Cyclopentane-forming di/sesterterpene synthases: widely distributed enzymes in bacteria, fungi, and plants
Abstract
Covering: 2000 to 2018
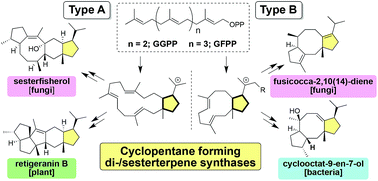
In the late 1960s, structurally unique fusicoccane- and ophiobolane-type di/sesterterpenes were isolated and their homologs were found to be widely distributed in various organisms. Nearly a half century later, the first terpene synthase PaFS was identified, which triggered the discovery of a number of di/sesterterpene synthases, which were named as cyclopentane-forming terpene synthases (CPF-TSs). In the past 10 years, CPF-TSs have emerged as a new type of class I terpene synthases, which afford di/sesterterpenes with characteristic polycyclic molecular skeletons; they catalyze two different types of cyclizations, defined as type A and B, which are relevant to the initial cyclization mode of a polyprenyl diphosphate. This review summarizes the characteristic features of CPF-TSs from various sources and detailed cyclization mechanisms; we have also discussed the structural diversification strategy of these novel enzymes.


 Please wait while we load your content...
Please wait while we load your content...