 Open Access Article
Open Access ArticleDiverse chemistry of the dianion [closo-B9H9]2−: synthesis and reactivity of its mono-anionic derivative [arachno-B9H12-4,8-Cl2]−†
Florian
Schlüter
a,
Eduard
Bernhardt
 *a and
Konstantin
Zhizhin
b
*a and
Konstantin
Zhizhin
b
aFakultät für Mathematik und Naturwissenschaften, Anorganische Chemie, Bergische Universität Wuppertal, Gaußstr. 20, 42119 Wuppertal, Germany. E-mail: edbern@uni-wuppertal.de; Fax: +49 202 439 3503
bKurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow 119991, Russia. E-mail: zhizhin@igic.ras.ru
First published on 23rd January 2018
Abstract
Attempted protonation of the dianion [closo-B9H9]2− under moisture-free conditions did not afford its mono-protonated form [closo-B9H10]−. The reaction of the former closo-borate with CH3COOH in dichloromethane yielded a monoanionic product [B2O(MeCO2)5]−. The treatment of [closo-B9H9]2− with HCl in dichloromethane afforded its arachno-derivative [arachno-B9H12-4,8-Cl2]− in a high yield. The experimental solution and quantum-chemically calculated 11B and 1H NMR spectra of the latter monoanion were found to be in a good agreement; its structure in the solid state was studied by the single crystal X-ray diffraction experiment for the crystal (PPh4)[arachno-B9H12-4,8-Cl2]·0.04HCl. The reaction of [arachno-B9H12-4,8-Cl2]− with liquid ammonia caused its quantitative conversion into the parent [closo-B9H9]2−.
Borates [closo-BnHn]2− (n > 4) are fundamental building blocks of the boron cluster chemistry (see for example1–4) and these borates with n = 6–12 were synthesized in 1959–1967.5–9 [closo-BnHn]2− have three-dimensional aromaticity, while the organic aromatic compounds have two-dimensional aromaticity10,11 (and literature cited therein). Among the [closo-BnHn]2− (n = 6–12), those with n = 7–9 are the least explored.
Borates with an arachno nine-vertex are described in literature since 1960.12–16 The existence of the monoanion [arachno-B9H14]− has been postulated by Lipscomb,1 while the first X-ray structural characterization in the form of its cesium(I) salt has been performed by Greenwood et al.;4,5 two possible conformations of this monoanion (a and b) are shown in Scheme 1.
 | ||
| Scheme 1 Known nine-vertex conformations of the monoanion arachno-B9H14− (a and b) and that of nido-B10H14 (c).17 | ||
Its i-configuration appears to be a deviation from Williams theory. The Williams theory postulates that for the generation of the arachno-boranes or carboranes from the nido-clusters, a vertex with the highest connectivity should be removed.18,19 The examples of the n-configuration of the monoanion [arachno-B9H14]− (in contrast to those of its i-configuration) are very rarely reported in literature. They include the dianion [n-B9H15]2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 as well as the organometallic and coordination compounds, such as [η6-(C6Me6)RuB8H14],20 [(dppe)Pt2B7H11]21 and [(PMe2Ph)2PtB7H10NHEt].14 The structure with i-geometry, on the other hand, is obtained by removing a corner with a lower connectivity of nido-B10H14 (Scheme 1c). Such geometry has been however observed for numerous members of the borates, including the dianion [i-B9H15]2−
11 as well as the organometallic and coordination compounds, such as [η6-(C6Me6)RuB8H14],20 [(dppe)Pt2B7H11]21 and [(PMe2Ph)2PtB7H10NHEt].14 The structure with i-geometry, on the other hand, is obtained by removing a corner with a lower connectivity of nido-B10H14 (Scheme 1c). Such geometry has been however observed for numerous members of the borates, including the dianion [i-B9H15]2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and a series of neutral coordination compounds [arachno-B9H13L].15 The borate nido-B10H14 has been also assigned to this geometry (see ref. 17 and references therein). The n-configuration has been observed17 only for one of its halogen-containing derivative, viz., [arachno-B9H12-4,8-Br2]−, which is a by-product of the synthesis of anti-B18H22 by the reaction of [nido-B9H12]− with HgBr2 in dichloromethane.17,22 In the present communication, we describe the products of the reactions of [closo-B9H9]2− with various acids and the reactivity of these products.
11 and a series of neutral coordination compounds [arachno-B9H13L].15 The borate nido-B10H14 has been also assigned to this geometry (see ref. 17 and references therein). The n-configuration has been observed17 only for one of its halogen-containing derivative, viz., [arachno-B9H12-4,8-Br2]−, which is a by-product of the synthesis of anti-B18H22 by the reaction of [nido-B9H12]− with HgBr2 in dichloromethane.17,22 In the present communication, we describe the products of the reactions of [closo-B9H9]2− with various acids and the reactivity of these products.
In the first stage of our investigation, we attempted to protonate [closo-B9H9]2− with acetic acid under anhydrous conditions. Dry acetic acid was added to the red-orange solution of (PPh4)2[closo-B9H9] in dichloromethane and then, the reaction mixture was left under pentane atmosphere for 3 days. This reaction resulted in the complete destruction of a polyhedral boron cluster, thus affording the monoanion [B2O(MeCO2)5]− as a major product, which crystallizes as (PPh4)[B2O(MeCO2)5] in colorless crystals.23 The heteroleptic products [B2O(MeCO2)5]− and B2O(MeCO2)4 were already detected spectroscopically few years ago.24–27 It should be noted that the homoleptic compounds [B(MeCO2)4]− and B(MeCO2)3 were not described in literature till date.
We also studied the reaction of (PPh4)2[closo-B9H9] with HCl in dichloromethane. Indeed, the bromine-containing compound [arachno-B9H12-4,8-Br2]− has been prepared6 using the reaction of the [nido-B9H12]− with HgBr2. The treatment of (PPh4)2[closo-B9H9] with purified gaseous HCl (the oxygen impurities were removed using three condensation–argon saturation cycles) in dichloromethane at −78 °C (the latter makes it possible to remove the remaining moisture impurities) under inert atmosphere afforded the chlorine-containing monoanion [arachno-B9H12-4,8-Cl2]− as per the following eqn (1):
| (PPh4)2[closo-B9H9] + 3HCl → (PPh4)[arachno-B9H12-4,8-Cl2] + (PPh4)Cl | (1) |
The calculated structure of this monoanion with a labeling scheme of its atoms is shown in Fig. 1.
 | ||
| Fig. 1 Labeling scheme of the atoms in the anion [arachno-B9H12-4,8-Cl2]− and its quantum-chemically calculated structure. | ||
The initial red-orange solution of (PPh4)2[closo-B9H9] in dichloromethane underwent an immediate decolouration after the addition of HCl. The resultant reaction mixture was carefully mixed with a five-fold volume of pentane and colorless crystals were formed after 3 days with high yield (83%).
The reaction of a suspension of (PPh4)[arachno-B9H12-4,8-Cl2] with liquid ammonia at r.t.19 resulted in the re-closing of this arachno-compound into the initial [closo-B9H9]2−: after 10 min, the initially colorless reaction mixture turned yellow, following which NH3 escaped from the mixture and the intensity of its color increased, thus indicating the formation of the closo-borate dianion according to eqn (2):
| 2(PPh4)[arachno-B9H12-4,8-Cl2] + 6NH3 → (PPh4)2[closo-B9H9] + (NH4)2[closo-B9H9] + 4NH4Cl | (2) |
The yield of the orange solid product, based on its NMR spectra, was quantitative.
The initial closo-borate compound and the products of its transformations were characterized using multinuclear NMR spectroscopy and by the single crystal X-ray diffraction study as; their 11B NMR spectra are shown in Fig. 2 and 3.
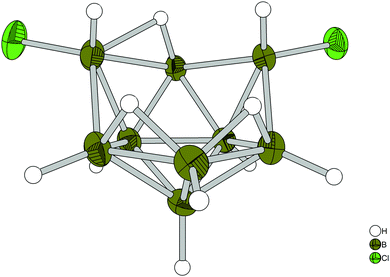 | ||
| Fig. 3 General view of the monoanion [arachno-B9H12-4,8-Cl2]− in the crystal (PPh4)[arachno-B9H12-4,8-Cl2]. Thermal ellipsoids are shown with 50% probability. | ||
In the spectrum b of new compound (PPh4)[arachno-B9H12-4,8-Cl2], the signals of its 11B nuclei split in the integral ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; the two chlorine-substituted boron atoms have a singlet character. An assignment of the above signals was made using the theoretically calculated 11B NMR values, which are also collected in Table 1.
1; the two chlorine-substituted boron atoms have a singlet character. An assignment of the above signals was made using the theoretically calculated 11B NMR values, which are also collected in Table 1.
| Assignment | δ 11B (ppm) | 1 J 11B–1H (Hz) | δ 1H (ppm) |
|---|---|---|---|
| a In parentheses: the calculated NMR parameters; 11B NMR data relative to BF3·OEt2 with δ11B = 0 ppm; δ11B = 101.63 − σ11B; 1H NMR data relative to Me4Si with δ1H = 0 ppm; δ1H = 31.97 − σ1H. b The averaged calculated δ11B. c The averaged calculated δ1H for the atoms H2, H4 and H8. | |||
| B5, B7 | 2.2 (−1.3)ab | 149 | 3.05 (2.53)bc |
| B6 | −4.9 (−14.5) | 153 | 2.86 (2.14) |
| B4, B8 | −8.6 (−10.0)b | −0.41 (1.13)bc | |
| B1, B3 | −10.9 (−17.3)b | 142 | 2.04 (1.58) b |
| B9 | −31.4 (−35.5) | 145 | 0.82 (0.32) |
| B2 | −49.1 (−56.3) | 152 | −0.41 (1.13)c |
| H10 | (−2.35) | ||
| H11 | (−3.20) | ||
| H12 | (−4.22) | ||
The bridging hydrogen atoms of [arachno-B9H12-4,8-Cl2]− were not detected at room temperature using 1H NMR method due to substantial broadening (due to the dynamic behavior)of the peaks. The same effect is also observed6 in its bromine-containing analog [arachno-B9H12-4,8-Br2]−. The calculated values of δ11B for the boron-based framework of [arachno-B9H12-4,8-Cl2]− were found to be in a good agreement with those experimentally observed (except that for the atom B6).
The colorless single crystals of the salt (PPh4)[arachno-B9H12-4,8-Cl2] were also characterized using the single crystal X-ray diffraction method;21 the molecular structure of its arachno-anion is shown in Fig. 3.
This structure is very similar to that of the parent anion [arachno-B9H14]−, having the same arrangement of both the backbone and the bridging protons. Two of the residual electron peaks observed in this spectrum were assigned to the HCl molecule with about 4% occupancy. In addition, peaks attributed to 3 μ-H and 2 endo-H could be found. The bridging proton H10 was evidenced to be deviated from the centrum of the corresponding boron–boron bond, while the same was not observed for the two other bridging protons. The bond distance B9–H10 (1.126 (12) Å) is smaller than that of B4–H10 (1.508 (13) Å). The quantum-chemical B3LYP/6-311G calculations20 showed that such arrangement of the bridging hydrogen atoms in the crystal (PPh4)[arachno-B9H12-4,8-Cl2] is energetically preferable by 5.83 kJ mol−1 as compared with its Cs-symmetric arrangement (Fig. 4).
 | ||
| Fig. 4 Quantum-chemically calculated structure of the monoanion [arachno-B9H12-4,8-Cl2]− having Cs symmetric arrangement. | ||
Such calculated Cs-symmetric structure contains two μ-H and three endo-H atoms and it was not experimentally detected using the NMR method.
Thus, the dianion [closo-B9H9]2− was found to react with gaseous HCl in dichloromethane under anhydrous conditions, affording its halogen-containing derivative [arachno-B9H12-4,8-Cl2]−, which undergoes re-closing with NH3 to form an initial closo-borate dianion.
Conflicts of interest
There are no conflicts to declare.References
- W. Preetz and G. Peters, Eur. J. Inorg. Chem., 1999, 1831–1846 CrossRef CAS.
- I. B. Sivaev, A. V. Prikaznov and D. Naoufal, Collect. Czech. Chem. Commun., 2010, 75, 1149–1199 CrossRef CAS.
- O. Volkov and P. Paetzold, J. Organomet. Chem., 2003, 680, 301–311 CrossRef CAS.
- I. B. Sivaev, V. I. Bregadse and S. Sjöberg, Collect. Czech. Chem. Commun., 2002, 67, 679–727 CrossRef CAS.
- M. F. Hawthorne and A. R. Pitochelli, J. Am. Chem. Soc., 1959, 81, 5519 CrossRef CAS.
- A. R. Pitochelli and M. F. Hawthorne, J. Am. Chem. Soc., 1960, 82, 3228–3229 CrossRef CAS.
- J. L. Boone, J. Am. Chem. Soc., 1964, 86, 5036 CrossRef CAS.
- F. Klanberg and E. L. Muetterties, Inorg. Chem., 1966, 5, 1955–1960 CrossRef CAS.
- F. Klanberg, D. R. Eaton, L. J. Guggenberger and E. L. Muetterties, Inorg. Chem., 1967, 6, 1271–1281 CrossRef CAS.
- J. Poater, M. Sola, C. Vinas and F. Teixidor, Angew. Chem., 2014, 126, 12387–12391 ( Angew. Chem., Int. Ed. , 2014 , 53 , 12191–12195 ) CrossRef.
- J. Poater, M. Sola, C. Vinas and F. Teixidor, Chem. – Eur. J., 2016, 22, 7437–7443 CrossRef CAS PubMed.
- F. E. Wang, P. G. Simpson and W. N. Lipscomb, J. Am. Chem. Soc., 1961, 83, 491–492 CrossRef CAS.
- F. E. Wang, P. G. Simpson and W. N. Lipscomb, J. Chem. Phys., 1961, 35, 1335–1339 CrossRef CAS.
- L. E. Benjamin, S. F. Stafiej and E. A. Takacs, J. Am. Chem. Soc., 1963, 85, 2674–2675 CrossRef CAS.
- N. N. Greenwood, H. J. Gysling, J. A. McGinnety and J. D. Owen, J. Chem. Soc., Chem. Commun., 1970, 505–506 RSC.
- N. N. Greenwood, H. J. Gysling, J. A. McGinnety and J. D. Owen, J. Chem. Soc., Dalton Trans., 1972, 986–989 RSC.
- J. Bould, R. Greatrex, J. D. Kennedy, D. L. Ormsby, M. G. S. Londesborough, K. L. F. Callaghan, M. Thornton-Pett, T. R. Spalding, S. J. Teat, W. Clegg, H. Fang, N. P. Rath and L. Barton, J. Am. Chem. Soc., 2002, 124, 7429–7439 CrossRef CAS PubMed.
- R. E. Williams, Inorg. Chem., 1971, 10, 210–214 CrossRef CAS.
- R. E. Williams, Adv. Inorg. Chem. Radiochem., 1976, 18, 67–142 CrossRef CAS.
- M. Bown, X. L. R. Fontaine, N. N. Greenwood, J. D. Kennedy and M. Thornton-Pett, J. Organomet. Chem., 1986, 315, C1–C4 CrossRef CAS.
- R. Macias, N. P. Rath and L. Barton, J. Chem. Soc., Chem. Commun., 1998, 1081–1082 RSC.
- D. F. Gaines, C. K. Nelson and G. A. Steehler, J. Am. Chem. Soc., 1984, 106, 7266–7267 CrossRef CAS.
- F. Schlüter, Die Chemie der closo-Borate [BnHn]2− (n = 6–9, 11) und [B21H18]−, Bergische Universität Wuppertal, Wuppertal, 2012, p. 194.
- R. G. Hayter, A. W. Laubengayer and P. G. Thompson, J. Am. Chem. Soc., 1957, 79, 4243–4244 CrossRef CAS.
- H.-A. Lehmann, G. Kessler, P. Ganecke and G. Nickl, Z. Anorg. Allg. Chem., 1965, 340, 16–22 CrossRef CAS.
- N. W. Alcock, V. M. Tracy and T. C. Waddington, J. Chem. Soc., Dalton Trans., 1976, 2238–2242 RSC.
- N. W. Alcock, V. M. Tracy and T. C. Waddington, J. Chem. Soc., Dalton Trans., 1976, 2243–2246 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1569828. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7nj04251e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |

