TGO possessed a high specific surface area and plenty of active groups could accelerate the natural affinitive binding to oxygen atoms in water molecules bonded to Gd species (Fig. S4†), thus resulting in an increased number of water molecules distributed on the TGO surface. To confirm our hypothesis, the T1 relaxation time of pure GO solution was measured (Fig. 4c). The results revealed that the T1 value of the solution gradually decreased as the GO concentration increased, indicating that GO could successfully affix water molecules and shorten the spin–spin time of the protons. Based on the average number of gadolinium atoms per FA-GCGLD nanosheet and the relaxivity per gadolinium atom, the relaxivity per FA-GCGLD nanosheet was approximately 2.7 × 104 mM−1 s−1. In addition, compared to Gd-DTPA and FA-GLD, the T1 relaxivity of FA-GCGLD was significantly enhanced.
The toxicity of imaging agents is also a key consideration for biomedical applications. Therefore, HepG2 and HeLa cells were used to evaluate the in vitro cytotoxicity of FA-GCGLD through the CCK-8 assay (Fig. S6†). When the cells were treated with FA-GCGLD in a wide range of concentrations for 24 and 48 h, the viability did not clearly decrease, indicating that FA-GCGLD had no significant cytotoxicity and showed good biocompatibility. The MR contrast enhancement of HepG2 cells administered with FA-FCGLD was then investigated. HepG2 cells treated with Gd-DTPA only slightly brightened as the concentration increased, with GCGLD showing similar results. However, FA-GCGLD caused a significant brightening of the cellular MR images, implying that FA-GCGLD could be effectively internalized by the HepG2 cells because of its folate-receptor targeting ability (Fig. S7a†). Furthermore, the reduction in T1 relaxation times observed from the relaxation time maps corroborated the T1-weighted image enhancements of HepG2 cells (Fig. S7c†). The HepG2 cells incubated with FA-GCGLD exhibited a significant decrease in the T1 relaxation time. Nevertheless, the cells incubated with Gd-DTPA and GCGLD particles showed only a slight decrease in the transverse relaxation time, further confirming that FA-GCGLD possessed an excellent targeting ability for HepG2 cells.
Drug release and cytotoxicity
DOX and COLC were effectively loaded onto FA-GCGLD via π–π stacking interactions, and the loading capacity (154%) was determined via UV-Vis spectroscopy. The drug release behavior of FA-GCGLD-DOX/COLC was investigated using UV-Vis spectroscopy under different pH conditions. As shown in Fig. 4d, the absorbance of COLC and DOX increased as the pH decreased, which indicated that the release of drugs could be effectively triggered by increasing the concentration of water protons. Fig. 4e and S5† show that the amount of drugs released was low (less than 5%) at neutral pH but markedly increased under acidic conditions, indicating that the release behavior of drugs was pH-dependent.Next, the viability of the HepG2 cells incubated with free DOX/COLC, GCGLD-DOX/COLC, and FA-GCGLD-DOX/COLC was measured to evaluate the applicability of the nanosystem for cancer treatment. All groups were found to exhibit a dose-dependent cytotoxic effect. However, compared with GCGLD-DOX/COLC, the HepG2 cells treated with FA-GCGLD-DOX/COLC showed higher apoptosis at 24 and 48 h (Fig. 4f and S8†). This result might be due to the effective internalization of FA-GCGLD-DOX/COLC by the HepG2 cells arising from the folate-receptor targeting interaction, facilitating the release of drug molecules and thus inducing cancer cell death.
Cell uptake and localization investigation
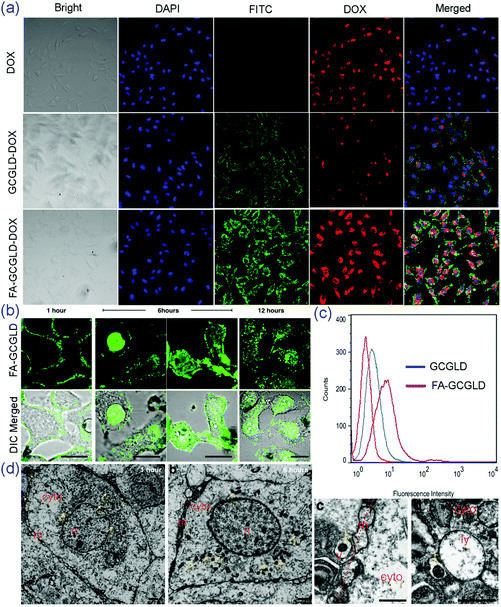
The intracellular localization of the particles in living cells was observed by confocal laser scanning microscopy (CLSM) (Fig. 5a). Fluorescein isothiocyanate (FITC) was rationally conjugated with FA-GCGLD-DOX/COLC to visualize the state of the particles in the cells. When HepG2 cells were treated with free DOX, only red fluorescence could be observed inside the cells because of the fast diffusion of DOX. Moreover, the HepG2 cells treated with GCGLD-DOX/COLC exhibited weak green and red fluorescence in the cytomembrane and cytoplasm, suggesting that minimal GCGLD-DOX/COLC was taken up by the cell and DOX was released in the cytoplasm. However, the HepG2 cells treated with FA-GCGLD-DOX/COLC showed stronger green and red fluorescence, indicating that FA-GCGLD-DOX/COLC possessed an excellent targeting ability and could be efficiently delivered into tumor cells. Furthermore, the fluorescence quantification analysis of HepG2 cells via flow cytometry indicated that there was considerably more FA-GCGLD-DOX/COLC in the cells than GCGLD-DOX/COLC. This result was consistent with the above-mentioned analysis (Fig. 5c).Subsequently, the precise subcellular localization and the dynamic entry of the FA-GCGLD nanosheets were evaluated via CLSM and transmission electron microscopy (TEM). The images obtained at 1 hour clearly showed that most of the FA-GCGLD nanosheets were closely associated with the cell plasma membrane and that negligible signal was observed from the intracellular region (Fig. 5b). With an increasing incubation time, FA-GCGLD crossed the cell membrane and penetrated into the cytosolic space. Notably, some FA-GCGLD nanosheets also permeated into the nucleus. Moreover, the images at 12 hours showed slightly less fluorescence remaining within the cells, implying a vesicular recycling process (e.g., exocytosis). In a parallel experiment, the TEM micrographs clearly exhibited the intracellular distribution of FA-GCGLD (Fig. 5d). Compared to an incubation time of 1 hour, the TEM images at an incubation time of 6 hours showed many nanoparticles in the cell. This result suggested that the cellular uptake of FA-GCGLD was time-dependent. In addition, the magnified images displayed abundant extracellular and intracellular vesicles that were closely associated with the plasma membrane (Fig. 5dc) and lysosomal/endosomal structures (Fig. 5dd), suggesting that the uptake mechanism of the FA-GCGLD nanosheets was endocytosis.
Bio-distribution, pharmacokinetics, and antitumor activity
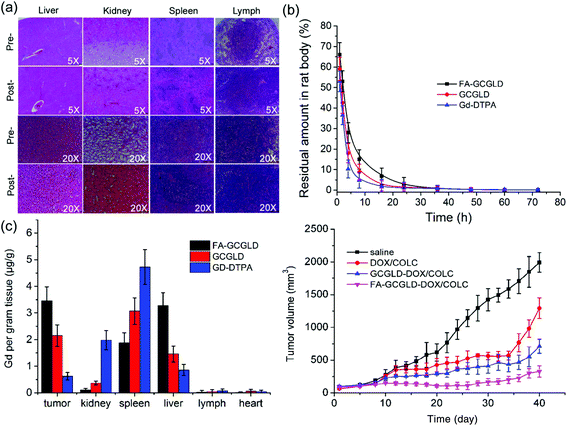
For a new theranostic agent, assessing its biocompatibility, pharmacokinetics and bio-distribution in vivo is necessary. The H&E images of various organs also showed no noticeable inflammation or damage after continuous injections of FA-GCGLD for a week (Fig. 6a), indicating that FA-GCGLD induced no obvious organ toxicity in the treated animals and possessed good biocompatibility. Next, the blood elimination kinetics of FA-GCGLD indicated that FA-GCGLD could be cleared (Fig. 6b) and left no residual material in the body. Moreover, FA-GCGLD possessed a longer retention time than GCGLD and Gd-DTPA, which would promote the delivery of FA-GCGLD into tumor tissue. To quantitatively assess the bio-distribution of the particles, hepatic cancer-bearing nude mice were injected with Gd-DTPA, GCGLD, and FA-GCGLD at a dose of 2 mg (Gd) kg−1 (animal body weight) via the tail vein. Significant accumulation of gadolinium in tumors appeared for GCGLD and FA-GCGLD compared with Gd-DTPA (Fig. 6c). In addition, the tumor accumulation of FA-GCGLD was significantly higher than that of GCGLD and Gd-DTPA by 2.6 and 8.9 times, respectively. This was due to the good tumor recognition ability of FA-GCGLD, which facilitated the effective accumulation of FA-GCGLD in the tumor. In addition, all particles (Gd-DTPA, GCGLD, and FA-GCGLD) were clearly accumulated in the kidney, liver, and spleen of the reticuloendothelial system.The inhibitory efficacy of the nanodrugs against tumors was evaluated in BALB/c nude mice (Fig. 6d). Mice injected with saline (control) showed rapid growth in their tumor size, suggesting that saline had no inhibitory effect on tumors. By contrast, DOX/COLC and GCGLD-DOX/COLC showed excellent inhibitory effects on tumors up to 34 days; however, at 34 days post-injection (p.i.), the tumor volume increased through explosive growth. This might be because the cancer cells generated gene mutations and thus possessed high drug resistance. Interestingly, the tumor volume of mice treated with FA-GCGLD-DOX/COLC was effectively inhibited and showed negligible growth throughout the whole treatment process, which could be attributed to the effective targeting ability of the particles and sustained drug release in the tumor tissue. These results indicated that the use of the targeted nanodrug prevented the emergence of tumor drug resistance and improved the cancer chemotherapeutic efficacy.
The results of the H&E staining of tumor biopsies treated with saline showed the presence of highly malignant hyperchromatic tumor cells on the slide (Fig. 7). However, the tumor cells on the slides treated with DOX/COLC, GCGLD-DOX/COLC, and FA-GCGLD-DOX/COLC showed different degrees of necrosis, and the malignant hyperchromatic area gradually decreased. Moreover, the extent of the necrotic areas was in the order of FA-GCGLD-DOX/COLC > GCGLD-DOX/COLC > DOX/COLC, whereas the size of the malignant hyperchromatic area was in the following sequence: FA-GCGLD-DOX/COLC < GCGLD-DOX/COLC < DOX/COLC. These results indicated that FA-GCGLD-DOX/COLC possessed the best therapeutic efficacy against tumor. In addition, the histological examination of the heart, kidney, liver, lung and spleen from mice treated with saline, GCGLD-DOX/COLC, or FA-GCGLD-DOX/COLC showed no or only minor histological changes, indicating that these samples were non-toxic or had minimal side effects on the body. In contrast, the samples treated with DOX/COLC presented obvious damage in the heart, kidney, liver, lung and spleen. Specifically, this damage consists of the following effects: (1) myocardial muscle cell lysis and inflammatory cell infiltration in the heart, (2) decrease in the glomerular interstitial space, (3) cloudy swelling of hepatocytes, (4) congestive change and mild thickening of the alveolar membrane, and (5) atrophy in the spleen. These results demonstrated that DOX/COLC could induce serious side effects on the body, whereas the FA-GCGLD nanosheet exhibited significantly enhanced therapeutic efficacy and reduced side effects.
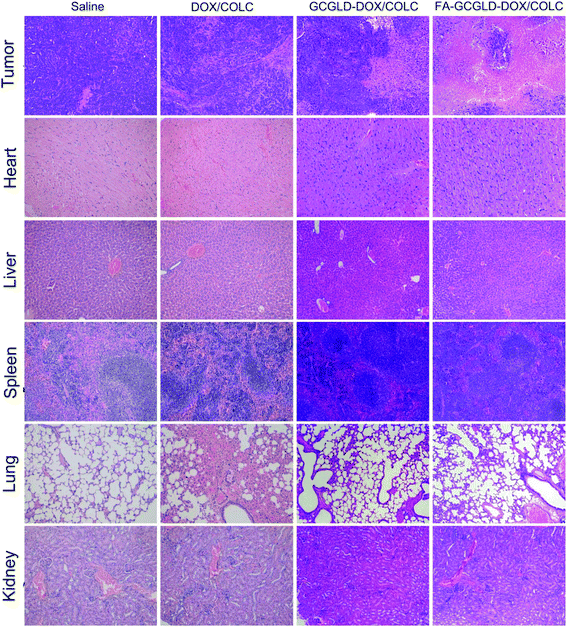 | ||
| Fig. 7 Pathological analysis of various organs in mice injected with different samples via the tail vein. | ||
T 1-Weighted MRI in vivo
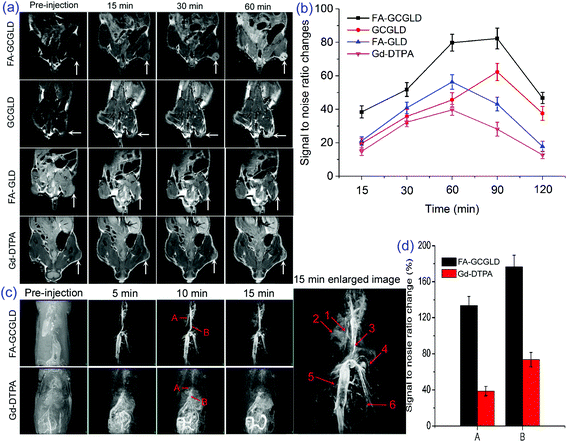
The MRI performance of FA-GCGLD was explored in vivo using a 9.4 T MR scanner. The T1 images of the regions of interest (ROIs) in tumors were acquired pre-injection and at 15, 30, 60, 90, and 120 min p.i. of Gd-DTPA, FA-GLD, GCGLD, and FA-GCGLD at a dose of 2 mg kg−1 (Fig. 8a). The T1-weighted images of tumor tissue show a slight brightness p.i. of Gd-DTPA or FA-GLD, but distinguishing between tumor and normal tissues was still challenging. In addition, for non-targeted GCGLD, the whole body of mice appeared bright, which made the observation of the tumor tissue difficult. Conversely, the tumor tissue of mice injected with FA-GCGLD was dramatically brightened and could be clearly observed, which could be attributed to the high relaxivity and excellent targeting ability of the FA-GCGLD nanosheet. The analysis of the MRI signal-to-noise ratio change (ΔSNR) in the tumor region indicated that the greatest ΔSNR of FA-GCGLD was up to 80.3% in T1 imaging at 90 min p.i. (Fig. 8b). By contrast, the corresponding ΔSNR values in the tumor area of Gd-DTPA and FA-GLD reached the maxima of only 39.5% and 56.6% at 60 min p.i., respectively. Additionally, a decrease in the ΔSNR value began at 60 min p.i. for Gd-DTPA and FA-GLD but commenced at 90 min p.i. for GCGLD and FA-GCGLD. These results indicated that among the systems tested, FA-GCGLD had the best T1 contrast enhancement effect and a longer blood circulation time, which are important attributes for the diagnosis of early tumor lesions.MR angiography investigation
The feasibility of FA-GCGLD as a potential MR angiography (MRA) CA was also explored. MRA is an important clinical tool used to detect various cardiovascular complications, including myocardial infarction and atherosclerotic plaque.38–40 Molecular CAs used for this diagnostic purpose are typically small in size (<2 nm diameters) and rapidly leak out of the vascular network in the circulatory system, resulting in poor steady-state imaging conditions and consequently poor resolution.41–43 To maintain high-resolution images within the vasculature, massive amounts of CAs are continuously administered throughout the MRI procedure, increasing the potential for toxicity. An MRA study was conducted on rats (average body weight of 220 g) intravenously injected with FA-GCGLD at a dose of 20 mg kg−1. A strong contrast in the vascular networks was observed at 5 min p.i., and excellent high-resolution images were obtained for up to 15 min (Fig. 8c). Prominent vascular details including the hepatic capillary, hepatic portal vein, inferior vena cava, proper hepatic artery, renal vein, and mesenteric artery were clearly distinguished. Importantly, contrast enhancement in the interstitial space of the rat body was not observed, indicating that FA-GCGLD was only present in the blood vessels and not within the interstitial space of the body. In contrast, the same dose of a commercial T1 contrast agent (Gd-DTPA) showed poor contrast in the vascular networks, and most of the Gd-DTPA molecules were absorbed or distributed in the intestine. An inherent reason for the observed results is the relatively low r1 value (2.45 mM−1 s−1) of Gd-DTPA.The ΔSNR values of FA-GCGLD within the hepatic portal vein and inferior vena cava at 15 min p.i. were 3.8-fold and 2.7-fold higher, respectively, than those of Gd-DTPA (Fig. 8d). Therefore, obtaining detailed diagnostic information from the vasculature using a single Gd-DTPA injection is difficult. FA-GCGLD could address many shortcomings of previous MRA CAs such as Gd-DTPA and achieve detailed vascular diagnostic imaging, which is particularly helpful in the context of a cardiovascular event. Therefore, the development of FA-GCGLD with a suitable size is likely to provide a new pool of molecules that might provide revolutionary solutions in the field of molecular imaging within a clinical setting.
Live subject statement
The mice used for the experiment were treated in accordance with the Ethics Committee Guidelines of the University of Science and Technology of China, and the study was approved by the Institutional Animal Care and Use Committee of National Tissue Engineering Center (Shanghai, China).Conclusions
In summary, a highly efficient MRA CA with a high Gd payload was developed for in vivo imaging and tumor therapy by conjugating TGO to a folic acid- and gadolinium-labeled dendrimer. Additionally, the release of anticancer drugs could be triggered by water protons, and the release rate increased as the pH decreased. The T1 relaxivity of FA-GCGLD was greatly enhanced to 11.6 mM−1 s−1 because of the ability of the FA-GCGLD nanosheet to affix water molecules, and enhance the contact efficiency between the Gd centers and water protons. In addition, cell assays indicated that FA-GCGLD-DOX/COLC could be effectively internalized by the HepG2 cells and then significant cell contrast enhancement and dramatic cell viability inhibition could be achieved. In vivo, the systemic delivery of FA-GCGLD-DOX/COLC decreased the tumor resistance against anticancer drugs and significantly inhibited the tumor growth. Moreover, FA-GCGLD was effectively accumulated in the tumor tissue, enhancing the accuracy of liver cancer diagnosis. These results demonstrated the diverse applications of FA-GCGLD for the accurate diagnosis and therapy of tumors. Moreover, when used for MRA, FA-GCGLD nanosheets with ultrahigh T1 relaxivity also clearly showed the vasculature network, indicating that FA-GCGLD might be a future candidate as a CA for the diagnosis of cardiovascular diseases.Author contributions
G. Z., D. Z. and Z. W. designed this study. G. Z. and R. D. prepared materials and carried out the corresponding characterization. X. T., X. Z. and R. D. carried out the cell experiments and the animal experiments. J. Q. and K. Z. performed magnetic resonance studies. W. H., Y. W. and J. H. analysed the histological data. G. Z., D. C., Z. W. and D. Z. analyzed and discussed the data. Z. W., G. Z., X. Z. and Y. W. are responsible for writing and editing the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
All authors acknowledge financial support from the National Natural Science Foundation of China (No. 21407151, 31370983, 31370983, 81371114, and 81271162), the Key Program of the Chinese Academy of Sciences (No. KSZD-EW-Z-022-05), the Science and Technology Service Programs of the Chinese Academy of Sciences (No. KFJ-STS-ZDTP-002 and KFJ-SW-STS-143), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2015385), the Science and Technology Project of Anhui Province (1604a0802082 and 1401045013), the Anhui Province Funds for Distinguished Young Scientists (1508085J08), the Key Projects of the Outstanding Young Talents in Colleges and Universities (No. gxyqZD2016058) and the Foundation of Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (2015-05).References
- A. Y. Louie, M. M. Huber, E. T. Ahrens, U. Rothbacher, R. Moats, R. E. Jacobs, S. E. Fraser and T. J. Meade, Nat. Biotechnol., 2000, 18, 321–325 CrossRef CAS PubMed.
- Z. P. Liang and P. C. Lauterbur, Principles of Magnetic Resonance Imaging: A Signal Processing Perspective, Wiley-IEEE Press, New York, 1999 Search PubMed.
- G. Zhang, R. Du, L. Zhang, D. Cai, X. Sun, Y. Zhou, J. Zhou, J. Qian, K. Zhong, K. Zheng, D. Kaigler, W. Liu, X. Zhang, D. Zou and Z. Wu, Adv. Funct. Mater., 2015, 25, 6101–6111 CrossRef CAS.
- M. F. Bellin, Eur. J. Radiol., 2006, 60, 314–323 CrossRef PubMed.
- F. G. Shellock and E. Kanal, J. Magn. Reson. Imaging, 1999, 10, 477–484 CrossRef CAS PubMed.
- J. S. Ananta, B. Godin, R. Sethi, L. Moriggi, X. Liu, R. E. Serda, R. Krishnamurthy, R. Muthupillai, R. D. Bolskar and L. Helm, Nat. Nanotechnol., 2010, 5, 815–821 CrossRef CAS PubMed.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- G. Zhang, J. Gao, J. Qian, L. Zhang, K. Zheng, K. Zhong, D. Cai, X. Zhang and Z. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 14192–14200 CAS.
- R. Anbazhagan, Y. A. Su, H. C. Tsai and R. J. Jeng, ACS Appl. Mater. Interfaces, 2016, 8, 1827–1835 CAS.
- S. Wen, Q. Zhao, X. An, J. Zhu, W. Hou, K. Li, Y. Huang, M. Shen, W. Zhu and X. Shi, Adv. Healthcare Mater., 2014, 3, 1568–1577 CrossRef CAS PubMed.
- Z. Xiong, Y. Wang, J. Zhu, Y. He, J. Qu, C. Effenberg, J. Xia, D. Appelhans and X. Shi, Biomater. Sci., 2016, 4, 1622–1629 RSC.
- S. Wen, K. Li, H. Cai, Q. Chen, M. Shen, Y. Huang, C. Peng, W. Hou, M. Zhu, G. Zhang and X. Shi, Biomaterials, 2013, 34, 1570–1580 CrossRef CAS PubMed.
- Y. Miyake, Y. Kimura, N. Orito, H. Imai, T. Matsuda, A. Toshimitsu and T. Kondo, Tetrahedron, 2015, 71, 4438–4444 CrossRef CAS.
- S. Wen, H. Liu, H. Cai, M. Shen and X. Shi, Adv. Healthcare Mater., 2013, 2, 1267–1276 CrossRef CAS PubMed.
- J. Zhu, Z. Xiong, M. Shen and X. Shi, RSC Adv., 2015, 5, 30286–30296 RSC.
- L. Sun, X. Li, X. Wei, Q. Luo, P. Guan, M. Wu, H. Zhu, K. Luo and Q. Gong, ACS Appl. Mater. Interfaces, 2016, 8, 10499–10512 CAS.
- A. Makino, Polym. J., 2014, 46, 783–791 CrossRef CAS.
- K. M. Au, Z. Lu, S. J. Matcher and S. P. Armes, Biomaterials, 2013, 34, 8925–8940 CrossRef CAS PubMed.
- X. Li, W. Zhao, X. Liu, K. Chen, S. Zhu, P. Shi, Y. Chen and J. Shi, Acta Biomater., 2016, 30, 378–387 CrossRef CAS PubMed.
- L. Hou, H. Zhang, Y. Wang, L. Wang, X. Yang and Z. Zhang, Int. J. Nanomed., 2015, 10, 4507–4520 CAS.
- N. Gong, H. Wang, S. Li, Y. Deng, X. Chen, L. Ye and W. Gu, Langmuir, 2014, 30, 10933–10939 CrossRef CAS PubMed.
- P. K. Avti, E. D. Caparelli and B. Sitharaman, J. Biomed. Mater. Res., Part A, 2013, 101, 3580–3591 CrossRef PubMed.
- J. Shi, B. Wang, Z. Chen, W. Liu, J. Pan, L. Hou and Z. Zhang, Pharm. Res., 2016, 33, 1472–1485 CrossRef CAS PubMed.
- H. W. Yang, C. Y. Huang, C. W. Lin, H. L. Liu, C. W. Huang, S. S. Liao, P. Y. Chen, Y. J. Lu, K. C. Wei and C. C. M. Ma, Biomaterials, 2014, 35, 6534–6542 CrossRef CAS PubMed.
- M. X. Zhang, Y. H. Cao, Y. Chong, Y. F. Ma, H. L. Zhang, Z. W. Deng, C. H. Hu and Z. J. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 13325–13332 CAS.
- J. J. Shi, L. Wang, J. Zhang, R. Ma, J. Gao, Y. Liu, C. F. Zhang and Z. Z. Zhang, Biomaterials, 2014, 35, 5847–5861 CrossRef CAS PubMed.
- J. B. Song, X. Y. Yang, O. Jacobson, L. Lin, P. Huang, G. Niu, Q. J. Ma and X. Y. Chen, ACS Nano, 2015, 9, 9199–9209 CrossRef CAS PubMed.
- C. H. Wu, D. Li, L. H. Wang, X. T. Guan, H. Yang, S. Li and Y. Y. Liu, Acta Biomater., 2017, 53, 631–642 CrossRef CAS PubMed.
- B. Tian, C. Wang, S. Zhang, L. Z. Feng and Z. Liu, ACS Nano, 2011, 5, 7000–7009 CrossRef CAS PubMed.
- L. Hou, Y. Yuan, J. Ren, Y. Zhang, Y. Wang, X. Shan, Q. Liu and Z. Zhang, J. Nanopart. Res., 2017, 19, 286 CrossRef.
- C. G. England, H. J. Im, L. Feng, F. Chen, S. A. Graves, R. Hernandez, H. Orbay, C. Xu, S. Y. Cho, R. J. Nickles, Z. Liu, D. S. Lee and W. Cai, Biomaterials, 2016, 100, 101–109 CrossRef CAS PubMed.
- Y. Wei, F. Zhou, D. Zhang, Q. Chen and D. Xing, Nanoscale, 2016, 8, 3530–3538 RSC.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. G. Penfield and R. F. Reilly, Nat. Clin. Pract. Nephrol., 2007, 3, 654–668 Search PubMed.
- C. A. Li, Nat. Mater., 2014, 13, 110–115 CrossRef CAS PubMed.
- P. Marckmann, L. Skov, K. Rossen, A. Dupont, M. B. Damholt, J. G. Heaf and H. S. Thomsen, J. Am. Soc. Nephrol., 2006, 17, 2359–2362 CrossRef PubMed.
- S. S. Gong, S. Y. Guang, F. Y. Ke and H. Y. Xu, China Meas. Test, 2016, 42, 39–44 Search PubMed.
- J. Sanz and Z. A. Fayad, Nature, 2008, 451, 953–957 CrossRef CAS PubMed.
- N. M. D. Wilke, C. Simm, J. Zhang, J. Ellermann, X. Ya, H. Merkle, G. Path, H. Lüdemann, R. J. Bache and K. Uurbil, Magn. Reson. Med., 1993, 29, 485–497 CrossRef CAS PubMed.
- K. Overoye-Chan, S. Koerner, R. J. Looby, A. F. Kolodziej, S. G. Zech, Q. Deng, J. M. Chasse, T. J. McMurry and P. Caravan, J. Am. Chem. Soc., 2008, 130, 6025–6039 CrossRef CAS PubMed.
- N. Lee and T. Hyeon, Chem. Soc. Rev., 2012, 41, 2575–2589 RSC.
- S. Flacke, S. Fischer, M. J. Scott, R. J. Fuhrhop, J. S. Allen, M. McLean, P. Winter, G. A. Sicard, P. J. Gaffney, S. A. Wickline and G. M. Lanza, Circulation, 2001, 104, 1280–1285 CrossRef CAS PubMed.
- H. Kobayashi and M. W. Brechbiel, Adv. Drug Delivery Rev., 2005, 57, 2271–2286 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and supporting results. See DOI: 10.1039/c7nr07957e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |