Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triterpenoids
Robert A.
Hill
 * and
Joseph D.
Connolly
* and
Joseph D.
Connolly
School of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bob.hill@glasgow.ac.uk
First published on 11th July 2018
Abstract
Covering 2014. Previous review: Nat. Prod. Rep., 2017, 34, 90–122
This review covers the isolation and structure determination of triterpenoids reported during 2014 including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, serratanes, isomalabaricanes and saponins; 374 references are cited.
1. Introduction
The interest in the pharmacological activities of triterpenoids continues to be very important.1 Several reviews have covered the anticancer effects of triterpenoids.2–9 Other activities that have been highlighted include anti-HIV,10–12 antiinflammatory,13 antiviral6 and against neurodegenerative disorders.14 As many of the active compounds are saponins there has been an interest in their synthesis15 and biosynthesis.16 Reviews have also appeared covering triterpenoids found in Astragalus species,17Gymnema sylvestre,18Panax species,19,20Sapindus species21 and Siraitia grosvenorii22 and plants of the Schisandraceae.23–25 Triterpenoid biosynthesis in plants26 and the mechanisms of oxidosqualene cyclases27 have also been covered.2. The squalene group
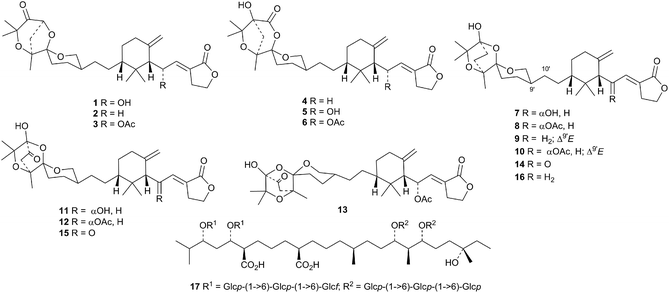
Two interesting series of polyisoprenoid derivatives, terreolides A 1–F 6 and saponaceolides H 7–P 15, have been reported from the previously unknown poisonous European mushroom Tricholoma terreum.28 The known saponaceolide B 16 was also obtained. The structures of terreolides A 1 and D 4 and saponaceolide B 16 were confirmed by X-ray crystallographic analyses. A complex polyisoprenoid glycoside, from the fruit of Lycium chinense, has been assigned the putative structure 17.293. The lanostane group
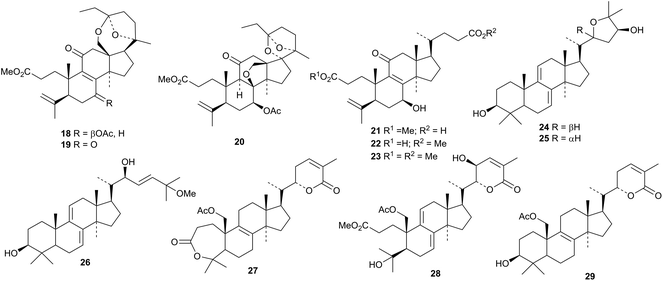
The flow of Ganoderma lanostanes continues.30Ganoderma boninense is the source of ganoboninketals A 18, B 19 and C 20.31 Fornicatins D 21, E 22 and F 23 and ganodercochlearins A 24, B 25 and C 26 are constituents of Ganoderma cochlear.32 The structure of ganodercochlearin B 25 was confirmed by X-ray analysis of the corresponding diacetate. Cultures of Ganoderma sp. KM01 produced ganodermalactones B 27, D 28 and E 29.33 The structure of ganodermalactone B 27 was confirmed by X-ray analysis and was shown to have the same structure as the previously reported colossolactone C, from Ganoderma colossum,34 however the pmr and cmr spectra for rings A and B are not in agreement.Other Ganoderma lanostanes include ganoderic acids XL130 and XL231, 20-hydroxyganoderic acid AM132, ganoderenic acid AM133 and ganoderesin C 34 from Ganoderma theaecolum,35 methyl lucidenate B 35 (ref. 36) and the butyl esters 36 and 37 (ref. 37) from the fruiting bodies of Ganoderma lucidum and the norlanostane 38 from the fruiting bodies of Ganoderma tropicum.38 The biological and pharmacological activities of ganoderic acid and lucidenic acid have been covered in a review.39
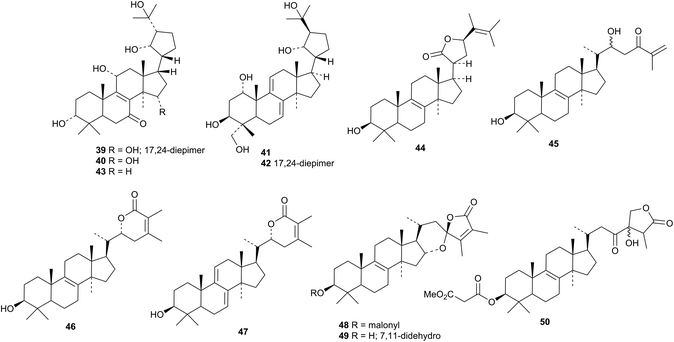
The mushroom Inonotus obliquus is a rich source of the 21,24-cyclolanostanes inonotusols A 39–E 43.40 They are accompanied by inonotusols F 44 and G 45. Inonotusols B 40, D 42 and E 43 have unusual configurations at C17 and inonotusol F 44 has an unusual configuration at C20 and methylation at C24. Further C24-methylated metabolites of Inonotus obliquus include inotolactones A 46 and B 47.41 Hexatenuins A 48, B 49 and C 50, from the fruiting body of Hexagonia tenuis, also have an extra carbon at C-24.42
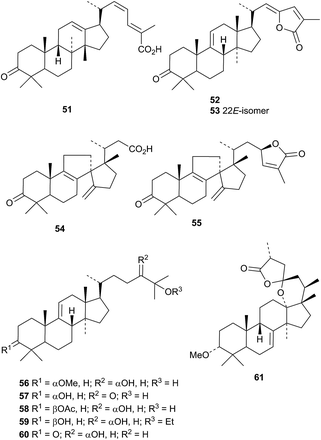
The protostane 51, from the bark of Garcinia ferrea, is accompanied by the lanostanes garciferolides A 52 and B 53.43 Two rearranged lanostanes 54 and 55 have been isolated from Abies nukiangensis together with compounds 56–59.44 The structures of 54 and the known 60 were confirmed by X-ray analyses. 3-O-Methylabiesatrine A 61 is a rearranged lanostane from Abies delavayi.45
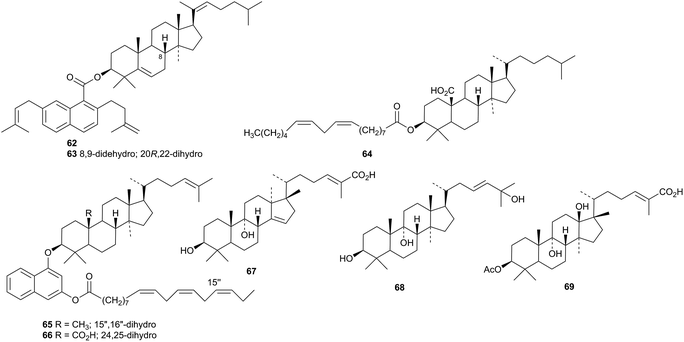
Two naphthalene esters, lanostenyl naphthoates A 62 and B 63 have been reported from the rhizomes of Acorus calamus.46 The related compounds 64–66 are found in the bark of Ficus religiosa.47 The mariesane derivative opaciniol B 67 and the lanostane opaciniol C 68 are constituents of Garcinia opaca.48 Opaciniol B 67 is the same as garcihombronane K isolated from Garcinia hombroniana in 2013.49 The rearranged lanostane 69 has been isolated from Garcinia hombroniana.50
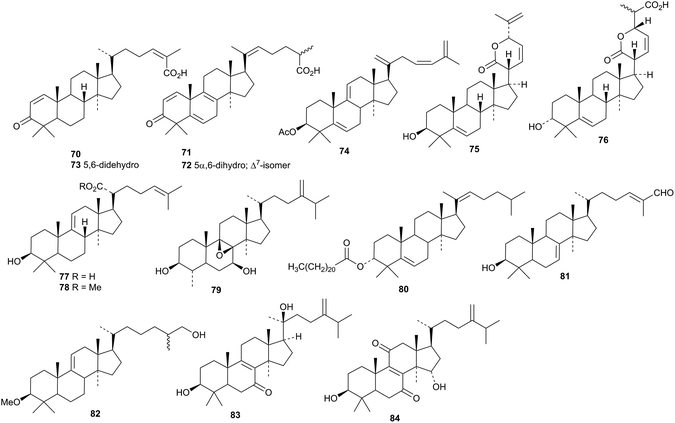
Other lanostanes include manglanostenoic acids A 70–D 73 from Mangifera indica var. Fazli,51 myrrhalanostenyl acetate 74, myrrhalanostenol 75, and myrrhalanostenoic acid 76 from the oleoresin of Commiphora myrrha,52 3β-hydroxylanosta-9(11),24-dien-21-oic acid 77 and its methyl ester 78 from Protorhus longifolia,53,54 the norlanostane 79 from Euphorbia bupleuroides,55 the ester 80 from the fruit of Cuminum cyminum,56 kiusianin A 81 from Tilia kiusiana57 and methyl ether 82 from Cymbopogon citratus.58 Compounds 83 and 84, from the branches and leaves of Polyalthia obliquei, were originally thought to be tirucallane derivatives but are now considered to be lanostanes.59,60 Lanostane saponins with known genins have been isolated from Cuminum cyminum56 and Panax ginseng.61,62
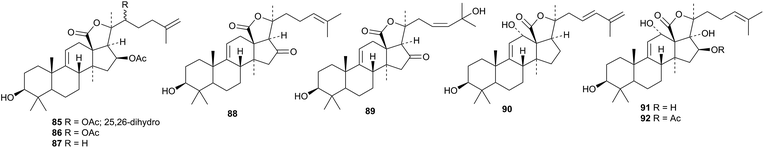
Investigations of several sea cucumbers have resulted in the identification of more holostane saponins, some with interesting pharmacological activities.63,64 Cladolosides A1–A6, from Vietnamese Cladolabes schmeltzii, have the new genins 85–89.65 Coustesides A–J are new saponins from Bohadschia cousteaui.66 Coustesides B and G have the new genin 90 and C and D the new genins 91 and 92, respectively. All the others have known genins.
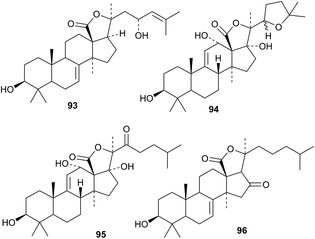
Variegatusides C–F have been isolated from Stichopus variegates.67 Only variegatuside C has a new genin 93. Holothurins D and E, with the new genin 94 and holothurinoside X, with the new genin 95, are constituents of Holothuria lessoni together with holothurinosides Y and Z with known genins.68 The new genin 96 has been reported for pseudocnoside A from Pseudocnus dubiosus leoninus.69 Holostane saponins with known genins include cucumariosides F1 and F2 from Eupentacta fraudatrix,70 kolgaosides A and B from Kolga hyalina,71 stichloroside F from Stichopus chloronotus72 and violaceusosides C, D, E and G from Pseudocolochirus violaceus.73
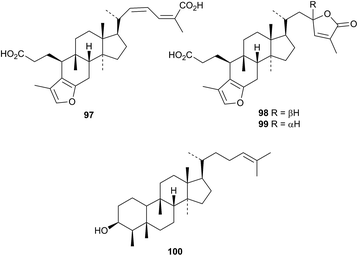
Cucurbitane triterpenoids of mushroom origin have been covered in a review.74 Three interesting furanoid cucurbitane derivatives, roseic acid 97 and roseolactones A 98 and B 99, have been isolated from Russula aurora and Russula minutula.75Empetrun nigrum var. japonicum is the source of the migrated cucurbitane nigrum-24-en-3β-ol 100.76
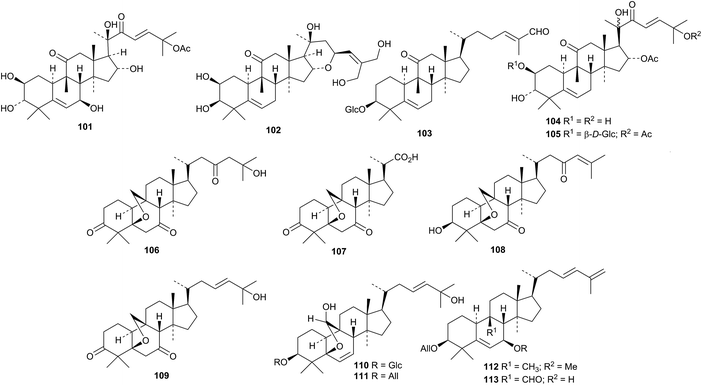
New cucurbitane derivatives isolated from Hemsleya species include 101 and 102 from Hemsleya amabilis77 and hemslepenside A 103, 16,25-di-O-acetylcucurbitacin F 2-O-β-D-glucopyranoside 104 and 16-O-acetylcucurbitacin F 105 from Hemsleya penxianensis.78 New cucurbitanes are still being found in the various parts of Momordica charantia.79 The fruit is the source of kuguacins T 106–W 109 (ref. 80) and charantosides D 110–G 113.81 The structure of kuguacin W 109 was confirmed by X-ray analysis.
The leaves and stem yielded karavilagenin F 114, karavilosides XII 115 and XIII 116 and momordicines VI 117, VII 118 and VIII 119.82 A separate investigation of the leaves led to the isolation of compounds 120 and 121.83 Two new glycosides were reported from the seeds, one with the new genin 122.84
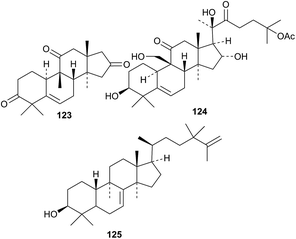
Kinoin D 123 is an octanorcucurbitane derivative from the roots of Ibervillea sonorae.85 Minor cucurbitane glycosides from Siraitia grosvenorii include 11-deoxymogrosides V and VI and 11-deoxyisomogroside V, all with known genins.86 23,24-Dihydrocucurbitacin C 124 is a new compound from Cucumus sativus.87 The unlikely stereochemistry of 125 has been proposed for a compound from the leaves and twigs of Euonymus alatus.88
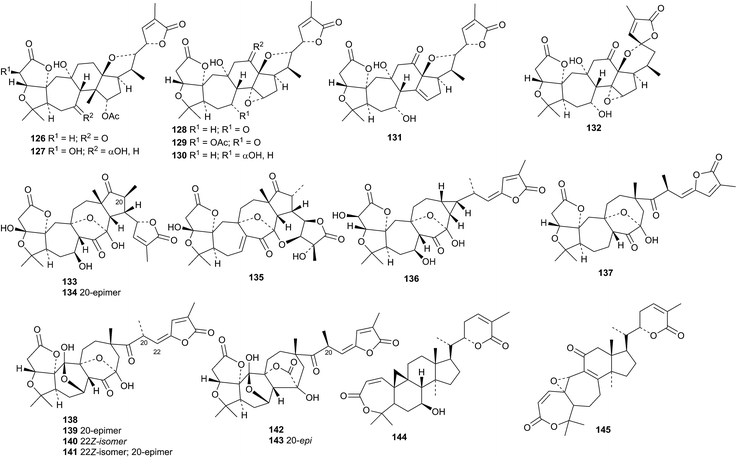
An impressive list of new compounds from Schisandra chinensis includes wuweizidilactones J 126–P 132, schindilactones I 133, J 134 and K 135, preschisanartanin N 136 and schisdilactone J 137.89 Lancifonins A 138–F 143 are new compounds from Schisandra lancifolia.90Schisandra sphenanthera is the source of schisphendilactones A 144 and B 145.91 The structures of lancifonin A 138 and schisphendilactone A 144 were confirmed by X-ray analyses.
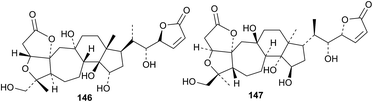
Other new compounds include heteroclitalactone N 146 from the stems of Kadsura heteroclitus92 and micrandiactone H 147 from Kadsura coccinea.93 The reported stereochemistry for micrandiactone H 147 is unusual and probably requires revision. The name of 147 should be changed to micrandilactone H in line with other compounds in the series.
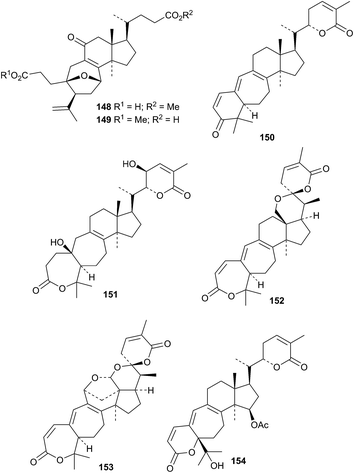
Cochlates A 148 and B 149 are cleaved cycloartane derivatives from Ganoderma cochlear.32 The structure of cochlate B 149 was confirmed by X-ray analysis. Cultures of Ganoderma sp. KM01 produce further cleaved derivatives ganodermalactones A 150, C 151, F 152 and G 153.33 The structures of ganodermalactones F 152 and G 153 were confirmed by X-ray analyses. The revised structure 154 has been assigned to colossolactone G, a further metabolite of these cultures.
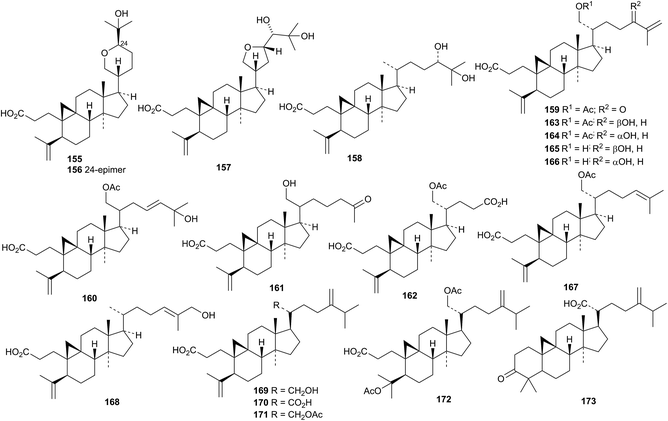
An impressive number of side-chain variations is to be found in the 3,4-secocycloartanes lithocarpic acids A 155–N 168 from Lithocarpus polystachyus.94 Lithocarpic acids O 169–S 173 are further examples from the same source.95 The structure of lithocarpic acid A 155 was confirmed by X-ray analysis.
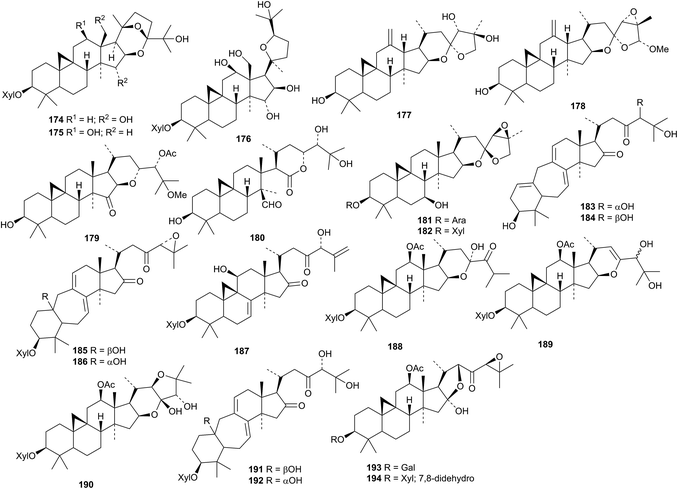
The three cycloartane xylosides 174–176, from Beesia calthaefolia, all have new genins.96 New compounds from Cimicifuga species include the rearranged cycloartanes yunnanterpene G 177 and 12,18-didehydro-26-methoxyacetol 178, isodahurinol 24-acetate 25-methyl ether 179, 15,16-secoshengmanol C 180 and the glycosides 181 and 182 from the aerial parts of Cimicifuga yunnanensis,97 cimifoetidanols A 183–H 190 and cimifoetidanosides A 191 and B 192 from the rhizomes of Cimicifuga foetida98 and glycosides 193 and 194 from the roots of Cimicifuga simplex.99 The structure of cimifoetidanol A 183 was confirmed by X-ray analysis.
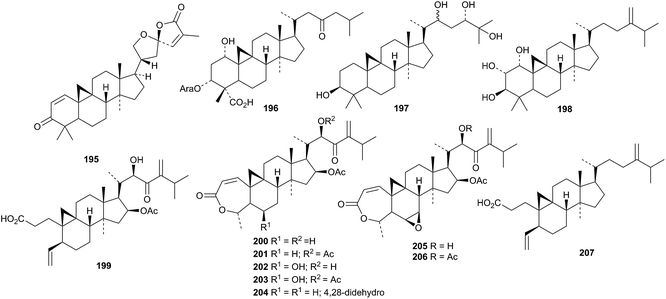
Two new cycloartanes 195 and 196 have been found in Kleinhovia hospita.100 The arabinoside 196 has a new genin. Two new glycosides, one with the new genin 197, have been reported from Landoltia punctata.101 Neomacrotriol 198 and the 29-norcycloartanes neomacroin 199, neomacrolactone 200 and related compounds 201–206 have been isolated from Neoboutonia macrocalyx.102 The related 29-nor-derivative 207 has been found in Cinnamosma fragrans.103
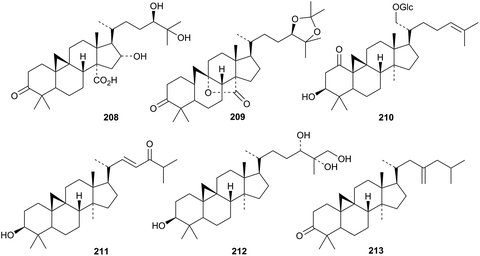
Other new cycloartanes include caloncobic acid C 208 and caloncobalactone C 209 from the leaves of Caloncoba glauca,104 the glucoside rhizostyloside 210, with a new genin, from Rhizophora stylosa,105 compound 211 from Cassia italica,106 and the tetrol 212 from the leaves and twigs of Walsura yunnanensis.107 The unusual 23-methylene structure 213 has been proposed for a constituent of Piper thomsonii.108
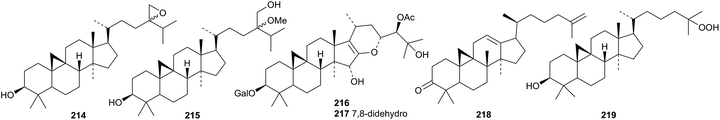
Compounds 214 and 215 have been isolated as mixtures of 24-epimers from Euphorbia fischeriana.109 Two glycosides 216 and 217 from the roots of Cimicifuga simplex are described as galactopyranosides but drawn as furanosides.99 Cycloarta-12,25-dien-3β-ol has been claimed as a constituent of Cameroonian brown propolis but the structure drawn is actually the 9(19)-cyclodammarane 218.110 25-Hydroperoxycycloartanol 219 is a constituent of Euphorbia bupleuroides.55
Cycloartane saponins with known genins include krugianoside A from Astragalus plumosus var. krugianus111 and riparsaponin from Homonoia riparia112 and saponins from Beesia calthaefolia,113Cimicifuga foetida114 and Euphorbia boissierana.115
4. The dammarane group
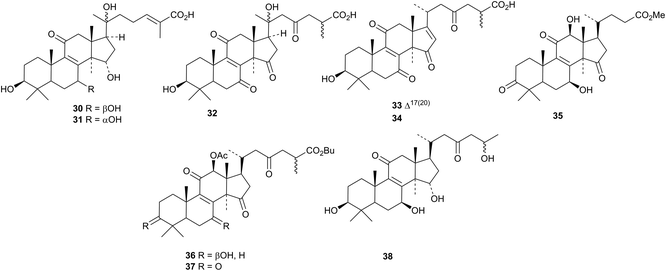
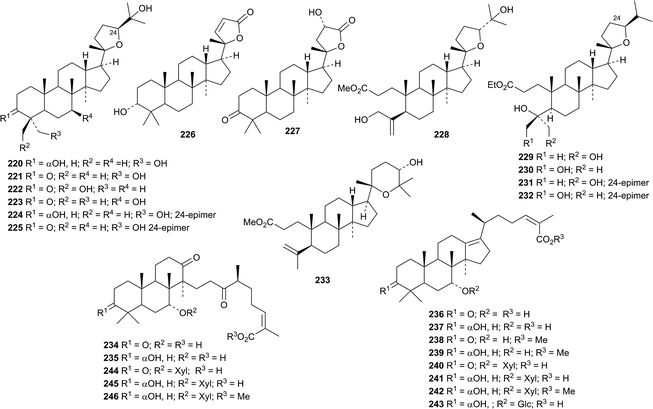
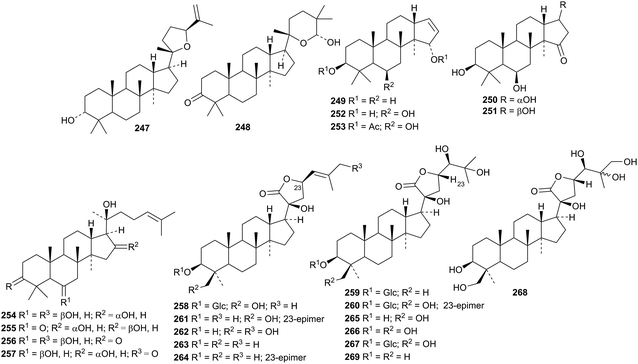
An interesting group of new dammaranes 220–233 has been reported from the stems of Dysoxylum binecteriferum.116 Several 13,17-secodammaranes are among the 20-epi-derivatives dysotriflorins A 234–M 246 from Dysoxylum densiflorum.117 The structure of dysotriflorin I 242 was confirmed by X-ray analysis.Dysomollisol 247 and dysomollisone 248 are constituents of the fruit of Dysoxylum mollissimum.118 Dysomollisone 248 has been assigned an unusual rearranged side-chain. The octanor-derivative rosanol A 249 has been found in the roots of Rosa rugosa.119 Horipenoids A 250–H 257, from Homonoia riparia, include several other octanor-derivatives.120 The structure of horipenoid E 254 was confirmed by X-ray analysis. Thirteen new dammarane saponins have been isolated from Gentianella azurea including the glucosides 258–260 whose structures were confirmed by X-ray analyses.121 Glucosides 258–260 have new genins. Further new genins 261–265 are found in the new dammarane saponins. The known gentirigenic acid and gentirigeoside A were also obtained from Gentianella azurea and their structures have been revised to 266 and 267, respectively, on the basis of X-ray analyses. The genins of the known gentirigeosides B and E were also revised to 268 and 269, respectively.
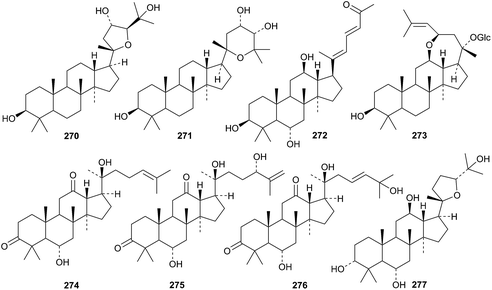
Phlomisumbrosides A and B are new glycosides from Phlomis umbrosa with the new genins 270 and 271.122 The 27-nordammarane 272 and the glucoside 273, with a known genin, are further constituents of the leaves of Panax ginseng.123 The three 3,12-diketones 274–276 were obtained from the same source.124 The epoxydammaranetetrol 277 has been isolated from the stems and leaves of American ginseng and given the erroneous name 3α-ocotillol (ocotillol is an epoxydammaranediol).125
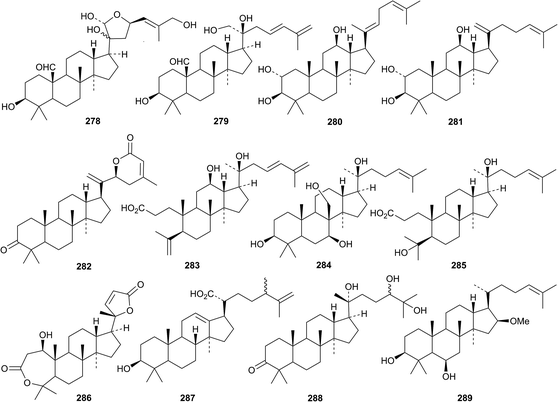
New compounds from Gynostemma pentaphyllum include two saponins with new genins 278 and 279,126 the saponins damulins C and D with the new genins 280 and 281 (ref. 127) and the keto-lactone 282.128 Cyclocarioside K, from Cyclocarya paliurus,129 and tubeimoside C, from Bolbostemma paniculatum,130 also have new genins, 283 and 284, respectively. Other new dammaranes include the ring A-cleaved derivative 285 from Aglaia abbreviata,131 deacetybrachycarpon-22-ene 286 from Cleome arabica,132 the 30-nor-24-methyl derivative floccosic acid 287 from Nepeta floccosa,133 the keto-triol 288 from the root bark of Ailanthus altissima134 and farmanol 289 from Nepeta suavis.135
New dammarane saponins with known genins include cyclocarioside J from Cyclocarya paliurus,136 jujubosides D and E (duplicate names) from Ziziphus jujuba,137 notoginsenosides FZ, LX and LY from Panax notoginseng138 and saponins from Aralia elata139 and Panax notoginseng.140 The pharmacological activities and production of saponins from Centella asiatica have been reviewed.
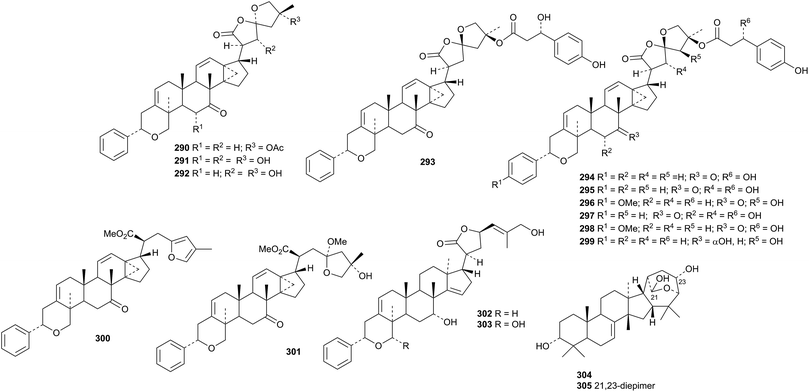
Fourteen new dichapetalin derivatives have been obtained from Dichapetalum gelonioides including 22-deoxydichapetalin P 290, 25-deacetyldichapetalins M 291 and P 292, dichapetalins T 293, U 294, V 295 and W 296, 6α-hydroxydichapetalin V 297, 22-deoxy-4′′-methoxydichapetalin V 298, 4′′-demethoxy-7-dihydrodichapetalin W 299, 7-dehydrodichapetalins E 300, G 301 and Q 302 and 29α-hydroxy-21-dehydrodichapetalin Q 303.141 The structure of 22-deoxydichapetalin P 290 was confirmed by X-ray analysis. The unusual 16,25-cyclised tirucallane structures 304 and 305 have been proposed for asperols A and B from Canarium asperum.142
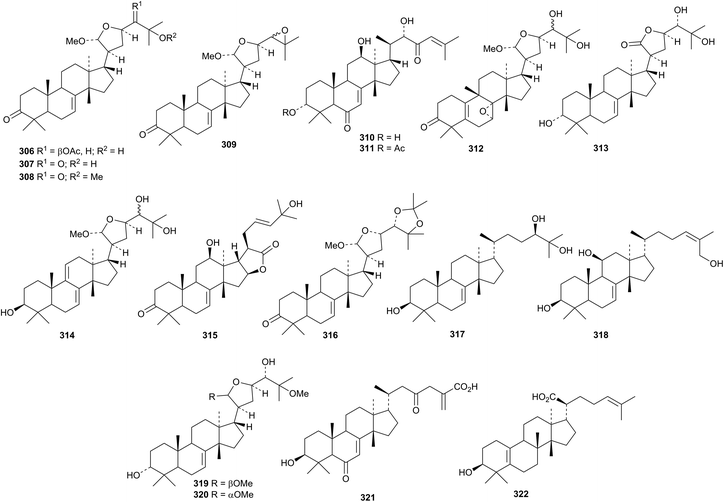
Other new tirucallane derivatives include the acetals 306–309 from Dysoxylum binectariferum,143 trichostemonol 310 and the corresponding 3-acetate 311, trichostemonate, from the stem bark of Walsura trichostemon,144,145 indicalilacols A 312–D 315 from the fruits of Azadirachta indica,146 toosendansin D 316 from Melia toosendan,147 compounds 317 and 318 from Celastrus stylosus,148 the 21-epimers paramignyols A 319 and B 320 from Paramignya scandens149 and dysoxylumin A 321 (duplicate name) from Dysoxylum densiflorum.150 The unusual structure 322 has been ascribed to a constituent of Melia azedarach.151
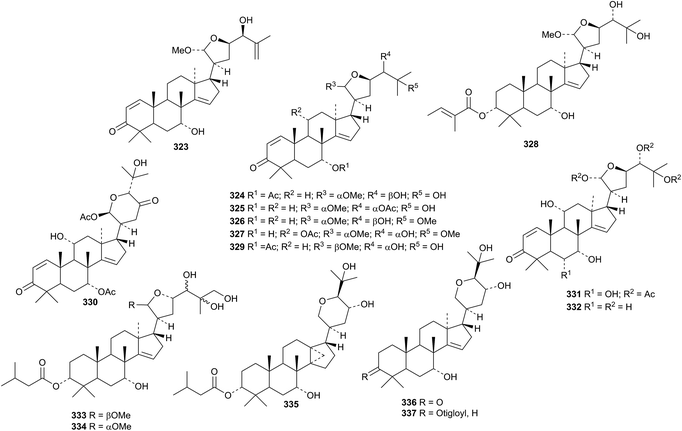
Xylogranatumines A 323–G 328 are new apotirucallenes from the Chinese mangrove Xylocarpus granatum.152 Other members of this class are represented by compounds 330–332 from the leaves of Walsura trichostemon,153 dictamnins A 333 and B 334, 21-epimers from the bark of Dictamnus dasycarpus,154 cedrodorols A 335 and B 336 from Cedrela odorata155 and compound 337 from Melia azedarach.156
4.1 Tetranortriterpenoids
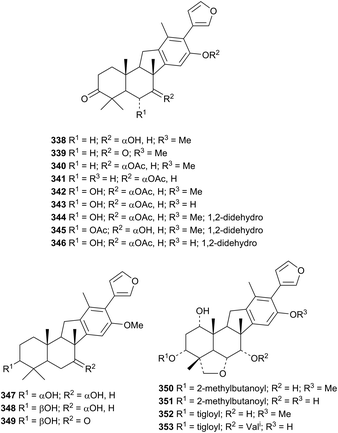
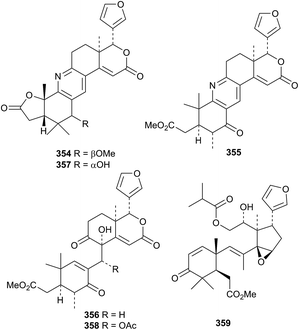
There is a strong interest in the biological activities of limonoids.157–160 Anticancer161,162 and pesticidal163 activities of limonoids have also been highlighted. The publication of new members of this class continues unabated. Walsucochinoids C 338–R 353, from Walsura cochinchinensis, form an interesting group of rearranged derivatives.164 The structures of walsuchinoids C 338 and L 347 were confirmed by X-ray analyses.Xylogranatopyridines A 354, B 355 and prexylogranatopyridine 356, from the Chinese mangrove Xylocarpus granatum, are closely related to the known xylogranatin F 357 and hainangranatumin D 358.165 The unusual 9,11-seco-derivative toonasecone A 359 has been isolated from Toona ciliata.166
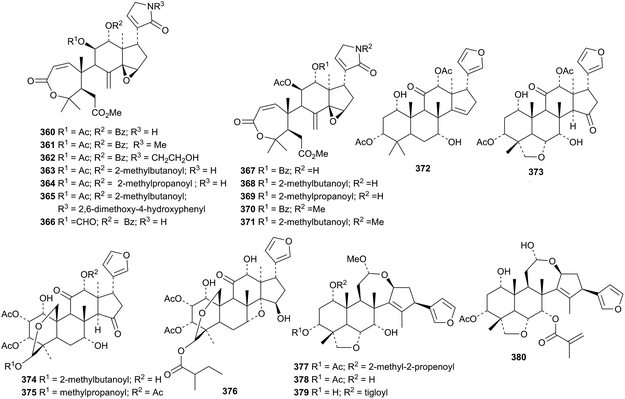
A series of amides, amooramides A 360–L 371, has been reported from the twigs and leaves of Amoora tsangii.167 The highly oxygenated tetranortriterpenoids 372–376, from the fruits of Melia toosendan, are accompanied by the ring-C cleaved derivatives 377–379.168 Compound 378 has also be isolated from Melia azedarach together with 380.169
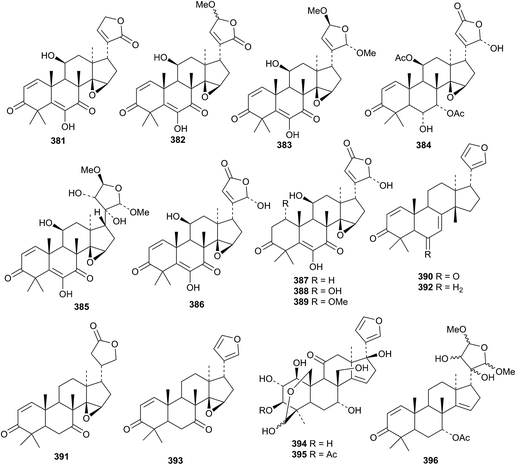
Nine new cedrelone derivatives, walsuranolide B 381, 11β-hydroxy-23-O-methylwalsuranolide 382, yunnanolides A 383 and B 384, yunnanol A 385, the isowalsuranolide derivatives 386–389, have been isolated from the leaves and twigs of Walsura yunnanensis.107 Dysoxylamins B 390, C 391 (duplicate names) and compounds 392 and 393 are constituents of Dysoxylum densiflorum.150 Other derivatives with intact skeletons include flexuosoids A 394 and B 395 from Phyllanthus flexuosus170 and compound 396 from the flowers of Azadirachta indica var. siamensis.171
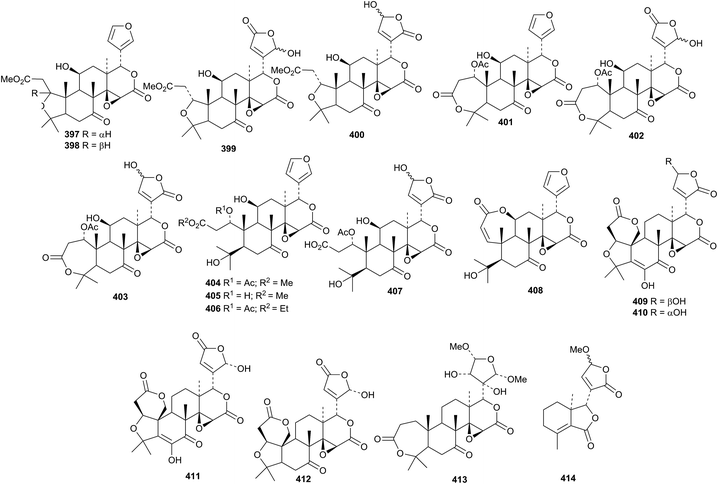
The A,D-seco derivatives clauemargines A 397–L 408 have been isolated from the stems of Clausena emarginata.172 The structure of clauemargine A 397 was confirmed by X-ray analysis. Other A,D-seco derivatives include euodirutaecins A 409 and B 410, as an inseparable mixture, evodirutaenin A 411 and shihulimonin A1 412 from the rhizomes of Coptis chinensis and Euodia rutaecarpa173 and kihadanin C 413 from the root bark of Dictamnus dasycarpus.174 23-O-Methyldasylactone B 414 is a further constituent of Dictamnus dasycarpus.174
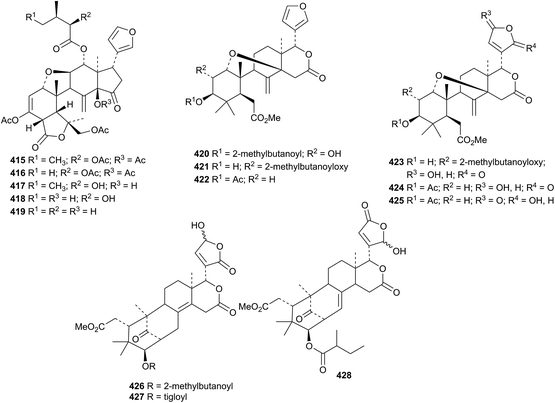
Ring B-cleaved derivatives are represented by aphagranols D 415–H 419 from the fruits of Aphanamixis grandifolia175 and the methyl angolensate derivatives cipaferens E 420–J 425 from the seeds of Cipadessa baccifera.176 The mexicanolide derivatives, cipaferens K 426, L 427 and M 428, were also isolated together with the known compounds cipadesin A and 2R-methylbutanoylproceranolide whose structures were confirmed by X-ray analyses.
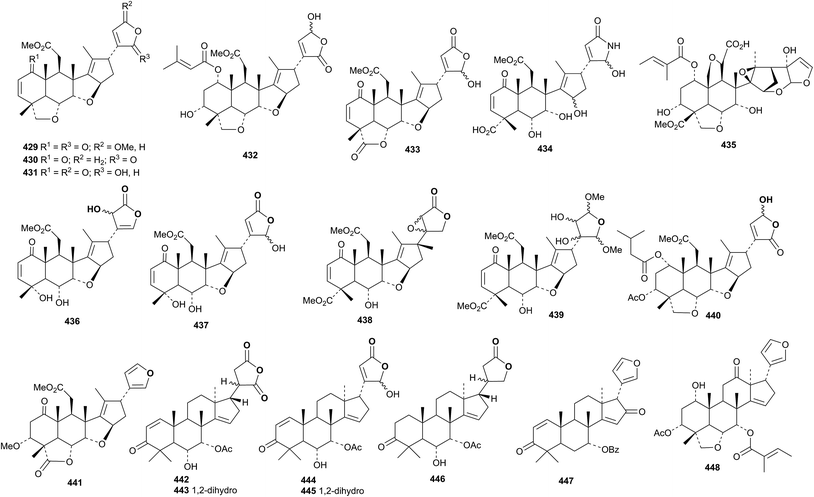
The seemingly endless investigations of the constituents of Azadirachta indica have, unsurprisingly, produced more unremarkable ring C-cleaved derivatives. These include the 28-deoxynimbolide derivative 429,177 compounds 430–432,178 nimbolide B 433 and nimbic acid B 434 (ref. 179) and compounds 435–437 from Azadirachta indica180 and compounds 438–441 from Azadirachta indica var. siamensis.181 The uncleaved derivatives 442–446 (ref. 180) and 447 and 448 (ref. 181) were also obtained.
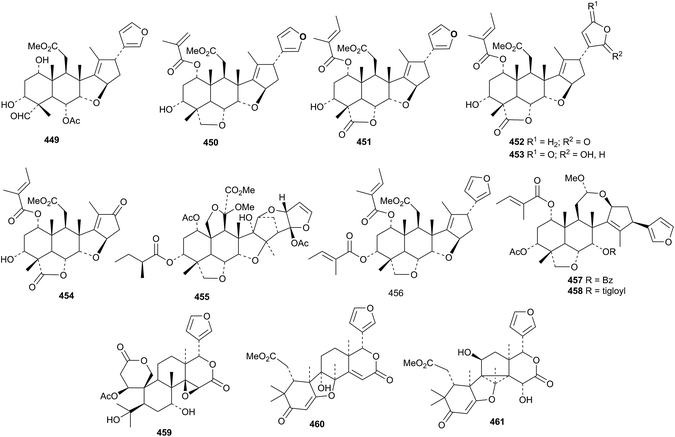
Three new ring C-cleaved derivatives 449–451, along with a host of known compounds, have been reported from the fruits of Melia azedarach.182 The leaves and bark are the source of the new derivatives 452–454 (ref. 183) while the meliacarpin derivative 455 was found in the leaves.156 Toosendansins A 456, B 457 and C 458 are constituents of Melia toosendan.147 The ichangin derivative 459, 9α-hydroxyhortolide A 460 and 11β-hydroxyhortolide C 461 were isolated from Hortia orcadia.184
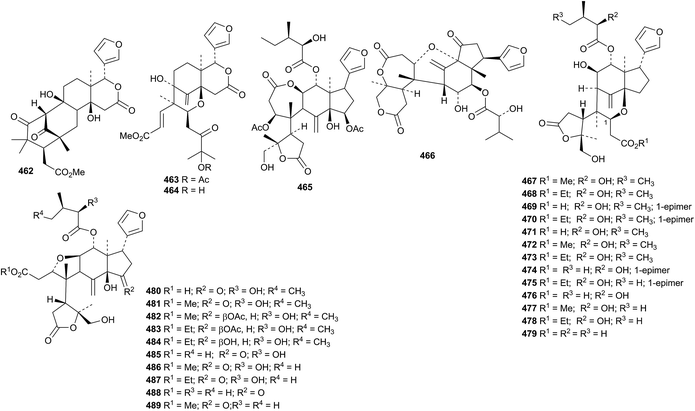
New skeletal variations of rearranged limonoids continue to appear. The structures of trichiconins A 462 and B 463, from Trichilia connaroides, were confirmed by X-ray analyses.185 They were accompanied by trichiconin C 464. Zaphaprinins A 465–Y 489 are modified prieurianin derivatives from the fruits of Aphanamixis grandifolia.186 The structures of zaphaprinins B 466, E 469 and P 480 were confirmed by X-ray analyses. Eight members of this group are ethyl esters and are presumably artefacts of the extraction process.
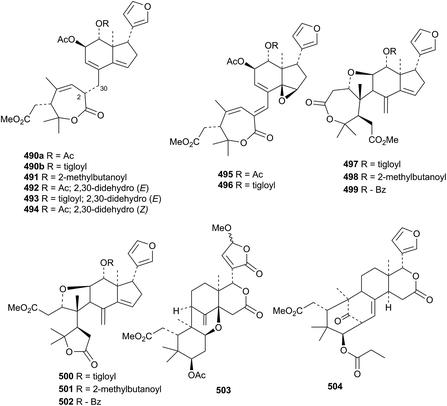
A similar group of compounds, aphanamixoids C 490a–P 502, has been reported from Aphanamixis polystachya.187 Cineracipadesin G 503 (ref. 188) and the swietenine derivative 504 (ref. 189) are constituents of Cipadessa cinerascens.
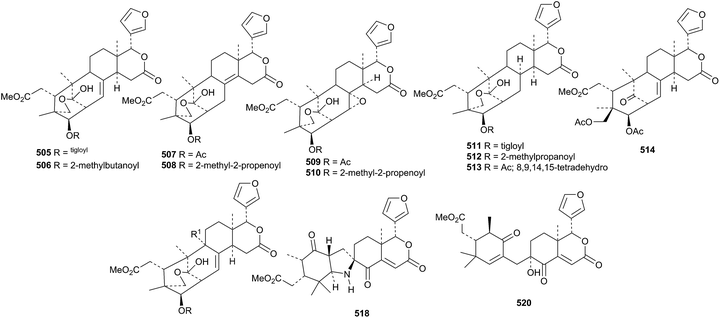
Compounds from an Indian Xylocarpus granatum include granatumins L 505–U 514 (ref. 190) and granatumins V 515–Y 518.191 The structures of granatumins L 505 and Y 518 were confirmed by X-ray analyses. Granatumins M 506 and V 515 have also been isolated from Xylocarpus granatum by another group and named xylomexicanins H and G, respectively.192 They occur with xylomexicanins E 519 and F 520 that were drawn with the wrong absolute configuration in the reference.
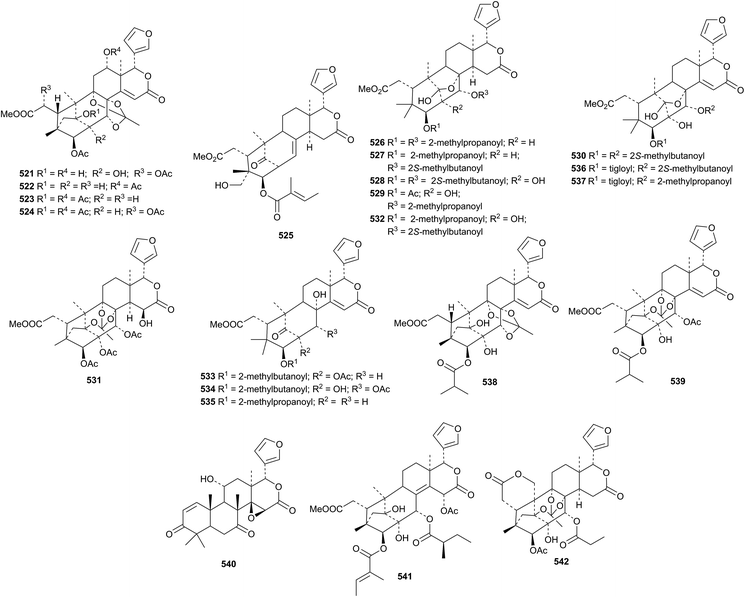
Other constituents of Xylocarpus granatum include the phragmalin derivatives 521–524 (ref. 193) and xylocartin C 525.194 Xylorumphins E 526–J 531 and 2-hydroxyxylorumphin F 532 are constituents of the seeds of Xylocarpus rumphii.195 The structure of xylorumphin G 528 was confirmed by X-ray analysis.
Carapanolides C 533–I 539 (ref. 196) and carapanolides J 540, K 541 and L 542 (ref. 197) have been reported from the seeds of Carapa guianensis. The structure of carapanolide F 536 was confirmed by X-ray analysis.
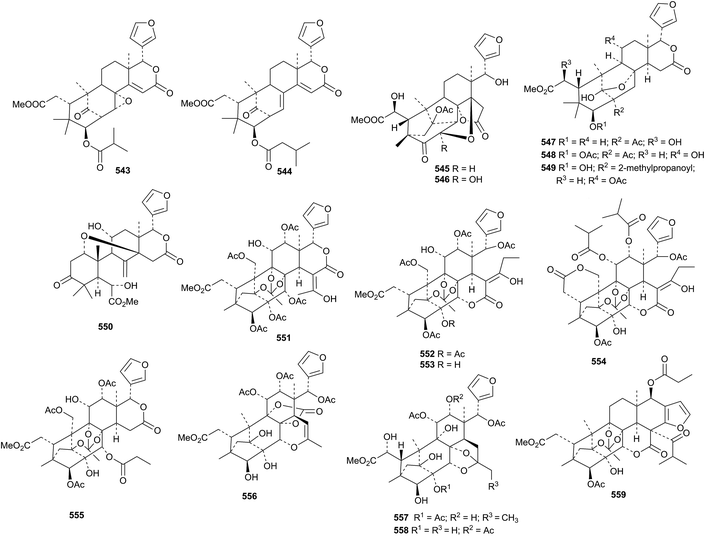
Further investigations of Khaya ivorensis have resulted in the isolation of 14,15-didehydroruageanin A 543 and 3-O-(3-methylbutanoyl)seneganolide A 544 (ref. 198) and ivorenoids A 545–F 550.199 Velutinasins A 551–H 558 are phragmalin derivatives from Chukrasia tabularis var. velutina.200 Velutinalide C 559 is another new compound from this source.201
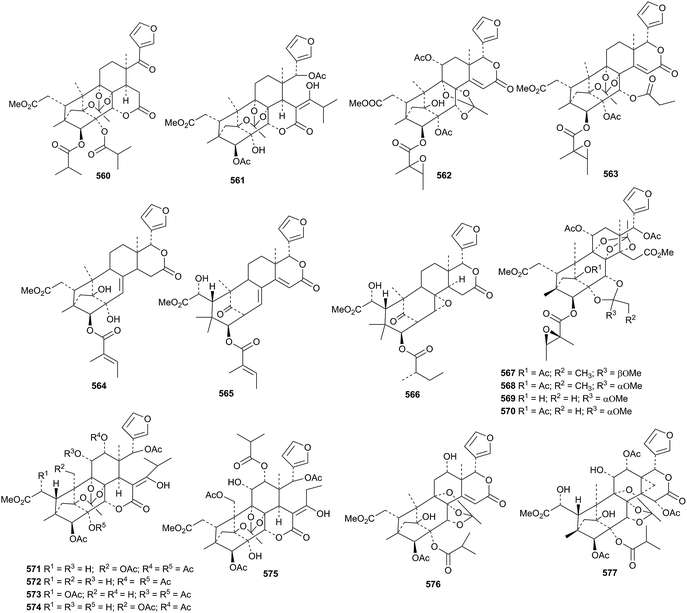
Neobeguea mahafalensis is the source of the phragmalin derivatives libiguin A 560 and libiguin B 561 (in equilibrium with its keto tautomer).202 These compounds are reported to have aphrodisiac properties. 2-Acetylsoymidin B 562 and soymidins D 563 and E 564 are constituents of Soymida febrifuga.203 Swielimonoids A 565–F 570 are additional constituents of Swietenia macrophylla seeds.204 Chukvelutilides I 571–O 577 are further new phragmalin derivatives from the seeds of Chukrasia tabularis.205 Synthetic studies have indicated that the biosynthesis of the 1,8,9-orthoester moiety in phragmalins involves intermediates with an ester at C-1.206
4.2 Quassinoids
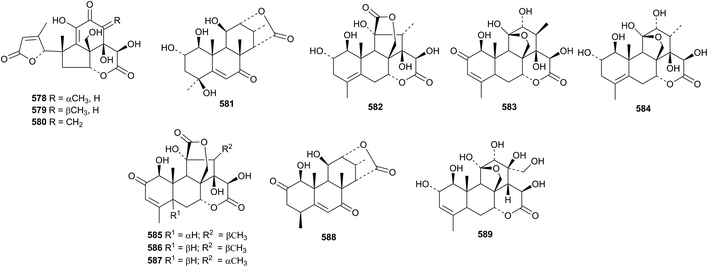
Eurylactones E 578, F 579 and G 580, eurycomalides D 581, and E 582 and 13α,18-dihydroeurycomanone 583 are new constituents of the roots of Eurycoma longifolia.207 Other constituents of Eurycoma longifolia include Δ4-14-hydroxyglaucarbol 584, 5-isoeurycomadilactone 585, eurycomadilactone 586 and 13-epieurycomadilactone 587 (ref. 208) and eurycomalide C 588.209 The structures of Δ4-14-hydroxyglaucarabol 584 and 5-isoeurycomadilactone 585 were confirmed by X-ray analyses. Shinjulactone O 589 has been isolated from the root bark of Ailanthus altissima.2105. The lupane group
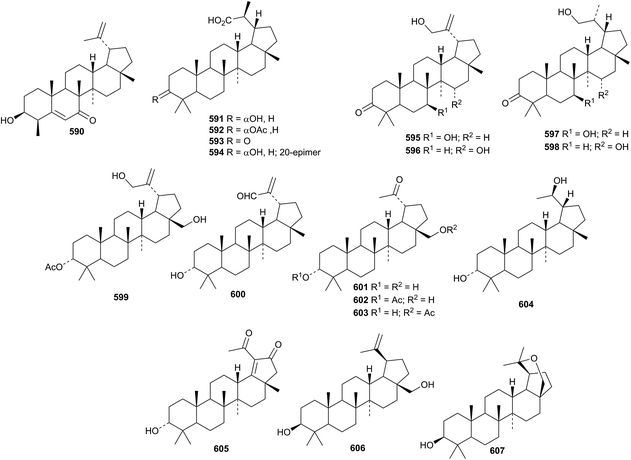
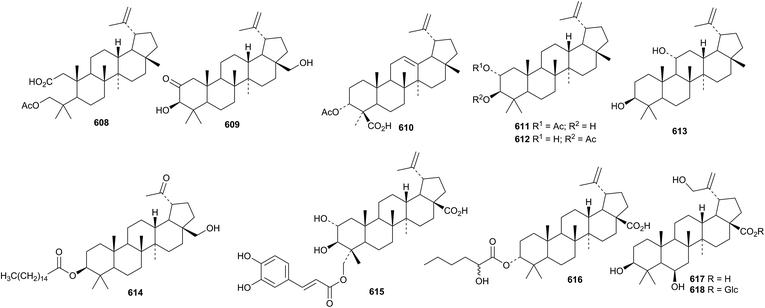
Betulinic acid has been reported to have a variety of pharmacological activities including antitumour activity.211,212 The first example of a 23-norlupane, 3-hydroxy-23-nor-5,20(29)-lupadien-7-one 590, has been reported from Lagerstroemia indica.213Euonymus carnosus is a prolific source of lupane triterpenoids. Fifteen new compounds 591–605, including the 30-nor-derivatives 601–605, have been isolated.214 The structure of 591 was confirmed by X-ray analysis. 19-Epibetulin 606 and 19-epi-20,28-epoxy-3-lupanol 607 have been reported from Hibiscus syriacus.215Other simple lupane derivatives include salacinins A 608 and B 609 from Salacia hainanensis,216 the acetates 610 from Boswellia sacra217 and 611 and 612 from Salvia viridis,218 20(29)-lupene-3β,11α-diol 613 together with its 3-palmitate and the 30-norlupane palmitate 614 from Saussurea phyllocephala,219 sorbanolic acid 615 from Sorbus lanata220 and the 2-hydroxyhexanoyl ester of 3-epibetulinic acid 616 from Dillenia indica.221 3β,6β,29-Trihydroxy-20(30)-lupen-28-oic acid 617 and its β-D-glucopyranosyl ester 618 are constituents of Licania cruegeriana.222 Lupane saponins with known genins include schekwangsiensides F and G from Schefflera kwangsiensis223 and saponins from Eryngium agavifolium.224
6. The oleanane group
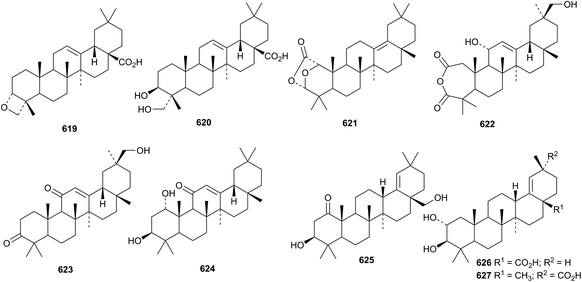
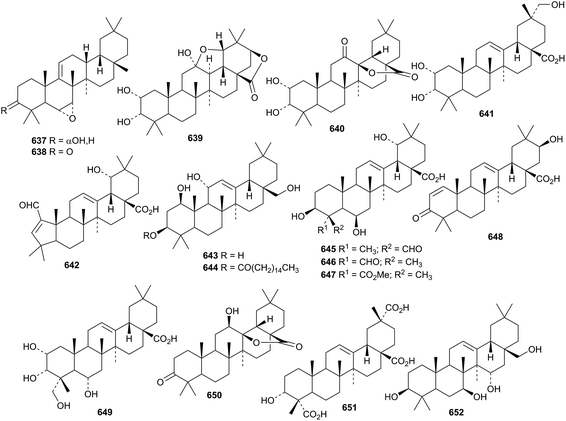
Oleanane triterpenoids and their saponins have a wide range of pharmacological activities.225–227 Oleanolic acid228–231 and maslinic acid232 have been well studied, particularly for their antitumour effects.Cyclocaric acid A, from Cyclocarya paliurus, was claimed to have the structure 619 with an oxetane ring.233 Synthesis of the oxetane 619 and re-examination of the original literature report indicates that cyclocaric acid A is identical with hederagenin 620.234 The 2,3-seco-derivative 621 has been found in Ligularia przewalskii,235 and the 2,3-seco anhydride 622 is a constituent of Microtropis fokiensis where it is accompanied by the intact oleananes 623, 624 and the 18-oleanene derivative 625.236 Further 18-oleanene derivatives include 2α,3β-dihydroxy-18-oleanen-28-oic acid 626 from Lawsonia inermis237 and the corresponding 29-oic acid 627 from Mentha suaveolens.238
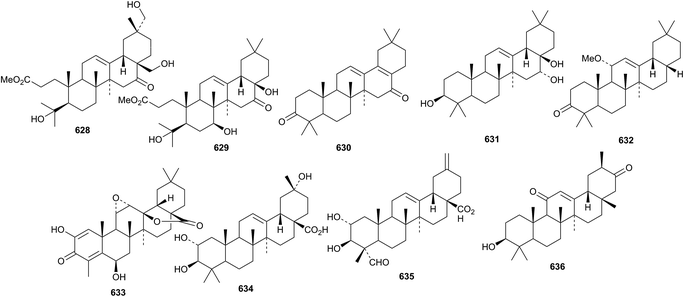
The 3,4-seco-derivatives camelliaoleans A 628 and B 629, together with the 28-noroleanane derivatives 630 and 631, have been isolated from Camellia japonica.239 Liquidaformone 632 is another 28-noroleanane from fruits of Liquidambar formosana.240 Asprellol C 633 is a 24-noroleanane from Ilex asprella,241 whereas the noroleananes 634 and 635 have been found in Akebia trifoliata242 and glyyunnansapogenin I 636 is from Glycyrrhiza yunnanensis.243
Two unusual 9(11)-oleanene derivatives 637 and 638, lacking a ketone at C-12, have been isolated from Boswellia ovalifiolata.244 Cannabifolin A 639, from Vitex negundo var. cannabifolia, has unusual cis-fused C/D rings.245 It is accompanied by cannabifolins E 640 and F 641. Rusaic acid B 642, from the roots of Rosa rugosa, has a contracted ring A.119
Other simple oleanane derivatives include 12-oleanene-1β,3β,11α,28-tetrol 643 and its 3-palmitate 644 from Saussurea phyllocephala,219 uncarinic acids F 645, G 646 and H 647 from Uncaria rhynchophylla,246 schekwangsiensin 648 from Schefflera kwangsiensis,223 glaucescic acid 649 from Terminalia glaucescens,247 the 28,13-olide 650 from Ekebergia capensis,248 3α-hydroxy-12-oleanene-23,28,29-trioic acid 651 from Acanthopanax gracilistylus249 and 12-oleanene-3β,7β,15α,28-tetrol 652 from Salvia argentea var. aurasiaca.250
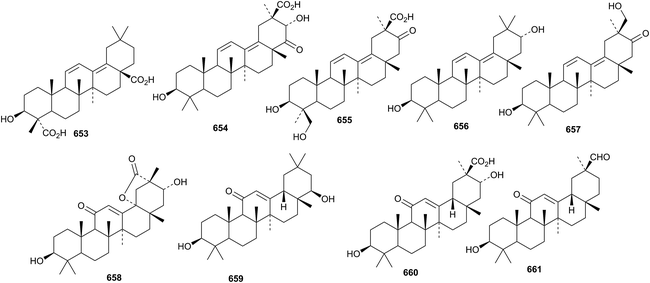
3β-Hydroxy-11,13(18)-oleanadiene-23,28-dioic acid 653, previously identified as the genin of saponarioside J, has been isolated from Anoectochilus elwesii.251 Yunganosides L, M, N1, N2, O and P are saponins from Glycyrrhiza yunnanensis with the new genins yunganogenins L 654–P 658.243 Licorice-saponins M3 and N4, from Glycyrrhiza glabra, have the new genin 659.252 Licorice-saponin M3 is the same as uralsaponin T that has been isolated from Glycyrrhiza uralensis together with uralsaponins P–S and W that have the new genins 660 and 661, respectively.253
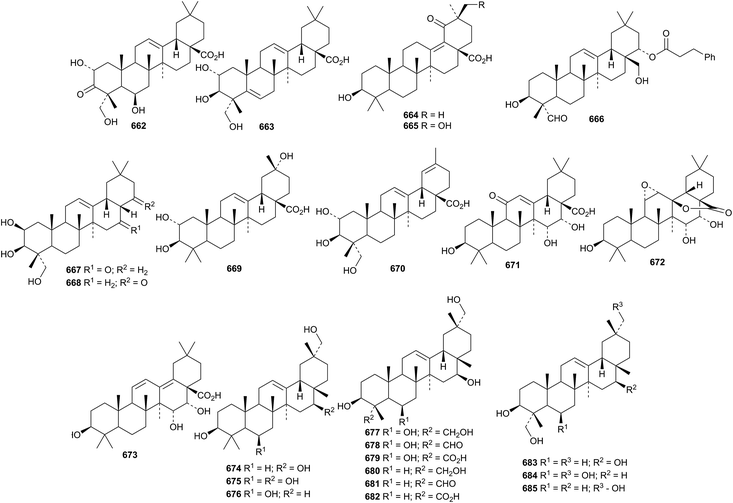
Other oleanane saponins with new genins include centellasaponins E and I, from Centela asiatica, with the genins 662 and 663,254 hippophosides A–D, from Hippophae rhamnoides ssp. sinensis, with the genins 664 and 665,255 oleiferasaponin B2, from Camellia oleifera, with the genin 666,256 tubeimosides A and B, from Bolbostemma paniculatum, with the genins 667 and 668,130 saponins from Akebia trifoliata with the noroleanane genins 669 and 670,257Entada phaseoloides with the genins 671–673,258Eclipata prostrata with the genin 12-oleanene-3β,16β,29-triol 674 (ref. 259) and Silphium asteriscus also with the genin 674 together with eleven related genins 675–685.260
New oleanane saponins with known genins that have been assigned trivial names are listed in Table 1.
| Trivial name | Plant species | Reference |
|---|---|---|
| Angulasaponins A–D | Vigna angularis | 261 |
| Aristatosides A–C | Cephalaria aristata | 262 |
| Bafouoside C | Cussonia bancoensis | 263 |
| Caspicaosides E–K | Gliditsia caspica | 264 |
| Celosins H–J (celosin I is a duplicate name) | Celosia argentea | 265 |
| Clematiunicinosides A–H | Clematis uncinata | 266 |
| Comastomasaponins A–H | Comastoma pedunculatum | 267 |
| Conyzasaponins R, S | Conyza japonica | 268 |
| Conyzasaponins T, U | Conyza japonica | 269 |
| Davisianosides A, B | Cephalaria davisiana | 270 |
| Flaccidosides V–VII | Anemone flaccida | 271 |
| Grindeliosides A–C | Grindelia argentina | 272 |
| Ilexasprellanosides D–F | Ilex asprella | 273 |
| Ilexpublesnin R | Ilex pubescens | 274 |
| Leptocarposide | Ludwigia leptocarpa | 275 |
| Licorice-saponin O4 | Glycyrrhiza glabra | 252 |
| Lobeliodosides A–D | Lysimachia lobelioides | 276 |
| Lonicerosides F–J | Lonicera japonica | 277 |
| Officinoterpenoside D | Rosmarinus officinalis | 278 |
| Oleiferosides A–H | Camellia oleifera | 279 |
| Oleiferoside B1 | Camellia oleifera | 256 |
| Paradoxosides A–E | Vitellaria paradoxa | 280 |
| Pittangretosides N, O, P | Pittosporum angustifolium | 281 |
| Pittangretosides A1, B1, J, K, M, Q–Z | Pittosporum angustifolium | 282 |
| Potentillanoside F | Potentilla anserina | 283 |
| Salbiges A, B | Salicornia herbacea | 284 |
| Sarconepaside C | Sarcopyramis nepalensis | 285 |
| Schefflesides A–H | Schefflera kwangsiensis | 286 |
| Schefflesides I–L | Schefflera kwangsiensis | 287 |
| Sieboldiisaponin B | Stachys sieboldii | 288 |
| Simenoside A | Gypsophila simonii | 289 |
| Schekwangsiensides A–E | Schefflera kwangsiensis | 223 |
| Uralsaponins M, N, O, U, V, Y | Glycyrrhiza uralensis | 253 |
| Yemuosides YM36, YM37 | Stauntonia chinensis | 290 |
| Yunganoside E3 | Glycyrrhiza yunnanensis | 243 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2.
| Plant species | Reference |
|---|---|
| Acacia auriculiformis | 291 |
| Anemone rivularis var. flore-minore | 292 |
| Callicarpa nudiflora | 293 |
| Centratherum anthelminticum | 294 |
| Clematis argentilucida | 295 |
| Croton lachnocarpus | 296 |
| Eclipta prostrata | 259 and 297 |
| Entada phaseoloides | 258 |
| Eryngium planum | 298 |
| Ganophyllum giganteum | 299 |
| Garcinia hanburyi | 300 |
| Gymnema sylvestre | 301 |
| Gypsophila arrostii var. nebulosi, Gypsophila bicolor | 302 |
| Manilkara hexandra | 303 |
| Melissa officinalis | 304 |
| Momordica charantia | 305 |
| Paonychia anatolica ssp. balansae | 306 |
| Patrinia scabra | 307 |
| Polycarpaea corymbosa var. eriantha | 308 |
| Polygala tenuifolia | 309 |
| Pycnanthemum flexuosum | 310 |
| Sapindus mukorossi | 311 |
| Silene rubicunda | 312 |
| Silphium asteriscus | 260 |
| Tremastelma palaestinum | 313 |
| Xanthoceras sorbifolia | 314 |
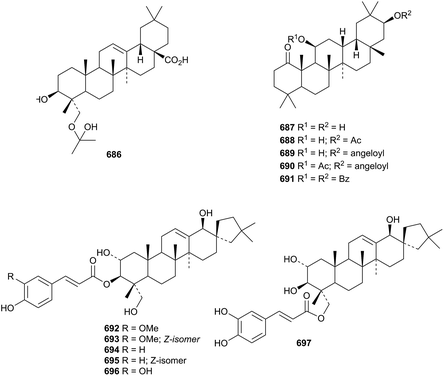
The unlikely acetone hemiacetal 686 has been reported as a constituent of Isodon adenantha.315 11,21-Dihydroxy-1-oleananone 687 and four esters 688–691 are claimed to be substituents of Coriandrum sativum.316 Other new oleanane esters include the 3-palmitoyl ester of 3β,28-dihydroxy-12-oleanen-11-one (procerenone) from Omphalocarpum procerum,317 and the oleoyl ester 12,18-oleanadien-3β-ol.318 Leonurusoleanolides E 692–J 697, from Leonurus japonicus, are further esters of the 19(18→17)-abeo-28-noroleanane phlomistetraol B.319
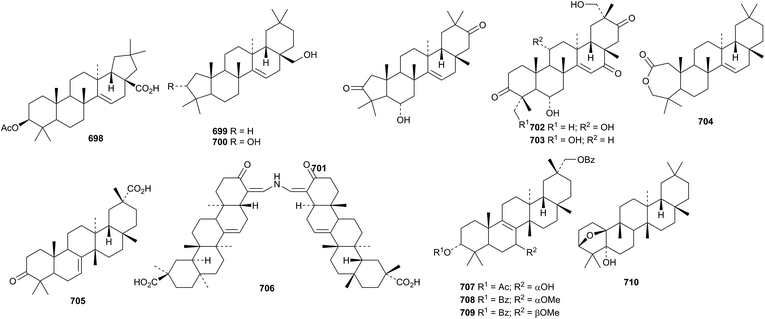
Malaytaxerate 698, from Sapium baccatum, is a ring-E contracted nortaraxerane derivative.320Davidia involucrata is the source of the ring-A contracted nortaraxeranes davinvolunols A 699 and B 700 and davinolunone A 701 together with the intact taraxeranes davinolunones B 702 and C 703.321 The structure of the 2,3-secotaraxerane pycanocarpine 704, from Pleiocarpa pycnantha, was established by X-ray analysis.322 A taraxerane saponin with a known genin has been isolated from the roots of Clematis argentilucida.323 The structures of both the multiflorane derivative turranoic acid 705 and turraenine 706, from a Turraea species, were also established by X-ray analysis.324 Turraenine 706 is an unusual nitrogen-containing dimeric normultiflorane. Three multiflorane esters 707–709 have been isolated from seeds of Cucurbita maxima.325 The glutinane derivative klodorol A 710, from Kleinia odora, is 5α-hydroxydebdropanoxide.326 The authors draw klodorol A 710 with incorrect stereochemistry at C-13 and C-14.
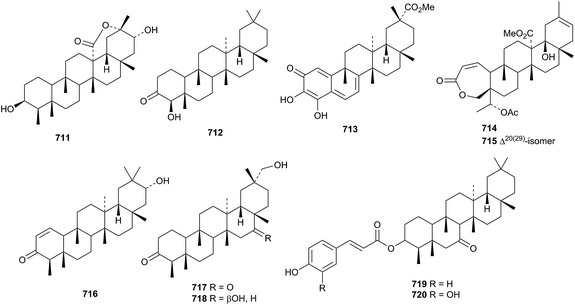
The structures of the friedelane triterpenoids found in Maytenus species have been summarised.327 Glaucalactone B 711 is a 29-norfriedelane 27,20-lactone from Caloncoba glauca104 and hainanenone 712 is a 23-norfriedelane derivative from Drypetes hainanensis.328 The 23,24-dinorfriedelane pristimerol 713, from Celastrus aculeatus, has been given the same name as the reduction product of pristimerin.329 Galphimines K 714 and L 715 are further 3,4-seco-derivatives from Galphimia glauca.330 Other friedelane derivatives include salacinin C 716 from Salacia hainanensis,216 the 3-ketones 717 and 718 from Maytenus robusta331 and the esters 718 and 720 from Drypetes hoanensis.332 The structure of 717 was confirmed by X-ray analysis. The known norfriedelane celastrol from Triperygium wilfordii has shown interesting antitumour activity.333
7. The ursane group
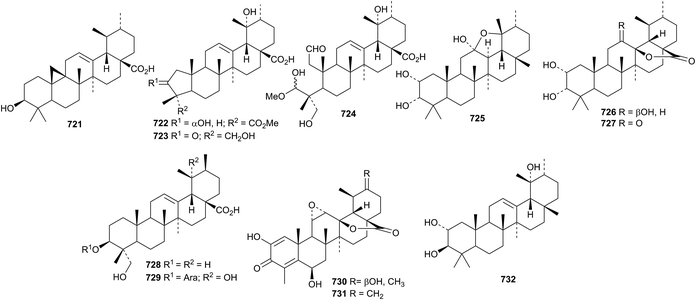
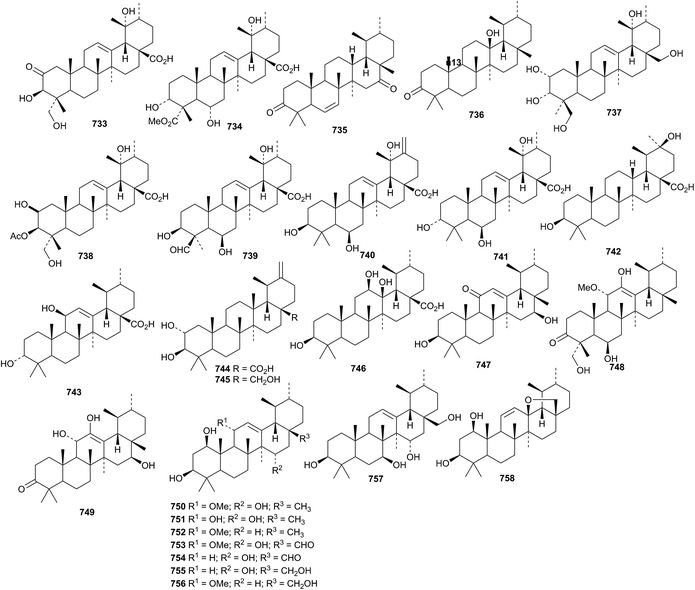
The antitumour activity of ursolic acid has been highlighted.334,335 The unusual 9,25-cyclo-12-ursen-3β-ol 721 has been reported from Cameroonian brown propolis.110 The ring-A modified ursanes davinvolunic acids A 722, B 723 and C 724 have been isolated from Davidia involucrata.336 Davinvolunic acid C 724 contains an unusual methyl hemiacetal. The leaves of Vitex negundo var. cannabifolia are the source of cannabifolins A 725, C 726 and D 727.245 The structure of cannabifolin A 725 was confirmed by X-ray analysis to have cis-fused rings C and D. Two ursane derivatives 728 and 729, with the unusual 20αH-configuration, have been identified in Ilex cornuta.337 Asprellols A 730 and B 731, from Ilex asprella, are 24-norursane derivatives.241 Urs-12-ene-2α,3β,19α-triol 732, from Terminalia arjuna, has been named torment.338Other simple ursane derivatives include cymosic acid 733 from Rosa cymosa,339 elatumic acid 734 from Omphalocarpum elatum,340 erandione 735 from Ricinus communis,341 klodorone A 736 from Kleinia odora,326 meyanthic acid 737 and urs-12-ene-2α,3α,19α,24,28-pentol 738 from Meyna (Vangueria) spinosa,342 uncarinic acids H 739 and I 740 from Uncaria rhynchophylla,246 3α,6β,19α-trihydroxyurs-12-en-28-oic acid 741 from Mitragyna diversifolia,343 3β,20β-dihydroxyursan-28-oic acid 742 from Malus domestica,344 3α,11β-dihydroxyurs-12-en-28-oic acid 743 from Gentiana veitchiorum,345 three compounds 744–746 from Zizyphus jujuba,346 three compounds 747–749 from Microtropis fokienensis236 and nine ursanes 750–758 from Salvia argentea var. aurasiaca.250
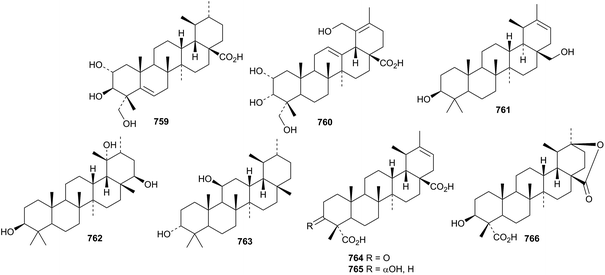
Centrellasaponin J, from Cenrella asiatica, has the new ursane genin 759.254 Further new ursane genins include 760 from Callicarpa nudiflora,293761 from Clematis argentilucida,323762 and 763 from Panax ginseng61 and 764–766 from Schefflera heptaphylla.347
Named ursane saponins with known genins include fagonoside A from Fagonia cretica,348 grandoside from Syzygium grande,349 ilexsaponins G and H from Ilex pubescens,350 ilexasprellanosides A–C from Ilex asprella,273 ilexpublesnins N–Q (Q is the same as zygoeichwaloside H isolated in 2007(ref. 351)) from Ilex pubescens,274 officinoterpenoside C from Rosmarinus officinalis,278 potentillanosides A–E283 and G352 from Potentilla anserina, sieboldiisaponins A353 and C288 from Stachys sieboldii, and zygofaboside C from Zygophyllum fabago.354 Unnamed saponins with known genins have been isolated from Eucommia ulmoides,355Ilex cornuta,356,357Melissa officinalis,304Schefflera heptaphylla347 and Vitex negundo.358
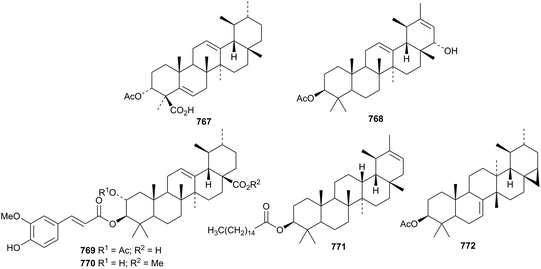
Further ursane esters include the acetate 767 from Boswellia sacra,217 the acetate 768 from Mentha suaveolens,238 ferulates 769 and 770 from Ampelopsis japonica.359 and the palmitate 771 from Inula cappa.360 Flaccidoside IV is a taraxastane saponin with a known genin from Anemone flaccida.271 The unusual 3β-acetoxy-22,28-cyclobauer-7-ene 772 has been identified in Ixeris chinensis.361
8. The hopane group
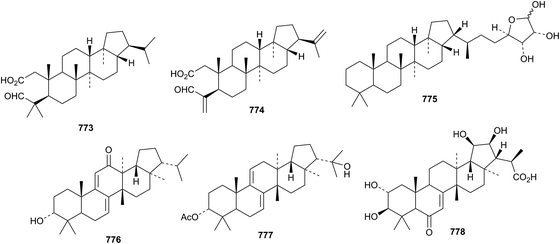
The 2,3-seco-21αH-hopane derivatives 773 and 774 have been isolated from Megacodon stylophorus.362 The structure of 773 was confirmed by X-ray analysis. Ribosylhopane 775, which has been postulated as a precursor of C35 bacteriohopanepolyols in Streptomyces coelicolor, has now been found in blocked mutants.363 Two fernane derivatives 776 and 777 have been reported from Lonicera quinquelocularis however the structures are drawn lacking C-28 in the reference.364 A fernane saponin, from Spergula fallax, has the new genin 778.3659. Miscellaneous compounds
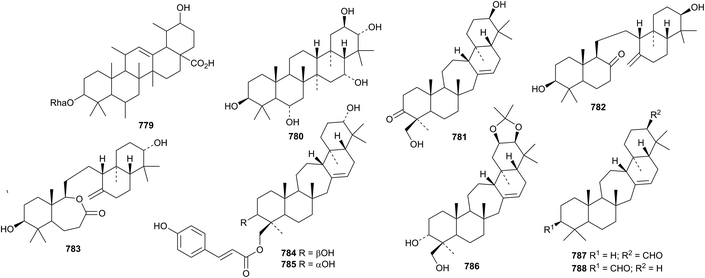
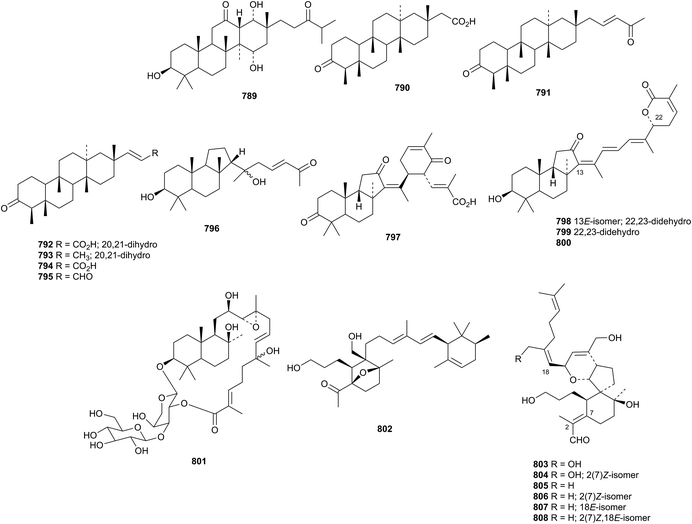
The unlikely structure 779 has been assigned to a triterpene rhamnoside from Sesbania aculeata.366 Three gammacerane saponins, from Spergula fallax, have the new genin 780.365Lycopodium japonicum is the source of the serratane derivatives lycojaponicuminols A 781–F 786 (ref. 367) and the formate esters 787 and 788.368Fatsioside A, from fruit of Fatsia japonica, has the new bacchane genin 789.369 The structure of the norshionane derivative astershionone A 790, from Aster tataricus, was established by X-ray analysis.370 The related astershionones B 791–F 795 were also isolated. Opaciniol A 796, from Garcinia opaca, is a hexnormalabaracane derivative.48 Further isomalabaricane derivatives, jaspiferins C 797–F 800 have been found in the South China Sea sponge Jaspis stellifera.371 Phyteujaposide 801 is an unusual polypodane cyclic saponin from Phyteuma japonicum.372 Further iridal triterpenoids from Iris species include irisgermanone 802 from Iris germanica373 and spirioiridotectals A 803–F 808 from Iris tectorum.374
10. Conflicts of interest
There are no conflicts of interest.11. References
- I. Podolak and Z. Janeczko, Mini-Rev. Org. Chem., 2014, 11, 280–291 CrossRef.
- J.-m. Li, F. Zheng, L.-j. Zhai and Z.-h. Xue, Zhongcaoyao, 2014, 45, 2265–2271 Search PubMed.
- W. Zhang, X. Men and P. Lei, J. Cancer Res. Ther., 2014, 10, 14–19 CrossRef PubMed.
- R. Tundis, F. Menichini and M. R. Loizzo, Stud. Nat. Prod. Chem., 2014, 41, 1–32 CrossRef.
- X.-J. Yan, L.-H. Gong, F.-Y. Zheng, K.-J. Cheng, Z.-S. Chen and Z. Shi, Drug Discovery Today, 2014, 19, 482–488 CrossRef PubMed.
- R. Paduch and M. Kandefer-Szerszen, Mini-Rev. Org. Chem., 2014, 11, 262–268 CrossRef.
- J. A. R. Salvador, A. S. Leal, D. P. S. Alho, B. M. F. Goncalves, A. S. Valdeira, V. I. S. Mendes and Y. Jing, Stud. Nat. Prod. Chem., 2014, 41, 33–73 Search PubMed.
- J.-R. Du, F.-Y. Long and C. Chen, Enzymes, 2014, 36, 95–130 Search PubMed.
- S. M. Kamble, S. N. Goyal and C. R. Patil, RSC Adv., 2014, 4, 33370–33382 RSC.
- B. Han and Z. Peng, J. Chem. Pharm. Res., 2014, 6, 438–443 Search PubMed.
- R. V. Patel and S. W. Park, Curr. Top. Med. Chem., 2014, 14, 1940–1966 CrossRef PubMed.
- L. Zhang, Q.-l. Li and L.-z. Li, Wujing Houqin Xueyuan Xuebao, Yixueban, 2014, 23, 263–268 Search PubMed.
- G.-S. Jeong and J.-S. Bae, Mini-Rev. Org. Chem., 2014, 11, 316–329 CrossRef.
- P. Ruszkowski and T. Bobkiewicz-Kozlowska, Mini-Rev. Org. Chem., 2014, 11, 307–315 CrossRef.
- L. Zu, Y. Zhao and G. Gu, J. Carbohydr. Chem., 2014, 33, 269–297 CrossRef.
- A. C. A. Yendo, F. de Costa, C. T. Da Costa, L. C. Colling, G. Gosmann and A. G. Fett-Neto, Mini-Rev. Org. Chem., 2014, 11, 292–306 CrossRef.
- X. Li, L. Qu, Y. Dong, L. Han, E. Liu, S. Fang, Y. Zhang and T. Wang, Molecules, 2014, 19, 18850–18880 CrossRef PubMed.
- G. Di Fabio, V. Romanucci, A. De Marco and A. Zarrelli, Molecules, 2014, 19, 10956–10981 CrossRef PubMed.
- H. N. Murthy, M. I. Georgiev, Y.-S. Kim, C.-S. Jeong, S.-J. Kim, S.-Y. Park and K.-Y. Paek, Appl. Microbiol. Biotechnol., 2014, 98, 6243–6254 CrossRef PubMed.
- W.-z. Yang, Y. Hu, W.-y. Wu, M. Ye and D.-a. Guo, Phytochemistry, 2014, 106, 7–24 CrossRef PubMed.
- G. Sachin, K. Dileep, M. Gopal and S. Shivali, J. Drug Delivery Ther., 2014, 4, 7–20 Search PubMed.
- J.-p. Cao, J. Tang, H.-s. Liu and X.-y. Qi, Shipin Gongye Keji, 2014, 35, 384–388 Search PubMed.
- Y.-p. Jin, S. Yan, J.-x. Liu, Y. Yang, Y.-s. Wang and Y.-p. Wang, Zhongcaoyao, 2014, 45, 137–143 Search PubMed.
- Y.-p. Jin, S. Yan, J.-x. Liu, Y. Yang, Y.-s. Wang and Y.-p. Wang, Zhongcaoyao, 2014, 45, 1643–1650 Search PubMed.
- Y.-p. Jin, S. Yan, J.-x. Liu and Y.-p. Wang, Zhongcaoyao, 2014, 45, 582–589 Search PubMed.
- R. Thimmappa, K. Geisler, T. Louveau, P. O'Maille and A. Osbourn, Annu. Rev. Plant Biol., 2014, 65, 225–257 CrossRef PubMed.
- I. Abe, Natural Poducts, 2014, 297–316 Search PubMed.
- X. Yin, T. Feng, J.-H. Shang, Y.-L. Zhao, F. Wang, Z.-H. Li, Z.-J. Dong, X.-D. Luo and J.-K. Liu, Chem.–Eur. J., 2014, 20, 7001–7009 CrossRef PubMed.
- N. A. Siddiqui, I.-M. Chung, P. Nagella, M. Ali, P. Alam and A. Ahmad, Asian J. Chem., 2014, 26, 6185–6188 CrossRef.
- Q. Xia, H. Zhang, X. Sun, H. Zhao, L. Wu, D. Zhu, G. Yang, Y. Shao, X. Zhang, X. Mao, L. Zhang and G. She, Molecules, 2014, 19, 17478–17535 CrossRef PubMed.
- K. Ma, J. Ren, J. Han, L. Bao, L. Li, Y. Yao, C. Sun, B. Zhou and H. Liu, J. Nat. Prod., 2014, 77, 1847–1852 CrossRef PubMed.
- X.-R. Peng, J.-Q. Liu, C.-F. Wang, X.-Y. Li, Y. Shu, L. Zhou and M.-H. Qiu, J. Nat. Prod., 2014, 77, 737–743 CrossRef PubMed.
- W. Lakornwong, K. Kanokmedhakul, S. Kanokmedhakul, P. Kongsaeree, S. Prabpai, P. Sibounnavong and K. Soytong, J. Nat. Prod., 2014, 77, 1545–1553 CrossRef PubMed.
- P. Kleinwaechter, N. Anh, T. T. Kiet, B. Schlegel, H.-M. Dahse, A. Haertl and U. Graefe, J. Nat. Prod., 2001, 64, 236–239 CrossRef.
- L.-Y. Liu, H. Chen, C. Liu, H.-Q. Wang, J. Kang, Y. Li and R.-Y. Chen, Fitoterapia, 2014, 98, 254–259 CrossRef PubMed.
- L. Yu, T. Yao, M. Wang, L.-x. Chen, F. Qiu and D. Wang, Zhongcaoyao, 2014, 45, 1363–1366 Search PubMed.
- D.-Z. Liu, Y.-Q. Zhu, X.-F. Li, W.-G. Shan and P.-F. Gao, Chem. Biodiversity, 2014, 11, 982–986 CrossRef PubMed.
- L.-l. Hu, Q.-y. Ma, S.-z. Huang, Z.-k. Guo, H.-x. Ma, J.-c. Guo, H.-f. Dai and Y.-x. Zhao, Phytochem. Lett., 2014, 7, 11–13 CrossRef.
- C.-L. Hsu and G.-C. Yen, Enzymes, 2014, 36, 33–56 Search PubMed.
- C. Liu, C. Zhao, H.-H. Pan, J. Kang, X.-T. Yu, H.-Q. Wang, B.-M. Li, Y.-Z. Xie and R.-Y. Chen, J. Nat. Prod., 2014, 77, 35–41 CrossRef PubMed.
- Y.-M. Ying, L.-Y. Zhang, X. Zhang, H.-B. Bai, D.-E. Liang, L.-F. Ma, W.-G. Shan and Z.-J. Zhan, Phytochemistry, 2014, 108, 171–176 CrossRef PubMed.
- A. Umeyama, C. Ohta, Y. Shino, M. Okada, Y. Nakamura, T. Hamagaki, H. Imagawa, M. Tanaka, A. Ishiyama, M. Iwatsuki, K. Otoguro, S. Omura and T. Hashimoto, Tetrahedron, 2014, 70, 8312–8315 CrossRef.
- D. A. Bui, M. K. Vu, H. D. Nguyen, L.-T. T. Nguyen, S. V. Dang and L.-H. D. Nguyen, Phytochem. Lett., 2014, 10, 123–126 CrossRef.
- Y.-L. Li, Y.-X. Gao, H.-Z. Jin, L. Shan, X.-S. Liang, X.-K. Xu, X.-W. Yang, N. Wang, A. Steinmetz, Z. Chen and W.-D. Zhang, Phytochemistry, 2014, 106, 116–123 CrossRef PubMed.
- X.-W. Yang, S.-M. Li, Y.-L. Li, L. Feng, Y.-H. Shen, S. Lin, J.-M. Tian, H.-W. Zeng, N. Wang, A. Steinmetz, Y. Liu and W.-D. Zhang, Phytochemistry, 2014, 105, 164–170 CrossRef PubMed.
- A. Suman, M. Ali, I. Rais and S. S. Husain, Chem. Nat. Compd., 2014, 50, 293–297 CrossRef.
- A. Ali, M. Jameel and M. Ali, Nat. Prod. J., 2014, 4, 248–253 Search PubMed.
- R. Mori, A. E. Nugroho, Y. Hirasawa, C. P. Wong, T. Kaneda, O. Shirota, A. H. A. Hadi and H. Morita, J. Nat. Med., 2014, 68, 186–191 CrossRef PubMed.
- S. Klaiklay, Y. Sukpondma, V. Rukachaisirikul and S. Phongpaichit, Phytochemistry, 2013, 85, 161–166 CrossRef PubMed.
- N. Jamila, M. Khairuddean, N. S. Yaacob, N. N. S. N. M. Kamal, H. Osman, S. N. Khan and N. Khan, Bioorg. Chem., 2014, 54, 60–67 CrossRef PubMed.
- S. H. Ansari, M. Ali and K. J. Naquvi, J. Saudi Chem. Soc., 2014, 18, 561–565 CrossRef.
- M. Shuaib, M. Ali and K. J. Naquvi, Int. J. Pharm. Pharm. Sci., 2014, 6, 372–375 Search PubMed.
- R. A. Mosa, M. L. Nhleko, T. V. Dladla and A. R. Opoku, J. Med. Plants Res., 2014, 8, 686–702 CrossRef.
- R. A. Mosa, J. J. Naidoo, F. S. Nkomo, S. E. Mazibuko, C. J. F. Muller and A. R. Opoku, Planta Med., 2014, 80, 1685–1691 CrossRef PubMed.
- S. Aichour, H. Haba, M. Benkhaled, D. Harakat and C. Lavaud, Phytochem. Lett., 2014, 10, 198–203 CrossRef.
- N. Chaudhary, S. S. Husain and M. Ali, J. Pharmacogn. Phytochem., 2014, 3, 149–154 Search PubMed.
- M. Shimada, M. Ozawa, K. Iwamoto, Y. Fukuyama, A. Kishida and A. Ohsaki, Chem. Pharm. Bull., 2014, 62, 937–941 CrossRef PubMed.
- M.-m. Zhang, L.-l. Sun, C. Li, W. Gao, J.-b. Yang, A.-g. Wang, Y.-l. Su and T.-f. Ji, Zhongguo Zhongyao Zazhi, 2014, 39, 1834–1837 Search PubMed.
- L.-K. Wang, C.-J. Zheng, X.-B. Li, G.-Y. Chen, C.-R. Han, W.-H. Chen and X.-P. Song, Molecules, 2014, 19, 7621–7628 CrossRef PubMed.
- L.-K. Wang, C.-J. Zheng, X.-B. Li, G.-Y. Chen, C.-R. Han, W.-H. Chen and X.-P. Song, Molecules, 2015, 20, 20268 CrossRef.
- I.-M. Chung, Y.-O. Kim, M. Ali, S.-H. Kim, I. Park, E.-H. Kim, Y.-S. Yang, H.-R. Park, E.-S. Son and A. Ahmad, Bioorg. Med. Chem. Lett., 2014, 24, 4203–4208 CrossRef PubMed.
- I.-M. Chung, S. Yang, S.-H. Kim and A. Ahmad, Asian J. Chem., 2014, 26, 7789–7791 CrossRef.
- J.-I. Park, H.-R. Bae, C. Gun Kim, V. A. Stonik and J.-Y. Kwak, Front. Chem., 2014, 2, 1–14 Search PubMed.
- D. L. Aminin, E. A. Pislyagin, E. S. Menchinskaya, A. S. Silchenko, S. A. Avilov and V. I. Kalinin, Stud. Nat. Prod. Chem., 2014, 41, 75–94 Search PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko, I. Y. Dolmatov, A. M. Savchenko and V. I. Kalinin, Nat. Prod. Commun., 2014, 9, 1421–1428 Search PubMed.
- M. Elbandy, J. R. Rho and R. Afifi, Eur. Food Res. Technol., 2014, 238, 937–955 CrossRef.
- X.-H. Wang, Y.-H. Yi, H. Han, L. Li, M.-X. Pan and Z.-R. Zou, Mar. Drugs, 2014, 12, 2004–2018 CrossRef PubMed.
- Y. Bahrami, W. Zhang, T. Chataway and C. Franco, Mar. Drugs, 2014, 12, 4439–4473 CrossRef PubMed.
- V. P. Careaga, C. Bueno, C. Muniain, L. Alché and M. S. Maier, Nat. Prod. Res., 2014, 28, 213–220 CrossRef PubMed.
- R. S. Popov, S. A. Avilov, A. S. Silchenko, A. I. Kalinovsky, P. S. Dmitrenok, B. B. Grebnev, N. V. Ivanchina and V. I. Kalinin, Biochem. Syst. Ecol., 2014, 57, 191–197 CrossRef.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, S. N. Fedorov, P. S. Dmitrenok, E. A. Yurchenko, V. I. Kalinin, A. V. Rogacheva and A. V. Gebruk, Nat. Prod. Commun., 2014, 9, 1259–1264 Search PubMed.
- P. T. Nguyen, B. T. T. Luyen, L. T. Vien, B. H. Tai, L. D. Dat, X. C. Nguyen, H. N. Nguyen, V. K. Phan, V. M. Chau and Y. H. Kim, Nat. Prod. Commun., 2014, 9, 615–618 Search PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, V. I. Kalinin, E. A. Yurchenko and S. S. Dautov, Nat. Prod. Commun., 2014, 9, 391–399 Search PubMed.
- D.-Z. Liu, Nat. Prod. Res., 2014, 28, 1099–1105 CrossRef PubMed.
- M. Clericuzio, G. Vidari, C. Cassino, L. Legnani and L. Toma, Eur. J. Org. Chem., 2014, 5462–5468 CrossRef.
- Y.-l. Zhang, R.-q. Mei, X. Liu, G.-m. Liu and B. Wu, Zhongcaoyao, 2014, 45, 2293–2298 Search PubMed.
- X.-B. Chen, G.-Y. Chen, J.-H. Liu, M. Lei, Y.-H. Meng, D.-A. Guo, X. Liu and L.-H. Hu, Fitoterapia, 2014, 94, 88–93 CrossRef PubMed.
- X. Xu, H. Bai, L. Zhou, Z. Deng, H. Zhong, Z. Wu and Q. Yao, Bioorg. Med. Chem. Lett., 2014, 24, 2159–2162 CrossRef PubMed.
- J. L. Perez, G. K. Jayaprakasha and B. S. Patil, ACS Symp. Ser., 2014, 1185, 51–78 CrossRef.
- J.-C. Chen, X.-X. Yuan, L. Zhou, J.-Q. Liu, Y. Nian, Z.-R. Li, Y. Li, M.-J. Xie and M.-H. Qiu, Helv. Chim. Acta, 2014, 97, 1546–1554 CrossRef.
- P. H. Yen, D. T. Dung, X. N. Nguyen, L. T. A. Hoang, D. T. T. Hang, D. T. H. Yen, T. C. Nguyen, N. K. Ban, V. M. Chau and V. K. Phan, Nat. Prod. Commun., 2014, 9, 383–386 Search PubMed.
- G.-T. Zhao, J.-Q. Liu, Y.-Y. Deng, H.-Z. Li, J.-C. Chen, Z.-R. Zhang, L. Zhou and M.-H. Qiu, Fitoterapia, 2014, 95, 75–82 CrossRef PubMed.
- Y.-B. Zhang, H. Liu, C.-Y. Zhu, M.-X. Zhang, Y.-L. Li, B. Ling and G.-C. Wang, J. Asian Nat. Prod. Res., 2014, 16, 358–363 CrossRef PubMed.
- L. Ma, A.-H. Yu, L.-L. Sun, W. Gao, M.-M. Zhang, Y.-L. Su, H. Liu, T.-F. Ji and D.-Z. Li, J. Asian Nat. Prod. Res., 2014, 16, 476–482 CrossRef PubMed.
- A. Jardón-Delgado, G. A. Magos-Guerrero and M. Martínez-Vázquez, Nat. Prod. Commun., 2014, 9, 15–16 Search PubMed.
- I. Prakash and V. S. P. Chaturvedula, Molecules, 2014, 19, 3669–3680 CrossRef PubMed.
- Z.-X. Qing, Y. Zhou, X.-B. Liu, P. Cheng and J.-G. Zeng, Nat. Prod. Res., 2014, 28, 1165–1170 CrossRef PubMed.
- E. J. Jeong, J.-Y. Bae, J.-R. Rho, Y. C. Kim, M.-J. Ahn and S. H. Sung, Bull. Korean Chem. Soc., 2014, 35, 2945–2949 CrossRef.
- Y.-M. Shi, L.-Y. Wang, X.-S. Zou, X.-N. Li, S.-Z. Shang, Z.-H. Gao, C.-Q. Liang, H.-R. Luo, H.-L. Li, W.-L. Xiao and H.-D. Sun, Tetrahedron, 2014, 70, 859–868 CrossRef.
- Y.-M. Shi, J. Yang, L. Xu, X.-N. Li, S.-Z. Shang, P. Cao, W.-L. Xiao and H.-D. Sun, Org. Lett., 2014, 16, 1370–1373 CrossRef PubMed.
- C.-Q. Liang, R.-H. Luo, J.-M. Yan, Y. Li, X.-N. Li, Y.-M. Shi, S.-Z. Shang, Z.-H. Gao, L.-M. Yang, Y.-T. Zheng, W.-L. Xiao, H.-B. Zhang and H.-D. Sun, Arch. Pharmacal Res., 2014, 37, 168–174 CrossRef PubMed.
- P. T. H. Minh, D. T. Lam, Q. T. Nguyen, N. T. Nguyen, V. P. Nhung, V. H. Nong, V. K. Phan, X. N. Nguyen, V. M. Chau, S. J. Park and S. H. Kim, Nat. Prod. Commun., 2014, 9, 373–374 Search PubMed.
- J.-h. Yeon, L. Cheng, Q.-q. He and L.-y. Kong, Zhongguo Tianran Yaowu, 2014, 12, 782–785 Search PubMed.
- H. Wang, R. Ning, Y. Shen, Z. Chen, J. Li, R. Zhang, Y. Leng and W. Zhao, J. Nat. Prod., 2014, 77, 1910–1920 CrossRef PubMed.
- R.-n. Ning, H.-m. Wang, Y. Shen, Z.-h. Chen, R.-j. Zhang, Y. Leng and W.-m. Zhao, Bioorg. Med. Chem. Lett., 2014, 24, 5395–5398 CrossRef PubMed.
- L.-H. Mu, H.-J. Li, D.-H. Guo, J.-Y. Zhao and P. Liu, Fitoterapia, 2014, 92, 41–45 CrossRef PubMed.
- N.-M. Bao, Y. Nian, G.-L. Zhu, W.-H. Wang, L. Zhou and M.-H. Qiu, Fitoterapia, 2014, 99, 191–197 CrossRef PubMed.
- J.-Y. Chen, P.-L. Li, X.-L. Tang, S.-J. Wang, Y.-T. Jiang, L. Shen, B.-M. Xu, Y.-L. Shao and G.-Q. Li, J. Nat. Prod., 2014, 77, 1997–2005 CrossRef PubMed.
- Y. Su, L. Wu, Q. Wang, B. Yang and H. Kuang, Bioorg. Med. Chem. Lett., 2014, 24, 5688–5691 CrossRef PubMed.
- J.-X. Mo, Y. Bai, B. Liu, C.-X. Zhou, L. Zou and L.-S. Gan, Helv. Chim. Acta, 2014, 97, 887–894 CrossRef.
- N. Wang, G. Xu, Y. Fang, T. Yang, H. Zhao and G. Li, Molecules, 2014, 19, 6623–6634 CrossRef PubMed.
- J. Namukobe, B. T. Kiremire, R. Byamukama, J. M. Kasenene, V. Dumontet, F. Guéritte, S. Krief, I. Florent and J. D. Kabasa, Phytochemistry, 2014, 102, 189–196 CrossRef PubMed.
- Y. Nomoto, L. Harinantenaina, S. Sugimoto, K. Matsunami and H. Otsuka, J. Nat. Med., 2014, 68, 513–520 CrossRef PubMed.
- J. D. Simo Mpetga, H.-P. He, X.-J. Hao, Y. Leng and P. Tane, Phytochem. Lett., 2014, 7, 52–56 CrossRef.
- P. T. T. Huong, C. N. Diep, N. Van Thanh, V. A. Tu, T. H. Hanh, N. T. Cuong, N. P. Thao, N. X. Cuong, D. T. Thao, T. H. Thai, N. H. Nam, N. K. Ban, P. Van Kiem and C. Van Minh, Nat. Prod. Commun., 2014, 9, 1255–1257 Search PubMed.
- G. A. Mohamed, Nat. Prod. Res., 2014, 28, 976–983 CrossRef PubMed.
- K.-L. Ji, P. Zhang, H.-B. Hu, S. Hua, S.-G. Liao and Y.-K. Xu, J. Nat. Prod., 2014, 77, 1764–1769 CrossRef PubMed.
- G. Rajeev and S. C. Jain, Res. J. Chem. Sci., 2014, 4, 7–11 Search PubMed.
- X. Kuang, W. Li, Y. Kanno, M. Mochizuki, Y. Inouye and K. Koike, Bioorg. Med. Chem. Lett., 2014, 24, 5423–5427 CrossRef PubMed.
- P. Sakava, E. Talla, M. Chelea, A. T. Tiabou, E. Z. o. Menkem, S. Laurent, L. Vander Elst, M. T. Fotsing, A. J. Y. Gbaweng, A. A. De Théodore and J. M. Tanyi, J. Appl. Pharm. Sci., 2014, 4, 1–9 Search PubMed.
- N. Denizli, I. Horo, D. Gülcemal, M. Masullo, M. Festa, A. Capasso, Ö. Koz, S. Piacente and Ö. Alankuş-Çalişkan, Fitoterapia, 2014, 92, 211–218 CrossRef PubMed.
- F. Xu, X. Zhao, L. Yang, X. Wang and J. Zhao, Molecules, 2014, 19, 13422–13431 CrossRef PubMed.
- L.-H. Mu, H.-J. Li, D.-H. Guo, J.-Y. Zhao and P. Liu, J. Nat. Med., 2014, 68, 604–609 CrossRef PubMed.
- D.-f. Zhu, Y. Nian, H.-y. Wang, Z.-r. Zhang, Y.-b. Song, R.-t. Li and M.-h. Qiu, Zhongguo Tianran Yaowu, 2014, 12, 294–296 Search PubMed.
- L. N. Gvazava and V. S. Kikoladze, Chem. Nat. Compd., 2014, 50, 1012–1015 CrossRef.
- H.-J. Yan, J.-S. Wang and L.-Y. Kong, J. Nat. Prod., 2014, 77, 234–242 CrossRef PubMed.
- A. E. Nugroho, T. Momota, R. Sugiura, M. Hanzawa, E. Yajima, Y. Nagakura, N. Yasuda, Y. Hirasawa, C. P. Wong, T. Kaneda, A. H. A. Hadi, H. Fukaya and H. Morita, Tetrahedron, 2014, 70, 9661–9667 CrossRef.
- C. Tantapakul, W. Maneerat, T. Sripisut and T. Ritthiwigrom, Nat. Prod. Commun., 2014, 9, 1553–1556 Search PubMed.
- N. P. Thao, B. T. T. Luyen, B. H. Tai, S. Y. Yang, S. H. Jo, N. X. Cuong, N. H. Nam, Y. I. Kwon, C. V. Minh and Y. H. Kim, Bioorg. Med. Chem. Lett., 2014, 24, 1192–1196 CrossRef PubMed.
- J.-H. Yu, Y. Shen, H.-B. Liu, Y. Leng, H. Zhang and J.-M. Yue, Org. Biomol. Chem., 2014, 12, 4716–4722 RSC.
- Y.-j. Huang, H. Lu, X.-l. Yu, S.-w. Zhang, W.-q. Wang, L.-y. Fen and L.-j. Xuan, J. Nat. Prod., 2014, 77, 1201–1209 CrossRef PubMed.
- M.-M. Ding, F.-L. Yan, J. Tan, Y.-X. Bai, X. Wang and Y.-X. Yang, Nat. Prod. Res., 2014, 28, 18–23 CrossRef PubMed.
- T.-L. Tran, Y.-R. Kim, J.-L. Yang, D.-R. Oh, T.-T. Dao and W.-K. Oh, Bioorg. Med. Chem., 2014, 22, 499–504 CrossRef PubMed.
- J.-L. Yang, T.-K.-Q. Ha, B. Dhodary, K.-H. Kim, J. Park, C.-H. Lee, Y.-C. Kim and W.-K. Oh, J. Nat. Prod., 2014, 77, 1615–1623 CrossRef PubMed.
- L. Han, M.-Y. Lin, Q. Zheng, H.-Y. Liu, H.-Y. Liu, G. Dong, J.-P. Liu and P.-Y. Li, Nat. Prod. Res., 2014, 28, 935–939 CrossRef PubMed.
- L. Shi, D.-H. Tan, Y.-E. Liu, M.-X. Hou and Y.-Q. Zhao, Helv. Chim. Acta, 2014, 97, 1333–1339 CrossRef.
- X.-L. Piao, S.-F. Xing, C.-X. Lou and D.-J. Chen, Bioorg. Med. Chem. Lett., 2014, 24, 4831–4833 CrossRef PubMed.
- N. Li, Z.-D. Tuo, S.-S. Xing, S.-Z. Qi, H.-S. Lee and L. Cui, Bull. Korean Chem. Soc., 2014, 35, 3122–3124 CrossRef.
- Y. Wu, Y.-Y. Li, X. Wu, Z.-Z. Gao, C. Liu, M. Zhu, Y. Song, D.-Y. Wang, J.-G. Liu and Y.-L. Hu, Biochem. Syst. Ecol., 2014, 57, 216–220 CrossRef.
- Y. Tang, J.-Q. Cao, W. Li, W. Li and Y.-Q. Zhao, Helv. Chim. Acta, 2014, 97, 268–277 CrossRef.
- Y. Ou, H.-B. Liu, Y. Ge, H. Li and H. Zhang, Chem. Nat. Compd., 2014, 50, 298–301 CrossRef.
- A. Ladhari, R. Haouala and M. DellaGreca, Chem. Nat. Compd., 2014, 50, 684–686 CrossRef.
- L. Ali, S. Ali, J. Hussain, A. Al-Harrasi, A. Latif Khan, S. Tasleem Hussain Shah and T. Shamim Rizvi, Helv. Chim. Acta, 2014, 97, 556–560 CrossRef.
- L.-f. Wang, J. Zhao, W.-z. Tang and X.-j. Wang, Zhongcaoyao, 2014, 45, 161–163 Search PubMed.
- F. Ullah Khan, J. Hussain, I. Ullah Khan, N. Muhammad, S. Badshah, M. W. Aslam, S. Jan, H. Khan, R. A. Khan and H. Khan, Asian J. Chem., 2014, 26, 119–121 CrossRef.
- Y. Liu, M.-Q. Zhang, X.-L. Li, T.-H. Xu, S.-X. Xie, Y.-J. Xu and D.-M. Xu, J. Asian Nat. Prod. Res., 2014, 16, 206–209 CrossRef PubMed.
- Z. Yu, X.-S. Wang and Z.-H. Hu, J. Asian Nat. Prod. Res., 2014, 16, 200–205 CrossRef PubMed.
- D. Li, J. Cao, X. Bi, X. Xia, W. Li and Y. Zhao, J. Ginseng Res., 2014, 38, 28–33 CrossRef PubMed.
- X. Wu, J. Zhang, G. Sun, Y. Tian, X. Sun, X. Zhang, X. Zhong and X. Xu, Rec. Nat. Prod., 2014, 8, 422–425 CrossRef PubMed.
- L. Qiu, Y. Jiao, G.-K. Huang, J.-Z. Xie, J.-H. Miao and X.-S. Yao, Helv. Chim. Acta, 2014, 97, 102–111 CrossRef.
- S.-X. Jing, S.-H. Luo, C.-H. Li, J. Hua, Y.-L. Wang, X.-M. Niu, X.-N. Li, Y. Liu, C.-S. Huang, Y. Wang and S.-H. Li, J. Nat. Prod., 2014, 77, 882–893 CrossRef PubMed.
- C. Y. Ragasa, O. B. Torres, D. D. Raga, E. H. Mandia, M.-J. Don and C.-C. Shen, Pharm. Lett., 2014, 6, 290–294 Search PubMed.
- J. Hu, Y. Song, H. Li, B. Yang, X. Mao, Y. M. Zhao and X. Shi, Fitoterapia, 2014, 99, 86–91 CrossRef PubMed.
- J. Sichaem, P. Siripong, S. Tip-pyang and J. Phaopongthai, Nat. Prod. Commun., 2014, 9, 367–368 Search PubMed.
- K. Phontree, J. Sichaem, S. Khumkratok, P. Siripong and S. Tip-pyang, Nat. Prod. Commun., 2014, 9, 1253–1254 Search PubMed.
- S.-i. Kurimoto, Y. Takaishi, F. A. Ahmed and Y. Kashiwada, Fitoterapia, 2014, 92, 200–205 CrossRef PubMed.
- L. Chen, J.-X. Zhang, B. Wang, S.-Z. Mu and X.-J. Hao, Fitoterapia, 2014, 97, 204–210 CrossRef PubMed.
- W.-G. Shan, Z.-L. Gao, Y.-M. Ying, J.-G. Xiang, F.-S. Wang and Z.-J. Zhan, Helv. Chim. Acta, 2014, 97, 1526–1530 CrossRef.
- N. H. T. Phan, N. T. D. Thuan, N. T. Ngoc, P. T. M. Huong, N. P. Thao, N. X. Cuong, N. Van Thanh, N. H. Nam, P. Van Kiem and C. Van Minh, Phytochem. Lett., 2014, 9, 78–81 CrossRef.
- J. Hu, Y. Song, H. Li, X. Mao, Y. Zhao, X. Shi and B. Yang, Phytochem. Lett., 2014, 10, 219–223 CrossRef.
- M. F. Khan, A. K. Rawat, B. Pawar, S. Gautam, A. K. Srivastava and D. S. Negi, Fitoterapia, 2014, 98, 98–103 CrossRef PubMed.
- Z.-F. Zhou, O. Taglialatela-Scafati, H.-L. Liu, Y.-C. Gu, L.-Y. Kong and Y.-W. Guo, Fitoterapia, 2014, 97, 192–197 CrossRef PubMed.
- J. Sichaem, S. Khumkratok, P. Siripong and S. Tip-pyang, J. Nat. Med., 2014, 68, 436–441 CrossRef PubMed.
- Y. Bai, X. Jin, X. Jia, W. Tang, X. Wang and Y. Zhao, Phytochem. Lett., 2014, 10, 118–122 CrossRef.
- W.-B. Wu, H. Zhang, S.-H. Dong, L. Sheng, Y. Wu, J. Li and J.-M. Yue, J. Asian Nat. Prod. Res., 2014, 16, 709–716 CrossRef PubMed.
- W.-M. Zhang, J.-Q. Liu, X.-R. Peng, L.-S. Wan, Z.-R. Zhang, Z.-R. Li and M.-H. Qiu, Nat. Prod. Bioprospect., 2014, 4, 157–162 CrossRef PubMed.
- R. Tundis, M. R. Loizzo and F. Menichini, Crit. Rev. Food Sci. Nutr., 2014, 54, 225–250 CrossRef PubMed.
- P. Jolanta, S. D. Jan, K. Agata and L. Stanislaw, Mini-Rev. Org. Chem., 2014, 11, 269–279 CrossRef.
- O. Tzakou, Pharmakeutike, 2014, 26, 6–16 Search PubMed.
- R. Romeo and M. A. Chiacchio, Med. Aromat. Plants--Ind. Profiles, 2014, 51, 427–448 Search PubMed.
- S. Nagini, Enzymes, 2014, 36, 131–147 Search PubMed.
- L. N. Bodduluru, E. R. Kasala, N. Thota, C. C. Barua and R. Sistla, Toxicol. In Vitro, 2014, 28, 1026–1035 CrossRef PubMed.
- E. S. Park, I. K. Bae, H. J. Jeon and S.-E. Lee, Entomol. Res., 2014, 44, 158–162 CrossRef.
- M.-L. Han, Y. Shen, Y. Leng, H. Zhang and J.-M. Yue, RSC Adv., 2014, 4, 19150–19158 RSC.
- Z.-F. Zhou, H.-L. Liu, W. Zhang, T. Kurtán, A. Mándi, A. Bényei, J. Li, O. Taglialatela-Scafati and Y.-W. Guo, Tetrahedron, 2014, 70, 6444–6449 CrossRef.
- J.-J. Xia, X.-Y. Li, S.-Z. Zhang, J.-Q. Liu, W.-M. Zhang, Y.-x. Yan, Z.-T. Ding and M.-H. Qiu, Tetrahedron Lett., 2014, 55, 2104–2106 CrossRef.
- G.-Y. Zhu, G. Chen, L. Liu, L.-P. Bai and Z.-H. Jiang, J. Nat. Prod., 2014, 77, 983–989 CrossRef PubMed.
- G.-Y. Zhu, L.-P. Bai, L. Liu and Z.-H. Jiang, Phytochemistry, 2014, 107, 175–181 CrossRef PubMed.
- Q. Jin, C. Lee, J. Woo Lee, J. Yeon Choi, J. Tae Hong, Y. Kim, M. Kyeong Lee and B. Yeon Hwang, Helv. Chim. Acta, 2014, 97, 1152–1157 CrossRef.
- J.-Q. Zhao, Y.-M. Wang, H.-T. Zhu, D. Wang, S.-H. Li, R.-R. Cheng, C.-R. Yang, Y.-F. Wang, M. Xu and Y.-J. Zhang, Nat. Prod. Bioprospect., 2014, 4, 233–242 CrossRef PubMed.
- W. Kitdamrongtham, K. Ishii, K. Ebina, J. Zhang, M. Ukiya, K. Koike, H. Akazawa, A. Manosroi, J. Manosroi and T. Akihisa, Chem. Biodiversity, 2014, 11, 73–84 CrossRef PubMed.
- H.-M. Xia, C.-J. Li, J.-Z. Yang, J. Ma, X.-G. Chen, D. Zhang, L. Li and D.-M. Zhang, J. Nat. Prod., 2014, 77, 784–791 CrossRef PubMed.
- P. Qian, H.-W. Jin and X.-W. Yang, J. Asian Nat. Prod. Res., 2014, 16, 333–344 CrossRef PubMed.
- L.-L. Wang, C.-S. Jiang, Y. Fu, F.-F. Chen, L.-F. Lan, H.-Y. Zhang and Y.-W. Guo, Helv. Chim. Acta, 2014, 97, 1301–1306 CrossRef.
- Y. Zhang, J.-S. Wang, Y.-C. Gu and L.-Y. Kong, Helv. Chim. Acta, 2014, 97, 1354–1364 CrossRef.
- B. Siva, B. Poornima, A. Venkanna, K. R. Prasad, B. Sridhar, V. Lakshma Nayak, S. Ramakrishna and K. S. Babu, Phytochemistry, 2014, 98, 174–182 CrossRef PubMed.
- L. Liu, Y.-L. Zhao, G.-G. Cheng, Y.-Y. Chen, X.-J. Qin, C.-W. Song, X.-W. Yang, Y.-P. Liu and X.-D. Luo, Nat. Prod. Bioprospect., 2014, 4, 335–340 CrossRef PubMed.
- M. Takagi, Y. Tachi, J. Zhang, T. Shinozaki, K. Ishii, T. Kikuchi, M. Ukiya, N. Banno, H. Tokuda and T. Akihisa, Chem. Biodiversity, 2014, 11, 451–468 CrossRef PubMed.
- H. Kato-Noguchi, M. A. Salam, O. Ohno and K. Suenaga, Molecules, 2014, 19, 6929–6940 CrossRef PubMed.
- M. J. Gualtieri, N. Malafronte, A. Vassallo, A. Braca, R. Cotugno, M. Vasaturo, N. De Tommasi and F. Dal Piaz, J. Nat. Prod., 2014, 77, 596–602 CrossRef PubMed.
- A. Manosroi, W. Kitdamrongtham, K. Ishii, T. Shinozaki, Y. Tachi, M. Takagi, K. Ebina, J. Zhang, J. Manosroi, R. Akihisa and T. Akihisa, Chem. Biodiversity, 2014, 11, 505–531 CrossRef PubMed.
- X. Pan, M. Matsumoto, Y. Nakamura, T. Kikuchi, J. Zhang, M. Ukiya, T. Suzuki, K. Koike, R. Akihisa and T. Akihisa, Chem. Biodiversity, 2014, 11, 987–1000 CrossRef PubMed.
- X. Pan, M. Matsumoto, Y. Nishimoto, E. Ogihara, J. Zhang, M. Ukiya, H. Tokuda, K. Koike, M. Akihisa and T. Akihisa, Chem. Biodiversity, 2014, 11, 1121–1139 CrossRef PubMed.
- V. G. P. Severino, S. D. L. de Freitas, P. A. C. Braga, M. R. Forim, M. F. das, G. F. da Silva, J. B. Fernandes, P. C. Vieira and T. Venâncio, Molecules, 2014, 19, 12031–12047 CrossRef PubMed.
- C.-P. Liu, J.-B. Xu, Y.-S. Han, M. A. Wainberg and J.-M. Yue, Org. Lett., 2014, 16, 5478–5481 CrossRef PubMed.
- Y. Zhang, J.-S. Wang, Y.-C. Gu, X.-B. Wang and L.-Y. Kong, Tetrahedron, 2014, 70, 6594–6606 CrossRef.
- J.-Y. Cai, D.-Z. Chen, S.-H. Luo, N.-C. Kong, Y. Zhang, Y.-T. Di, Q. Zhang, J. Hua, S.-X. Jing, S.-L. Li, S.-H. Li, X.-J. Hao and H.-P. He, J. Nat. Prod., 2014, 77, 472–482 CrossRef PubMed.
- L.-R. Fu, Q.-Y. Ma, S.-Z. Huang, H.-F. Dai, Z.-K. Guo, Z.-F. Yu and Y.-X. Zhao, J. Asian Nat. Prod. Res., 2014, 16, 1054–1059 CrossRef PubMed.
- X.-Y. Wang, C.-M. Yuan, G.-H. Tang, T. Zou, F. Guo, J.-H. Liao, H.-Y. Zhang, G.-Y. Zuo, G.-X. Rao, Q. Zhao, X.-J. Hao and H.-P. He, J. Asian Nat. Prod. Res., 2014, 16, 795–799 CrossRef PubMed.
- M.-Y. Li, Q. Xiao, T. Satyanandamurty and J. Wu, Chem. Biodiversity, 2014, 11, 262–275 CrossRef PubMed.
- W. Chen, L. Shen, M. Li, Q. Xiao, T. Satyanandamurty and J. Wu, Fitoterapia, 2014, 94, 108–113 CrossRef PubMed.
- Y.-B. Wu, X. Qing, C.-H. Huo, H.-M. Yan, Q.-W. Shi, F. Sauriol, Y.-C. Gu and H. Kiyota, Tetrahedron, 2014, 70, 4557–4562 CrossRef.
- Z.-F. Zhou, L.-Y. Kong, T. Kurtán, H.-L. Liu, A. Mándi, J. Li, Y.-C. Gu and Y.-W. Guo, Planta Med., 2014, 80, 949–954 Search PubMed.
- Y. Wu, Y. Bai, X. Guo, J. Qi, M. Dong, F. Sauriol, Q. Shi, Y. Gu and C. Huo, Chem. Nat. Compd., 2014, 50, 314–316 CrossRef.
- C. Sarigaputi, D. Sommit, T. Teerawatananond and K. Pudhom, J. Nat. Prod., 2014, 77, 2037–2043 CrossRef PubMed.
- T. Inoue, Y. Matsui, T. Kikuchi, Y. In, O. Muraoka, T. Yamada and R. Tanaka, Fitoterapia, 2014, 96, 56–64 CrossRef PubMed.
- Y. Matsui, T. Kikuchi, T. Inoue, O. Muraoka, T. Yamada and R. Tanaka, Molecules, 2014, 19, 17130–17140 CrossRef PubMed.
- K.-L. Ji, S.-G. Liao, X.-L. Zheng, Z. Na, H.-B. Hu, P. Zhang and Y.-K. Xu, Molecules, 2014, 19, 3004–3011 CrossRef PubMed.
- W.-B. Wu, H. Zhang, H.-C. Liu, S.-H. Dong, Y. Wu, J. Ding and J.-M. Yue, Tetrahedron, 2014, 70, 3570–3575 CrossRef.
- F. Zhang, C.-R. Zhang, X. Tao, J. Wang, W.-S. Chen and J.-M. Yue, Bioorg. Med. Chem. Lett., 2014, 24, 3791–3796 CrossRef PubMed.
- H.-L. Liu, X.-L. Chen, W. Xiao and Y.-W. Guo, Helv. Chim. Acta, 2014, 97, 1445–1451 CrossRef.
- S. Razafimahefa, F. Mutulis, I. Mutule, E. Liepinsh, M. Dambrova, H. Cirule, B. Svalbe, S. Yahorava, A. Yahorau, B. Rasolondratovo, P. Rasoanaivo and J. E. S. Wikberg, Planta Med., 2014, 80, 306–314 CrossRef PubMed.
- P. A. Yadav, G. Suresh, M. S. A. Rao, G. Shankaraiah, P. Usha Rani and K. S. Babu, Bioorg. Med. Chem. Lett., 2014, 24, 888–892 CrossRef PubMed.
- Y.-B. Cheng, Y.-T. Chien, J.-C. Lee, C.-K. Tseng, H.-C. Wang, I. W. Lo, Y.-H. Wu, S.-Y. Wang, Y.-C. Wu and F.-R. Chang, J. Nat. Prod., 2014, 77, 2367–2374 CrossRef PubMed.
- J.-L. Yin, X. Fang, E.-D. Liu, C.-M. Yuan, S.-F. Li, Y. Zhang, H.-P. He, S.-L. Li, Y.-T. Di and X.-J. Hao, Planta Med., 2014, 80, 1304–1309 CrossRef PubMed.
- S.-M. Hu, J. Luo, L. Yang and L.-Y. Kong, Tetrahedron Lett., 2014, 55, 815–817 CrossRef.
- S. Park, N. X. Nhiem, P. V. Kiem, C. V. Minh, B. H. Tai, N. Kim, H. H. Yoo, J.-H. Song, H.-J. Ko and S. H. Kim, Bioorg. Med. Chem. Lett., 2014, 24, 3835–3840 CrossRef PubMed.
- D. Meng, X. Li, L. Han, L. Zhang, W. An and X. Li, Fitoterapia, 2014, 92, 105–110 CrossRef PubMed.
- T. V. A. Tran, C. Malainer, S. Schwaiger, A. G. Atanasov, E. H. Heiss, V. M. Dirsch and H. Stuppner, J. Nat. Prod., 2014, 77, 483–488 CrossRef PubMed.
- X.-L. Yang, Y.-L. Yuan, D.-M. Zhang, F. Li and W.-C. Ye, Nat. Prod. Res., 2014, 28, 1432–1437 CrossRef PubMed.
- J.-e. Yi, J. Wu, L.-x. Wen, W. Xia, R.-c. Zhu, W.-w. Jiang and Z.-l. Tan, Zhongcaoyao, 2014, 45, 2118–2124 Search PubMed.
- R. Csuk, Expert Opin. Ther. Pat., 2014, 24, 913–923 CrossRef PubMed.
- S. Jeelani and M. A. Khuroo, Chem. Nat. Compd., 2014, 50, 681–683 CrossRef.
- J. Zhou, C.-J. Li, J.-Z. Yang, J. Ma, Y. Li, X.-Q. Bao, X.-G. Chen, D. Zhang and D.-M. Zhang, J. Nat. Prod., 2014, 77, 276–284 CrossRef PubMed.
- L.-S. Shi, C.-H. Wu, T.-C. Yang, C.-W. Yao, H.-C. Lin and W.-L. Chang, Fitoterapia, 2014, 97, 184–191 CrossRef PubMed.
- M.-H. Yu, Z.-F. Shi, B.-W. Yu, E.-H. Pi, H.-Y. Wang, A.-J. Hou and C. Lei, Fitoterapia, 2014, 98, 143–148 CrossRef PubMed.
- L. Ali, J. Hussain, A. Al-Rawahi and A. Al-Harrasi, Rec. Nat. Prod., 2014, 8, 407–411 Search PubMed.
- S. Rungsimakan and M. G. Rowan, Phytochemistry, 2014, 108, 177–188 CrossRef PubMed.
- J. Hu, Y. Song, B. Yang, X. Zuo, X. Mao and X. Shi, Arch. Pharmacal Res., 2014, 37, 1515–1521 CrossRef PubMed.
- A. Latif, S. H. Hussain, M. Ali, M. Arfan, M. Ahmed, R. J. Cox, T. J. Simpson and G. Uddin, Rec. Nat. Prod., 2014, 8, 19–24 Search PubMed.
- P. S. Ghosh, I. S. Sarma, N. Sato, Y. Harigaya and B. Dinda, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53B, 1284–1287 Search PubMed.
- O. Estrada, W. Contreras, G. Acha, E. Lucena, W. Venturini, A. Cardozo and C. Alvarado-Castillo, Molecules, 2014, 19, 21215–21225 CrossRef PubMed.
- Y. Wang, C.-L. Zhang, Y.-F. Liu, D. Liang, H. Luo, Z.-Y. Hao, R.-Y. Chen and D.-Q. Yu, Planta Med., 2014, 80, 215–222 Search PubMed.
- C. B. Colloca, L. A. Espinar and V. E. Sosa, Nat. Prod.: Indian J., 2014, 10, 61–68 Search PubMed.
- C. Guang, J. Chen, S. Sang and S. Cheng, J. Agric. Food Chem., 2014, 62, 8247–8255 CrossRef PubMed.
- K. Jatczak and G. Grynkiewicz, Acta Biochim. Pol., 2014, 61, 227–243 Search PubMed.
- N. R. Parikh, A. Mandal, D. Bhatia, K. S. Siveen, G. Sethi and A. Bishayee, Phytochem. Rev., 2014, 13, 793–810 CrossRef PubMed.
- A. Paszel-Jaworska, A. Romaniuk and M. Rybczynska, Mini-Rev. Org. Chem., 2014, 11, 330–342 CrossRef.
- M. K. Shanmugam, X. Dai, A. P. Kumar, B. K. H. Tan, G. Sethi and A. Bishayee, Cancer Lett., 2014, 346, 206–216 CrossRef PubMed.
- Q.-F. Luo, J.-H. Liu and L. Chen, Mini-Rev. Org. Chem., 2014, 11, 355–361 CrossRef.
- X. Huang and Y. Yang, Guoji Zhongliuxue Zazhi, 2014, 41, 96–99 Search PubMed.
- G. Lozano-Mena, M. Sánchez-González, M. E. Juan and J. M. Planas, Molecules, 2014, 19, 11538–11559 CrossRef PubMed.
- R. Zhong, R. Shu, X. Ni, C. Xu and L. Li, Yaoxue Xuebao, 1996, 31, 398–400 Search PubMed.
- M. E. Wright, J. Byrd, C. He and N. Dunlap, J. Nat. Prod., 2014, 77, 2566–2569 CrossRef PubMed.
- S.-J. Liu, Z.-X. Liao, C. Liu, G.-Y. Yao and H.-S. Wang, Phytochem. Lett., 2014, 9, 11–16 CrossRef.
- I.-H. Chen, Y.-C. Du, T.-L. Hwang, I. F. Chen, Y.-H. Lan, H.-F. Yen, F.-R. Chang and Y.-C. Wu, Molecules, 2014, 19, 4608–4623 CrossRef PubMed.
- J. Zhang, J. Liu, B. Xu, Y. Zhuang, M. Yosikawa and B. Yin, Asian J. Chem., 2014, 26, 4521–4522 Search PubMed.
- E.-S. A. El-Kashoury, H. I. El-Askary, Z. A. Kandil, S. M. Ezzat, M. A. Salem and A. A. Sleem, J. Med. Plants Res., 2014, 8, 747–755 CrossRef.
- M. N. Uddin, G. Sharma, J.-L. Yang, H. S. Choi, S.-I. L. Lim, K. W. Kang and W. K. Oh, Phytochemistry, 2014, 103, 99–106 CrossRef PubMed.
- H.-j. Shang, W.-j. Wang, D.-y. Li, H.-m. Hua and Z.-l. Li, Zhongcaoyao, 2014, 45, 1207–1210 Search PubMed.
- K. Jiang, J.-Q. Bai, J. Chang, J.-J. Tan, S.-J. Qu, H.-F. Luo, C.-H. Tan and D.-Y. Zhu, Helv. Chim. Acta, 2014, 97, 64–69 CrossRef.
- J. Wang, Q.-L. Xu, M.-F. Zheng, H. Ren, T. Lei, P. Wu, Z.-Y. Zhou, X.-Y. Wei and J.-W. Tan, Molecules, 2014, 19, 4301–4312 CrossRef PubMed.
- S. Ji, Q. Wang, X. Qiao, H.-c. Guo, Y.-f. Yang, T. Bo, C. Xiang, D.-a. Guo and M. Ye, J. Pharm. Biomed. Anal., 2014, 90, 15–26 CrossRef PubMed.
- R. Chib, M. Kumar, M. Rizvi, S. Sharma, A. Pandey, S. Bani, S. S. Andotra, S. C. Taneja and B. A. Shah, RSC Adv., 2014, 4, 8632–8637 RSC.
- M.-M. Li, X.-Q. Su, J. Sun, Y.-F. Gu, Z. Huang, K.-W. Zeng, Q. Zhang, Y.-F. Zhao, D. Ferreira, J. K. Zjawiony, J. Li and P.-F. Tu, J. Nat. Prod., 2014, 77, 2248–2254 CrossRef PubMed.
- Y.-b. Zhang, W.-z. Yang, C.-l. Yao, R.-h. Feng, M. Yang, D.-a. Guo and W.-y. Wu, Fitoterapia, 2014, 96, 39–47 CrossRef PubMed.
- O. Aiyelaagbe, O. Olaoluwa, I. Oladosu and S. Gibbons, Rec. Nat. Prod., 2014, 8, 7–11 Search PubMed.
- B. N. Irungu, J. A. Orwa, A. Gruhnojic, P. A. Fitzpatrick, G. Landberg, F. Kimani, J. Midiwo, M. Erdélyi and A. Yenesew, Molecules, 2014, 19, 14235–14246 CrossRef PubMed.
- Z.-Y. Wu, Y.-B. Zhang, K.-K. Zhu, C. Luo, J.-X. Zhang, C.-R. Cheng, R.-H. Feng, W.-Z. Yang, F. Zeng, Y. Wang, P.-P. Xu, J.-L. Guo, X. Liu, S.-H. Guan and D.-A. Guo, J. Nat. Prod., 2014, 77, 2342–2351 CrossRef PubMed.
- H. Lakhal, A. Kabouche, A. Alabdul Magid, L. Voutquenne-Nazabadioko, D. Harakat and Z. Kabouche, Phytochemistry, 2014, 102, 145–151 CrossRef PubMed.
- J. Cai, L. Zhao, E. Zhu and J. Guo, Nat. Prod. Res., 2014, 28, 2163–2168 CrossRef PubMed.
- J.-H. Wei, Y.-F. Zheng, C.-Y. Li, Y.-P. Tang and G.-P. Peng, J. Asian Nat. Prod. Res., 2014, 16, 1044–1053 CrossRef PubMed.
- W. Song, L. Si, S. Ji, H. Wang, X.-m. Fang, L.-y. Yu, R.-y. Li, L.-n. Liang, D. Zhou and M. Ye, J. Nat. Prod., 2014, 77, 1632–1643 CrossRef PubMed.
- Y. Shao, D.-W. Ou-Yang, W. Gao, L. Cheng, X.-X. Weng and D.-Y. Kong, Helv. Chim. Acta, 2014, 97, 992–998 CrossRef.
- C. Chen, W. Gao, L. Cheng, Y. Shao and D.-Y. Kong, J. Asian Nat. Prod. Res., 2014, 16, 231–239 CrossRef PubMed.
- H. Zhou, C. Z. Wang, J. Z. Ye and H. X. Chen, Phytochem. Lett., 2014, 8, 46–51 CrossRef.
- K. Matsuzaki, K. Murano, Y. Endo and S. Kitanaka, Nat. Prod. Commun., 2014, 9, 1695–1698 Search PubMed.
- H. Xiong, X. Ding, X.-z. Yang, G.-z. Yang and Z.-n. Mei, Planta Med., 2014, 80, 710–718 CrossRef PubMed.
- F.-M. Xi, C.-T. Li, J. Han, S.-S. Yu, Z.-J. Wu and W.-S. Chen, Bioorg. Med. Chem., 2014, 22, 6515–6522 CrossRef PubMed.
- M. Masullo, L. Calabria, D. Gallotta, C. Pizza and S. Piacente, Phytochemistry, 2014, 97, 70–80 CrossRef PubMed.
- Y. Jiang, K.-W. Zeng, B. David and G. Massiot, Phytochemistry, 2014, 107, 111–118 CrossRef PubMed.
- N. B. Sarikahya, Phytochem. Lett., 2014, 8, 149–155 CrossRef.
- B. K. Ponou, R. N. Nono, R. B. Teponno, A. L. Tapondjou, M.-A. Lacaille-Dubois, L. Quassinti, M. Bramucci and L. Barboni, Phytochem. Lett., 2014, 10, 255–259 CrossRef.
- F. R. Melek, I. A. A. Kassem, T. Miyase and W. Fayad, Phytochemistry, 2014, 100, 110–119 CrossRef PubMed.
- X. Pang, H.-X. Yan, Z.-F. Wang, M.-X. Fan, Y. Zhao, X.-T. Fu, C.-Q. Xiong, J. Zhang, B.-P. Ma and H.-Z. Guo, J. Asian Nat. Prod. Res., 2014, 16, 240–247 CrossRef PubMed.
- S.-G. Li, X.-J. Huang, M.-M. Li, M. Wang, R.-B. Feng, W. Zhang, Y.-L. Li, Y. Wang and W.-C. Ye, Chem. Pharm. Bull., 2014, 62, 35–44 CrossRef PubMed.
- B.-S. Cui, Y.-Q. Qiao, Y. Yuan, L. Tang, H. Chen, Y. Li and S. Li, Planta Med., 2014, 80, 1647–1656 CrossRef PubMed.
- S.-l. Yan, Y.-f. Su, X.-y. Xie, C.-y. Guo, M. Que and L. Chen, Zhongcaoyao, 2014, 45, 23–27 Search PubMed.
- L. Qiao, Y.-f. Su, C.-y. Guo, X.-y. Xie, M. Que and L. Chen, Zhongcaoyao, 2014, 45, 1211–1218 Search PubMed.
- P. Kayce, N. Boke Sarikahya and S. Kirmizigul, Phytochem. Lett., 2014, 10, 324–329 CrossRef.
- X.-J. Huang, J.-Q. Tang, M.-M. Li, Q. Liu, Y.-L. Li, C.-L. Fan, H. Pei, H.-N. Zhao, Y. Wang and W.-C. Ye, J. Asian Nat. Prod. Res., 2014, 16, 910–921 CrossRef PubMed.
- N. P. Alza, E.-M. Pferschy-Wenzig, S. Ortmann, N. Kretschmer, O. Kunert, G. N. Rechberger, R. Bauer and A. P. Murray, Chem. Biodiversity, 2014, 11, 311–322 CrossRef PubMed.
- Y. Lei, S.-P. Shi, Y.-L. Song, D. Bi and P.-F. Tu, Chem. Biodiversity, 2014, 11, 767–775 CrossRef PubMed.
- Y. Zhou, K. Zeng, J. Zhang, N. Li, X. Chai, Y. Jiang and P. Tu, Fitoterapia, 2014, 97, 98–104 CrossRef PubMed.
- F. D. Mabou, P. L. F. Tebou, D. Ngnokam, D. Harakat and L. Voutquenne-Nazabadioko, Magn. Reson. Chem., 2014, 52, 32–36 CrossRef PubMed.
- Q.-J. Li, Z. Zhu, X.-S. Yang and X.-J. Hao, Helv. Chim. Acta, 2014, 97, 839–846 CrossRef.
- M. Kuroda, T. Shizume and Y. Mimaki, Chem. Pharm. Bull., 2014, 62, 92–96 CrossRef PubMed.
- Y. Zhang, T. A. Adelakun, L. Qu, X. Li, J. Li, L. Han and T. Wang, Fitoterapia, 2014, 99, 78–85 CrossRef PubMed.
- X. Li, J. Zhao, C. Peng, Z. Chen, Y. Liu, Q. Xu, I. A. Khan and S. Yang, Planta Med., 2014, 80, 590–598 CrossRef PubMed.
- J. Zhang, M. Kurita, T. Shinozaki, M. Ukiya, K. Yasukawa, N. Shimizu, H. Tokuda, E. T. Masters, M. Akihisa and T. Akihisa, Phytochemistry, 2014, 108, 157–170 CrossRef PubMed.
- C. Bäcker, K. Jenett-Siems, K. Siems, M. Wurster, A. Bodtke and U. Lindequist, Z. Naturforsch., C: J. Biosci., 2014, 69, 191–198 Search PubMed.
- C. Bäcker, K. Jenett-Siems, K. Siems, M. Wurster, A. Bodtke, T. H. J. Niedermeyer and U. Lindequist, Z. Naturforsch., B: J. Chem. Sci., 2014, 69, 1026–1044 Search PubMed.
- T. Morikawa, K. Ninomiya, K. Imura, T. Yamaguchi, Y. Akagi, M. Yoshikawa, T. Hayakawa and O. Muraoka, Phytochemistry, 2014, 102, 169–181 CrossRef PubMed.
- Y. Zhao, X. Wang, H. Wang, T. Liu and Z. Xin, Food Chem., 2014, 151, 101–109 CrossRef PubMed.
- W.-b. Wei, Y.-j. Huang, H.-j. Cong, S.-w. Zhang, D.-r. Pan and L.-j. Xuan, Phytochem. Lett., 2014, 8, 101–104 CrossRef.
- Y. Wang, L. Wang, W.-J. Wang, X.-Q. Zhang, H.-Y. Tian, Q.-W. Zhang, Y.-L. Li and W.-C. Ye, Carbohydr. Res., 2014, 385, 65–71 CrossRef PubMed.
- C.-Q. Wang, Y. Wang, W.-J. Wang, L. Wang and W.-C. Ye, Phytochem. Lett., 2014, 10, 268–271 CrossRef.
- H. K. Cho, C. S. Kim, W. S. Suh, K. H. Kim and K. R. Lee, Heterocycles, 2014, 89, 2619–2626 CrossRef.
- I. Arslan, Chem. Biodiversity, 2014, 11, 445–450 CrossRef PubMed.
- Y. Chen, F. Yang, S. Wang, D.-b. Wang, J. Xu and G.-z. Yang, Bull. Korean Chem. Soc., 2014, 35, 1212–1214 CrossRef.
- N. Asati and R. N. Yadava, Int. J. Pharm. Res. Bio-Sci., 2014, 3, 341–349 Search PubMed.
- X. Wang, M. Wang, M. Xu, Y. Wang, H. Tang and X. Sun, Molecules, 2014, 19, 2121–2134 CrossRef PubMed.
- B. Huang, H.-Z. Fu, W.-K. Chen, Y.-H. Luo and S.-C. Ma, Chem. Pharm. Bull., 2014, 62, 695–699 CrossRef PubMed.
- B. K. Mehta, D. Mehta and H. Misra, J. Indian Chem. Soc., 2014, 91, 1583–1590 Search PubMed.
- M. Zhao, N. Ma, F. Qiu, W.-L. Hai, H.-F. Tang, Y. Zhang and A.-D. Wen, Planta Med., 2014, 80, 942–948 CrossRef PubMed.
- Z.-H. Pan, D.-S. Ning, J.-L. Liu, B. Pan and D.-P. Li, Nat. Prod. Res., 2014, 28, 48–51 CrossRef PubMed.
- F.-M. Xi, C.-T. Li, J.-L. Mi, Z.-J. Wu and W.-S. Chen, Nat. Prod. Res., 2014, 28, 35–40 CrossRef PubMed.
- M. Kowalczyk, M. Masullo, B. Thiem, S. Piacente, A. Stochmal and W. Oleszek, Nat. Prod. Res., 2014, 28, 653–660 CrossRef PubMed.
- E. G. Montes, A.-C. Mitaine-Offer, J. M. Amaro-Luis, T. Paululat, C. Delaude, L. Pouysegu, S. Quideau, L. B. Rojas, S. Delemasure, P. Dutartre and M.-A. Lacaille-Dubois, Phytochemistry, 2014, 98, 236–242 CrossRef PubMed.
- H.-M. Wang, Q.-F. Liu, Y.-W. Zhao, S.-Z. Liu, Z.-H. Chen, R.-J. Zhang, Z.-Z. Wang, W. Xiao and W.-M. Zhao, J. Asian Nat. Prod. Res., 2014, 16, 20–28 CrossRef PubMed.
- Y. Liu, T.-h. Xu, M.-q. Zhang, X. Li, Y.-j. Xu, H.-y. Jiang, T.-h. Liu and D.-m. Xu, Zhongguo Tianran Yaowu, 2014, 12, 300–304 Search PubMed.
- D. Pertuit, S. Avunduk, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, T. Paululat, S. Delemasure, P. Dutartre and M.-A. Lacaille-Dubois, Phytochemistry, 2014, 102, 182–188 CrossRef PubMed.
- J. Y. Eskander, E. G. Haggag, M. R. El-Gindi and M. M. Mohamedy, Med. Chem. Res., 2014, 23, 717–724 CrossRef.
- M. A. Tantry, G. A. Bhat, A. Idris, J. A. Dar, S. Yousef Al Omar, K. Z. Masoodi, B. A. Ganai, A. N. Kamili and A. S. Shawl, Helv. Chim. Acta, 2014, 97, 1497–1506 CrossRef.
- L. Ma, A.-H. Yu, L.-L. Sun, W. Gao, M.-M. Zhang, Y.-L. Su, H. Liu and T. Ji, Molecules, 2014, 19, 2238–2246 CrossRef PubMed.
- D. Gülcemal, M. Masullo, Ö. Alankuş-Çalişkan and S. Piacente, Fitoterapia, 2014, 92, 274–279 CrossRef PubMed.
- F. Feng, X.-y. Xu, F.-l. Liu, W.-y. Liu and N. Xie, Zhongguo Tianran Yaowu, 2014, 12, 43–46 Search PubMed.
- M. J. Manase, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, S. Delemasure, P. Dutartre and M.-A. Lacaille-Dubois, Phytochemistry, 2014, 100, 150–155 CrossRef PubMed.
- M. Kuroda, T. Shizume and Y. Mimaki, Nat. Prod. Commun., 2014, 9, 379–382 Search PubMed.
- T. Murata, M. Nakano, T. Miyase and F. Yoshizaki, Chem. Pharm. Bull., 2014, 62, 608–612 CrossRef PubMed.
- X.-M. Zhang, D.-P. Yang, Z.-Y. Xie, X. Xue, L.-P. Zhu, D.-M. Wang and Z.-M. Zhao, Nat. Prod. Res., 2014, 28, 1058–1064 CrossRef PubMed.
- Q. Wu, G. Tu and H. Fu, J. Chin. Pharm. Sci., 2014, 23, 246–250 Search PubMed.
- G. Şenel, D. Gülcemal, M. Masullo, S. Piacente and T. Karayildirim, Chem. Biodiversity, 2014, 11, 408–418 CrossRef PubMed.
- W. Li and X. Li, Chem. Nat. Compd., 2014, 50, 100–102 CrossRef.
- X.-L. Zhou, C.-J. Xiao, L.-B. Wu, B. Huang, X. Dong and B. Jiang, J. Asian Nat. Prod. Res., 2014, 16, 555–564 CrossRef PubMed.
- M. Iqbal, M. Bilal, R. Iqbal, M. Akram, I. B. Baloch and M. K. Baloch, Journal of Chemistry, 2014, 396436 Search PubMed.
- R. F. Ngamgwe, R. Yankam, J. R. Chouna, C. Lanz, J. Furrer, S. Schurch, M. Kaiser, B. N. Lenta, S. Ngouela, E. Tsamo and R. Brenneisen, Iran. J. Pharm. Res., 2014, 13, 1425–1430 Search PubMed.
- C. Berto, F. Maggi, P. C. B. Nya, A. Pettena, I. Boschiero and S. Dall'Acqua, Nat. Prod. Commun., 2014, 9, 1691–1694 Search PubMed.
- M. Ye, J. Xiong, J.-J. Zhu, J.-L. Hong, Y. Zhao, H. Fan, G.-X. Yang, G. Xia and J.-F. Hu, J. Nat. Prod., 2014, 77, 178–182 CrossRef PubMed.
- R. L. M. Al Muqarrabun, N. Ahmat, S. R. S. Aris, N. Norizan, N. Shamsulrijal, F. Z. M. Yusof, M. N. Suratman, M. I. M. Yusof and F. Salim, Nat. Prod. Res., 2014, 28, 1003–1009 CrossRef PubMed.
- Q.-W. Tan, M.-A. Ouyang, Q.-J. Chen and Z.-J. Wu, Molecules, 2014, 19, 17619–17631 CrossRef PubMed.
- O. A. Omoyeni, M. Meyer, E. Iwuoha, I. Green and A. A. Hussein, Molecules, 2014, 19, 3389–3400 CrossRef PubMed.
- M. Zhao, N. Ma, F. Qiu, X. Tian, Y. Zhang, H. Tang and X. Liu, Fitoterapia, 2014, 97, 234–240 CrossRef PubMed.
- V. E. Rasamison, L. H. Rakotondraibe, C. Slebodnick, P. J. Brodie, M. Ratsimbason, K. TenDyke, Y. Shen, L. M. Randrianjanaka and D. G. I. Kingston, Org. Lett., 2014, 16, 2626–2629 CrossRef PubMed.
- T. Kikuchi, S. Ueda, J. Kanazawa, H. Naoe, T. Yamada and R. Tanaka, Molecules, 2014, 19, 4802–4813 CrossRef PubMed.
- N. M. Al Musayeib, R. A. Mothana, S. R. M. Ibrahim, A. A. El Gamal and S. M. Al-Massarani, Nat. Prod. Res., 2014, 28, 1142–1146 CrossRef PubMed.
- D.-b. Pu, Y. Gao, R.-t. Li and H.-z. Li, Bopuxue Zazhi, 2014, 31, 437–447 Search PubMed.
- D. Chen, X. Cheng, Y. Sun and P. Liu, Chem. Nat. Compd., 2014, 50, 93–96 CrossRef.
- W.-H. Tang, S.-T. Bai, L. Tong, W.-J. Duan, J.-W. Su, J.-X. Chen and Y. Xie, Biochem. Syst. Ecol., 2014, 54, 78–82 CrossRef.
- M. González-Cortazar, M. Herrera-Ruiz, A. Zamilpa, E. Jiménez-Ferrer, S. Marquina, L. Álvarez and J. Tortoriello, Planta Med., 2014, 80, 90–96 Search PubMed.
- G. Faria de Sousa, D. C. F. Soares, W. d. N. Mussel, N. F. E. Pompeu, G. D. d. F. Silva, S. A. Vieira Filho and L. P. Duarte, J. Braz. Chem. Soc., 2014, 25, 1338–1345 Search PubMed.
- S. Wittayalai, C. Mahidol, V. Prachyawarakorn, H. Prawat and S. Ruchirawat, Phytochemistry, 2014, 99, 121–126 CrossRef PubMed.
- J. A. R. Salvador, R. C. Santos, S. A. C. Figueiredo and Y. Jing, Mini-Rev. Org. Chem., 2014, 11, 400–407 CrossRef.
- Y.-l. Sun and H. Luo, Yixue Zongshu, 2014, 20, 656–658 Search PubMed.
- L.-l. Zang, B.-n. Wu, Y. Lin, J. Wang, L. Fu and Z.-y. Tang, Chin. J. Integr. Med., 2014, 20, 72–79 CrossRef PubMed.
- Q.-W. Tan, M.-A. Ouyang and B. Gao, Molecules, 2014, 19, 4897–4906 CrossRef PubMed.
- W.-L. Wang, X. Zhou, Y.-L. Liu, Q.-M. Xu, X.-R. Li and S.-L. Yang, J. Asian Nat. Prod. Res., 2014, 16, 175–180 CrossRef PubMed.
- R. Hossain, R. Sultana, A. Adhikari, M. I. Choudhary, Y. Ali and S. Zaman, Nat. Prod. Commun., 2014, 9, 371–372 Search PubMed.
- X.-P. Wu, X.-P. Zhang, G.-X. Ma, M. Han, M.-D. Xu, H.-F. Wu, X.-Y. Huang, Z. Huang, J.-S. Yang, J.-Q. Yuan, X.-D. Xu and X.-M. Zhong, J. Asian Nat. Prod. Res., 2014, 16, 422–425 CrossRef PubMed.
- L. P. Sandjo, C. G. Fru, V. Kuete, F. Nana, S. O. Yeboah, R. Mapitse, B. M. Abegaz, T. Efferth, T. Opatz and B. T. Ngadjui, Z. Naturforsch., C: J. Biosci., 2014, 69, 276–282 Search PubMed.
- P. Srivastava, Jyotshna, N. Gupta, A. K. Maurya and K. Shanker, Nat. Prod. Res., 2014, 28, 306–311 CrossRef PubMed.
- P. Rudrapaul, N. Das, U. C. De and B. Dinda, Phytochem. Lett., 2014, 9, 7–10 CrossRef.
- X.-f. Cao, J.-s. Wang, P.-r. Wang and L.-y. Kong, Zhongguo Tianran Yaowu, 2014, 12, 628–631 Search PubMed.
- Q.-Q. He, L. Yang, J.-Y. Zhang, J.-N. Ma and C.-M. Ma, J. Food Sci., 2014, 79, C1970–C1983 CrossRef PubMed.
- H.-P. Yang, S. Que, Y.-P. Shi and L.-T. Ban, J. Chem. Pharm. Res., 2014, 6, 1986–1990 Search PubMed.
- A. Qiao, Y. Wang, L. Xiang, Z. Zhang and X. He, Fitoterapia, 2014, 98, 137–142 CrossRef PubMed.
- C. Wu, Y.-H. Duan, W. Tang, M.-M. Li, X. Wu, G.-C. Wang, W.-C. Ye, G.-X. Zhou and Y.-L. Li, Fitoterapia, 2014, 92, 127–132 CrossRef PubMed.
- M. I. Anjum, E. Ahmed, A. Sharif, A. Jabbar, A. Malik, T. Hussain, Z. H. Farooqi and A. Nawaz, Asian J. Chem., 2014, 26, 7386–7388 Search PubMed.
- M. N. Samy, S. Sugimoto, K. Matsunami, H. Otsuka and M. S. Kamel, Phytochem. Lett., 2014, 10, 86–90 CrossRef.
- L. Li, L.-S. Feng and Y.-X. He, J. Asian Nat. Prod. Res., 2014, 16, 830–835 CrossRef PubMed.
- S. A. Sasmakov, Z. M. Putieva and U. Lindequist, Pharmazie, 2007, 62, 957–959 Search PubMed.
- Y. Liu, L. Cheng, Q.-q. He, J.-h. Yeon and D.-y. Kong, Zhongcaoyao, 2014, 45, 2742–2747 Search PubMed.
- H. K. Cho, C. S. Kim, K. W. Woo and K. R. Lee, Bull. Korean Chem. Soc., 2014, 35, 1553–1555 CrossRef.
- S. S. Khan, A. Khan, A. Khan, U. Farooq, A. Ahmed, A. Zahoor, V. Uddin Ahmad, B. Sener and N. Kucukboyaci, Rec. Nat. Prod., 2014, 8, 354–359 Search PubMed.
- Y.-x. Ding, T.-y. Wang, Y.-w. Zhang, Y.-m. Huang, L. Ma, D.-d. Li, D.-q. Dou and Q. Li, Zhongguo Zhongyao Zazhi, 2014, 39, 4225–4229 Search PubMed.
- W. Wang, J. Zhao, S. Li, Y. Lu, Y. Liu, Q. Xu, X. Li, I. A. Khan and S. Yang, Fitoterapia, 2014, 99, 40–47 CrossRef PubMed.
- S. Li, J. Zhao, Y. Liu, Z. Chen, Q. Xu, I. A. Khan and S. Yang, J. Agric. Food Chem., 2014, 62, 488–496 CrossRef PubMed.
- J. Chen, C.-l. Fan, Y. Wang and W.-c. Ye, Zhongguo Tianran Yaowu, 2014, 12, 218–221 Search PubMed.
- J. Mi, C. Wu, C. Li, F. Xi, Z. Wu and W. Chen, Nat. Prod. Res., 2014, 28, 52–56 CrossRef PubMed.
- Y. Wu, Y. Yang, M. Dong, F. Sauriol, Q. Shi, Y. Gu and C. Huo, Chem. Nat. Compd., 2014, 50, 850–852 CrossRef.
- Q.-H. Wang, N.-R.-C.-K.-T. Han, N.-Y.-T. Dai, X.-L. Wu, W.-Q. Tai, J.-S. Wu and R.-J. Wu, Z. Naturforsch., B: J. Chem. Sci., 2014, 69, 1021–1025 Search PubMed.
- C. Liu, Z.-X. Liao, S.-J. Liu, L.-J. Ji and H.-F. Sun, Planta Med., 2014, 80, 936–941 CrossRef PubMed.
- W. Liu, E. Sakr, P. Schaeffer, H. M. Talbot, J. Donisi, T. Härtner, E. Kannenberg, E. Takano and M. Rohmer, ChemBioChem, 2014, 15, 2156–2161 CrossRef PubMed.
- D. Khan, M. Afzal, S. Woodward and S. Khan, Rec. Nat. Prod., 2014, 8, 121–127 Search PubMed.
- A. I. Hamed, M. Masullo, L. Pecio, D. Gallotta, U. A. Mahalel, S. Pawelec, A. Stochmal and S. Piacente, J. Nat. Prod., 2014, 77, 657–662 CrossRef PubMed.
- S. Sharma, S. K. Chattopadhyay, M. Singh, D. U. Bawankule and S. Kumar, Phytochemistry, 2014, 100, 132–140 CrossRef PubMed.
- Y. Zhang, P. Yi, Y. Chen, Z.-n. Mei, X. Hu and G.-z. Yang, Fitoterapia, 2014, 96, 95–102 CrossRef PubMed.
- X. Wang, D. Yu and S. Yu, Chin. J. Chem., 2014, 32, 1007–1010 CrossRef.
- S. Yu, X. Ye, W. Xin, K. Xu, X.-Y. Lian and Z. Zhang, Planta Med., 2014, 80, 315–320 CrossRef PubMed.
- W.-B. Zhou, G.-Z. Zeng, H.-M. Xu, W.-J. He, Y.-M. Zhang and N.-H. Tan, Fitoterapia, 2014, 93, 98–104 CrossRef PubMed.
- D.-J. Jin, S.-A. Tang, G.-S. Xing, W.-J. Zhao, C. Zhao, H.-Q. Duan and W.-H. Lin, J. Asian Nat. Prod. Res., 2014, 16, 427–433 CrossRef PubMed.
- C. S. Kim, O. W. Kwon, S. Y. Kim, K. H. Kim and K. R. Lee, Heterocycles, 2014, 89, 1913–1922 CrossRef.
- O. Potterat, C. Herzog, M. Raith, S. N. Ebrahimi and M. Hamburger, Helv. Chim. Acta, 2014, 97, 32–38 CrossRef.
- C.-L. Zhang, Y. Wang, Y.-F. Liu, G. Ni, D. Liang, H. Luo, X.-Y. Song, W.-Q. Zhang, R.-Y. Chen, N.-H. Chen and D.-Q. Yu, J. Nat. Prod., 2014, 77, 411–415 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |