Review on nanoscale Bi-based photocatalysts
Rongan
He
 ab,
Difa
Xu
ab,
Difa
Xu
 ab,
Bei
Cheng
ab,
Bei
Cheng
 a,
Jiaguo
Yu
a,
Jiaguo
Yu
 *a and
Wingkei
Ho
*a and
Wingkei
Ho
 *c
*c
aState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China. E-mail: jiaguoyu@yahoo.com
bHunan Province Key Laboratory of Applied Environmental Photocatalysis, Changsha University, Changsha 410022, China
cDepartment of Science and Environmental Studies, The Education University of Hong Kong, Tai Po, N. T. Hong Kong, P. R. China. E-mail: keithho@ied.edu.hk
First published on 9th May 2018
Abstract
Nanoscale Bi-based photocatalysts are promising candidates for visible-light-driven photocatalytic environmental remediation and energy conversion. However, the performance of bulk bismuthal semiconductors is unsatisfactory. Increasing efforts have been focused on enhancing the performance of this photocatalyst family. Many studies have reported on component adjustment, morphology control, heterojunction construction, and surface modification. Herein, recent topics in these fields, including doping, changing stoichiometry, solid solutions, ultrathin nanosheets, hierarchical and hollow architectures, conventional heterojunctions, direct Z-scheme junctions, and surface modification of conductive materials and semiconductors, are reviewed. The progress in the enhancement mechanism involving light absorption, band structure tailoring, and separation and utilization of excited carriers, is also introduced. The challenges and tendencies in the studies of nanoscale Bi-based photocatalysts are discussed and summarized.
Conceptual insightsPhotocatalytic technology is effective for environmental remediation and energy conversion. Nano Bi-based photocatalysts are an important family of visible-light-excitable photocatalysts. Therefore, enhancing the activity of these photocatalysts by adjusting their nanostructure is one of the hot topics in photocatalysis. In this paper, we have reviewed the recent development on the improvement of the performance of nanoscale Bi-based photocatalysts to provide general and new insights into this family. |
Introduction
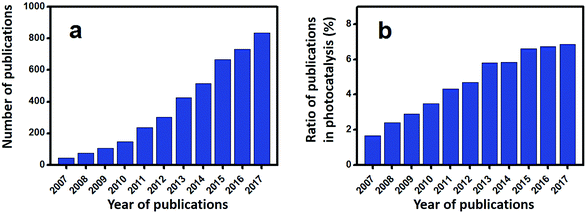
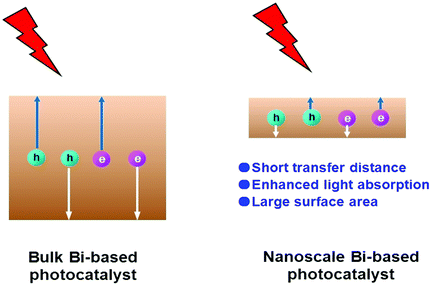
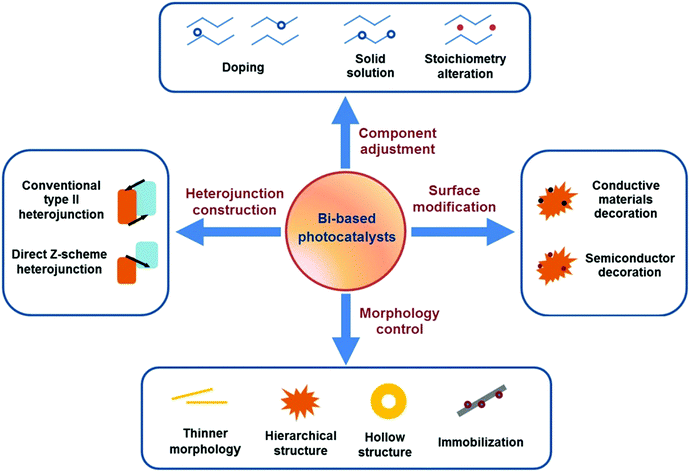
Escalating serious environment and energy crises have resulted in a growing demand for effective environmental remediation and energy conversion techniques. These needs can be addressed using photocatalysis, which is an increasingly developed photochemical strategy. In a photocatalytic procedure, electrons and holes are generated from semiconductors under light irradiation and participate in reduction and oxidization reactions, respectively. Thus, photocatalysis promotes redox reactions, such as pollutant degradation, water oxidization, H2 evolution, CO2 reduction and N2 fixation.1–19 Consequently, photocatalysis is regarded as a green technique for removing organic pollutants and producing fuel or electricity. Given that visible-light energy constitutes about 43% of solar energy, visible-light-responsive photocatalysts are preferred in photocatalysis and photoelectrocatalysis.Up to now, many types of semiconductors, including metal oxides (TiO2, ZnO, Ag2O, Fe2O3, Cu2O, Ta2O5), metal sulfides (ZnS, CdS, MoS2, Bi2S3), multi-component oxides (Bi2WO6, InTaO4, BiVO4, Ag3VO4, SrTiO3), metal selenides (MoSe2, CdSe), metal phosphides (Ni2P), metal phosphates (Ag3PO4), metal halides and oxyhalides (AgBr, BiOBr) and metal-free materials (SiC, g-C3N4, and Si), have been employed as photocatalysts.3,4,20–24 Among them, those that possess a band gap of Eg > 3 eV are called wide-band-gap photocatalysts, such as TiO2, ZnO, ZnS, SrTiO3, and KTaO3. In contrast, those with a band gap of Eg ≤ 3 eV are called visible-light-responsive photocatalysts, such as Ag2O, Bi2WO6, InTaO4, CoO, BiVO4, Fe2O3, Cu2O, Ag3VO4, TaON, CdS, Ta3N5, CdSe, Bi2S3, SiC, g-C3N4, and Si. Bi-Based photocatalysts are important visible-light-responsive photocatalysts and have recently attracted rapidly increasing attention, shown not only from the number of publications, but also from the ratio of publications in the field of photocatalysis (see Fig. 1a and b). Considering the stability of Bi3+, most studies have focused on Bi3+-containing compounds, such as Bi2O3,25 BiVO4,26 Bi4Ti3O12,27 Bi12TiO20,28 Bi2O2CO3,29 Bi2WO6,30 BiPO4,31 BiFeO3,32 BiOX (X = Cl, Br, I),33 Bi3TiNbO9,34 and Bi0.5K0.5TiO3.35 The majority of these compounds possess a layered structure and plate-like appearance. Bi5+-Containing compounds, such as LiBiO3, NaBiO3, and KBiO3, can also be excited by visible light. However, such types are less reported than the others because of the instability of Bi5+. In Bi(III) compounds, the hybridized O 2p and Bi 6s2 orbitals may cause an upshift of the valence bands (VBs).33 Therefore, the band gaps of Bi compounds are usually smaller than 3.0 eV and can be excited by visible light. Bi-based photocatalysts are regarded as promising candidates for removing toxins from water and air3,36–38 as well as for the production of fuel.2,39–41 However, the photocatalytic performance of bulk Bi-based semiconductors is not as high as those of nanoscale Bi-based photocatalysts, because the photogenerated electrons and holes of these materials have not been easily exploited and utilized. Also, bulk photocatalysts have weaker light absorption and smaller surface areas than nanoscale photocatalysts (see Fig. 2). Numerous attempts have been made to enhance bulk Bi-based semiconductors to achieve ideal photocatalytic activity. These studies have focused on nanoscale component adjustment, morphology control, heterojunction construction, and surface modification2,37,38,42 (Fig. 3).
Component adjustment
The band structure parameters, such as VB, conduction band (CB), and band gap (Eg), are crucial to the photocatalyst activity. Furthermore, component adjustments, such as doping, solid-solution preparation and stoichiometry alteration, are effective methods that can be used to tune the band structures of bismuthal semiconductors. Therefore, suitable component adjustment is favorable for bismuthal photocatalysts.Doping
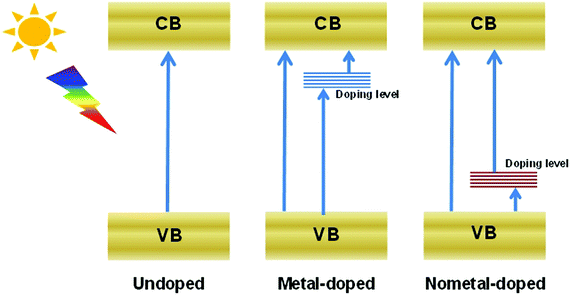
Heteroatom doping is widely adopted to increase the visible-light absorption of photocatalysts, because this process can generate a doping level between the CB and VB (Fig. 4). Consequently, the energy required to excite electrons decreases, and the light response of semiconductors increases.43–47 Doping can also improve the charge transmission properties of semiconductors and lead to the augmented transfer efficiency of carriers. Doping is commonly employed to enhance the activity of Bi-based semiconductors.Many bismuthal compounds are visible-light responsive. Thus, doping has been performed more frequently to enhance the visible-light absorption of bismuthal compounds with a wider band-gap, such as in Bi2O2CO3,48 BiPO4,49 BiOCl,50 and Bi3TiNbO9.34 For example, the band gap of Bi3TiNbO9 can be reduced from 3.1 eV to 2.6 eV when it is doped with a 10% molar ratio of Ni2+.34 Zheng et al.51 found that the band gap of Bi2WO6 can be narrowed by doping a small amount of Br with the surfactant cetyltrimethylammonium bromide (CTAB) during preparation. Doping by up to approximately 3% can also narrow the band gap of Bi2MoO6 from 2.96 eV to 2.69 eV and consequently improve the performance of Bi2MoO6 in the photocatalytic degradation of Rhodamine B (RhB).52 Zhang et al.53 studied the effects of doping on the electronic structures and optical properties of BiOCl using first-principle calculations. Studies have also proposed that codoping with Sb and I can substantially narrow the band gaps and increase the light absorption of BiOCl because of the difference in the electronegativities between the Sb/I and Bi/Cl atoms.
Doping can further narrow the band gap of visible-light-responsive bismuthal compounds.54,55 However, the improvement resulting from a narrowed band gap is limited, because visible light response is not the main barrier to the photocatalytic performance of bismuthal compounds. Low utilization efficiency of the generated carriers is the primary issue to be solved for these bismuthal photocatalysts. In this case, doping can also enhance the photocatalyst activity if the dopant can reduce impedance or improve charge separation. For instance, F-doped β-Bi2O3 has been shown to exhibit higher photocatalytic activity than that of pure β-Bi2O3 in the degradation of methyl orange (MO).56 This can be attributed to the higher separation efficiency of electron–hole pairs and lower VB position (indicating stronger oxidation capacity). B-Doping can increase the photocatalytic activity of BiOBr on the inactivity of Escherichia coli, which is attributed to the role of B as the electron acceptor for the effective separation of electrons and holes.57 Such a role may have been caused by the empty p-orbital and unpaired electrons in B. Br doping could reduce the effective mass of electrons in Bi2WO6, by which the transfer and separation of the carrier can be facilitated.58 Aurivillius-type Ce-doped SrBi2Ta2O9 fabricated by Senthil and co-workers59 exhibited a higher photocatalytic activity than SrBi2Ta2O9 for H2 generation. The improvement comes from the enhanced separation of electrons and holes through a dipole moment derived from the bonding of Ce4+ with the surrounding oxygen atoms. Zhang and colleagues60 found that carbon doping increases the internal electric field by 126-fold as a result of the enlarged differences in the electrostatic potentials between the [Bi3O4] and [Cl] layers induced by the carbon doping (Fig. 5). Consequently, the separation of holes and electrons was improved. Er-Doped Bi24O31Br10,61 carbon-doped Bi2WO6,62 Mo-doped BiVO4,63 and Er/Yb codoped BiVO464 also showed enhanced photocatalytic activities relative to those of the original bismuthal compounds due to increased carrier transfer and separation.
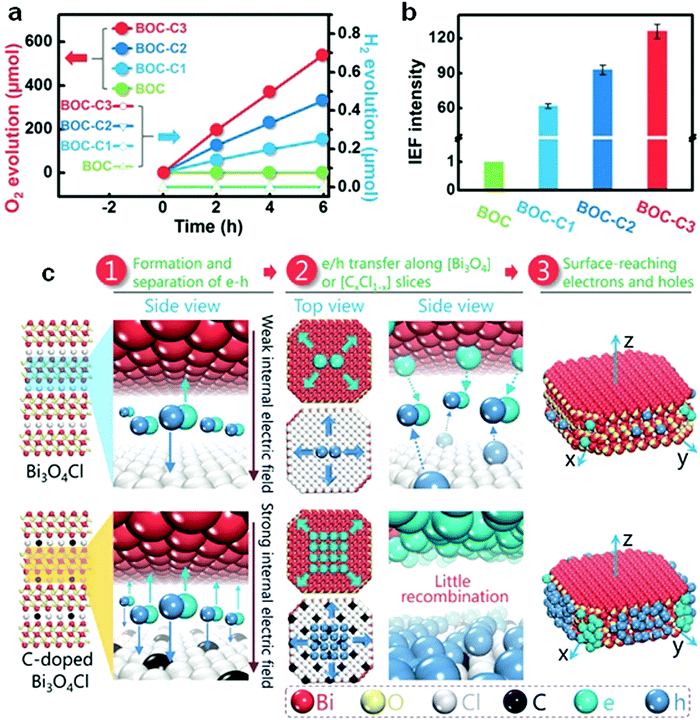 | ||
| Fig. 5 (a) The performance of visible-light-driven oxygen and hydrogen evolution; (b) internal electric field intensity of C-doped Bi3O4Cl (assuming the intensity of BOC to be “1”); and (c) schematic diagram of the carrier migration of pure and C-doped Bi3O4Cl; BOC-C1, BOC-C2, and BOC-C3 present C-doped Bi3O4Cl with carbon concentrations of 0.92%, 1.86%, and 3.16%, respectively. Reproduced with permission from ref. 60. Copyright 2016 John Wiley and Sons. | ||
Anion and mixed-valence cation dopants can also improve the photocatalytic performance of bismuthal semiconductors by facilitating the transfer and separation of carriers. For instance, doping with IO3− can increase the activity of BiOI in the photocatalytic degradation of MO because of the key role played by IO3− in improving the transfer efficiency of the carriers.65 In-Doped BiOI exhibited augmented activity in the photocatalytic degradation of p-chloroaniline, because the introduced In resulted in the formation of a doping energy level that acted as a scavenger of photo-induced electrons and consequently prevented the recombination of charges.66 Jiang et al.67 fabricated Sn4+-doped Bi2S3 through a solvothermal method, in which Sn4+ acted as an electron scavenger by interconversion between Sn4+ and Sn2+ and facilitated O2 reduction. Consequently, the photocatalytic performance of Sn4+-doped Bi2S3 was enhanced. Similarly, the dopant Tb acted as an electron-trapping agent through interconversion between Tb4+ and Tb3+ and improved the photocatalytic activity of Bi2MoO6 in RhB degradation.68
Although the above conventional doping of heteroatoms can enhance the activities of bismuthal compounds, the introduced hetero element may alter the space lattice and cause additional stress. This process suppresses the introduction of the dopant and limits the improvements of visible-light absorption and carrier transfer derived from doping. Hence, doping by substituting the original element, rather than interstitial doping, is desired.
For example, when Bi and Ti in Bi4Ti3O12 are partially replaced by Cr, the doped Bi4Ti3O12 exhibits a higher activity in H2 generation because of the considerably narrowed band gap and enlarged light absorption of Bi4Ti3O12.69 The cosubstitution of Gd and Mn in BiFeO3 nanoparticles (NPs) reduces the band gap and electrical resistivity of BiFeO3.70 The band gap of BiOCl was narrowed when Bi3+ was partially substituted by Sn2+, and this process consequently endowed BiOCl with increased photocatalytic activity for degrading RhB.71 When O in BiVO4 was partially substituted by F through a solid–vapor reaction, the photoelectrochemical water oxidation on BiVO4 was strongly promoted.72 This effect may be attributed to the more negative flat band potential (an upshift of approximately 0.1 V) and increased carrier density resulting from the doping of F. The moderate upshift of the flat band potential makes the consumption of electrons easier, which is favorable to the holes in the VB for oxidation reactions. Bi3FeMo2O12 was found to be superior to Bi3GaMo2O12 in the photocatalytic degradation of RhB under visible-light irradiation. This result was due to the narrower band gap of Bi3FeMo2O12 (2.3 eV) than that of Bi3GaMo2O12 (2.95 eV) as a result of the contribution of Fe 3d orbitals to the CB.73 Nevertheless, not all elemental substitutions lead to a reduced band gap in bismuthal compounds. If Bi and Fe in BiFeO3 NPs are cosubstituted with Ca and Ti using a sol–gel method, the band gap will increase upon an increase in the amount of Ca and Ti.74
Elemental substitution can also improve the separation of carriers and thus boost the performance of photocatalysts. Gu et al.75 obtained Ce-doped BiVO4 by substituting Bi3+ with Ce ions in a homogeneous precipitation synthesis. The introduction of Ce enhanced the photocatalytic performance of MB and phenol removal by effectively hindering the recombination of photo-generated carriers. BiV0.8Mo0.2O4 exhibited an improved photoelectrochemical activity in water oxidation because of the more efficient charge transport and electron–hole separation derived from the formation of cation vacancies (Bi5+)/oxygen interstitials as a result of high Mo doping.76
Solid solution
Fabricating solid solutions is an effective technique to adjust the band structure of photocatalysts by hybridizing two or more crystalline phases at the atomic scale. A solid solution can be regarded as a special doped semiconductor, in which the anions or cations of the host semiconductor are selectively replaced by introduced ones over the whole range of the composition.77 By adjusting the concentration of cation and/or anion substitution (by controlling the components and composition), the band structure of a solid solution can be tuned.78,79 Although the aforementioned doping methods can enhance the performance of bismuthal photocatalysts to some extent, the shortcomings of doping are obvious. The formed doping levels are generally discrete (Fig. 3), and the dopant may also act as the recombination center of carriers and increase the number of recombination sites for electrons and holes.78,79 Contrary to doping, solid solutions contain no discrete energy levels. The band structures of solid solutions are uniform, and their band gaps usually fall in the region between those of original semiconductors (Fig. 6). Consequently, the electron excitation can be improved with a lower increase in carrier recombination.Bismuth oxyhalide solid solutions are the most frequently studied among Bi-based solid solutions because of the similar structures of BiOX (X = Cl, Br, I) and easy fabrication of solid solutions comprising these compounds. Hence, several studies have also considered BiOX as a kind of heterooxyhalide-substituted bismuth oxyhalide.80 Furthermore, along with the ratio of oxyhalides, the absorption edges of bismuth oxyhalide solid solutions usually vary gradually between the absorption edges of the pristine compounds (Fig. 7).81 This phenomenon renders the tailoring of the band structure as highly convenient. For example, hierarchical Bi-rich Bi4O5BrxI2−x solid-solution nanosheets can be prepared via a precursor method and show improved photocatalytic activity for CO2 conversion and Cr(VI) removal.82 Flower-like BiOCl0.5Br0.5 hierarchical solid solutions can be fabricated through reacting Bi2O3 with a mixed solution of KCl and KBr at room temperature in the presence of glacial acetic acid and exhibit enhanced photocatalytic activity for RhB degradation under visible-light irradiation, compared to pure BiOCl and BiOBr.83 Similarly, 3D flower-like BiOClxBr1−x,84 BiOClxIy,85 and BiOCl1−xBrx hierarchical microspheres86 have also been prepared by simple one-pot methods and have exhibited better activities than pure BiOCl, BiOBr, and BiOI. When the three latter halogens were introduced during fabrication, ternary solid solutions BiOClxBryIz (x + y + z = 1) could be obtained, and the as-prepared solid solutions generally possessed a controllable band gap and highly enhanced visible-light photocatalytic activity.87 F-Containing bismuth oxyhalide solid solutions are less reported in solid-solution preparations because of the partial miscibility of BiOF1−xYx, as proposed by Zhao et al.88 They examined the properties of BiOX1−xYx (X, Y = F, Cl, Br, I) solid solutions through density functional theory calculations. The results showed that several properties of BiOCl1−xBrx, BiOBr1−xIx, and BiOCl1−xIx solid solutions changed almost linearly with x, while the properties of BiOF1−xYx (Y = Cl, Br, I) solid solutions are partially miscible. In addition, the related calculated data were difficult to fit. Therefore, this behavior has been attributed to the partial miscibility of BiOF1−xYx.
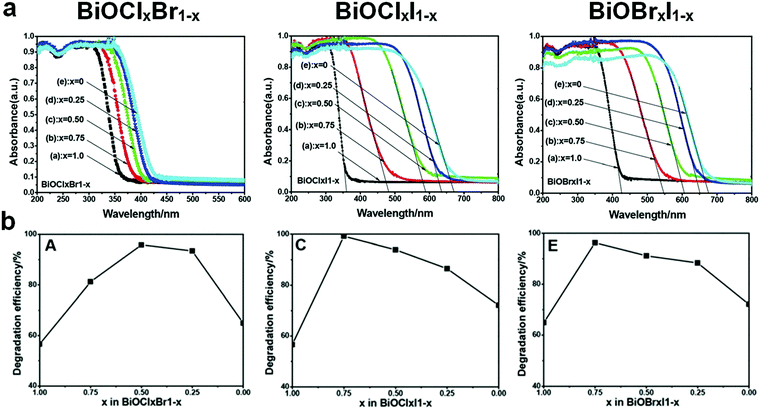 | ||
| Fig. 7 (a) UV-visible light diffuse reflectance spectra and (b) RhB degradation efficiency of BiOClxBr1−x, BiOClxI1−x, and BiOBrxI1−x solid solutions. Reproduced with permission from ref. 81. Copyright 2013 Royal Society of Chemistry. | ||
In addition to bismuth oxyhalides, other Bi-containing solid solutions were found to be more efficient than pure bismuthal compounds for visible-light-driven photocatalysis. For instance, Terebilenko et al.89 fabricated Bi1−x/3V1−xMoxO4 solid solutions for photocatalytic water oxidation. The resulting solid solutions possessed the same band gap of 2.25 eV (narrower than that of BiVO4), which endowed such solid solutions with high visible-light absorption. A Sr0.9Bi0.1Ti0.9Fe0.1O3 solid solution synthesized by Lu et al.90 performed more effectively than SrTiO3 in H2 production under full-range irradiation (λ ≥ 250 nm). This finding was attributed to the suitable band gap of the former compound. The resulting band gap can be tuned by adjusting the ratio of Bi to Sr (see Fig. 8).
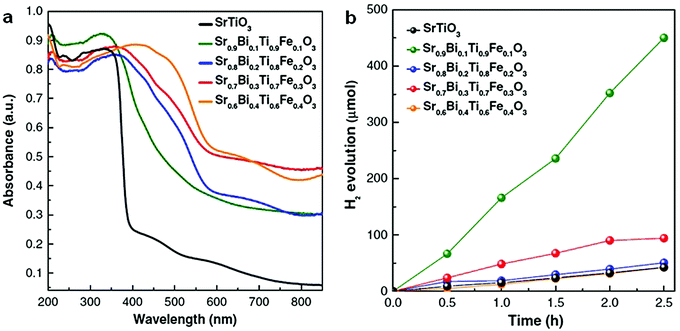 | ||
| Fig. 8 (a) UV-visible diffuse reflectance spectra and (b) photocatalytic H2 production of Sr0.9Bi0.1Ti0.9Fe0.1O3 solid solutions under full-range irradiation (λ ≥ 250 nm). Reproduced with permission from ref. 90. Copyright 2017 Elsevier. | ||
Stoichiometry alteration
The layered structures of bismuthal compounds render the component adjustment convenient. Even without introducing a foreign element, the band structures can also be tuned by changing the stoichiometry of the bismuthal semiconductor. In this field, similar to solid solutions, bismuth oxyhalides have been extensively studied because the band gap, VB, and CB of oxygen-rich (bismuth-rich) or oxygen-deficient compounds usually changes gradually along with the atomic ratio. Consequently, the compounds can be enhanced by stoichiometric variation. In a bismuth oxyhalide, both the O 2p and X np (n = 3, 4, and 5 for X = Cl, Br, and I, respectively) states have a major contribution to the VB, while the Bi 6p states dominate the CB. When the O content increases, the O 2p states of bismuth oxyiodide become more significant than the X np state in the VB, therefore the band gap energies of O-rich bismuth oxyiodides are closer to those of Bi2O3.91 That is, the VB can be tuned by changing the O/X ratio.The band gap of BiOI is very narrow, but its CB is less negative, and its VB is less positive. These characteristics result in the low reduction and oxidation capacities of BiOI. Consequently, the CB upshift and VB downshift become more favorable, but excessive shift in the CB and VB may result in a wide band gap, which is unfavorable for photocatalysis. That is, a moderate upshift of CB and downshift of VB are required. Generally, the VB of bismuth oxyiodides downshifts to a more positive level because of the increased contribution of the O 2p states, while the CB upshifts to a more negative position owing to the additional contribution of I 5p, thus the band gap of the bismuth oxyiodides increases with an increase in the bismuth and oxygen content. Hence, bismuth oxyiodides with moderate overdoses of bismuth and oxygen, such as Bi4O5I2 or Bi6O9I3, are more advisable. For instance, Bi4O5I2 nanosheets (fabricated using a solvothermal method) exhibited a higher activity than BiOI in the photocatalytic degradation of RhB because of the more positive VB of the former.92 For BiOCl, narrowing the wide band gap is the primary aim of the atomic ratio adjustment. Oxygen-rich bismuth oxychlorides, such as Bi24O31Cl10 (Eg = 2.71 eV, ref. 93) and Bi12O17Cl2 (Eg = 2.57 eV, ref. 94), possess narrower band gaps and can be excited by visible light. Therefore, the visible-light photocatalytic performances of these bismuth oxychlorides are better than that of BiOCl. Moreover, Cl-rich bismuth oxychloride was also found to possess a narrower band gap. For instance, the band gap of Bi12O15Cll6 nanosheets fabricated by Wang et al.95 was estimated to be 2.36 eV, and the photocatalytic performance of Bi12O15Cll6 nanosheets for bisphenol A (BPA) removal was superior to that of BiOCl nanosheets. Studies on oxygen-rich bismuth oxybromides have mainly focused on Bi4O5Br2 because of its narrow band gap. In reports by Xia and co-workers,96 Bi4O5Br2 nanosheets fabricated using a solvothermal method were shown to possess a narrower band gap (2.37 eV) and lower carrier transfer resistance than those of BiOBr (2.82 eV) and were more effective than BiOBr in the photocatalytic degradation of BPA. For similar reasons, Bi4O5Br2 showed much higher activity than BiOBr in the photocatalytic degradation of resorcinol97 and ciprofloxacin (CIP)98 under visible light. Furthermore, Bi4O5Br2 nanosheets were also discovered to be more suitable than BiOBr nanosheets in the photoreduction of CO2.99 These results are due to the CB position of Bi4O5Br2 being higher than that of BiOBr and thus more favorable for the reduction reaction of CO2.
The band structures of other Bi compounds, such as BiVO4, Bi2SiO5, and Bi2O3, can also be altered by varying the stoichiometry for activity enhancement. Batool et al.100 found that Bi4(SiO4)3 nanofibers possess lower impedance and exhibit higher photocatalytic performance than Bi2SiO5 nanofibers in the degradation of MO. Huang and co-workers101 prepared Bi4V2O11 through a solvothermal method. The synthesized Bi4V2O11 had a narrower band gap (2.15 eV) than that of BiVO4 (2.4 eV) and showed high water oxidation activity under 300 W Xe lamp irradiation. In Kalyan's research, a BiO2−x phase with a narrow band gap (1.84 eV) formed on the surface of a Bi2O3 photoanode during photoelectrochemical testing in the presence of KOH and H2O2.102 The formed BiO2−x phase improved the photocurrent because of its narrow band gap.
Morphology control
The photocatalytic properties of semiconductors are highly dependent on their morphologies and sizes.103 High specific surface area, low thickness, and hierarchical and hollow structures can increase light absorption and accessibility of photocatalysts. Such properties enable the accelerated migration of carriers to the surface and reduce the dosage of photocatalysts. Accordingly, the performance of Bi-based photocatalysts can be enhanced by building ultrathin and hierarchical and hollow structures or by immobilization on substrates with specific morphologies.Thin morphologies
Thickness greatly affects the activities of bismuthal compounds because of the influence of such a variable on the efficiency of light absorption and the distance of the photo-generated carriers from the surfaces. A smaller sized bismuthal compound usually leads to a higher specific surface area and better photocatalytic and photoelectrochemical performance.104,105 Considering unique layered plate-like structures, the exposed-facet ratio of bismuthal compounds usually changes along with the thickness of the compounds. Therefore, the thickness effect has often been discussed in related reports. Thinner structures can endow bismuthal compounds with a higher specific surface area, increased light absorption efficiency, and enabled utilization of photo-generated electrons and holes. Ultimately, such properties may enhance photocatalytic activity. With a decrease in the thickness, the surface energy of the nanoplates would also largely increase, single plates become thermodynamically unstable and these nanoplates prefer to stack together into one larger particle to reduce surface energy during growth. As a result, many Bi-based photocatalysts usually possess a granular appearance comprising nanoplates. The thin morphology discussed in this section refers to these nanoplates.Chen and colleagues106 found that reducing the thickness of BiOBr nanosheets can significantly increase the exposed (001) facets and the photocatalytic activity of the BiOBr nanosheets. The enhanced photocatalytic activity of the BiOBr nanosheets was ascribed mainly to the enhanced absorption of visible light and improved separation efficiency of charge carriers due to the ultrathin structure. Li et al.107 proposed that the photoactivity of the BiOBr nanosheets depends on nanosheet thickness. Similarly, reducing the thickness of Bi2O2CO3 [accompanied by an increase in the (001) facets] improved its photocatalytic activity in the degradation of RhB,108 and the activities of Bi2WO6 were augmented along with a reduction in thickness.109
Thinner structures sometimes result in additional facet and structure differences, which affects the performance of bismuthal photocatalysts. For example, increasing the exposure of (001) facets (accompanied by a reduction in the thickness) can improve the efficiency of RhB degradation on Bi2Fe4O9 nanoplates, because a larger exposed (001) surface can provide more Fe3+ cations to generate more hydroxyl radicals.110 A reduction in the thickness can also improve the photocatalytic performance of BiVO4 in O2 evolution, owing to the increased ratio of reduction functional (010) facets versus dominating oxidation functional (110) facets (Fig. 9).111 Hu et al.112 noted that reducing the thickness of BiTaO4 not only increased the specific surface area but also caused upshifts in the VB and CB. Density of states (DOS) calculations showed that the energy levels of the VB and CB of the (010) facets of BiTaO4 are more negative than those of bulk BiTaO4. The (010) facets in BiTaO4 increase along with a reduction in the thickness, therefore, thinner BiTaO4 single-crystal nanoplates possess more {010} facets and higher VB and CB positions.
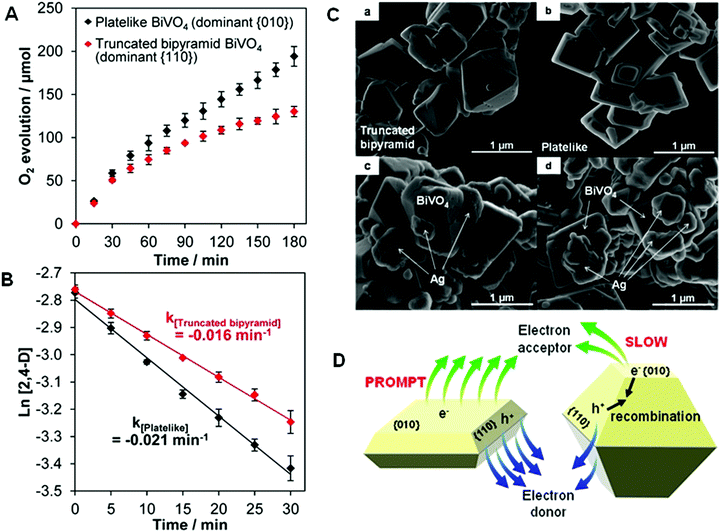 | ||
| Fig. 9 (A) Photocatalytic O2 evolution from an AgNO3 solution; (B) pseudo-first-order-fitted photocatalytic degradation curves of 2,4-dichlorophenoxyacetic acid (2,4-D) on truncated bipyramid (red) and plate-like (black) BiVO4 under visible light (λ > 420 nm); SEM images of bare (C-a) truncated bipyramid and (C-b) plate-like BiVO4 and the corresponding Ag-deposited (C-c) truncated bipyramid and (C-d) plate-like BiVO4. (D) Schematic diagram of the promotion induced by increasing the size of the (010) facets in BiVO4. Reproduced with permission from ref. 111. Copyright 2016 American Chemical Society. | ||
When the thickness of a bismuthal semiconductor is reduced to below 10 nm, the specific surface area, light absorption, and carrier migration will be further enhanced. Sometimes, new characteristics might also arise. Ultrathin square-like BiOCl nanosheets (3–7 nm in thickness) have been shown to exhibit a higher activity than thicker nanosheets in the photocatalytic degradation of RhB due to a larger specific surface area.113 Di et al.114 reported the preparation of ultrathin BiOI nanosheets and their composites, which displayed a uniform thickness of approximately 0.9 nm (Fig. 10), approaching the thickness of one [I–Bi–O–Bi–I] unit (0.9149 nm). The CB position of the BiOI nanosheets was determined to be −0.95 eV (NHE), negative to reduce O2 to ˙O2−. This characteristic is beneficial for H2 generation or CO2 reduction. However, the researchers did not provide results on the reduction application, although their reports on the surface modification of BiOI nanosheets still exhibited improved activity for the photocatalytic degradation of RhB. The ultrathin H1.78Sr0.78Bi0.22Nb2O7 nanosheets (Fig. 11) prepared by calcination exhibited 5.5 and 26.2 times higher activity than H1.78Sr0.78Bi0.22Nb2O7 plates and SrBi2Nb2O9 platelets for photocatalytic H2 generation, respectively. This result can be ascribed to the higher separation efficiency of the photo-generated carriers and specific surface area.115
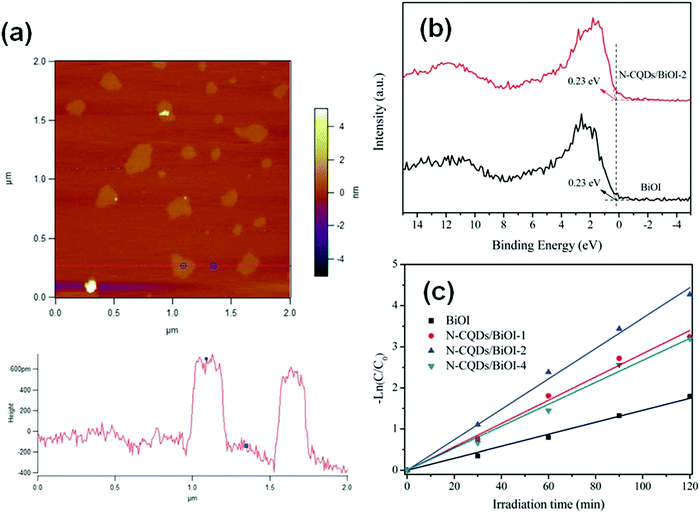 | ||
| Fig. 10 (a) Atomic force microscopy (AFM) image and corresponding height of the atomically thin BiOI nanosheets; (b) valence-band X-ray photoelectron spectra and (c) pseudo-first-order-fitted kinetic curves (for the RhB degradation) of pure BiOI and N-carbon quantum dot (CQD) decorated BiOI. Reproduced with permission from ref. 114. Copyright 2016 Royal Society of Chemistry. | ||
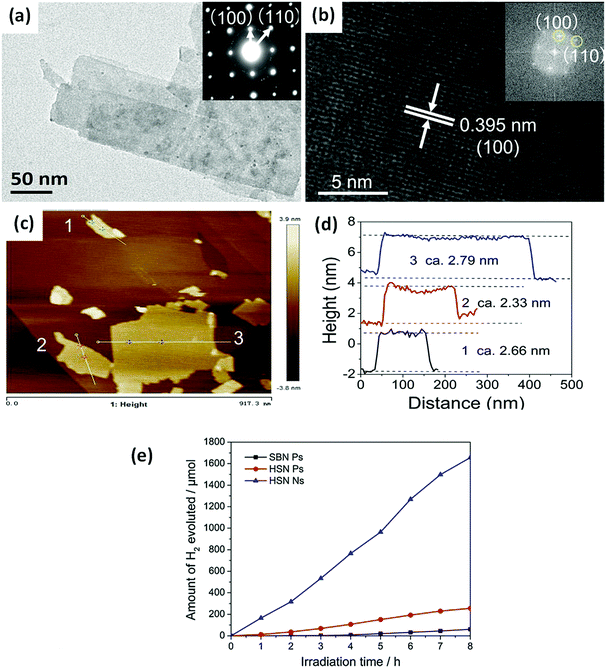 | ||
| Fig. 11 (a) Transmission electron microscopy (TEM) and (b) high-resolution TEM (HRTEM) images of H1.78Sr0.78Bi0.22Nb2O7 nanosheets (HSN Ns); (c) AFM image; (d) corresponding height of the HSN Ns; and (e) the H2 evolution curves of the SrBi2Nb2O9 platelets (SBN Ps), H1.78Sr0.78Bi0.22Nb2O7 platelets (HSN Ps), and HSN Ns. Reproduced with permission from ref. 115. Copyright 2017 Elsevier. | ||
Preparation of fabric or quantum sized materials is another method for obtaining thin morphologies and high specific surface areas. Bharathkumar et al.116 fabricated BiFeO3 fibers using an electrospinning method. The obtained BiFeO3 fibers then exhibited improved relative photocatalytic activity compared with that of BiFeO3 particles for degrading RhB, due to their high specific surface area. Zhao and co-workers117 prepared metal Bi nanofibers using an aqueous reduction procedure and found that Bi nanofibers exhibited a higher photocatalytic activity than Bi nanoplates and NPs in degrading RhB. Yang et al.118 fabricated bismuth oxide formate (HCOOBiO) nanowires (Fig. 12) with a diameter of 20 nm and a length of up to several hundreds of micrometers in N,N-dimethylformamide (DMF) solution via a solvothermal route. These HCOOBiO nanowires showed a higher activity than HCOOBiO microspheres in the photocatalytic degradation of MO. In the studies of Lu and colleagues,119 BiVO4 nanofibers were prepared using an electrospinning and calcination method and showed a higher efficiency than BiVO4 rods in the photocatalytic removal of MB under visible-light irradiation. Additionally, Bi2S3 with a high length-to-width ratio was found to be highly effective in the photocatalytic reduction of CO2 to CH3OH.120 The high length-to-width ratio not only increases the specific surface area but also enlarges the band gap (by a downshift of the VB and an upshift of the CB) owing to the increased influence of the surface state resulting from the smaller size. And as it happens, the CB is upshifted to a level sufficient enough for CO2 reduction. Bismuth monoxide quantum dots (QDs) obtained using a hydrothermal method exhibited high efficiency in the photocatalytic reduction of N2.121 The ammonia production rate reached 1226 μmol g−1 h−1 in the absence of a sacrificial agent and a cocatalyst. As Bi5O7Br was tailored into nanotubes with a diameter of 5 nm, its CB (−1.14 eV) was more negative than that of BiOBr. Though such a CB is still not thermodynamically accessible for direct free N2 fixation, N2 fixation was achieved because the formed oxygen vacancy in samples injects trapped photogenerated electrons directly into the chemically adsorbed N2 and weakens the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond.122
N triple bond.122
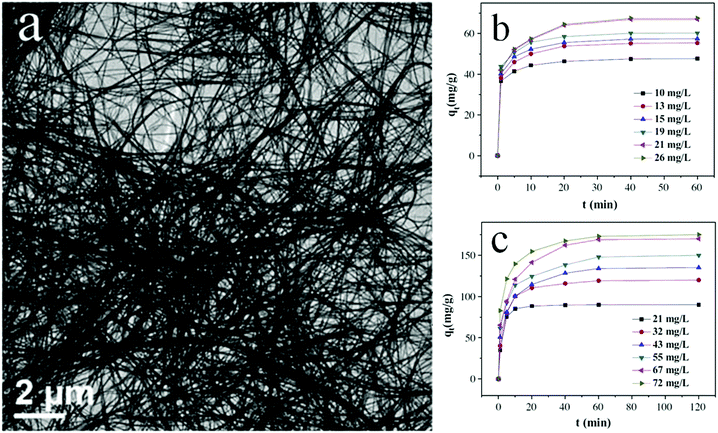 | ||
| Fig. 12 Scanning electron microscopy (SEM) images (a) of ultra-long HCOOBiO nanowires. Removal of (b) MO and (c) Cr(VI) at various initial concentrations from ultra-long HCOOBiO nanowires. Reproduced with permission from ref. 118. Copyright 2015 Elsevier. | ||
Hierarchical structures
Photocatalysts with hierarchical structures usually exhibit better performances than bulk photocatalysts. Such superiority is due to the high specific surface area, efficient light harvesting, accessibility, and easy transport of the reactants of the former catalysts. Yang et al.123 accomplished the early work on hierarchical ordered oxides. Since then, the design and synthesis of hierarchical-structured nanomaterials have attracted significant attention.124–126Specific surface area is one of the most important factors that results in the higher activity of bismuthal compounds. Generally, photocatalysts with a hierarchical structure possess relative higher specific surface areas than bulk photocatalysts or individual rods and plates, and work better in photocatalytic reactions. For example, grain-like Bi24O31Br10 hierarchical architectures (Fig. 13) fabricated using a solvothermal method in the presence of starch also showed improved photocatalytic activity in the degradation of RhB.127 Qin and co-workers128 prepared BiYO3 with a high specific surface area using a hydrothermal method with soft templates. The BiYO3 prepared using disodium ethylenediaminetetraacetate (EDTA) as a template possessed the highest specific surface area and photocatalytic activity for reducing CO2. Jia et al.129 found that hierarchical Bi2S3 produced using a hydrothermal method [with cetyltrimethylammonium bromide (CTAB) as a surfactant] exhibited higher photocatalytic activity than Bi2S3 nanorods in the degradation of RhB. This performance was ascribed to the synergetic effect of the shape, surface area, band gap, crystallinity, and size resulting from the 3D hierarchical structure. Han's group130 fabricated flower-like δ-Bi2O3 by thermally treating bismuthyl acetate CH3COO(BiO), which was prepared from Bi2O3 and glacial acetic acid (HAc) or Bi(NO3)3·5H2O and HAc. The obtained δ-Bi2O3 showed superior activity over that of δ-Bi2O3 sheets in the photocatalytic degradation of RhB. Zhou et al.131 prepared uniform cylindrical BiFeO3 samples by adjusting the ratio of HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH to 8
NaOH to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 in the hydrothermal process. The uniform cylindrical BiFeO3 exhibited enhanced photocatalytic activities for removing RhB under visible-light irradiation, because the uniform structures favored charge transport and increased the accessibility to organic matter. Li et al.132 prepared BiOBr strips accumulated from tiny pieces using a solvothermal method with bismuth subsalicylate as a template. They found that the BiOBr strips showed an enhanced performance for the photocatalytic degradation of RhB. Furthermore, the hierarchical structure of bismuthal oxyiodide obviously promoted the performance of the compound in phenol removal.133
12 in the hydrothermal process. The uniform cylindrical BiFeO3 exhibited enhanced photocatalytic activities for removing RhB under visible-light irradiation, because the uniform structures favored charge transport and increased the accessibility to organic matter. Li et al.132 prepared BiOBr strips accumulated from tiny pieces using a solvothermal method with bismuth subsalicylate as a template. They found that the BiOBr strips showed an enhanced performance for the photocatalytic degradation of RhB. Furthermore, the hierarchical structure of bismuthal oxyiodide obviously promoted the performance of the compound in phenol removal.133
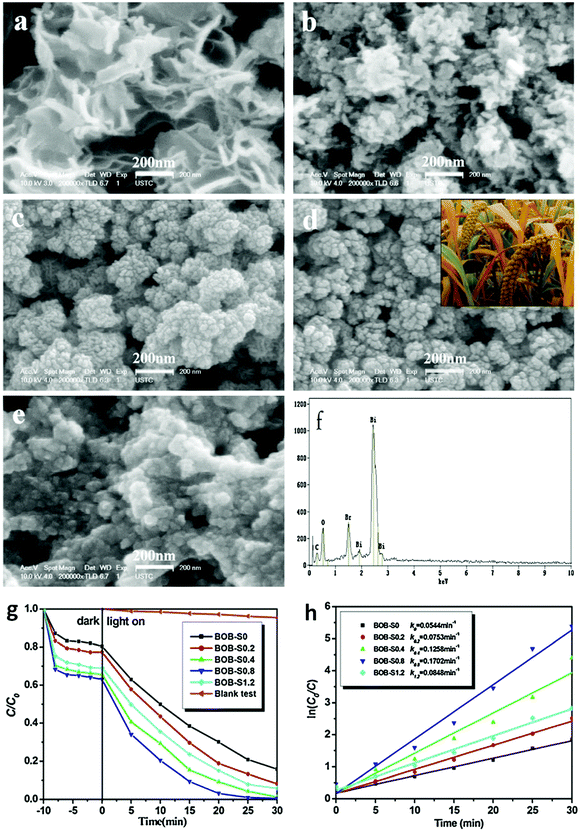 | ||
| Fig. 13 SEM images of Bi24O31Br10 hierarchical architectures (BOB) (a) BOB-S0, (b) BOB-S0.2, (c) BOB-S0.4, (d) BOB-S0.8 (grain-like), and (e) BOB-S1.2 (e.g., -S1.2 indicates that the mass of starch used was 1.2 g); (f) EDS pattern of BOB-S0.8; (g) photocatalytic degradation curves of RhB; and (h) pseudo-first-order-fitted kinetic curves over Bi24O31Br10 samples under visible-light irradiation. Reproduced with permission from ref. 127. Copyright 2016 Elsevier. | ||
Even in the construction of a hierarchical structure, the morphologies of bismuthal photocatalysts can be further tuned to different forms by adjusting the solvent, pH, and surfactants, as well as by adding capping agents. For instance, Wang et al.134 synthesized 3D hierarchical flower-like α-Bi2O3 microspheres with various morphologies (Fig. 14) in an ethanol–water mixture. They heated the mixture in an oil bath at 95 °C, and the morphology was controlled by adjusting the amount of added glycerol and oleic acid (capping agents). Sarka et al.135 fabricated Bi2S3 in different shapes (Fig. 15) using a solvothermal method with various solvents. The performance of Bi2S3 nanocrystals in removing MB was better than those of the Bi2S3 nanorods and nanoplates due to the higher specific surface area and suitable band structure of the Bi2S3 nanocrystals. Zhao et al.136 altered the morphologies of BiVO4 from olive-shaped to primrose-like, leaf-shaped, or strip-like morphologies (Fig. 16), by adjusting the concentration of glycerol in the aqueous solution during template-free solvothermal synthesis. In addition to solvent control, the morphologies of BiVO4 can also be modified by pH adjustment. Besides this, the hierarchical morphology alteration of BiVO4 can also be achieved through adjusting the pH.137 The relationship between pH and the morphologies of BiVO4 is illustrated in Fig. 17. If NaHCO3 is added in the solid-state synthesis of BiVO4, the morphology can be turned from polyhedral to a rice-like shape, by increasing the nucleation rate and hindering the continual growth of the original product particles.138 In the preparation of Bi2WO6, its morphology can be tuned from accumulated sheets to a complex morphology by adjusting the molecular weight of the added polyvinylpyrrolidone (PVP).139 Examples of such complex morphology include flower-like, red blood cell-like, and square pillar-like morphologies. Bi2WO6 with a thin sheet-like appearance showed the best photocatalytic performance in the decomposition of RhB under visible-light irradiation.
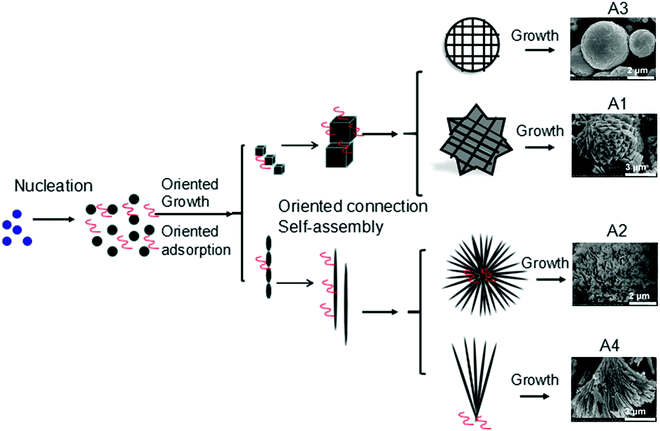 | ||
| Fig. 14 3D hierarchical flower-like α-Bi2O3 microspheres fabricated using different solvents: A1 (0.5 mL of glycerol), A2 (1 mL of glycerol), A3 (1 mL of glycerol and 1 mL of oleic acid), and A4 (1 mL of glycerol and 2 mL of oleic acid). Reproduced with permission from ref. 134. Open Access 2016 World Scientific. | ||
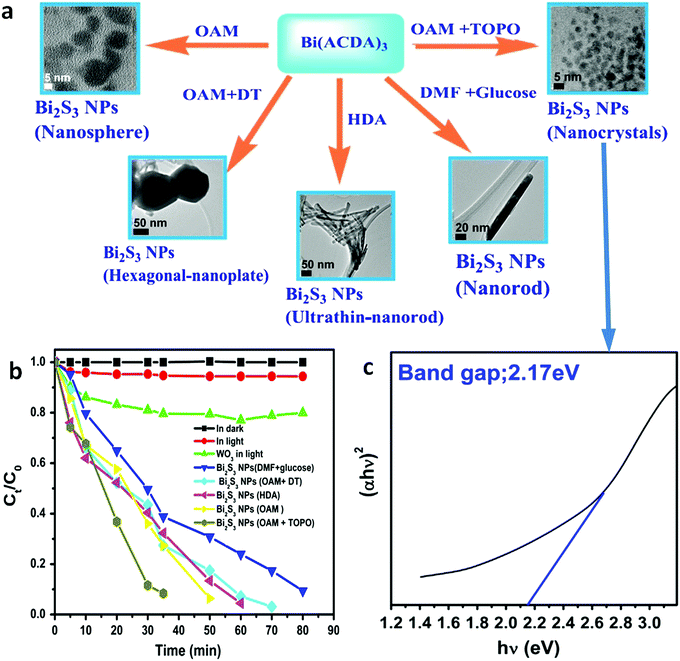 | ||
| Fig. 15 (a) Synthesis pathways of Bi2S3 NPs with tuned morphologies from Bi(ACDA)3 (ACDA = 2-aminocyclopentene-1-dithiocarboxylic acid radical) using solvent comprising glucose, oleylamine (OAM), hexadecylamine (HDA), dodecanethiol (DT), trioctylphosphine oxide (TOPO), and dimethylformamide (DMF); (b) photocatalytic degradation curves of MB under visible-light irradiation and (c) the Tauc plot of the Bi2S3 NPs using a mixed solvent composed of OAM and TOPO. Reproduced with permission from ref. 135. Copyright 2016 Elsevier. | ||
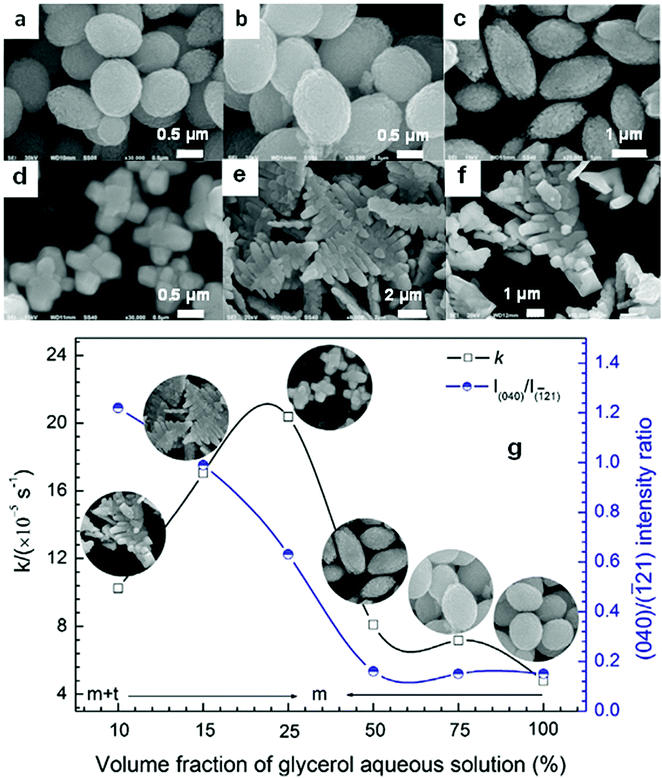 | ||
Fig. 16 SEM images of the BiVO4 samples prepared with the following volume fractions of glycerol aqueous solution: (a) 100%, (b) 75%, (c) 50%, (d) 25%, (e) 15%, and (f) 10%; (g) relationship between the glycerol fraction, the (040)/(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 21) intensity ratio, the morphology, and the photocatalytic performance of the BiVO4 samples. Reproduced with permission from ref. 136. Copyright 2016 Elsevier. 21) intensity ratio, the morphology, and the photocatalytic performance of the BiVO4 samples. Reproduced with permission from ref. 136. Copyright 2016 Elsevier. | ||
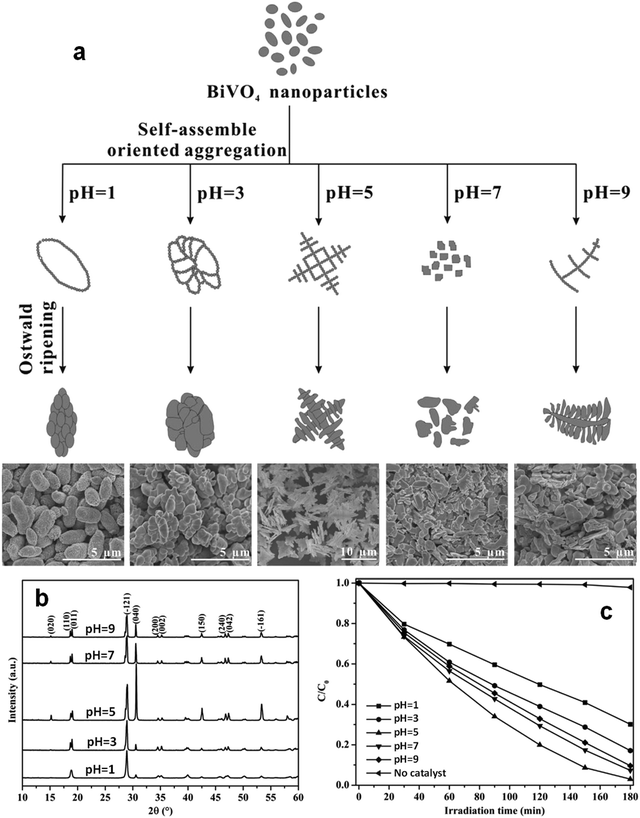 | ||
| Fig. 17 (a) Schematic diagram of the possible formation mechanism and (b) powder X-ray diffraction patterns (PXRD) patterns of BiVO4 samples with various morphologies prepared at different pH values; (c) photocatalytic activities of the BiVO4 samples in the degradation of MB under visible light. Reproduced with permission from ref. 137. Copyright 2016 Elsevier. | ||
Hollow and porous structures
The hierarchical structure of Bi compounds can be transformed to a hollow structure, which is more preferable for improving the photocatalytic performance, using a suitable method. For instance, Qian et al.140 fabricated mesoporous Bi4Ti3O12 by calcinating a mixture of Bi(NO3)3 and tetrabutyl titanate. The product had a markedly higher specific surface area (109.4 m2 g−1) and exhibited a better performance than that of the bulk compound. Gong and co-workers141 prepared porous BiVO4 by electrospinning and calcination in the presence of PVP. The obtained porous BiVO4 had a larger specific surface area and enhanced photocatalytic activity for the degradation of MB. Kashfi-Sadabad et al.142 synthesized Sm-doped Bi2MoO6 hollow spheres by developing a solvothermal pathway in the presence of pluronic P123 copolymer. During the synthesis, no spherical structures formed in the absence of P123 (Fig. 18b). At a low dosage of P123, the products became solid spheres without hollow structures (Fig. 18c). Hollow spheres formed gradually and became enlarged upon further P123 addition. Finally, Bi2MoO6 hollow spheres with diameters of 1–1.5 μm and a thickness of approximately 100 nm (Fig. 18d) were obtained when the P123 amount reached 3 g. The formation of these spheres is illustrated in Fig. 18a. Hollow spherical bismuthal photocatalysts can also be fabricated using a solvothermal method in the absence of a template. Zhou and colleagues143 fabricated hollow Bi2WO6 microspheres (Fig. 19) using a template-free solvothermal route with a mixed solvent composed of ethylene glycol (EG) and ethanol. The well-defined hollow Bi2WO6 microspheres exhibited considerably better photocatalytic activity in the degradation of RhB than Bi2WO6 with other morphologies under visible-light irradiation. Li et al.144 synthesized hollow Bi2MoO6 spheres using a solvothermal route in a Bi2MoO6 precursor solution. The obtained yolk shell-like Bi2MoO6 displayed markedly higher efficiency in removing RhB. Such improvement was attributed to the hollow structure accompanied by enhanced light absorption, increased specific surface area, and augmented charge transfer. If a mixed solution containing glycerol, ethanol, and deionized water was adopted, hollow Bi2MoO6 was still obtained during the solvothermal synthesis.145 Multishell hollow structures are more attractive than other structural types because of their higher surface area and unique nested structure, which ensure highly efficient light utilization. Zong et al.146 achieved Bi–V–O heterostructured multishell hollow spheres (composed of interconnected BiVO4 and Bi4V2O11) (Fig. 20) using NaOH-treated carbonaceous microsphere templates and a suitable heating rate. The double-shell Bi–V–O hollow spheres were more effective than their single-shell counterparts in removing MB under visible-light irradiation because of the multiple light reflections and effective light utilization achieved through the multishell hollow structure.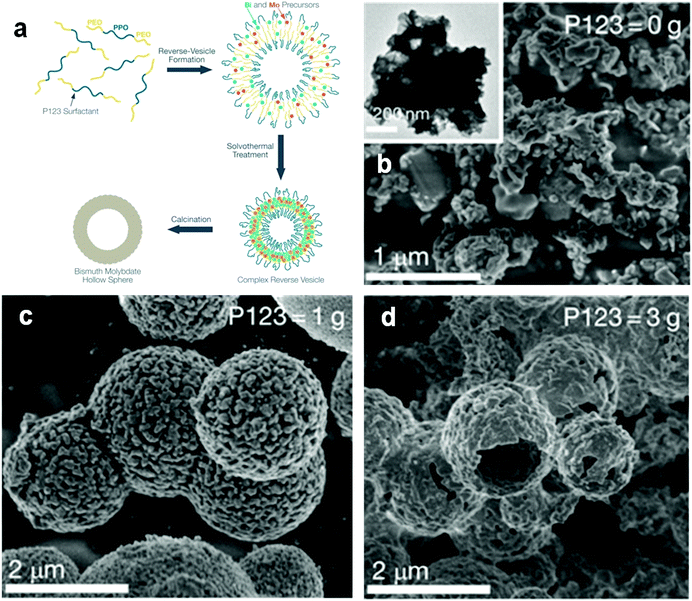 | ||
| Fig. 18 (a) Schematic diagram of the formation of hollow-structured Bi2MoO6 and SEM images of Bi2MoO6 fabricated (b) without P123, (c) with 1 g of P123, and (d) with 3 g of P123. Reproduced with permission from ref. 142. Copyright 2016 American Chemical Society. | ||
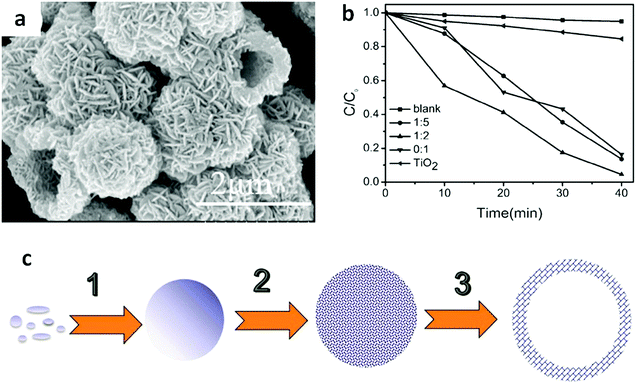 | ||
Fig. 19 (a) SEM images of Bi2WO6 hollow spheres prepared using a mixed solvent of EG and ethanol (EA) (VEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEA = 1 VEA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 160 °C for 2 h; (b) photocatalytic degradation curves of RhB on Bi2WO6 samples prepared using solvents with different VEG 2) at 160 °C for 2 h; (b) photocatalytic degradation curves of RhB on Bi2WO6 samples prepared using solvents with different VEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEA ratios, and (c) schematic diagram of the formation of hollow structures, involving the formation (1), surface roughening (2) and hollowing (3) of amorphous solid microspheres. Reproduced with permission from ref. 143. Open Access 2016 MDPI. VEA ratios, and (c) schematic diagram of the formation of hollow structures, involving the formation (1), surface roughening (2) and hollowing (3) of amorphous solid microspheres. Reproduced with permission from ref. 143. Open Access 2016 MDPI. | ||
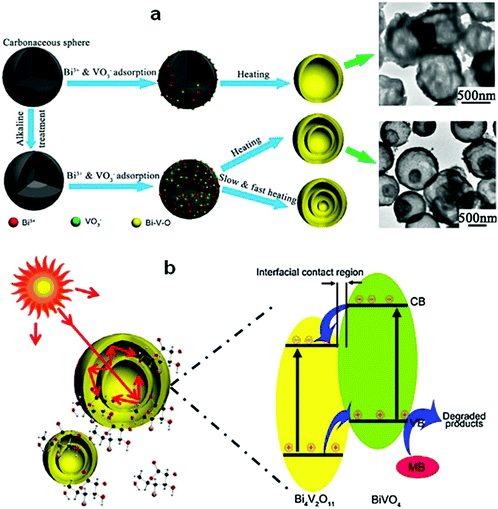 | ||
| Fig. 20 (a) Schematic diagram of the formation of Bi–V–O multishell hollow spheres and (b) photoabsorption and photocatalytic mechanism of the Bi–V–O multishell hollow spheres. Reproduced with permission from ref. 146. Copyright 2017 Elsevier. | ||
Hollow structures are not restricted to hollow spheres. Hollow tubes and cross-linked net-like structures are also favorable morphologies for bismuthal photocatalysts. These alternative structures can be constructed with assistance from directed surfactant agents or substrates. For example, Bi2O2CO3 nanotubes (Fig. 21) can grow on a graphene substrate through hydrolysis by adjusting the pH to 10.5 using urea.147 In the presence of CTAB (as a morphology director), BiVO4 can grow into nanotubes during hydrothermal synthesis.148 The formed BiVO4 nanotubes exhibit improved activity relative to that of BiVO4 nanorods in the photocatalytic degradation of MO. When dual templates of polystyrene latex spheres (PS) and P123 are adopted in the preparation of Bi2O3/TiO2 composites (sol–gel method), a series of 3D-ordered macroporous profiles are produced (Fig. 22).149 The composites present a significantly higher activity than P25 and Bi2O3 in the photocatalytic removal of crystal violet. Such an advantage can be ascribed to the formed porous structure and heterojunction. Lee et al.150 fabricated a type of porous β-Bi2O3 from Bi(NO3)3 using a rapid phase and surface transformation with structure-guided combustion waves. The compound showed a higher photocatalytic performance in the degradation of RhB than those of α-Bi2O3 rods and Bi(NO3)3·H2O rods.
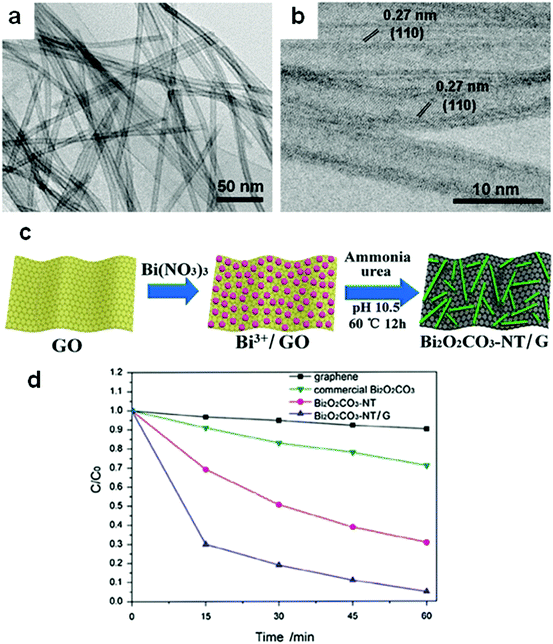 | ||
| Fig. 21 (a) TEM and (b) HRTEM images and (c) formation mechanism of Bi2O2CO3 nanotubes on a graphene substrate; (d) photocatalytic degradation behavior of samples toward reactive red (X-3B). Reproduced with permission from ref. 147. Copyright 2017 Elsevier. | ||
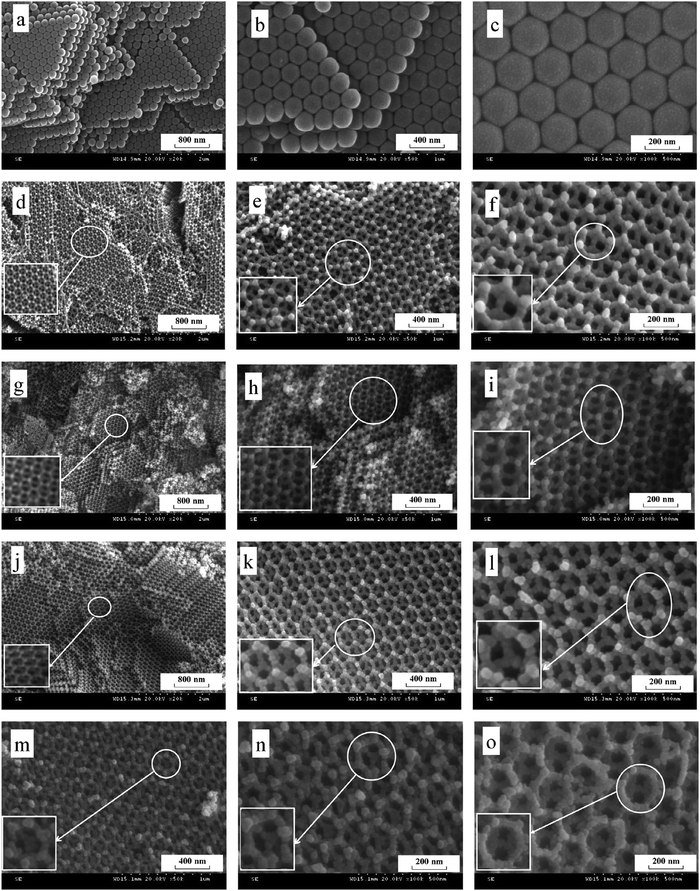 | ||
Fig. 22 SEM images of (a–c) polystyrene latex spheres, (d–f) 3DOM Bi2O3/TiO2-1 (nB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nTi = 0.015 nTi = 0.015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (g–i) 3DOM Bi2O3/TiO2-2 (nBi 1), (g–i) 3DOM Bi2O3/TiO2-2 (nBi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nTi = 0.03 nTi = 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (j–l) 3DOM Bi2O3/TiO2-3 (nBi 1), (j–l) 3DOM Bi2O3/TiO2-3 (nBi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nTi = 0.06 nTi = 0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (m–o) 3DOM Bi2O3/TiO2-4 (nBi 1) and (m–o) 3DOM Bi2O3/TiO2-4 (nBi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nTi = 0.09 nTi = 0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reproduced with permission from ref. 149. Copyright 2015 Elsevier. 1). Reproduced with permission from ref. 149. Copyright 2015 Elsevier. | ||
Immobilization
During an application, the undesired aggregation of NPs would seriously affect the performance of Bi-based photocatalysts, so an effective separation method should be employed to reduce the aggregation. The cyclic utilization of Bi-based photocatalysts should also be considered. Immobilization has been considered as an effective means to solve these two issues. Furthermore, with the aid of substrate materials, the profile of bismuthal photocatalysts can be tuned to achieve photocatalytic reactions. Hence, suitable immobilizations have been explored for improving the performance of Bi-based photocatalysts.Immobilization requires the increased dispersion of bismuthal photocatalysts, which increases the availability of photocatalysts and improves the attachment between the photocatalysts and reactants. Wang and co-workers151 prepared a BiOCl film on a Bi plate using a room-temperature reaction, and the product exhibited a high photocatalytic activity in the degradation of RhB because of the good dispersion of the formed BiOCl nanonuclei. Sonawane and colleagues152 immobilized Bi2O3 on bentonite using an intercalation method. The obtained Bi2O3/bentonite composite exhibited improved performance relative to the separate use of Bi2O3 and bentonite in the photocatalytic degradation of RhB because of the increased adsorption capacity brought about by the use of bentonite. Similarly, Wang et al.153 immobilized phosphotungstic acid-modified BiOBr on the surface of a zeolite. This process endowed the composites with enhanced photocatalytic activity for the degradation of MO. Li et al.154 immobilized BiOI on diatomite and obtained composites with improved photocatalytic performance in the degradation of RhB. Tong and co-workers155 used nickel foam as a substrate to immobilize a Bi2WO6/TiO2 composite and increase the efficiency of Bi2WO6/TiO2 in the photocatalytic degradation of RhB. Shi et al.156 found that the performance of BiVO4 in the photocatalytic removal of tetracycline hydrochloride could be enhanced when supported on palygorskite. Zhang and colleagues157 demonstrated that Bi4Ti3O12 immobilized on SiO2 spheres could more effectively decolorize Brilliant Red-X3B because of the good dispersion and smaller size of Bi4Ti3O12.
If the substrates are semiconductor materials, a heterojunction will form and facilitate the separation of electrons and holes. Mu and co-workers158 deposited spindle-like BiVO4 on TiO2 nanofibers (Fig. 23) using a solvothermal method, and the composite exhibited enhanced visible-light activity than that of a mechanical mixture of BiVO4 and TiO2 in the photocatalytic degradation of RhB. This result could be ascribed not only to the thorough dispersal of the BiVO4 NPs by immobilization, but also to the improved separation of electrons and holes by the formed heterojunction between BiVO4 and TiO2. Zhang and co-workers159 reported the enhanced performance of BiOCl nanosheets in the photocatalytic degradation of RhB by immobilizing the BiOCl sheets on TiO2 arrays over FTO (Fig. 24). This effect was achieved, because the reflection within the ordered array structure endowed the composites with enhanced light absorption. Moreover, the heterojunction between BiOCl and TiO2 significantly decreased the charge transfer resistance and facilitated the separation of photo-generated holes and electrons. Kumar et al.160 prepared Bi2S3/TiO2 composites from Bi2S3 nanotubes and TiO2 NPs. The acquired composites showed improved photocatalytic performance in the degradation of amaranth.
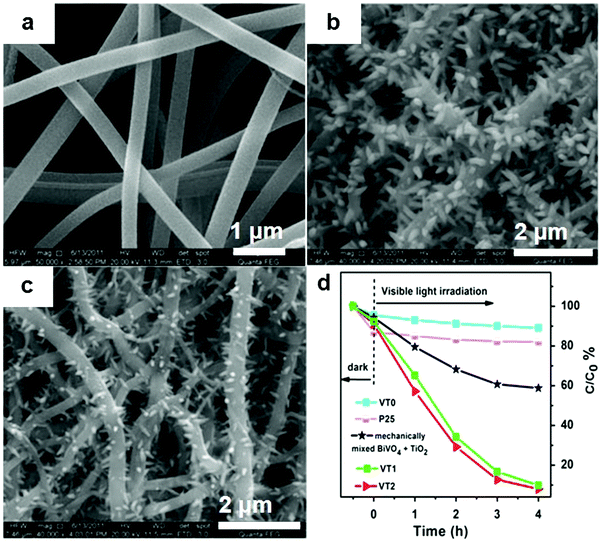 | ||
| Fig. 23 SEM images of (a) TiO2 nanofibers (VT0), (b and c) BiVO4 immobilized on TiO2 nanofibers using a low (VT1) and high (VT2) concentration of raw material solution, and (d) the degradation curves of RhB over different photocatalysts under visible light. Reproduced with permission from ref. 158. Copyright 2016 Elsevier. | ||
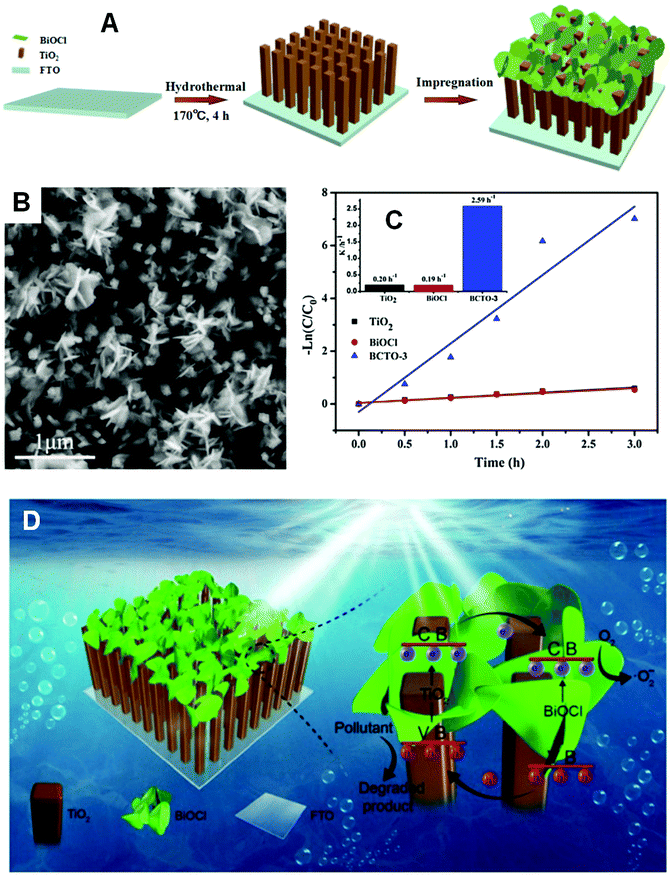 | ||
| Fig. 24 (A) Schematic diagram of BiOCl/TiO2 composite formation; (B) FESEM images of a BiOCl/TiO2 composite (BCTO-3); (C) kinetic curves of RhB photocatalytic degradation on TiO2, BiOCl, and BCTO-3; (D) schematic diagram of the reaction mechanism over the immobilized BiOCl/TiO2 composite under visible light. Reproduced with permission from ref. 159. Copyright 2017 Elsevier. | ||
Conductive substrates not only affect the morphologies of the composites, but also act as electron acceptors. For example, when a BiOCl sheet was immobilized on carbon fibers, the performance of the sheet in the photocatalytic degradation of 4-nitrophenol was enhanced due to the good dispersion of BiOCl nanoplates and the electron trapping role of the carbon fibers.161 Likewise, Weng and co-workers162 loaded Bi4Ti3O12 on carbon fibers using a hydrothermal method with carbon-fiber-supported TiO2 nanosheets as a precursor. The prepared composite exhibited better performance than that of Bi4Ti3O12 nanosheets in the photocatalytic degradation of MO. Di et al.163 deposited small Bi4O5Br2 nanosheets on multi-walled carbon nanotubes (MWCNTs) via an ionic liquid-assisted solvothermal method and found that the activity of the composites in the photocatalytic degradation of tetracycline hydrochloride was obviously improved.
Immobilization plays a more important role in gaseous reactions than in other reactions, because well-dispersed photocatalysts are crucial to the external and internal diffusions of gaseous molecules. Dong's group164 immobilized Bi NPs on g-C3N4 and observed that an NO removal efficiency of 60.8% was achieved when Bi NPs of 12 nm were decorated on g-C3N4. This efficiency was higher than that of g-C3N4 (38.6%). Meanwhile, Dong's group165 deposited metal Bi NPs on TiO2 for NO removal and noted that Bi can act as a cocatalyst, similar to a noble metal, due to surface plasmon resonance and can effectively photoactivate TiO2 during NO oxidation under visible-light irradiation. Xia et al.166 loaded BiOI on porous Al2O3 using a hydrothermal method and observed that the obtained BiOI/Al2O3 composite exhibited photocatalytic performance by simultaneously eliminating gaseous NO and SO2.
Heterojunction construction
Heterojunction construction is an effective pathway for enhancing the performance of photocatalysts, because photo-generated electrons and holes can be effectively separated by a heterojunction.29,42,167–175 For most Bi-based photocatalysts, a narrow band gap ensures that electrons can be excited by visible light. However, the excited electrons recombine with holes soon after. Therefore, heterojunction construction plays a key role in improving the performance of Bi-based photocatalysts. Bi-Based heterojunctions include conventional and Z-scheme heterojunctions. Among the conventional heterojunctions, the type II junction is the most common one, while in Z-scheme systems, the newly-emerged direct Z-scheme heterojunction appears to be the most effective junction structure used for exploring the capacity of photo-generated carriers.Conventional type II heterojunctions
Conventional heterojunctions are the most commonly reported in Bi-based photocatalysts, and the two main carrier migrations are illustrated in Fig. 25. In the type I mode, photo-induced holes migrate from the lower VB of semiconductor B (SC-B) to the higher VB of semiconductor A (SC-A), whereas electrons migrate from the higher CB of SC-B to the lower CB of SC-A. During the process, electrons and holes migrate in the same direction, but electrons and holes migrate at different speeds due to their different effective mass or other factors. Consequently, holes and electrons are separated. In the type II mode, holes migrate from the lower VB of SC-B to the higher VB of SC-A, while electrons migrate from the higher CB of SC-A to the lower CB of SC-B. During the process, electrons and holes migrate in opposite directions, resulting in their separation. In any of these two heterojunctions, the electrons and holes can be separated, and the photocatalytic activity of the photocatalysts can be enhanced.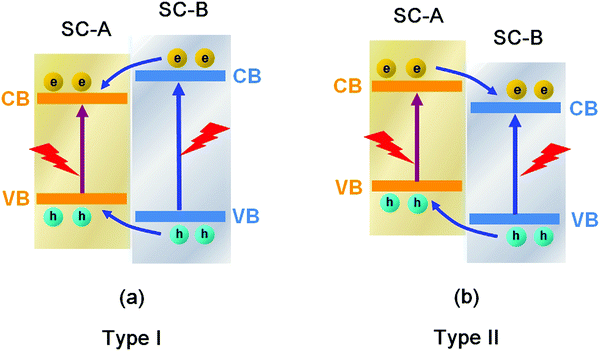 | ||
| Fig. 25 Schematic diagram of the carrier migrations in conventional (a) type I and (b) type II heterojunctions between semiconductor A (SC-A) and semiconductor B (SC-B). | ||
Among the different types of heterojunctions, the type II junction is the most reported one. This kind of heterojunction usually forms between bismuthal compounds and semiconductors with a narrow band gap, when the CB and VB of one semiconductor are lower than that of the coupled one. For example, Zhu and co-workers176 reported the fabrication of a binary Bi and non-Bi Bi4Ti3O12/CeO2 composite by coupling Bi4Ti3O12 with CeO2 using a molten salt and ion-impregnation method. The obtained Bi4Ti3O12/CeO2 composite exhibited enhanced activity in the photocatalytic degradation of BPA. This phenomenon can be attributed to the important role of the heterojunction between Bi4Ti3O12 and CeO2, which facilitates carrier separation (Fig. 26). In addition, Fan et al.177 constructed a binary Bi-based Bi2MoO6/BiOI composite heterojunction (Fig. 27a and b) using an anion exchange method with BiOI as a precursor. The as-prepared Bi2MoO6/BiOI exhibited improved photocatalytic activities in the degradation of RhB (Fig. 27c). The sample with a Mo/I molar ratio of 50% exhibited the best activity under visible light excitation due to the formation of a type II heterojunction between Bi2MoO6 and BiOI (Fig. 27d). This type of charge migration and separation were also observed for heterojunctions formed between ZnO/BiOI and BiOCl/Bi3PO4 couples.178,179 If three matched bismuthal semiconductors are coupled together, a ternary heterojunction such as Bi2S3/Bi2O3/Bi2O2CO3 can be constructed that exhibits a better performance.180 The enhancement in the activity of Bi2S3/Bi2O3/Bi2O2CO3 is ascribed to the increased light absorption and efficient charge separation by the double type II heterojunction (Fig. 28). Type II carrier migrations can also occur in homojunctions. In a homojunction, the interface between two homogenous semiconductors forms due to different phase structures.181,182
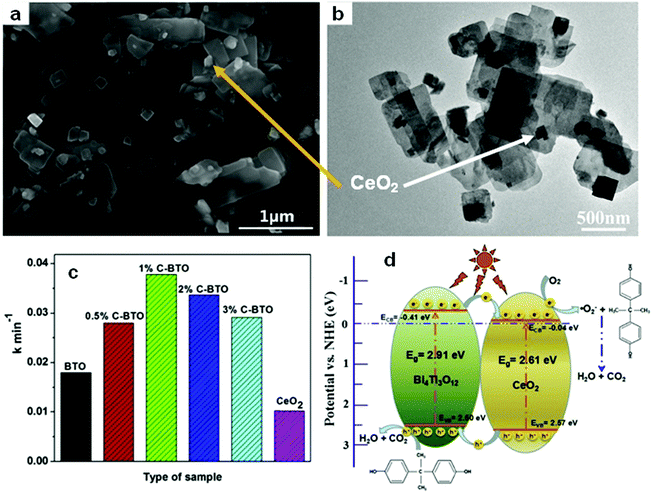 | ||
| Fig. 26 (a) SEM and (b) TEM images of CeO2/Bi4Ti3O12 composites (1%C–BTO); (c) comparison of pseudo-first-order kinetic constants of different photocatalysts for BPA; and (d) a schematic illustration of the charge migration in the 1%C–BTO composites under light irradiation. Reproduced with permission from ref. 176. Copyright 2016 Elsevier. | ||
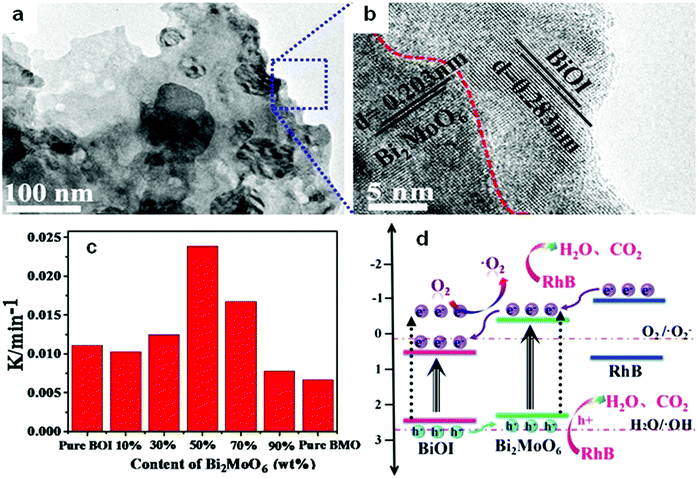 | ||
| Fig. 27 (a) TEM images and (b) the corresponding HRTEM images of Bi2MoO6/BiOI composites (BMO/BOI = 50%); (c) reaction rate constants for RhB degradation on BiOI (BOI), Bi2MoO6 (BMO) and BMO/BOI; and (d) a schematic diagram showing the charge transfer of a BMO/BOI heterostructure. Reproduced with permission from ref. 177. Open Access 2016 World Scientific. | ||
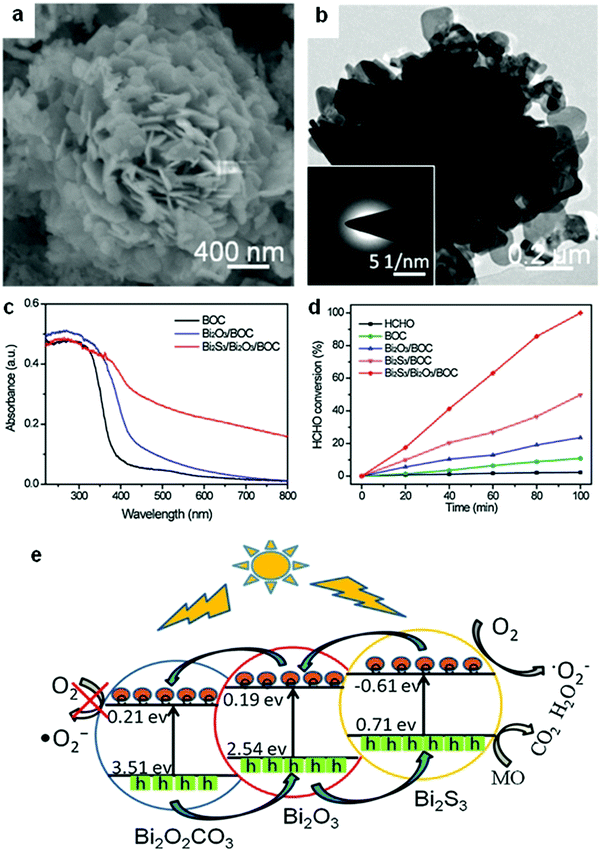 | ||
| Fig. 28 (a) SEM and (b) TEM images of a ternary Bi2S3/Bi2O3/Bi2O2CO3 composite photocatalyst; (c) UV-visible absorption spectra of Bi2O2CO3(BOC), Bi2O3/BOC, and Bi2S3/Bi2O3/BOC; (d) comparison of the photocatalytic HCHO removal of Bi2O2CO3(BOC), Bi2O3/BOC, Bi2S3/BOC, and Bi2S3/Bi2O3/BOC; and (e) a schematic diagram showing the charge-transfer process in ternary Bi2S3/Bi2O3/Bi2O2CO3 double heterojunctions. Reproduced with permission from ref. 180. Copyright 2016 Elsevier. | ||
Numerous preparation strategies for conventional heterojunctions (mainly Type II) have been reported, and most of these techniques have revealed enhanced photocatalytic activities (Table 1). Nevertheless, conventional heterojunctions have evident limitations. Photo-generated electrons migrate from a more negative CB to a less negative CB and the holes from a more positive VB to a less positive VB. This type of migration for electrons and holes reduces the redox capacity of the photocatalyst. Consequently, any improvement in the performance of the photocatalysts will be significantly limited.
| Bismuthal compound | Coupled material | Method | Application | Ref. |
|---|---|---|---|---|
| Rhodamine B, RhB; methylene blue, MB; methyl orange, MO; bisphenol A, BPA; ciprofloxacin, CIP; PEDOT:PSS = Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate). | ||||
| Binary Bi and non-Bi heterojunction | ||||
| Bi2O3 | FeVO4 | Grounding and calcination | Malachite green | 186 |
| Bi2O3 | TiO2 | Hydrothermal synthesis and calcination | MB | 187 |
| Bi2O3 | g-C3N4 | Self-assembly | RhB | 188 |
| Bi2O3 | g-C3N4 | Mixing-calcination | RhB | 189 |
| Bi2S3 | ZnS | Cation exchange | MB | 190 |
| Bi2S3 | ZnS | Solvothermal method | H2 generation | 191 |
| Bi2S3 | Pd4S | Thermal reduction and cation exchange | Atrazine | 192 |
| Bi2O2CO3 | CdS | Reflux | MB | 193 |
| Bi2Ti2O7 nanosheets | TiO2 fibers | Hydrothermal method | RhB | 194 |
| Bi12TiO20 | g-C3N4 | Hydrothermal method | Gaseous HCHO | 195 |
| Bi4Ti3O12 | g-C3N4 | Ball milling | Acid orange II (AO-7) | 196 |
| Bi4Ti3O12 | CeO2 | Molten salt and ion impregnation | BPA | 176 |
| Bi2O2CO3 | g-C3N4 | Calcination | Dibutyl phthalate and MO | 197 |
| Bi2O2CO3 | Ag3PO4 | Hydrothermal method and precipitation | RhB | 198 |
| BiFeO3 | g-C3N4 | Hydrothermal method | Guaiacol | 199 |
| BiFeO3 | CuO | Hydrothermal synthesis and impregnation | MO | 200 |
| Bi2Sn2O7 | In2O3 | Impregnation | RhB | 201 |
| BiOCOOH | Ag2O | Solvothermal deposition | RhB and p-chlorophenol | 202 |
| BiVO4 | TiO2 fibers | Hydrothermal method | Brilliant red X-3B | 203 |
| BiVO4 | 3DOM TiO2 | Hydrothermal method | RhB | 204 |
| BiVO4 | g-C3N4 | Ultrasonic assembly | CO2 reduction | 205 |
| BiVO4 | CeO2 | Coprecipitation and subsequent annealing | RhB | 206 |
| BiVO4 | Ag2O | Impregnation and evaporation | MO | 207 |
| BiVO4 | ZnO | Annealing of mixture | RhB | 208 |
| BiVO4 | SnO2 | Hydrothermal method | MB | 209 |
| BiVO4 | Co3O4 | Drop-casting method | Water oxidation | 210 |
| BiVO4/FTO | PEDOT:PSS | Electrodeposition | Photo-photocurrent | 211 |
| Bi2WO6 | TiO2 | Hydrothermal method | RhB and MO | 212 |
| Bi2WO6 | TiO2 fibers | Hydrothermal method | RhB and phenol | 213 |
| Bi2WO6(001) | TiO2(001) | Hydrothermal method | MB | 214 |
| Bi2WO6 | WO3 | Hydrothermal method | RhB | 215 |
| Bi2WO6 | α-Fe2O3 | Electrospinning with sintering | RhB | 216 |
| Bi2WO6 | CeO2 | Hydrothermal method | RhB | 217 |
| Bi2WO6 | AgCl | Hydrothermal method | RhB | 218 |
| Bi2WO6 | Ag2O | Chemical precipitation | RhB | 219 |
| Bi2WO6 | CdS and ZnS | Surface functionalization | RhB | 220 |
| Bi2MoO6 | TiO2 | Solvothermal method | Phenol and nitrobenzene | 221 |
| Bi2MoO6 | g-C3N4 | Liquid chemisorption and thermal treatment | RhB | 222 |
| Bi2MoO6 | Ag3VO4 | Hydrothermal method and precipitation | RhB | 223 |
| Bi2MoO6 | AgBr | Precipitation–deposition | RhB | 224 |
| Bi2MoO6 | AgI | Precipitation–deposition | RhB and BPA | 225 |
| Bi2MoO6 | MoO3 | Chemical vapor deposition | O2 evolution and glycerol oxidation | 226 |
| Bi2MoO6 | MoO3 | Hydrothermal method | Photoanode | 227 |
| Bi2SiO5 | AgI | Precipitation | Acid red G and gaseous HCHO | 228 |
| BiOCl | g-C3N4 | Solvothermal method | RhB | 229 |
| BiOCl | CuS | Hydrothermal method | RhB | 230 |
| BiOCl | TiO2 | Solvothermal method | Benzene | 231 |
| BiOBr | N doped graphene | Wet chemical method | Chlorpyrifos detection | 232 |
| BiOBr | CoFe2O4 | Solvothermal method | Congo red | 233 |
| BiOBr | NiFe2O4 | Hydrothermal method | MB | 234 |
| p-Type BiOBr | n-Type La2Ti2O7 | Refluxed in oil bath | RhB | 235 |
| BiOBr | CdWO4 | Hydrothermal synthesis and precipitation | RhB | 159 |
| BiOBr | CeO2 | Precipitation–deposition | RhB | 236 |
| BiOBr | Ag3PO4 | Precipitation–deposition | RhB | 237 |
| BiOI | TiO2 nanotube | Impregnating-hydroxylation | MO | 238 |
| BiOI | TiO2 fibers | Successive ionic layer adsorption and reaction | MO | 239 |
| BiOI | Fe2O3 | In situ hydrolysis | RhB | 240 |
| BiOI | La(OH)3 | Chemical impregnation | NO | 241 |
| BiOX (X = Cl, Br, I) | AgX (X = Cl, Br and I) | Precipitation | RhB | 242 |
| Bi4O5I2 | g-C3N4 | Solvothermal method using an ionic liquid | RhB and endocrine | 243 |
| Binary Bi-based heterojunctions | ||||
| α-Bi2O3/β-Bi2O3 | Solid-state reaction | Indigo carmine and RhB | 244 | |
| α-Bi2O3/β-Bi2O3 | In situ phase transformation by calcination | RhB | 245 | |
| Bi2O3-Bi2S3 | Hydrothermal method | RhB | 184 | |
| Bi2O3/BiVO4 | Solvothermal synthesis followed by annealing | Phenol | 246 | |
| Bi2O3/BiVO4 | Hydrothermal method | RhB | 247 | |
| Bi2O3 QDs/BiVO4 fibers | Direct heat treatment | RhB | 248 | |
| Bi2WO6/Bi2O3 | Solid-state reaction | RhB | 249 | |
| Bi2O3/BiOCl | Alkaline treatment | MO | 250 | |
| β-Bi2O3/BiOI | In situ reaction | MO | 251 | |
| Bi5O7I/Bi2O3 | Chemical etching | Malachite green | 252 | |
| Bi2S3/BiOCl | Solvothermal synthesis | Salicylic acid, RhB | 253 | |
| Bi2S3/Bi2WO6 | Anion exchange approach | RhB | 254 | |
| Bi2S3/Bi2WO6 | Hydrothermal method | Reduction of Cr(VI) | 255 | |
| Bi2S3/Bi4Ti3O12 nanofibers | In situ ion exchange | RhB | 256 | |
| Bi2S3/Bi2O2CO3 | Anion exchange | Gaseous NO | 257 | |
| Bi2S3/Bi2O2CO3 hollow microspheres | One-pot room temperature route | RhB | 258 | |
| Bi2Ti2O7/Bi4Ti3O12 | One-step molten salt method | MO, RhB | 259 | |
| Bi12TiO20/Bi2WO6 | Hydrothermal method | RhB | 260 | |
| BiVO4/Bi2O2CO3 | Hydrothermal method | RhB | 261 | |
| BiPO4/BiOBr | Mixing in solvent | Gaseous o-dichlorobenzene | 262 | |
| Bi2MoO6/BiVO4 | Spin-coating | Photoelectrode | 263 | |
| Bi2MoO6/BiPO4 | Hydrothermal method | RhB | 264 | |
| Bi2WxMo1−xO6/BiOCl | Solvothermal method | RhB | 265 | |
| Bi3.64Mo0.36O6.55/Bi2MoO6 | Hydrothermal method | RhB | 266 | |
| BiOI/BiVO4 | Coprecipitation | Pseudomonas aeruginosa | 267 | |
| BiOI/BiVO4 | Precipitation–deposition | MO | 268 | |
| BiOCl/BiVO4 | Coprecipitation method | RhB | 269 | |
| BiOCl/Bi12O17Cl2 | Hydrothermal method | MO | 270 | |
| BiOI/Bi2MoO6 | Precipitation–deposition | BPA | 271 | |
| Bi2MoO6/BiOI | hydrothermal method | RhB | 177 | |
| Bi24O31Cl10/BiOCl | Phase transformation by annealing | Conversion of benzyl alcohol | 272 | |
| Bi4O5I2/Bi5O7I | In situ phase transformation | BPA and RhB | 273 | |
| Bi4O5I2/Bi5O7I | Hydrothermal method | Propylparaben | 274 | |
| Bi2O2CO3/BiOI | Pore impregnation | RhB | 275 | |
| Bi2O4-Decorated BiOBr | Alkali posttreatment assisted light irradiation | MO | 276 | |
| Monoclinic BiVO4/tetragonal BiVO4 | Hydrothermal method | RhB | 277 | |
| BiVO4/Bi4V2O11 | Precursor conversion | Photoelectrodes | 278 | |
| Ternary heterojunctions | ||||
| Bi2O3/Bi2S3/MoS2 | Hydrothermal method | O2 evolution and MB degradation | 279 | |
| AgI/BiOI–Bi2O3 | Etching-deposition | Cr(VI) reduction | 280 | |
| BiOClx/BiOBry/BiOIz | Electrospinning and the sol–gel methods | Trichloroethylene | 281 | |
| Bi2S3/Bi2O3/Bi2O2CO3 | Heat treatment and ion exchange | HCHO, MO, and phenol | 180 | |
| Bi7O9I3/AgI/AgIO3 | Chemical deposition | MO and gaseous NO | 282 | |
| Bi7O9I3/Bi5O7I/g-C3N4 | Hydrothermal method | Crystal violet | 283 | |
| BiOBr/Co(OH)2/PVP | Solvothermal synthesis | MO | 284 | |
Given the limited improvement of a simple conventional heterojunction, several strategies combining heterojunction and morphology control, such as the preparation of a MoS2/Bi12O17Cl2 2D bilayer heterostructure183 and a Bi2S3 nanorods/Bi2O3 microtubes composite,184 have been developed to further enhance the performance of bismuthal photocatalysts. In our previous work, we found that heterojunctions can form between two adjacent (001) and (110) facets of BiOI, whereas their CBs and VBs are located in different positions. Consequently, BiOI with a hierarchical morphology and an optimal ratio of (001) and (110) facets was found to possess the highest photocatalytic activity.185 However, the combination of heterojunction and morphology control is still unable to overcome the inherent limitations of a conventional heterojunction, although this characteristic results in the better performance of as-prepared photocatalysts.
Direct Z-scheme heterojunctions
In 2013, Yu et al. proposed a direct Z-scheme heterojunction concept to explain the enhancement in the photocatalytic activity of a TiO2/g-C3N4 composite photocatalyst.285 This direct Z-scheme heterojunction is different from conventional liquid-phase Z-scheme heterojunctions and all-solid-state Z-scheme heterojunctions. The direct Z-scheme heterojunction does not require an electron medium.286–290 The transfer of charge carriers in this heterojunction is through the built-in electric field between the interface of SC-A and SC-B (Fig. 29a). However, the transfer of charge carriers in the liquid-phase (Fig. 29b) and all-solid-state (Fig. 29c) Z-scheme heterojunction is through the use of ions in the solution and noble metal NPs as electron conductors, respectively.291–295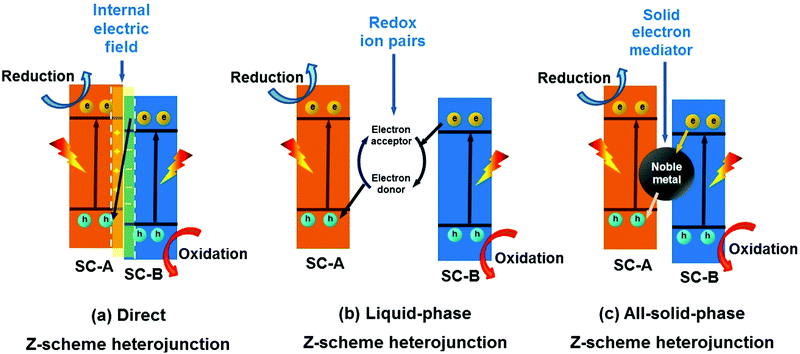 | ||
| Fig. 29 Schematic illustration of (a) direct Z-scheme, (b) liquid-phase Z-scheme and (c) all-solid-state Z-scheme heterojunctions (SC represents semiconductor). | ||
The structure of a direct Z-scheme heterojunction is similar to that of a type-II heterojunction (Fig. 25b) but with different charge carrier transport mechanisms. In a typical type-II heterojunction photocatalyst, SC-A has higher CB and VB positions than SC-B. Under light irradiation, the electrons in SC-A and holes in SC-B will transfer to the CB of SC-B and VB of SC-A, respectively. This process results in the spatial separation of the electron–hole pairs. However, the reduction ability of photo-generated electrons in the CB of SC-B and the oxidation ability of holes in the VB of SC-A are greatly reduced.
In contrast, the direct Z-scheme heterojunction exhibits a completely different mechanism of charge carrier migration. A photo-generated electron with a low reduction potential in SC-B will recombine with a photo-generated hole with a low oxidation potential in SC-A (Fig. 29a). Finally, the photo-generated electron and hole will remain in the CB of SC-A and VB of SC-B, respectively. The direct Z-scheme heterojunction will result in the spatial separation of useful charge carriers, remove useless charge carriers, and optimize the redox ability of the SC-A and SC-B composition system. Therefore, an enhanced photocatalytic performance can be observed in a direct Z-scheme heterojunction photocatalyst. In addition, the transfer mechanism of the charge carriers in a direct Z-scheme heterojunction is also different from that of conventional liquid-phase and all solid-state Z-scheme heterojunctions. No electron conductor is needed in a direct Z-scheme heterojunction, and the charge carrier can directly migrate using the built-in field at the SC-A and AC-B interfaces. Moreover, the fabrication cost of the direct Z-scheme photocatalyst is greatly reduced. Significantly, the direct Z-scheme heterojunction offers at least two obvious merits, namely, a low fabrication cost and high redox ability.
Many Bi-based direct Z-scheme heterojunction photocatalysts have been prepared and reported. For example, Liu et al.296 prepared a CdTe/Bi2S3 direct Z-scheme heterojunction photocatalyst using an electrostatic adsorption and self-assembly method. The obtained CdTe/Bi2S3 exhibited higher photocurrent responses and microcystin-LR sensitivity than the individual CdTe and Bi2S3 nanorod components. Meanwhile, Dai and colleagues297 formed a g-C3N4/Bi2MoO6 direct Z-scheme heterojunction between g-C3N4 and Bi2MoO6 using a hydrothermal method. This g-C3N4/Bi2MoO6 photocatalyst exhibited a higher activity than the individual g-C3N4 and Bi2MoO6 components in the photocatalytic degradation of MB (Fig. 30). Ullah and co-workers298 reported the synthesis of a Se/BiVO4 direct Z-scheme heterojunction photocatalyst and its higher photocurrent density than that of its individual Se and BiVO4 components. Huang and colleagues299 demonstrated a direct Z-scheme electron migration mechanism between g-C3N4 and BiOIO3 in their prepared g-C3N4/BiOIO3 composite under UV-visible light irradiation. The photocatalytic activity of the g-C3N4/BiOIO3 composite was shown to be markedly higher than those of g-C3N4 and mechanically mixed g-C3N4/BiOIO3 for the removal of RhB. We recently fabricated a Bi2O3/g-C3N4 direct Z-scheme heterojunction photocatalyst using an in situ room-temperature approach, including photoreduction deposition of Bi3+ and subsequent air-oxidation of the resultant metallic Bi.300 In the prepared composite, Bi2O3 quantum dots were uniformly distributed on the surface of g-C3N4 sheets (Fig. 31a), and the photocatalytic activity of Bi2O3/g-C3N4 was found to be higher than those of pure Bi2O3 and g-C3N4 in the photocatalytic degradation of phenol under visible light (Fig. 31b). This phenomenon can be attributed to the formed direct Z-scheme heterojunction (Fig. 31c). More reports on direct Z-scheme heterojunctions are listed in Table 2 for reference.
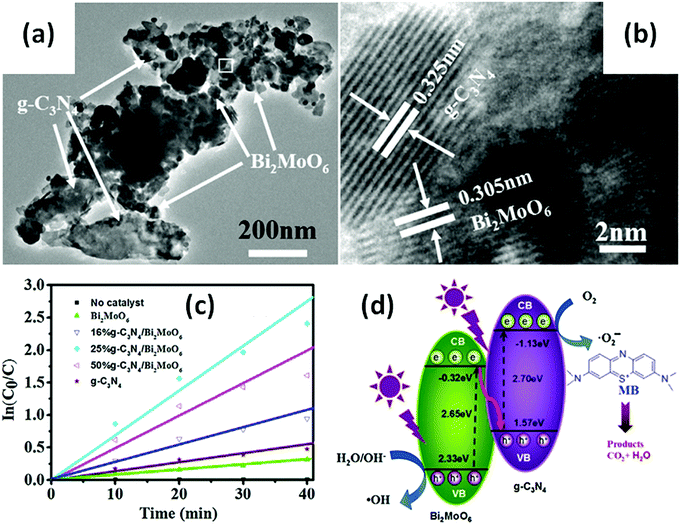 | ||
| Fig. 30 (a) TEM and (b) HRTEM images of a g-C3N4/Bi2MoO6 composite; (c) pseudo first-order fitted kinetic curves of MB degradation on g-C3N4/Bi2MoO6 composites (25%g-C3N4/Bi2MoO6); and (d) a schematic illustration of electron and hole migration in a g-C3N4/Bi2MoO6 direct Z-scheme heterojunction. Reproduced with permission from ref. 297. Copyright 2015 Elsevier. | ||
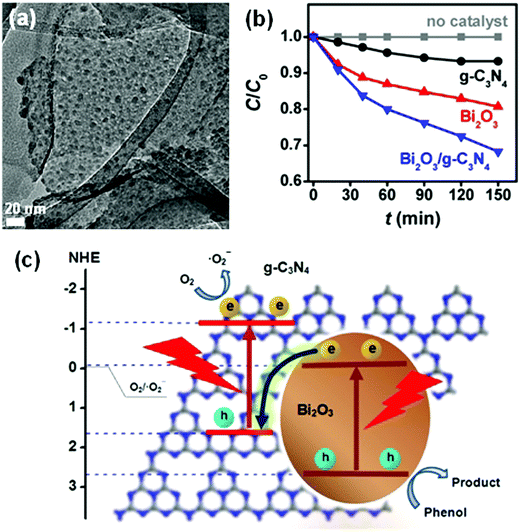 | ||
| Fig. 31 (a) TEM images of a Bi2O3/g-C3N4 composite; (b) comparison of the photocatalytic performance of Bi2O3, g-C3N4, and Bi2O3/g-C3N4 in the degradation of phenol; and (c) the photocatalytic mechanism of a Bi2O3/g-C3N4 direct Z-scheme heterojunction. Reproduced with permission from ref. 300. Copyright 2018 Elsevier. | ||
| Bismuthal compound | Coupled material | Method | Application | Ref. |
|---|---|---|---|---|
| Rhodamine B, RhB; methylene blue, MB; methyl orange, MO; bisphenol A, BPA; ciprofloxacin, CIP. | ||||
| Bi2O3 | g-C3N4 | Ball milling and heat treatment | MB | 301 |
| Bi2Sn2O7 | g-C3N4 | High-temperature solid-state reaction | MB and acid red 18 | 302 |
| Bi2MoO6 | g-C3N4 | Hydrothermal method | MB | 297 |
| BiOBr | g-C3N4 | Reflux process | RhB and BPA | 303 |
| BiOIO3 | g-C3N4 | Hydrothermal method | MO, RhB, and dichlorophenol | 299 |
| BiOI | CdS | Hydrothermal method | RhB | 304 |
| Bi2WO6 | MoS2 | Hydrothermal method | RhB | 305 |
| Bi2MoO6 | CuO, Co3O4, NiO | Precipitation | RhB | 306 |
| Bi2O3 | NaNbO3 | Ball milling method | RhB | 307 |
| BiVO4 | Se film | Chemical vapor deposition | Photocurrent enhancement | 298 |
| BiVO4 | SiC | Precipitation followed by calcination | O2 evolution | 308 |
| BiOBr | Bi2MoO6 | Two-step coprecipitation method | RhB and CIP | 309 |
| BiO1−xBr | Bi2O2CO3 | Solvothermal method | CIP | 310 |
| BiPO4 | Bi2O2(OH)(NO3) | Hydrothermal method | Dichlorophenol | 311 |
| MoS2/BiOI/AgI | Precipitation | RhB | 312 | |
| BiVO4/ZnIn2S4/g-C3N4 | Impregnation and calcination | Congo red and metronidazole | 313 | |
Surface modification
Surface modification is an effective and widely used method for enhancing the activity of a photocatalyst.314 It also plays an important role in improving the performance of Bi-based photocatalysts. An extensive range of materials can be adopted to modify surfaces. Carbon-based materials, metals, ions, polymers, and semiconductors can be used to modify bismuthal photocatalysts. If a semiconductor is used as a modifier, a nanosized heterojunction will be formed, and the carriers will transfer faster in the tiny heterojunction than in bulk junctions because of the larger interface ratio. For example, the dandelion-like MoS2-decorated Bi2S3 prepared by Li et al.315 exhibited improved photocatalytic performance in the degradation of RhB due to the tiny heterojunctions formed between MoS2 and Bi2S3 (Fig. 32). Further to this, SnO2 modified BiVO4 was found to be able to reduce CO2 to CH4, with the introduced SnO2 acting as a redox reaction platform capable of accepting the photogenerated electrons from BiVO4 nanoplates during the conversion of CO2.316 AgBr, graphene-like BN (g-BN), and some bismuthal compounds with a narrow band gap have also been reported as semiconductor modifiers for Bi-based photocatalysts (Table 3).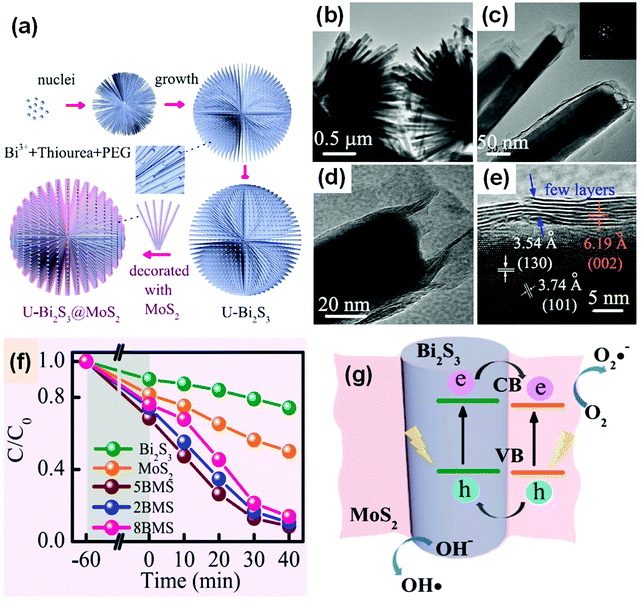 | ||
| Fig. 32 (a) Schematic illustration of the formation of MoS2-decorated Bi2S3 (BMS); (b–d) TEM and (e) HRTRM images of MoS2-decorated Bi2S3; (f) comparison of the photocatalytic performance of RhB on MoS2, Bi2S3 and BMS (2MBS, 5MBS, and 8MBS present in molar ratios of Mo4+-to-Bi3+ of 20%, 50%, and 80%) under visible-light irradiation; and (g) a schematic diagram of the charge transfer at a MoS2/Bi2S3 heterojunction. Reproduced with permission from ref. 315. Open Access 2017 Springer Nature. | ||
| Modifier | Bi-Based material | Method | Target material of enhanced performance | Ref. |
|---|---|---|---|---|
| GO: graphene oxide; RGO: reduced graphene oxide; MOF: metal–organic framework; CQDs: carbon quantum dots; GQDs: graphene quantum dots; Rh6G: Rhodamine 6G; MB: methylene blue; PEDOT:PSS: poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate). | ||||
| Semiconductor | ||||
| MoS2 | Bi2S3 | Hydrothermal method | RhB | 315 |
| g-C3N4 | Bi2O4 | Hydrothermal method | MO | 335 |
| CdS | BiVO4 | Solvothermal method | H2 generation | 336 |
| Cu2O | Bi2WO6 | Interfacial self-assembly | MB | 337 |
| AgBr QDs | Bi2WO6 | Precipitation–deposition | MB and phenol | 338 |
| g-BN | BiOI | Solvothermal method | RhB | 339 |
| g-BN | BiOCl | Microwave-assisted method | Cr(VI) reduction | 340 |
| g-BN | BiPO4 | Solvothermal method | Enrofloxacin | 341 |
| Bi2O3 QDs | BiVO4 | Calcination | RhB | 248 |
| Bi2O4 | BiOBr | Alkali posttreatment under light irradiation | MO | 276 |
| BiOI | Bi2MoO6 | Deposition–precipitation | MO | 342 |
| BiOBr | BiPO4 | Hydrothermal method | MB | 343 |
| Carbon material | ||||
| Carbon | Bi4Ti3O12 | Coprecipitation | MO | 344 |
| Carbon | Bi2MoO6 | Two-step hydrothermal method | RhB | 345 |
| Carbon microspheres | BiFeO3 | Hydrothermal method | RhB | 346 |
| Carbon microspheres | Bi0.5Dy0.5VO4 | Self-assembly | H2 generation | 347 |
| Carbon nanotubes | Bi4O5Br2 | Solvothermal method | Tetracycline hydrochloride and RhB | 163 |
| Carbon dots | BiOI | Immersion | MO | 348 |
| CQDs | Bi20TiO32 | Oil bath | Isoproturon | 349 |
| CQDs | Bi4O5I2 | Solvothermal method | RhB | 350 |
| CQDs | BiOI | Hydrothermal method | RhB | 351 |
| GO | BiVO4 | Hydrothermal method | RhB | 352 |
| GO | BiOCl | Two-step synthesis | RhB | 353 |
| GO | BiOxIy | Hydrothermal method | Crystal violet | 354 |
| GO | BiFeO3 | Ultrasonic treatment | BPA | 355 |
| GO | Bi2O2CO3 | Precipitation | Gaseous NO | 356 |
| GO | Bi2WO6 | Liquid nitrogen freezing and freeze-drying dehydration | RhB | 323 |
| GO | BiOI | Self-assembly | Phenol | 357 |
| RGO | BiOI | Hydrothermal method | MO | 358 |
| RGO | BiOBr | Two-step hydrothermal method | Higher activity for MO than for RhB | 359 |
| RGO | Bi25FeO40 | Hydrothermal method | MO | 360 |
| RGO | Bi2WO6 | Wrapped | RhB | 361 |
| RGO | Bi2S3 | Hydrothermal method | 2,4-Dichlorophenol | 362 |
| RGO | BiPO4 | Solvothermal method | Chlorpyrifos detection | 363 |
| Graphene | Ag/BiOBr0.2I0.8 | Combined solvothermal method and photodeposition | RhB | 364 |
| Graphene | BiOCl0.7Br0.3 | Solvothermal method | RhB | 365 |
| Graphene | Bi2O2CO3 | Hydrothermal method | RhB | 366 |
| Ag and graphene | Bi2WO6 | Hydrothermal method followed by photodeposition | RhB | 367 |
| GQDs | Bi2MoO6 | Self-assembly | BPA, MB, TC, CIP, and phenol | 324 |
| Au and RGO | Bi2MoO6 | Solvothermal synthesis and photochemical reduction | RhB | 368 |
| Ag and graphene | Bi2Fe4O9 | Multistep synthesis | MB | 369 |
| Metal and ion | ||||
| Pd | Bi2MO6 | Photoreduction | Phenol | 370 |
| Pd | Bi2WO6 | Chemical deposition | RhB | 371 |
| Pd | BiOBr | Solvothermal method | RhB and CIP | 372 |
| Pd | m-BiVO4/BiOBr | Reduction in the dark | Polychlorinated biphenyls | 373 |
| Pt | BiOBr | Photodeposition | p-Nitrophenol | 374 |
| Pt | BiFeO3 | Impregnation and thermal reduction | MO | 375 |
| Pt | Bi2WO6 | Chemical reduction | Rhodamine 6G | 376 |
| Pt | Bi2WO6/TiO2 | Photodeposition | CO2 reduction | 377 |
| Au | BiOCl | Photodeposition | Formaldehyde | 378 |
| Au | BiVO4 | Pulsed laser deposition | Congo red and water splitting | 379 |
| Au | BiPO4 | Solvothermal method | CO oxidation | 380 |
| Au | Bi2O2CO3 | Hydrothermal method | NO | 381 |
| Ag | BiFeO3 film | Sol spin coating and annealing | O2 evolution | 382 |
| Ag | BiOBr | Solvothermal method | Tetracycline hydrochloride | 383 |
| Ag | BiVO4 | Hydrothermal method | Reduced electron-transfer resistance | 384 |
| Ag | Bi4Ti3O12 | Sonochemical method | RhB | 385 |
| Ag | Bi2WO6 | Irradiation | RhB | 386 |
| Bi | Bi2WO6 | In situ reduction | Phenol | 319 |
| Bi | Bi2WO6 nanorod | Hydrothermal reaction | Rh6G | 387 |
| Bi | Bi2MoO6 | Solvothermal reduction | Rh6G | 388 |
| Bi | BiPO4 | Solvothermal treatment | MB | 389 |
| Bi | BiOBr | Reduction reaction at room temperature | MO | 390 |
| Bi | BiOI/CNFs | Solvothermal method | MO | 391 |
| Cr | Bi4Ti3O12 | Sol–gel hydrothermal process | H2 generation | 69 |
| Ni | BiOI | Photo-assisted deposition | Reducing Cr(VI) to Cr(III) | 392 |
| Fe(III) | Bi2O2CO3 | Impregnation | MO | 393 |
| Fe | Bi2Ti2O7 | Precipitation | MO | 394 |
| Fe2O3 | Macroporous BiVO4 | Impregnation | 4-Nitrophenol | 395 |
| CoOx | BiFeO3 | Photodeposition | O2 evolution | 396 |
| Rh(III) | BiOCl | Impregnation | Gaseous acetaldehyde (Vis) | 397 |
| Organic material | ||||
| Polythiophene | Bi2MoO6 | In situ chemical oxidative polymerization | RhB | 326 |
| Polyaniline | Bi2O2CO3 | Low-temperature chemical method | RhB | 327 |
| PVP | BiOBr | Solvothermal method | RhB | 328 |
| MIL-101 (MOF) | BiVO4 | Hydrothermal method | RhB | 398 |
| Ionic liquid | BiPO4 | Hydrothermal method | RhB | 399 |
| Surface defect | ||||
| Bi defects | Bi6S2O15 | Hydrothermal method | Phenol | 330 |
| Dimer vacancy | Bi2WO6 | Solvothermal method | Diclofenac | 332 |
| Defect | BiPO4 | Ball milling | MB | 400 |
Coupling bismuthal photocatalysts with conductive materials is another effective surface modification strategy. The conductive materials usually act as electron acceptors to promote the migration of photo-generated electrons and consequently facilitate the separation of carriers. Metals, carbon-based materials, conductive polymers, and metallic Bi have been adopted as electron scavengers to enhance the photocatalytic activities of bismuthal compounds (Table 3). Commonly employed noble metals include Au, Ag, Pt, and Pd. Hirakawa et al.317 obtained a composite by loading Au NPs on BiVO4. The prepared Au/BiVO4 exhibited an enhanced production of H2O2 from water than that of pure BiVO4 under visible-light irradiation due to the selective two-electron reduction of O2 by Au. In addition to noble metals, other metals can also serve as electron trapping agents. Park and co-workers318 decorated Ni NPs on W-doped BiVO4 nanofibers and obtained a fibrous composite (Ni@NiO/W:BiVO4 NFs). The process was followed by a calcination method, during which Ni NPs were partly oxidized to NiO. The as-prepared composite exhibited a higher photocurrent and O2 evolution than W-doped BiVO4 nanofibers and Pt-decorated fibers in the photocatalytic oxidation of water. The enhancement was attributed to the unique structure of the Ni@NiO decoration, in which the Ni metal traps the photo-generated electrons and NiO accepted holes. Moreover, the metal Bi-decorated Bi2WO6 exhibited a photocatalytic efficiency three times higher than that of pure Bi2WO6 in the degradation of phenol under visible light, because metal Bi not only acts as an electron acceptor, but also promotes the separation of carriers.319 Finally, the surface plasmon resonance of Bi was also embodied in the Bi/BiOBr composite, which possessed a higher activity for NO oxidation than that of pure BiOBr.320
Carbon materials, such as carbon dots (CDs), CNTs, graphene, graphene oxide (GO), reduced graphene oxide (RGO), carbon quantum dots (CQDs), and graphene quantum dots (GQDs), can be used as modifiers. Among these materials, graphene-based materials are more favorable because of their special graphitic structure and ideal conductivity.321 Priya et al.322 loaded a Bi2O3/BiOCl heterojunction on a prepared graphene sand composite using a wet impregnation method. The obtained composite showed an improved performance over that of Bi2O3/BiOCl for the mineralization of ampicillin and oxytetracycline. The improvement was attributed to the electron trapping role of the graphitic carbon on the graphene sand composite. When Bi2WO6 microspheres were wrapped with GO using a freeze-drying dehydration method, they exhibited a higher activity than that of pure Bi2WO6 on the photocatalytic degradation of RhB due to the improved separation of the electrons and holes by GO.323 GQDs possess a small particle size and high specific surface area. Therefore, GQDs are more favorable for the surface modification of photocatalysts. For example, Bi2MoO6 decorated with GQDs via a self-assembly approach, showed a remarkable increase in photocatalytic degradation efficiency of different target pollutants, including BPA, MB, TC, CIP, and phenol.324 Nevertheless, GO or RGO materials may suffer from instability in long-term reactions. Subramanian and colleagues325 found that RGO coupled with Bi2Ti2O7 might suffer from decomposition and result in a reduced performance of the composite after long-term illumination in an oxidation environment (Fig. 33a and b). The degradation of RGO was attributed to the oxidation by the formed ˙OH (Fig. 33c). Several conductive polymers, such as polyaniline, polypyrrole, and polythiophene, can play the role of electron scavenger and promote the electron migration in bismuthal photocatalysts.326–328 However, the conductivities of these polymers are much weaker than those of noble metal and carbon materials. Thus, the related research is less reported. Usually, amorphous carbon is not suitable for electron trapping because of its poor conductivity. Nevertheless, it also plays a positive role in the enhancement of photocatalytic activity due to it enhancing the visible light absorption and carrier separation of the photocatalyst.329
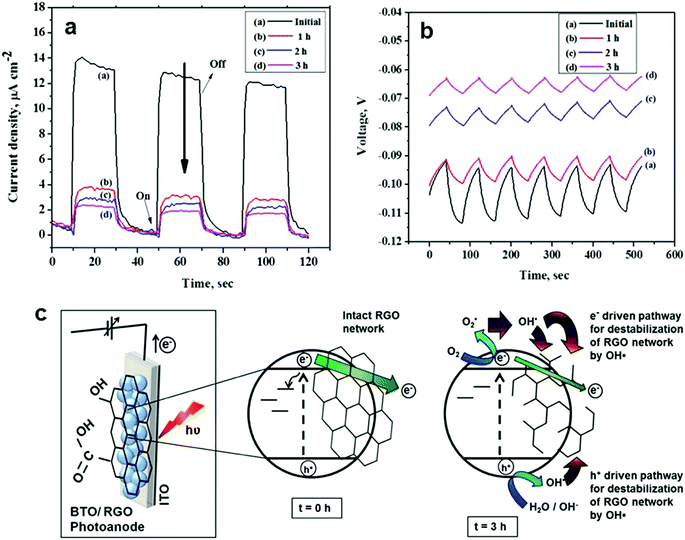 | ||
| Fig. 33 (a) I/t and (b) V/t curves of the Bi2Ti2O7/RGO composite (BTO/RGO) during long-term light irradiation and (c) the degradation mechanism of the RGO on a BTO/RGO film under light illumination for 3 h in an air-equilibrated solution. Reproduced with permission from ref. 325. Copyright 2016 Electrochemical Society. | ||
Introducing vacancies or elements with different chemical valence states may also improve the performance of photocatalysts. Wang et al.330 introduced vacancies into the surface of Bi6S2O15 nanowires by increasing the molar ratio of Na2SO4 and Bi(NO3)3 from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during hydrothermal synthesis. The light absorption of vacancy Bi6S2O15 nanowires at 370–500 nm was stronger than that of pure Bi6S2O15 nanowires. Vacancy Bi6S2O15 nanowires also showed improved photocatalytic activity for the degradation of phenol and MB. The enhancement was attributed to the improved visible-light response induced by the surface vacancies (Fig. 34). Zhang and colleagues331 treated BiVO4 at different temperatures under different atmospheres. The sample treated at 973 K in N2 exhibited the best activity for the formation of EG from aldehyde. The enhancement was attributed to the formation of V4+. Bi2WO6 with a large fraction of {100} high-energy facets showed relatively high efficiency in the photocatalytic degradation of diclofenac, because “Bi–O” dimer vacancy pairs formed on the {100} facets could bring about a narrower band gap and less photoexcited charge recombination.332 Moderate oxygen-deficient defects in BiOBr also play an indispensable role for superior photocatalytic CO2 reduction, by acting as the active sites for CO2 adsorption and activation, trapping photogenerated electrons and thus impact upon the recombination of the electron–hole pairs.333 Oxygen vacancies were also found to have a positive influence on the performance of BiOCl on photocatalytic CO2 reduction because oxygen vacancies can induce exciton dissociation and provide more photo-induced electrons for CO2 reduction.334
1 during hydrothermal synthesis. The light absorption of vacancy Bi6S2O15 nanowires at 370–500 nm was stronger than that of pure Bi6S2O15 nanowires. Vacancy Bi6S2O15 nanowires also showed improved photocatalytic activity for the degradation of phenol and MB. The enhancement was attributed to the improved visible-light response induced by the surface vacancies (Fig. 34). Zhang and colleagues331 treated BiVO4 at different temperatures under different atmospheres. The sample treated at 973 K in N2 exhibited the best activity for the formation of EG from aldehyde. The enhancement was attributed to the formation of V4+. Bi2WO6 with a large fraction of {100} high-energy facets showed relatively high efficiency in the photocatalytic degradation of diclofenac, because “Bi–O” dimer vacancy pairs formed on the {100} facets could bring about a narrower band gap and less photoexcited charge recombination.332 Moderate oxygen-deficient defects in BiOBr also play an indispensable role for superior photocatalytic CO2 reduction, by acting as the active sites for CO2 adsorption and activation, trapping photogenerated electrons and thus impact upon the recombination of the electron–hole pairs.333 Oxygen vacancies were also found to have a positive influence on the performance of BiOCl on photocatalytic CO2 reduction because oxygen vacancies can induce exciton dissociation and provide more photo-induced electrons for CO2 reduction.334
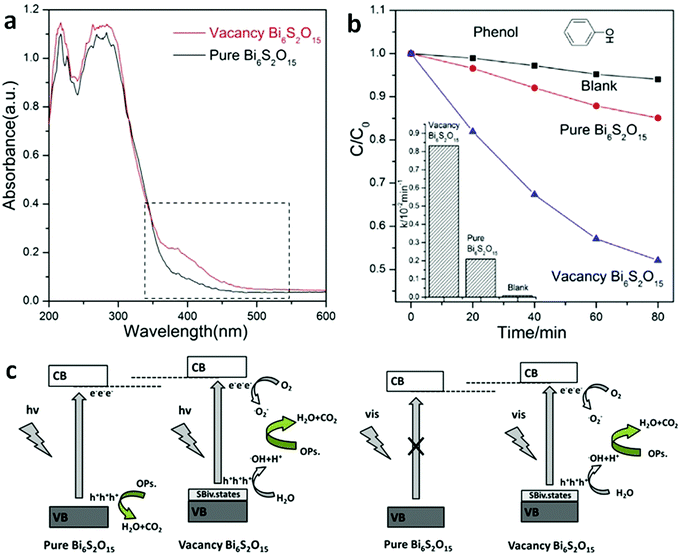 | ||
| Fig. 34 (a) UV-visible diffuse reflectance spectra of pure Bi6S2O15 and vacancy Bi6S2O15 nanowires; (b) degradation rate constant k of pure Bi6S2O15 and vacancy Bi6S2O15 nanowire photocatalysts; (c) schematic diagram of the carrier separation and photocatalytic reaction of pure Bi6S2O15 and vacancy Bi6S2O15 nanowires under UV-visible irradiation. Reproduced with permission from ref. 330. Copyright 2015 Elsevier. | ||
Conclusions and prospects
A layered structure and excellent visible-light response endow Bi-based photocatalysts with excellent photocatalytic activity and promising prospects for application in the fields of environmental science and energy. Studies centered on Bi-based photocatalysts will be beneficial for the future development of photocatalysts. Considerable work has been performed to control the composition, morphology, and structure of these catalysts to improve the photocatalytic performance of Bi-based photocatalysts. These photocatalysts are used for the visible-light photocatalytic degradation of organic pollutants, H2 generation, water splitting, and CO2 reduction. Although significant progress has been achieved, great efforts are still required to further explore the advantages of Bi-based photocatalysts as visible-light photocatalysts. The following five aspects deserve special attention:(1) Previous studies on Bi-based photocatalysts have mainly focused on the photocatalytic degradation of organic pollutants. Few studies have reported on the application of these photocatalysts in photocatalytic H2 generation, water splitting, and CO2 reduction. Several investigations have focused on BiVO4. The main obstacle in the study of Bi-based photocatalysts is the less negative CB and the poor reducing abilities of bismuthal compounds. More bismuthal materials should be extensively explored to overcome these obstacles. The construction of nanosized direct Z-scheme heterojunctions by coupling bismuthal semiconductors with semiconductors that have a more negative CB is a promising method for exploring Bi-based photocatalysts for reduction applications.
(2) Bi-based photocatalyst photodegradation has been primarily evaluated in aqueous solutions instead of under gaseous conditions. The latter condition is favorable for the use of Bi-based photocatalysts because visible light can be more fully utilized in the gas phase. The removal of formaldehyde and benzene from air is important for environmental remediation and indoor air purification. Therefore, extending the application of Bi-based photocatalysts to the degradation of gaseous contaminants is meaningful.
(3) The aim of photocatalysis is to solve serious environmental and energy problems in the world. We hope that this review can further stimulate the research into and application of Bi-based photocatalytic materials in the near future. We believe that the understanding and knowledge on Bi-based photocatalysts can be greatly enhanced and the experimental and characterization methods improved.
(4) Theoretical simulations can provide new insight into understanding the photocatalytic mechanism and relationship between the microstructure and performance. The theoretical investigations on Bi-based photocatalytic materials should be further strengthened, which will contribute to a deep understanding on the surfaces, interfaces, nanostructures and activity at the atomic and molecular level of the material properties.
(5) Although Bi-based photocatalysts have been continuously investigated over the past ten years, their photocatalytic performance is still far from the requirements for practical application. More research work is still needed to reach their real application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (21573170, 51320105001, U1705251 and 21433007), the Self-determined and Innovative Research Funds of SKLWUT (2017-ZD-4), and the Natural Science Foundation of Hubei Province of China (No. 2015CFA001). The project was also supported by the Internal Research Grant (RG 11/2016-2017R), and the EdUHK and General Research Fund (GRF 18301117) by RGC of the HK government. The work was also supported by the Natural Science Foundation of Hunan Province of China (2018JJ2457).References
- K. Maeda, K. Teramura, D. L. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef PubMed.
- Z. Huang, L. Pan, J. Zou, X. Zhang and L. Wang, Nanoscale, 2014, 6, 14044 RSC.
- P. Reddy, P. Reddy, E. Kwon, K. H. Kim, T. Akter and S. Kalagara, Environ. Int., 2016, 91, 94 CrossRef PubMed.
- P. Zhou, J. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920 CrossRef PubMed.
- O. Ola and M. M. Maroto-Valer, J. Photochem. Photobiol., C, 2015, 24, 16 CrossRef.
- J. Low, B. Cheng and J. Yu, Appl. Surf. Sci., 2017, 392, 658 CrossRef.
- S. Cao and J. Yu, J. Photochem. Photobiol., C, 2016, 27, 72 CrossRef.
- J. Low, J. Yu and W. Ho, J. Phys. Chem. Lett., 2015, 6, 4244 CrossRef PubMed.
- Q. Xiang, B. Cheng and J. Yu, Angew. Chem., Int. Ed., 2015, 54, 11350 CrossRef PubMed.
- C. Trellu, E. Mousset, Y. Pechaud, D. Huguenot, E. D. van Hullebusch, G. Esposito and M. A. Oturan, J. Hazard. Mater., 2016, 306, 149 CrossRef PubMed.
- S. Cao, F. F. Tao, Y. Tang, Y. Li and J. Yu, Chem. Soc. Rev., 2016, 45, 4747 RSC.
- W. Ong, Z. Lin and K. Domen, Part. Part. Syst. Charact., 2018, 35, 1700371 CrossRef.
- K. H. Leong, A. Abd Aziz, L. C. Sim, P. Saravanan, M. Jang and D. Bahnemann, Beilstein J. Nanotechnol., 2018, 9, 628 CrossRef PubMed.
- X. Chen, N. Li, Z. Kong, W. Ong and X. Zhao, Mater. Horiz., 2018, 5, 9 RSC.
- W. Ong, Front. Mater., 2017, 4, 11 Search PubMed.
- Y. W. Phuan, W. Ong, M. N. Chong and J. D. Ocon, J. Photochem. Photobiol., C, 2017, 33, 54 CrossRef.
- Z. Li, X. Meng and Z. Zhang, J. Photochem. Photobiol., C, 2018, 35, 39 CrossRef.
- W. Ong, L. Tan, S. Chai, S. Yong and A. R. Mohamed, ChemSusChem, 2014, 7, 690 CrossRef PubMed.
- W. Ong, L. Tan, Y. H. Ng, S. Yong and S. Chai, Chem. Rev., 2016, 116, 7159 CrossRef PubMed.
- E. Rahmanian, R. Malekfar and M. Pumera, Chem. – Eur. J., 2018, 24, 18 CrossRef PubMed.
- W. Han, Z. Li, Y. Li, X. Fan, F. Zhang, G. Zhang and W. Peng, Front. Chem., 2017, 5, 84 CrossRef PubMed.
- D. Zeng, W. Xu, W. J. Ong, J. Xu, H. Ren, Y. Chen, H. Zheng and D. L. Peng, Appl. Catal., B, 2018, 221, 47 CrossRef.
- D. Zeng, L. Xiao, W. J. Ong, P. Wu, H. Zheng, Y. Chen and D. L. Peng, ChemSusChem, 2017, 10, 4624 CrossRef PubMed.
- S. Zeng, P. Kar, U. K. Thakur and K. Shankar, Nanotechnology, 2018, 29, 052001 CrossRef PubMed.
- Y. Wu, Y. Chaing, C. Huang, S. Wang and H. Yang, Dyes Pigm., 2013, 98, 25 CrossRef.
- H. Fan, T. Jiang, H. Li, D. Wang, L. Wang, J. Zhai, D. He, P. Wang and T. Xie, J. Phys. Chem. C, 2012, 116, 2425 Search PubMed.
- H. He, J. Yin, Y. P. Li, Y. Zhang, H. Qiu, J. Xu, T. Xu and C. Wang, Appl. Catal., B, 2014, 156, 35 CrossRef.
- A. E. Nogueira, E. Longo, E. R. Leite and E. R. Camargo, Ceram. Int., 2015, 41, 12073 CrossRef.
- Z. Ni, Y. Sun, Y. Zhang and F. Dong, Appl. Surf. Sci., 2016, 365, 314 CrossRef.
- L. Zhang and Y. Zhu, Catal. Sci. Technol., 2012, 2, 694 Search PubMed.
- Y. Zhu, Y. Liu, Y. Lv, Q. Ling, D. Liu and Y. Zhu, J. Mater. Chem. A, 2014, 2, 13041 Search PubMed.
- T. Gao, Z. Chen, Q. Huang, F. Niu, X. Huang, L. Qin and Y. Huang, Rev. Adv. Mater. Sci., 2015, 40, 97 Search PubMed.
- H. F. Cheng, B. B. Huang and Y. Dai, Nanoscale, 2014, 6, 2009 RSC.
- L. Xu, Y. Wan, H. Xie, Y. Huang, X. Qiao, L. Qin and H. J. Seo, J. Am. Ceram. Soc., 2016, 99, 3964 CrossRef.
- H. B. Luong, H. T. Le Thi, V. C. Nguyen, T. K. Nguyen, V. T. Duong, V. T. Trinh and D. D. Dang, Mater. Lett., 2016, 164, 631 CrossRef.
- X. Zhang, Y. Yang, H. Li, X. Zou and Y. Wang, Prog. Chem., 2016, 28, 1550 Search PubMed.
- R. He, S. Cao and J. Yu, Acta Phys.-Chim. Sin., 2016, 32, 2841 Search PubMed.
- R. He, S. Cao, P. Zhou and J. Yu, Chin. J. Catal., 2014, 35, 989 CrossRef.
- X. Jin, L. Ye, H. Xie and G. Chen, Coord. Chem. Rev., 2017, 349, 84 CrossRef.
- B. Lamm, B. J. Trzesniewski, H. Doscher, W. A. Smith and M. Stefik, ACS Energy Lett., 2018, 3, 112 CrossRef.
- J. Di, J. Xia, H. Li, S. Guo and S. Dai, Nano Energy, 2017, 41, 172 CrossRef.
- L. Chen, J. He, Y. Liu, P. Chen, C. Au and S. Yin, Chin. J. Catal., 2016, 37, 780 CrossRef.
- N. Serpone and A. V. Emeline, J. Phys. Chem. Lett., 2012, 3, 673 CrossRef PubMed.
- W. Yu, J. Zhang and T. Peng, Appl. Catal., B, 2016, 181, 220 CrossRef.
- M. S. Akple, J. Low, Z. Qin, S. Wageh, A. A. Alghamdi, J. Yu and S. Liu, Chin. J. Catal., 2015, 36, 2127 CrossRef.
- K. Qi, B. Cheng, J. Yu and W. Ho, J. Alloys Compd., 2017, 727, 792 CrossRef.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179 CrossRef.
- J. Zai, F. Cao, N. Liang, K. Yu, Y. Tian, H. Sun and X. Qian, J. Hazard. Mater., 2017, 321, 464 CrossRef PubMed.
- J. Di, J. Chen, M. Ji, Q. Zhang, L. Xu, J. Xia and H. Li, Chem. Eng. J., 2017, 313, 1477 CrossRef.
- M. Gao, D. Zhang, X. Pu, H. Li, W. Li, X. Shao, D. Lv, B. Zhang and J. Dou, Sep. Purif. Technol., 2016, 162, 114 CrossRef.
- H. Zheng, W. Guo, S. Li, R. Yin, Q. Wu, X. Feng, N. Ren and J. Chang, Catal. Commun., 2017, 88, 68 CrossRef.
- P. Dumrongrojthanath, T. Thongtem, A. Phuruangrat and S. Thongtem, Res. Chem. Intermed., 2016, 42, 5087 CrossRef.
- L. Zhang, Z. Tang, W. Lau, W. Yin, S. Hu and L. Liu, Phys. Chem. Chem. Phys., 2017, 19, 20968 RSC.
- W. Meng, R. Hu, J. Yang, Y. Du, J. Li and H. Wang, Chin. J. Catal., 2016, 37, 1283 CrossRef.
- J. Han, G. Zhu, M. Hojamberdiev, J. Peng and P. Liu, J. Mater. Sci., 2016, 51, 2057 CrossRef.
- Z. Liang, Y. Cao, Y. Li, J. Xie, N. Guo and D. Jia, Appl. Surf. Sci., 2016, 390, 78 CrossRef.
- D. Wu, S. Yue, W. Wang, T. An, G. Li, H. Y. Yip, H. Zhao and P. K. Wong, Appl. Catal., B, 2016, 192, 35 CrossRef.
- Y. Wei, X. Wei, S. Guo, Y. Huang, G. Zhu and J. Zhang, Mater. Sci. Eng., B, 2016, 206, 79 CrossRef.
- V. Senthil, T. Badapanda, A. Chithambararaj, A. C. Bose and S. Panigrahi, Int. J. Hydrogen Energy, 2016, 41, 22856 CrossRef.
- J. Li, L. Cai, J. Shang, Y. Yu and L. Zhang, Adv. Mater., 2016, 28, 4059 CrossRef PubMed.
- Z. S. Liu, Z. L. Liu, J. L. Liu, J. W. Zhang, T. F. Zhou and X. Ji, Mater. Res. Bull., 2016, 76, 256 CrossRef.
- D. Liu, J. Huang, L. Cao, X. Tao and B. Zhang, J. Mater. Sci.: Mater. Electron., 2016, 27, 2473 CrossRef.
- H. Jung, S. Y. Chae, H. Kim, B. K. Min and Y. J. Hwang, Catal. Commun., 2016, 75, 18 CrossRef.
- L. Shan and Y. Liu, J. Mol. Catal. A: Chem., 2016, 416, 1 CrossRef.
- Y. Huang, H. Li, W. Fan, F. Zhao, W. Qiu, H. Ji and Y. Tong, ACS Appl. Mater. Interfaces, 2016, 8, 27859 Search PubMed.
- H. Li, Z. Yang, J. Zhang, Y. Huang, H. Ji and Y. Tong, Appl. Surf. Sci., 2017, 423, 1188 CrossRef.
- Y. Jiang, J. Hu and J. Li, RSC Adv., 2016, 6, 39810 RSC.
- H. Li, W. Li, S. Gu, F. Wang and H. Zhou, Catal. Sci. Technol., 2016, 6, 3510 Search PubMed.
- Z. Chen, X. Jiang, C. Zhu and C. Shi, Appl. Catal., B, 2016, 199, 241 CrossRef.
- M. Hasan, M. A. Basith, M. A. Zubair, M. S. Hossain, R. Mahbub, M. A. Hakim and M. F. Islam, J. Alloys Compd., 2016, 687, 701 CrossRef.
- X. Han, S. Dong, C. Yu, Y. Wang, K. Yang and J. Sun, J. Alloys Compd., 2016, 685, 997 CrossRef.
- B. Anke, M. Rohloff, M. G. Willinger, W. Hetaba, A. Fischer and M. Lerch, Solid State Sci., 2017, 63, 1 CrossRef.
- X. Nie, W. Wulayin, T. Song, M. Wu and X. Qiao, Appl. Surf. Sci., 2016, 387, 351 CrossRef.
- V. Kumar and S. Singh, Appl. Surf. Sci., 2016, 386, 78 CrossRef.
- S. Gu, W. Li, F. Wang, H. Li and H. Zhou, Catal. Sci. Technol., 2016, 6, 1870 Search PubMed.
- R. P. Antony, T. Baikie, S. Y. Chiam, Y. Ren, R. R. Prabhakar, S. K. Batabyal, S. C. J. Loo, J. Barber and L. H. Wong, Appl. Catal., A, 2016, 526, 21 CrossRef.
- B. Liu, J. Li, W. Yang, X. Zhang, X. Jiang and Y. Bando, Small, 2017, 13, 1701998 CrossRef PubMed.
- I. Tsuji, H. Kato, H. Kobayashi and A. Kudo, J. Am. Chem. Soc., 2004, 126, 13406 CrossRef PubMed.
- T. Jing and Y. Dai, Acta Phys.-Chim. Sin., 2017, 33, 295 Search PubMed.
- H. Huang, X. Li, X. Han, N. Tian, Y. Zhang and T. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 3673 RSC.
- K. Ren, J. Liu, J. Liang, K. Zhang, X. Zheng, H. Luo, Y. Huang, P. Liu and X. Yu, Dalton Trans., 2013, 42, 9706 RSC.
- Y. Bai, L. Ye, T. Chen, P. Wang, L. Wang, X. Shi and P. K. Wong, Appl. Catal., B, 2017, 203, 633 CrossRef.
- J. Zhang, Q. Han, J. Zhu and X. Wang, J. Colloid Interface Sci., 2016, 477, 25 CrossRef PubMed.
- T. Wu, X. Li, D. Zhang, F. Dong and S. Chen, J. Alloys Compd., 2016, 671, 318 CrossRef.
- Y. Zhang, X. Sun, G. Yang, Y. Zhu, H. Si, J. Zhang and Y. Li, Mater. Sci. Semicond. Process., 2016, 41, 193 CrossRef.
- Q. Qin, Y. Guo, D. Zhou, Y. Yang and Y. Guo, Appl. Surf. Sci., 2016, 390, 765 CrossRef.
- X. Sun, Y. Zhang, C. Li, Z. Zhang, Z. Peng, H. Si, J. Zhang and Y. Li, J. Alloys Compd., 2015, 638, 254 CrossRef.
- Z. Zhao, Q. Liu and W. Dai, Sci. Rep., 2016, 6, 31449 CrossRef PubMed.
- K. V. Terebilenko, K. L. Bychkov, V. N. Baumer, N. S. Slobodyanik, M. V. Pavliuk, A. Thapper, I. I. Tokmenko, I. M. Nasieka and V. V. Strelchuk, Dalton Trans., 2016, 45, 3895 RSC.
- L. Lu, M. Lv, G. Liu and X. Xu, Appl. Surf. Sci., 2017, 391, 535 CrossRef.
- X. Xiao, C. Liu, R. Hu, X. Zuo, J. Nan, L. Li and L. Wang, J. Mater. Chem., 2012, 22, 22840 RSC.
- J. Xia, M. Ji, J. Di, B. Wang, S. Yin, M. He, Q. Zhang and H. Li, J. Alloys Compd., 2017, 695, 922 CrossRef.
- X. Jin, L. Ye, H. Wang, Y. Su, H. Xie, Z. Zhong and H. Zhang, Appl. Catal., B, 2015, 165, 668 CrossRef.
- F. Chang, J. Luo, X. Wang, Y. Xie, B. Deng and X. Hu, J. Colloid Interface Sci., 2015, 459, 136 CrossRef PubMed.
- C. Wang, X. Zhang, X. Song, W. Wang and H. Yu, ACS Appl. Mater. Interfaces, 2016, 8, 5320 Search PubMed.
- J. Xia, Y. Ge, J. Di, L. Xu, S. Yin, Z. Chen, P. Liu and H. Li, J. Colloid Interface Sci., 2016, 473, 112 CrossRef PubMed.
- R. Li, F. Xie, J. Liu, Y. Wang, Y. Wang, X. Zhang and C. Fan, Dalton Trans., 2016, 45, 9182 RSC.
- J. Di, J. Xia, M. Ji, S. Yin, H. Li, H. Xu, Q. Zhang and H. Li, J. Mater. Chem. A, 2015, 3, 15108 Search PubMed.
- L. Ye, X. Jin, C. Liu, C. Ding, H. Xie, K. H. Chu and P. K. Wong, Appl. Catal., B, 2016, 187, 281 CrossRef.
- S. S. Batool, S. Hassan, Z. Imran, K. Rasool, M. Ahmad and M. A. Rafiq, Int. J. Environ. Sci. Technol., 2016, 13, 1497 CrossRef.
- Z. Jiang, Y. Liu, M. Li, T. Jing, B. Huang, X. Zhang, X. Qin and Y. Dai, Sci. Rep., 2016, 6, 22727 CrossRef PubMed.
- K. C. Chitrada, R. Gakhar, D. Chidambaram, E. Aston and K. S. Raja, J. Electrochem. Soc., 2016, 163, H546 CrossRef.
- M. Maisano, M. V. Dozzi and E. Selli, J. Photochem. Photobiol., C, 2016, 28, 29 CrossRef.
- S. Y. Jeong, K. S. Choi, H. Shin, T. L. Kim, J. Song, S. Yoon, H. W. Jang, M. Yoon, C. Jeon, J. Lee and S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 505 Search PubMed.
- H. L. Tan, R. Amal and Y. H. Ng, ACS Appl. Mater. Interfaces, 2016, 8, 28607 Search PubMed.
- F. Duan, X. Wang, T. Tan and M. Chen, Phys. Chem. Chem. Phys., 2016, 18, 6113 RSC.
- H. Li, T. Hu, J. Liu, S. Song, N. Du, R. Zhang and W. Hou, Appl. Catal., B, 2016, 182, 431 CrossRef.
- L. Yang, G. Zhang, H. Wang, X. Bai, X. Shen, J. Liu and D. Gao, CrystEngComm, 2016, 18, 3683 RSC.
- T. Hu, H. Li, R. Zhang, N. Du and W. Hou, RSC Adv., 2016, 6, 31744 RSC.
- T. Wu, L. Liu, M. Pi, D. Zhang and S. Chen, Appl. Surf. Sci., 2016, 377, 253 CrossRef.
- H. L. Tan, X. Wen, R. Amal and Y. H. Ng, J. Phys. Chem. Lett., 2016, 7, 1400 CrossRef PubMed.
- Y. Hu, G. Chen, C. Li, Y. Zhou, J. Sun, S. Hao and Z. Han, J. Mater. Chem. A, 2016, 4, 5274 Search PubMed.
- X. Li, C. Zhu, Y. Song, D. Du and Y. Lin, RSC Adv., 2017, 7, 10235 RSC.
- J. Di, J. Xia, M. Ji, L. Xu, S. Yin, Z. Chen and H. Li, J. Mater. Chem. A, 2016, 4, 5051 Search PubMed.
- B. Zhang, Y. Li, J. Luo, H. Zhao, J. Zhao, G. Dong, Y. Zhu and C. Wang, Appl. Surf. Sci., 2017, 391, 499 CrossRef.
- S. Bharathkumar, M. Sakar and S. Balakumar, J. Phys. Chem. C, 2016, 120, 18811 Search PubMed.
- Y. Li, Y. Zhao, G. Wu and J. Zhao, Inorg. Chem., 2016, 55, 4897 CrossRef PubMed.
- L. Yang, Q. Han, X. Wang and J. Zhu, Chem. Eng. J., 2015, 262, 169 CrossRef.
- Z. Liu, Q. Lu, C. Wang, J. Liu and G. Liu, J. Alloys Compd., 2015, 651, 29 CrossRef.
- J. Jin and T. He, Appl. Surf. Sci., 2017, 394, 364 CrossRef.
- S. Sun, Q. An, W. Wang, L. Zhang, J. Liu and W. A. I. Goddard, J. Mater. Chem. A, 2017, 5, 201 Search PubMed.
- S. Wang, X. Hai, X. Ding, K. Chang, Y. Xiang, X. Meng, Z. Yang, H. Chen and J. Ye, Adv. Mater., 2017, 29, 1701774 CrossRef PubMed.
- P. Yang, T. Deng, D. Zhao, P. Feng, D. Pine, B. F. Chmelka, G. M. Whitesides and G. D. Stucky, Science, 1998, 282, 2244 CrossRef PubMed.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603 RSC.
- J. Fu, B. Zhu, C. Jiang, B. Cheng, W. You and J. Yu, Small, 2017, 13, 1603938 CrossRef PubMed.
- L. Qi, B. Cheng, W. Ho, G. Liu and J. Yu, ChemNanoMat, 2015, 1, 58 CrossRef.
- Z. Liu, J. Liu, Z. Liu, J. Niu and P. Feng, Mater. Res. Bull., 2016, 81, 119 CrossRef.
- Z. Qin, H. Tian, T. Su, H. Ji and Z. Guo, RSC Adv., 2016, 6, 52665 RSC.
- T. Jia, X. Wang, F. Long, J. Li, Z. Kang, F. Fu, G. Sun and J. Chen, Crystals, 2016, 6, 140 CrossRef.
- J. Zhang, Q. Han, X. Wang, J. Zhu and G. Duan, Mater. Lett., 2016, 162, 218 CrossRef.
- M. Zhou, W. Li, Y. Du, D. Kong, Z. Wang, Y. Meng, X. Sun, T. Yan, D. Kong and J. You, J. Nanopart. Res., 2016, 18, 346 CrossRef.
- H. Li, J. Liu, T. Hu, N. Du, S. Song and W. Hou, Mater. Res. Bull., 2016, 77, 171 CrossRef.
- R. He, S. Cao, J. Yu and Y. Yang, Catal. Today, 2016, 264, 221 CrossRef.
- Y. Wang, Z. Li, H. Yu and C. Feng, Funct. Mater. Lett., 2016, 9, 1650059 CrossRef.
- A. Sarka, A. B. Ghosh, N. Saha, D. N. Srivastava, P. Paul and B. Adhikary, J. Colloid Interface Sci., 2016, 483, 49 CrossRef PubMed.
- G. Zhao, W. Liu, J. Li, Q. Lv, W. Li and L. Liang, Appl. Surf. Sci., 2016, 390, 531 CrossRef.
- L. Chen, J. Wang, D. Meng, X. Wu, Y. Wang and E. Zhong, Mater. Lett., 2016, 162, 150 CrossRef.
- Z. Liang, Y. Cao, H. Qin and D. Jia, Mater. Res. Bull., 2016, 84, 397 CrossRef.
- Q. Zhang, J. Chen, Y. Xie, M. Wang and X. Ge, Appl. Surf. Sci., 2016, 368, 332 CrossRef.
- K. Qian, Z. Jiang, H. Shi, W. Wei, C. Zhu and J. Xie, Mater. Lett., 2016, 183, 303 CrossRef.
- M. Wang, X. Xi, C. Gong, X. L. Zhang and G. Fan, Mater. Res. Bull., 2016, 74, 258 CrossRef.
- R. Kashfi-Sadabad, S. Yazdani, A. Alemi, D. H. Tran, R. Ramprasad and M. T. Pettes, Langmuir, 2016, 32, 10967 CrossRef PubMed.
- Y. Zhou, X. Meng, L. Tong, X. Zeng and X. Chen, Energies, 2016, 9, 764 CrossRef.
- J. Li, X. Liu, Z. Sun and L. Pan, Ceram. Int., 2015, 41, 8592 CrossRef.
- L. Hou, H. Hua, L. Gan and C. Yuan, Mater. Lett., 2015, 159, 35 CrossRef.
- L. Zong, P. Cui, F. Qin, K. Zhao, Z. Wang and R. Yu, Mater. Res. Bull., 2017, 86, 44 CrossRef.
- Y. Tang, C. Yang, K. Li, F. Jing, R. Liu, D. Wu and J. Jia, J. Colloid Interface Sci., 2017, 491, 273 CrossRef PubMed.
- Y. Ying, F. Tao, T. Hong and L. Wang, Mater. Sci. Semicond. Process., 2015, 32, 82 CrossRef.
- L. Li, X. Huang, J. Zhang, W. Zhang, F. Ma, Z. Xiao, S. Gai, D. Wang and N. Li, J. Colloid Interface Sci., 2015, 443, 13 CrossRef PubMed.
- R. Y. Lee, H. Hwang, T. H. Kim and W. Choi, ACS Appl. Mater. Interfaces, 2016, 8, 3366 Search PubMed.
- X. Zhang, R. Li, Y. Wang, X. Zhang, Y. Wang and C. Fan, Mater. Lett., 2016, 174, 126 CrossRef.
- S. P. Patil, B. Bethi, G. H. Sonawane, V. S. Shrivastava and S. Sonawane, J. Ind. Eng. Chem., 2016, 34, 356 CrossRef.
- Q. Wang, D. Jiao, Y. Bai, J. Zhong, L. Zhao, X. Yong, J. Tong and J. Li, Mater. Lett., 2015, 161, 267 CrossRef.
- B. Li, H. Huang, Y. Guo and Y. Zhang, Appl. Surf. Sci., 2015, 353, 1179 CrossRef.
- Q. Tong, Y. Dong, X. Wang, P. Yan and D. He, NANO, 2015, 10, 1550077 CrossRef.
- Y. Shi, Y. Hu, L. Zhang, Z. Yang, Q. Zhang, H. Cui, X. Zhu, J. Wang, J. Chen and K. Wang, Appl. Clay Sci., 2017, 137, 249 CrossRef.
- L. Li, Z. Ma, F. Bi, H. Li, W. Zhang and H. He, J. Adv. Oxid. Technol., 2016, 19, 310 Search PubMed.
- Z. Guo, P. Li, H. Che, G. Wang, C. Wu, X. Zhang and J. Mu, Ceram. Int., 2016, 42, 4517 CrossRef.
- Y. Shen, X. Yu, W. Lin, Y. Zhu and Y. Zhang, Appl. Surf. Sci., 2017, 399, 67 CrossRef.
- S. Kumar, S. Sharma, S. Sood, A. Umar and S. K. Kansal, Ceram. Int., 2016, 42, 17551 CrossRef.
- M. Zhang, C. Shao, X. Zhang and Y. Liu, CrystEngComm, 2015, 17, 7276 RSC.
- B. Weng, F. Xu and F. Yu, Mater. Lett., 2015, 145, 70 CrossRef.
- J. Di, M. Ji, J. Xia, X. Li, W. Fan, Q. Zhang and H. Li, J. Mol. Catal. A: Chem., 2016, 424, 331 CrossRef.
- G. Jiang, X. Li, M. Lan, T. Shen, X. Lv, F. Dong and S. Zhang, Appl. Catal., B, 2017, 205, 532 CrossRef.
- Z. Zhao, W. Zhang, X. Lv, Y. Sun, F. Dong and Y. Zhang, Environ. Sci.: Nano, 2016, 3, 1306 RSC.
- D. Xia, L. Hu, C. He, W. Pan, T. Yang, Y. Yang and D. Shu, Chem. Eng. J., 2015, 279, 929 CrossRef.
- S. Wang, J. Yun, B. Luo, T. Butburee, P. Peerakiatkhajohn, S. Thaweesak, M. Xiao and L. Wang, J. Mater. Sci. Technol., 2017, 33, 1 Search PubMed.
- X. Zhang, Y. Liu, Q. Zhang and Y. Zhou, Prog. Chem., 2016, 28, 1560 Search PubMed.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150 CrossRef PubMed.
- R. Marschall, Adv. Funct. Mater., 2014, 24, 2421 CrossRef.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234 RSC.
- X. Meng and Z. Zhang, J. Mol. Catal. A: Chem., 2016, 423, 533 CrossRef.
- B. N. Nunes, L. F. Paula, Í. A. Costa, A. E. H. Machado, L. G. Paterno and A. O. T. Patrocinio, J. Photochem. Photobiol., C, 2017, 32, 1 CrossRef.
- D. Li and W. Shi, Chin. J. Catal., 2016, 37, 792 CrossRef.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Alghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- Y. Liu, G. Zhu, J. Gao, M. Hojamberdiev, H. Lu, R. Zhu, X. Wei and P. Liu, J. Alloys Compd., 2016, 688, 487 CrossRef.
- L. Fan, B. Wei, L. Xu, Y. Liu, W. Cao, N. Ma and H. Gao, NANO, 2016, 11, 1650095 CrossRef.
- M. Yan, Y. Hua, F. Zhu, W. Gu, J. Jiang, H. Shen and W. Shi, Appl. Catal., B, 2017, 202, 518 CrossRef.
- M. Zhang, J. Qin, P. Yu, B. Zhang, M. Ma, X. Zhang and R. Liu, Beilstein J. Nanotechnol., 2018, 9, 789 CrossRef PubMed.
- Y. Huang, W. Fan, B. Long, H. Li, F. Zhao, Z. Liu, Y. Tong and H. Ji, Appl. Catal., B, 2016, 185, 68 CrossRef.
- Y. Guo, P. Wang, J. Qian, J. Hou, Y. Ao and C. Wang, Catal. Sci. Technol., 2018, 8, 486 Search PubMed.
- C. Lv, G. Chen, X. Zhou, C. Zhang, Z. Wang, B. Zhao and D. Li, ACS Appl. Mater. Interfaces, 2017, 9, 23748 Search PubMed.
- J. Li, G. Zhan, Y. Yu and L. Zhang, Nat. Commun., 2016, 7, 11480 CrossRef PubMed.
- L. Chen, J. He, Q. Yuan, Y. Liu, C. Au and S. Yin, J. Mater. Chem. A, 2015, 3, 1096 Search PubMed.
- R. He, J. Zhang, J. Yu and S. Cao, J. Colloid Interface Sci., 2016, 478, 201 CrossRef PubMed.
- X. Liu and Y. Kang, Mater. Lett., 2016, 164, 229 CrossRef.
- Y. Huang, Y. Wei, J. Wang, D. Luo, L. Fan and J. Wu, Appl. Surf. Sci., 2017, 423, 119 CrossRef.
- X. Dang, X. Zhang, Y. Chen, X. Dong, G. Wang, C. Ma, X. Zhang, H. Ma and M. Xue, J. Nanopart. Res., 2015, 17, 93 CrossRef.
- Y. Li, S. Wu, L. Huang, H. Xu, R. Zhang, M. Qu, Q. Gao and H. Li, J. Phys. Chem. Solids, 2015, 76, 112 CrossRef.
- D. Xiong, G. Huang, B. Zhou, Q. Yan, A. Pan and W. Huang, J. Colloid Interface Sci., 2016, 464, 103 CrossRef PubMed.
- M. Nawaz, J. Photochem. Photobiol., A, 2017, 332, 326 CrossRef.
- L. Long, A. Zhang, Y. Huang, X. Zhang and H. Yu, J. Mater. Chem. A, 2015, 3, 4301 Search PubMed.
- Y. Ao, L. Xu, P. Wang, C. Wang, J. Hou and J. Qian, Dalton Trans., 2015, 44, 11321 RSC.
- D. Zhou, H. Yang, Y. Tu, Y. Tian, Y. Cai, Z. Hu and X. Zhu, Nanoscale Res. Lett., 2016, 11, 193 CrossRef PubMed.
- H. Sun, J. Li, G. Zhang and N. Li, J. Mol. Catal. A: Chem., 2016, 424, 311 CrossRef.
- Y. Guo, J. Li, Z. Gao, X. Zhu, Y. Liu, Z. Wei, W. Zhao and C. Sun, Appl. Catal., B, 2016, 192, 57 CrossRef.
- W. Shan, Y. Hu, Z. Bai, M. Zheng and C. Wei, Appl. Catal., B, 2016, 188, 1 CrossRef.
- Y. Hu, C. Dong, K. Wu, S. Xia, X. Li and X. Wei, Mater. Lett., 2015, 147, 69 CrossRef.
- J. An, G. Zhang, R. Zheng and P. Wang, J. Environ. Sci., 2016, 48, 218 CrossRef PubMed.
- F. Niu, D. Chen, L. Qin, N. Zhang, J. Wang, Z. Chen and Y. Huang, ChemCatChem, 2015, 7, 3279 CrossRef.
- Y. Xing, W. Que, X. Yin, Z. He, X. Liu, Y. Yang, J. Shao and L. B. Kong, Appl. Surf. Sci., 2016, 387, 36 CrossRef.
- S. Li, K. Xu, S. Hu, W. Jiang, J. Zhang, J. Liu and L. Zhang, Appl. Surf. Sci., 2017, 397, 95 CrossRef.
- N. Bao, Z. Yin, Q. Zhang, S. He, X. Hu and X. Miao, Ceram. Int., 2016, 42, 1791 CrossRef.
- M. Zalfani, B. van der Schueren, Z. Hu, J. C. Rooke, R. Bourguiga, M. Wu, Y. Li, G. Van Tendeloo and B. Su, J. Mater. Chem. A, 2015, 3, 21244 Search PubMed.
- Y. Huang, M. Fu and T. He, Acta Phys.-Chim. Sin., 2015, 31, 1145 Search PubMed.
- J. Xu, W. Wang, J. Wang and Y. Liang, Appl. Surf. Sci., 2015, 349, 529 CrossRef.
- J. Li, M. Cui, Z. Guo, Z. Liu and Z. Zhu, Mater. Lett., 2015, 151, 75 CrossRef.
- G. Joshi, J. K. Pandey, S. Singh and R. Sharma, Korean J. Chem. Eng., 2017, 34, 500 CrossRef.
- J. Yin, S. Huang, Z. Jian, Z. Wang and Y. Zhang, Mater. Sci. Semicond. Process., 2015, 34, 198 CrossRef.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356 CrossRef PubMed.
- K. Trzcinski, M. Szkoda, K. Siuzdak, M. Sawczak and A. Lisowska-Oleksiak, Appl. Surf. Sci., 2016, 388, 753 CrossRef.
- C. Yang, Y. Huang, F. Li and T. Li, J. Mater. Sci., 2016, 51, 1032 CrossRef.
- X. Sun, H. Zhang, J. Wei, Q. Yu, P. Yang and F. Zhang, Mater. Sci. Semicond. Process., 2016, 45, 51 CrossRef.
- J. Zhang, L. Huang, L. Yang, Z. Lu, X. Wang, G. Xu, E. Zhang, H. Wang, Z. Kong, J. Xi and Z. Ji, J. Alloys Compd., 2016, 676, 37 CrossRef.
- Z. Zhu, Y. Yan and J. Li, J. Mater. Sci., 2016, 51, 2112 CrossRef.
- X. Liu, Q. Lu and C. Wang, Mater. Lett., 2015, 154, 81 CrossRef.
- S. Issarapanacheewin, K. Wetchakun, S. Phanichphant, W. Kangwansupamonkon and N. Wetchakun, Mater. Lett., 2015, 156, 28 CrossRef.
- J. Li, C. Yu, W. Fang, L. Zhu, W. Zhou and Q. Fan, Chin. J. Catal., 2015, 36, 987 CrossRef.
- L. Chen, H. Hua, Q. Yang and C. Hu, Appl. Surf. Sci., 2015, 327, 62 CrossRef.
- R. Tang, H. Su, S. Duan, Y. Sun, L. Li, X. Zhang, S. Zeng and D. Sun, RSC Adv., 2015, 5, 41949 RSC.
- J. Li, X. Liu, Z. Sun and L. Pan, J. Colloid Interface Sci., 2016, 463, 145 CrossRef PubMed.
- W. Chen, G. Duan, T. Liu, S. Chen and X. Liu, Mater. Sci. Semicond. Process., 2015, 35, 45 CrossRef.
- S. Jonjana, A. Phuruangrat, S. Thongtem, O. Wiranwetchayan and T. Thongtem, Mater. Lett., 2016, 180, 93 CrossRef.
- S. Jonjana, A. Phuruangrat, T. Thongtem and S. Thongtem, Mater. Lett., 2016, 172, 11 CrossRef.
- M. Xu and W. Zhang, Eur. J. Inorg. Chem., 2016, 826 CrossRef.
- A. Dittmer, J. Menze, B. Mei, J. Strunk, H. S. Luftman, R. Gutkowski, I. E. Wachs, W. Schuhmann and M. Muhler, J. Phys. Chem. C, 2016, 120, 18191 Search PubMed.
- S. N. Lou, J. Scott, A. Iwase, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2016, 4, 6964 Search PubMed.
- Z. Wan and G. Zhang, J. Mater. Chem. A, 2015, 3, 16737 Search PubMed.
- S. Yin, J. Di, M. Li, Y. Sun, J. Xia, H. Xu, W. Fan and H. Li, J. Mater. Sci., 2016, 51, 4769 CrossRef.
- X. Wang, H. Hu, S. Chen, K. Zhang, J. Zhang, W. Zou and R. Wang, Mater. Chem. Phys., 2015, 158, 67 CrossRef.
- X. Wang, Q. Ni, D. Zeng, G. Liao, Y. Wen, B. Shan and C. Xie, Appl. Surf. Sci., 2016, 396, 590 CrossRef.
- D. Jiang, X. Du, D. Chen, Y. Li, N. Hao, J. Qian, H. Zhong, T. You and K. Wang, Carbon, 2016, 102, 10 CrossRef.
- R. Jiang, H. Y. Zhu, J. B. Li, F. Q. Fu, J. Yao, S. T. Jiang and G. M. Zeng, Appl. Surf. Sci., 2016, 364, 604 CrossRef.
- X. Li, L. Wang, L. Zhang and S. Zhuo, Appl. Surf. Sci., 2017, 419, 586 CrossRef.
- Y. Ao, K. Wang, P. Wang, C. Wang and J. Hou, Appl. Catal., B, 2016, 194, 157 CrossRef.
- X. Wen, C. Zhang, C. Niu, L. Zhang, G. Zeng and X. Zhang, Catal. Commun., 2017, 90, 51 CrossRef.
- O. Mehraj, N. A. Mir, B. M. Pirzada and S. Sabir, Appl. Surf. Sci., 2015, 332, 419 CrossRef.
- G. Dai, J. Yu and G. Liu, J. Phys. Chem. C, 2011, 115, 7339 Search PubMed.
- K. Wang, C. Shao, X. Li, F. Miao, N. Lu and Y. Liu, Materials, 2016, 9, 90 CrossRef PubMed.
- O. Mehraj, B. M. Pirzada, N. A. Mir, M. Z. Khan and S. Sabir, Appl. Surf. Sci., 2016, 387, 642 CrossRef.
- Y. Sun, X. Xiao, X. Dong, F. Dong and W. Zhang, Chin. J. Catal., 2017, 38, 217 CrossRef.
- S. Lee, Y. Park, D. Pradhan and Y. Sohn, J. Ind. Eng. Chem., 2016, 35, 231 CrossRef.
- J. Xia, M. Ji, J. Di, B. Wang, S. Yin, Q. Zhang, M. He and H. Li, Appl. Catal., B, 2016, 191, 235 CrossRef.
- T. A. Gadhi, A. Hernandez-Gordillo, M. Bizarro, P. Jagdale, A. Tagliaferro and S. E. Rodil, Ceram. Int., 2016, 42, 13065 CrossRef.
- L. Shan, G. Wang, D. Li, X. San, L. Liu, L. Dong and Z. Wu, Dalton Trans., 2015, 44, 7835 RSC.
- M. Mao, F. Chen, C. Zheng, J. Ning, Y. Zhong and Y. Hu, J. Alloys Compd., 2016, 688, 1080 CrossRef.
- Y. Cheng, H. Wang, Y. Zhu, F. Liao, Z. Li and J. Li, J. Mater. Sci.: Mater. Electron., 2015, 26, 1268 CrossRef.
- C. Lv, G. Chen, J. Sun, C. Yan, H. Dong and C. Li, RSC Adv., 2015, 5, 3767 RSC.
- D. Peng, Z. Zou, F. Long, J. He and T. Zhang, Ionics, 2016, 22, 2347 CrossRef.
- Q. Zhao, X. Liu, Y. Xing, Z. Liu and C. Du, J. Mater. Sci., 2017, 52, 2117 CrossRef.
- S. Han, J. Li, K. Yang and J. Lin, Chin. J. Catal., 2015, 36, 2119 CrossRef.
- L. Cheng and Y. Kang, Catal. Commun., 2015, 72, 16 CrossRef.
- Y. Mi, H. Li, Y. Zhang, R. Zhang and W. Hou, Appl. Surf. Sci., 2017, 423, 1062 CrossRef.
- X. Li, Y. Li, J. Shen and M. Ye, Ceram. Int., 2016, 42, 3154 CrossRef.
- A. Rauf, M. S. A. S. Shah, G. H. Choi, U. B. Humayoun, D. H. Yoon, J. W. Bae, J. Park, W. Kim and P. J. Yoo, ACS Sustainable Chem. Eng., 2015, 3, 2847 CrossRef.
- Y. Liu, M. Zhang, L. Li and X. Zhang, Catal. Commun., 2015, 60, 23 CrossRef.
- F. Dong, X. Feng, Y. Zhang, C. Gao and Z. Wu, RSC Adv., 2015, 5, 11714 RSC.
- Y. Wu, Q. Han, L. Wang, X. Wang and J. Zhu, Mater. Chem. Phys., 2017, 187, 72 CrossRef.
- Y. Liu, G. Zhu, J. Peng, J. Gao, C. Wang and P. Liu, J. Mater. Sci.: Mater. Electron., 2017, 28, 2172 CrossRef.
- X. Zhang, L. Zhang, J. Hu, C. Pan and C. Hou, Appl. Surf. Sci., 2015, 346, 33 CrossRef.
- P. Madhusudan, J. Ran, J. Zhang, J. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286 CrossRef.
- X. Zou, Y. Dong, X. Zhang, Y. Cui, X. Ou and X. Qi, Appl. Surf. Sci., 2017, 391, 525 CrossRef.
- Y. Ma, Y. Jia, L. Wang, M. Yang, Y. Bi and Y. Qi, Phys. Chem. Chem. Phys., 2016, 18, 5091 RSC.
- X. Lin, D. Liu, X. Guo, N. Sun, S. Zhao, L. Chang, H. Zhai and Q. Wang, J. Phys. Chem. Solids, 2015, 76, 170 CrossRef.
- Q. W. Cao, Y. F. Zheng and X. C. Song, Ceram. Int., 2016, 42, 14533 CrossRef.
- Z. Dai, F. Qin, H. Zhao, F. Tian, Y. Liu and R. Chen, Nanoscale, 2015, 7, 11991 RSC.
- Z. Xiang, Y. Wang, D. Zhang and P. Ju, J. Ind. Eng. Chem., 2016, 40, 83 CrossRef.
- T. He, D. Wu and Y. Tan, Mater. Lett., 2016, 165, 227 CrossRef.
- C. Feng, D. Wang, B. Jin and Z. Jiao, RSC Adv., 2015, 5, 75947 RSC.
- L. Hao, H. Huang, Y. Guo, X. Du and Y. Zhang, Appl. Surf. Sci., 2017, 420, 303 CrossRef.
- T. Yan, M. Sun, H. Liu, T. Wu, X. Liu, Q. Yan, W. Xu and B. Du, J. Alloys Compd., 2015, 634, 223 CrossRef.
- X. Liu, Y. Su, Q. Zhao, C. Du and Z. Liu, Sci. Rep., 2016, 6, 28689 CrossRef PubMed.
- H. Huang, K. Xiao, T. Zhang, F. Dong and Y. Zhang, Appl. Catal., B, 2017, 203, 879 CrossRef.
- S. Tu, M. Lu, X. Xiao, C. Zheng, H. Zhong, X. Zuo and J. Nan, RSC Adv., 2016, 6, 44552 RSC.
- Y. Si, J. Li, J. Zhong, J. Zeng, S. Huang, W. Yuan, M. Li and J. Ding, Curr. Appl. Phys., 2016, 16, 240 CrossRef.
- D. Wu, L. Ye, S. Yue, B. Wang, W. Wang, H. Y. Yip and P. K. Wong, J. Phys. Chem. C, 2016, 120, 7715 Search PubMed.
- H. Qian, M. Lai, X. Huang, W. Wang, C. Xu, H. Yong, Y. Wen and Y. Zhou, Surf. Rev. Lett., 2016, 23, 1650032 CrossRef.
- W. S. Dos Santos, M. Rodriguez, A. S. Afonso, J. P. Mesquita, L. L. Nascimento, A. O. T. Patrocinio, A. C. Silva, L. C. A. Oliveira, J. D. Fabris and M. C. Pereira, Sci. Rep., 2016, 6, 31406 CrossRef PubMed.
- J. Ke, J. Liu, H. Sun, H. Zhang, X. Duan, P. Liang, X. Li, M. O. Tade, S. Liu and S. Wang, Appl. Catal., B, 2017, 200, 47 CrossRef.
- Q. Wang, X. Shi, E. Liu, J. C. Crittenden, X. Ma, Y. Zhang and Y. Cong, J. Hazard. Mater., 2016, 317, 8 CrossRef PubMed.
- Y. Zhang, M. Park, H. Y. Kim, B. Ding and S. Park, Appl. Surf. Sci., 2016, 384, 192 CrossRef.
- C. Zeng, Y. Hu, Y. Guo, T. Zhang, F. Dong, X. Du, Y. Zhang and H. Huang, Appl. Catal., B, 2016, 194, 62 CrossRef.
- S. Chou, C. Chen, Y. Dai, J. Lin and W. W. Lee, RSC Adv., 2016, 6, 33478 RSC.
- W. Li, P. Li, Y. Liu, B. Zhang, H. Zhang, W. Geng and Q. Zhang, ChemCatChem, 2015, 7, 4163 CrossRef.
- J. Yu, S. Wang, J. Low and W. Xiao, Phys. Chem. Chem. Phys., 2013, 15, 16883 RSC.
- J. Low, C. Jiang, B. Cheng, S. Wageh, A. A. Al-Ghamdi and J. Yu, Small Methods, 2017, 1, 1700080 CrossRef.
- W. Yu, J. Chen, T. Shang, L. Chen, L. Gu and T. Peng, Appl. Catal., B, 2017, 219, 693 CrossRef.
- K. Qi, B. Cheng, J. Yu and W. Ho, Chin. J. Catal., 2017, 38, 1936 CrossRef.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532 CrossRef.
- J. Liu, B. Cheng and J. Yu, Phys. Chem. Chem. Phys., 2016, 18, 31175 RSC.
- A. J. Bard, J. Photochem., 1979, 10, 59 CrossRef.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. D. Sharp, A. Kudo, T. Yamada and K. Domen, Nat. Mater., 2016, 15, 611 CrossRef PubMed.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, Nat. Mater., 2006, 5, 782 CrossRef PubMed.
- Y. Sasaki, H. Kato and A. Kudo, J. Am. Chem. Soc., 2013, 135, 5441 CrossRef PubMed.
- M. Wang, Q. Han, L. Li, L. Tang, H. Li, Y. Zhou and Z. Zou, Nanotechnology, 2017, 28, 274002 CrossRef PubMed.
- Q. Liu, J. Huan, N. Hao, J. Qian, H. Mao and K. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 18369 Search PubMed.
- J. Lv, K. Dai, J. Zhang, L. Geng, C. Liang, Q. Liu, G. Zhu and C. Chen, Appl. Surf. Sci., 2015, 358, 377 CrossRef.
- S. N. F. M. Nasir, H. Ullah, M. Ebadi, A. A. Tahir, J. S. Sagu and M. A. M. Teridi, J. Phys. Chem. C, 2017, 121, 6218 Search PubMed.
- W. Wang, H. Cheng, B. Huang, X. Liu, X. Qin, X. Zhang and Y. Dai, J. Colloid Interface Sci., 2015, 442, 97 CrossRef PubMed.
- R. He, J. Zhou, H. Fu, S. Zhang and C. Jiang, Appl. Surf. Sci., 2018, 430, 273 CrossRef.
- J. Zhang, Y. Hu, X. Jiang, S. Chen, S. Meng and X. Fu, J. Hazard. Mater., 2014, 280, 713 CrossRef PubMed.
- X. Zhao, J. Yu, H. Cui and T. Wang, J. Photochem. Photobiol., A, 2017, 335, 130 CrossRef.
- C. Liu, Q. Wu, M. Ji, H. Zhu, H. Hou, Q. Yang, C. Jiang, J. Wang, L. Tian, J. Chen and W. Hou, J. Alloys Compd., 2017, 723, 1121 CrossRef.
- C. Song, Y. Feng, W. Shi and C. Liu, CrystEngComm, 2016, 18, 7796 RSC.
- F. Wang, W. Li, S. Gu, H. Li, X. Wu, C. Ren and X. Liu, J. Photochem. Photobiol., A, 2017, 335, 140 CrossRef.
- H. Li, T. Hu, R. Zhang, J. Liu and W. Hou, Appl. Catal., B, 2016, 188, 313 CrossRef.
- S. Chen, Y. Hu, L. Ji, X. Jiang and X. Fu, Appl. Surf. Sci., 2014, 292, 357 CrossRef.
- D. Wang, Z. Guo, Y. Peng and W. Yuan, Chem. Eng. J., 2015, 281, 102 CrossRef.
- S. Wang, X. Yang, X. Zhang, X. Ding, Z. Yang, K. Dai and H. Chen, Appl. Surf. Sci., 2017, 391, 194 CrossRef.
- J. Ding, Z. Dai, F. Qin, H. Zhao, S. Zhao and R. Chen, Appl. Catal., B, 2017, 205, 281 CrossRef.
- G. Liu, S. You, H. Huang, M. Ma and N. Ren, Chemosphere, 2017, 171, 702 CrossRef PubMed.
- M. J. Islam, D. A. Reddy, N. S. Han, J. Choi, J. K. Song and T. K. Kim, Phys. Chem. Chem. Phys., 2016, 18, 24984 RSC.
- W. Jo and T. S. Natarajan, J. Colloid Interface Sci., 2016, 482, 58 CrossRef PubMed.
- W. Fang, M. Xing and J. Zhang, J. Photochem. Photobiol., C, 2017, 32, 21 CrossRef.
- M. Li, J. Wang, P. Zhang, Q. Deng, J. Zhang, K. Jiang, Z. Hu and J. Chu, Sci. Rep., 2017, 7, 42484 CrossRef PubMed.
- K. Hu, Z. Li, S. Chen, J. Bian, Y. Qu, J. Tang and L. Jing, Part. Part. Syst. Charact., 2018, 35, 1700320 CrossRef.
- H. Hirakawa, S. Shiota, Y. Shiraishi, H. Sakamoto, S. Ichikawa and T. Hirai, ACS Catal., 2016, 6, 4976 CrossRef.
- K. R. Yoon, J. W. Ko, D. Youn, C. B. Park and I. Kim, Green Chem., 2016, 18, 944 RSC.
- S. Yu, Y. Zhang, M. Li, X. Du and H. Huang, Appl. Surf. Sci., 2017, 391, 491 CrossRef.
- X. Dong, W. Zhang, Y. Sun, J. Li, W. Cen, Z. Cui, H. Huang and F. Dong, J. Catal., 2018, 357, 41 CrossRef.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Small, 2016, 12, 6640 CrossRef PubMed.
- B. Priya, P. Raizada, N. Singh, P. Thakur and P. Singh, J. Colloid Interface Sci., 2016, 479, 271 CrossRef PubMed.
- J. Zhai, H. Yu, H. Li, L. Sun, K. Zhang and H. Yang, Appl. Surf. Sci., 2015, 344, 101 CrossRef.
- Y. Hao, X. Dong, X. Wang, S. Zhai, H. Ma and X. Zhang, J. Mater. Chem. A, 2016, 4, 8298 Search PubMed.
- J. Selvaraj, S. Gupta, R. Anand, S. Fiechter and V. R. Subramanian, J. Electrochem. Soc., 2016, 163, H147 CrossRef.
- Z. Zhang, T. Zheng, J. Xu and H. Zeng, J. Mater. Sci., 2016, 51, 3846 CrossRef.
- Z. Zhao, Y. Zhou, F. Wang, K. Zhang, S. Yu and K. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 730 Search PubMed.
- Y. Li, Z. Wang, B. Huang, Y. Dai, X. Zhang and X. Qin, Appl. Surf. Sci., 2015, 347, 258 CrossRef.
- Q. Hao, R. Wang, H. Lu, C. Xie, W. Ao, D. Chen, C. Ma, W. Yao and Y. Zhu, Appl. Catal., B, 2017, 219, 63 CrossRef.
- J. Wang, W. Jiang, D. Liu, Z. Wei and Y. Zhu, Appl. Catal., B, 2015, 176, 306 CrossRef.
- Z. Shen, S. Xie, W. Fan, Q. Zhang, Z. Xie, W. Yang, Y. Wang, J. Lin, X. Wu, H. Wan and Y. Wang, Catal. Sci. Technol., 2016, 6, 6485 Search PubMed.
- G. Zhang, Z. Hu, M. Sun, Y. Liu, L. Liu, H. Liu, C. Huang, J. Qu and J. Li, Adv. Funct. Mater., 2015, 25, 3726 CrossRef.
- X. Y. Kong, W. P. C. Lee, W. Ong, S. Chai and A. R. Mohamed, ChemCatChem, 2016, 8, 3074 CrossRef.
- Z. Ma, P. Li, L. Ye, Y. Zhou, F. Su, C. Ding, H. Xie, Y. Bai and P. K. Wong, J. Mater. Chem. A, 2017, 5, 24995 Search PubMed.
- H. Y. Wang, Z. S. Liu, Y. L. Zhao, J. N. Niu and P. Z. Feng, Mater. Res. Bull., 2017, 89, 253 CrossRef.
- F. Q. Zhou, J. C. Fan, Q. J. Xu and Y. L. Min, Appl. Catal., B, 2017, 201, 77 CrossRef.
- L. Liu, L. Ding, Y. Liu, W. An, S. Lin, Y. Liang and W. Cui, Appl. Surf. Sci., 2016, 364, 505 CrossRef.
- D. Wang, L. Guo, Y. Zhen, L. Yue, G. Xue and F. Fu, J. Mater. Chem. A, 2014, 2, 11716 Search PubMed.
- D. Liu, Z. Jiang, C. Zhu, K. Qian, Z. Wu and J. Xie, Dalton Trans., 2016, 45, 2505 RSC.
- H. Xu, Z. Wu, M. Ding and X. Gao, Mater. Des., 2017, 114, 129 CrossRef.
- Z. Chen, X. Chen, J. Di, Y. Liu, S. Yin, J. Xia and H. Li, J. Colloid Interface Sci., 2017, 492, 51 CrossRef PubMed.
- F. Chen, C. Niu, Q. Yang, X. Li and G. Zeng, Ceram. Int., 2016, 42, 2515 CrossRef.
- W. An, W. Cui, Y. Liang, J. Hu and L. Liu, Appl. Surf. Sci., 2015, 351, 1131 CrossRef.
- J. Chen, W. Mei, C. Liu, C. Hu, Q. Huang, N. Chen, J. Chen, R. Zhang and W. Hou, Mater. Lett., 2016, 172, 184 CrossRef.
- Y. Sun, J. Wu, T. Ma, P. Wang, C. Cui and D. Ma, Appl. Surf. Sci., 2017, 403, 141 CrossRef.
- C. Wu, F. Zhang and X. Zhou, J. Mater. Sci.: Mater. Electron., 2015, 26, 7496 CrossRef.
- Q. Wang, D. Jiao, Y. Wu, H. Guo, H. She, J. Li, J. Zhong, F. Wang and J. Tong, Int. J. Hydrogen Energy, 2016, 41, 16032 CrossRef.
- B. Long, Y. Huang, H. Li, F. Zhao, Z. Rui, Z. Liu, Y. Tong and H. Ji, Ind. Eng. Chem. Res., 2015, 54, 12788 CrossRef.
- R. Xie, L. Zhang, H. Xu, Y. Zhong, X. Sui and Z. Mao, Chem. Eng. J., 2017, 310, 79 CrossRef.
- M. Ji, J. Xia, J. Di, B. Wang, S. Yin, L. Xu, J. Zhao and H. Li, J. Colloid Interface Sci., 2016, 478, 324 CrossRef PubMed.
- J. Di, J. Xia, M. Ji, L. Xu, S. Yin, Q. Zhang, Z. Chen and H. Li, Carbon, 2016, 98, 613 CrossRef.
- C. Yu, S. Dong, J. Zhao, X. Han, J. Wang and J. Sun, J. Alloys Compd., 2016, 677, 219 CrossRef.
- Y. Zhang, M. Park, H. Kim and S. Park, J. Alloys Compd., 2016, 686, 106 CrossRef.
- S. Chou, W. Chung, L. Chen, Y. Dai, W. Lin, J. Lin and C. Chen, RSC Adv., 2016, 6, 82743 RSC.
- T. Soltani and B. Lee, Chem. Eng. J., 2016, 306, 204 CrossRef.
- W. Zhang, F. Dong and W. Zhang, Appl. Surf. Sci., 2015, 358, 75 CrossRef.
- R. He, S. Cao, D. Guo, B. Cheng, S. Wageh, A. A. Al-Ghamdi and J. Yu, Ceram. Int., 2015, 41, 3511 CrossRef.
- R. Vinoth, S. G. Babu, R. Ramachandran and B. Neppolian, Appl. Surf. Sci., 2017, 418, 163 CrossRef.
- X. Yu, J. Shi, L. Feng, C. Li and L. Wang, Appl. Surf. Sci., 2017, 396, 1775 CrossRef.
- X. Wang, W. Mao, Q. Wang, Y. Zhu, Y. Min, J. Zhang, T. Yang, J. Yang, X. Li and W. Huang, RSC Adv., 2017, 7, 10064 RSC.
- H. Wang, Y. Liang, L. Liu, J. Hu and W. Cui, Appl. Surf. Sci., 2017, 392, 51 CrossRef.
- Y. Chen, G. Tian, G. Mao, R. Li, Y. Xiao and T. Han, Appl. Surf. Sci., 2016, 378, 231 CrossRef.
- J. Qian, Z. Yang, C. Wang, K. Wang, Q. Liu, D. Jiang, Y. Yan and K. Wang, J. Mater. Chem. A, 2015, 3, 13671 Search PubMed.
- H. Liu, Z. Chen and Y. Wang, J. Photochem. Photobiol., A, 2016, 326, 30 CrossRef.
- G. Tang, J. Dong, K. Wu, W. Liang, D. Zhou, S. Ma, H. Tang and C. Li, Ceram. Int., 2016, 42, 5607 CrossRef.
- P. Madhusudan, J. Yu, W. Wang, B. Cheng and G. Liu, Dalton Trans., 2012, 41, 14345 RSC.
- J. Low, J. Yu, Q. Li and B. Cheng, Phys. Chem. Chem. Phys., 2014, 16, 1111 RSC.
- J. Bi, W. Fang, L. Li, X. Li, M. Liu, S. Liang, Z. Zhang, Y. He, H. Lin, L. Wu, S. Liu and P. K. Wong, J. Alloys Compd., 2015, 649, 28 CrossRef.
- Z. Hu, S. K. Lua and T. Lim, ACS Sustainable Chem. Eng., 2015, 3, 2726 CrossRef.
- X. Meng and Z. Zhang, Appl. Surf. Sci., 2017, 392, 169 CrossRef.
- J. Zhang, T. Chen, H. Lu, Z. Yang, F. Yin, J. Gao, Q. Liu and Y. Tu, Appl. Surf. Sci., 2017, 404, 282 CrossRef.
- J. Di, J. X. Xia, S. Yin, H. Xu, L. Xu, Y. G. Xu, M. Q. He, H. M. Li and J. G. Wang, Mater. Technol., 2015, 30, 113 CrossRef.
- M. Palmai, E. M. Zahran, S. Angaramo, S. Balint, Z. Paszti, M. R. Knecht and L. G. Bachas, J. Mater. Chem. A, 2017, 5, 529 Search PubMed.
- W. Guo, Q. Qin, L. Geng, D. Wang, Y. Guo and Y. Yang, J. Hazard. Mater., 2016, 308, 374 CrossRef PubMed.
- F. Niu, D. Chen, L. Qin, T. Gao, N. Zhang, S. Wang, Z. Chen, J. Wang, X. Sun and Y. Huang, Sol. Energy Mater. Sol. Cells, 2015, 143, 386 CrossRef.
- Y. Yu, S. Lu and S. Bao, J. Nanopart. Res., 2015, 17, 323 CrossRef.
- S. Murcia-Lopez, V. Vaiano, M. C. Hidalgo, J. A. Navio and D. Sannino, Photochem. Photobiol. Sci., 2015, 14, 678 Search PubMed.
- X. Yan, X. Zhu, R. Li and W. Chen, J. Hazard. Mater., 2016, 303, 1 CrossRef PubMed.
- N. V. Chien, W. S. Chang, J. Chen, K. Tsai, W. Tzeng, Y. Lin, H. Kuo, H. Liu, K. Chang, W. Chou, C. Wu, Y. Chen, C. Luo, Y. Hsu and Y. Chu, Nano Energy, 2015, 15, 625 CrossRef.
- P. Zhang, H. Yu, J. Li, H. Zhao, B. Zhu, W. Huang and S. Zhang, RSC Adv., 2016, 6, 15304 RSC.
- R. Wang, X. Li, W. Cui, Y. Zhang and F. Dong, New J. Chem., 2015, 39, 8446 RSC.
- P. Yilmaz, D. Yeo, H. Chang, L. Loh and S. Dunn, Nanotechnology, 2016, 27, 345402 CrossRef PubMed.
- J. Di, J. Xia, M. Ji, B. Wang, S. Yin, Y. Huang, Z. Chen and H. Li, Appl. Catal., B, 2016, 188, 376 CrossRef.
- J. Li, J. Zhou, H. Hao and Z. Zhu, Mater. Lett., 2016, 170, 163 CrossRef.
- D. P. Dutta and A. K. Tyagi, Mater. Res. Bull., 2016, 74, 397 CrossRef.
- Q. Wu, Y. Cui, L. Yang, G. Zhang and D. Gao, Sep. Purif. Technol., 2015, 142, 168 CrossRef.
- S. Lu, Y. Yu, S. Bao and S. Liao, RSC Adv., 2015, 5, 85500 RSC.
- M. Jin, S. Lu, L. Ma and M. Gan, Appl. Surf. Sci., 2017, 396, 438 CrossRef.
- F. Tian, H. Zhao, G. Li, Z. Dai, Y. Liu and R. Chen, ChemSusChem, 2016, 9, 1579 CrossRef PubMed.
- Y. Guo, Y. Zhang, N. Tian and H. Huang, ACS Sustainable Chem. Eng., 2016, 4, 4003 CrossRef.
- Y. Chen, B. Liu, J. Chen, D. Chen, X. Yan, W. Xiao, L. Ge, M. Tu, Q. Wang and Z. Wang, Mater. Lett., 2015, 161, 289 CrossRef.
- E. S. Baeissa, Desalin. Water Treat., 2016, 57, 28939 CrossRef.
- S. Liu, P. Deng, G. Dai, Y. Liang and S. Zhang, Ceram. Int., 2016, 42, 10094 CrossRef.
- W. Ragsdale, S. Gupta, K. Conard, S. Delacruz and V. R. Subramanian, Appl. Catal., B, 2016, 180, 442 CrossRef.
- K. Zhang, Y. Liu, J. Deng, S. Xie, H. Lin, X. Zhao, J. Yang, Z. Han and H. Dai, Appl. Catal., B, 2017, 202, 569 CrossRef.
- S. J. A. Moniz, D. Pugh, C. S. Blackman, J. Tang and C. J. Carmalt, Cryst. Growth Des., 2016, 16, 3818 Search PubMed.
- J. Hu, X. Wu, C. Huang, W. Fan and X. Qiu, Appl. Surf. Sci., 2016, 387, 45 CrossRef.
- Y. Xu, M. Lv, H. Yang, Q. Chen, X. Liu and F. Wei, RSC Adv., 2015, 5, 43473 RSC.
- H. Lv, J. Guang, Y. Liu, H. Tang, P. Zhang, Y. Lu and J. Wang, RSC Adv., 2015, 5, 100625 RSC.
- Y. Zhu, Q. Ling, Y. Liu, H. Wang and Y. Zhu, Appl. Catal., B, 2016, 187, 204 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |