Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mesocrystals for photocatalysis: a comprehensive review on synthesis engineering and functional modifications
Shaodong
Sun
 *a,
Xiaojing
Yu
*a,
Qing
Yang
a,
Zhimao
Yang
b and
Shuhua
Liang
*a
*a,
Xiaojing
Yu
*a,
Qing
Yang
a,
Zhimao
Yang
b and
Shuhua
Liang
*a
aShaanxi Province Key Laboratory for Electrical Materials and Infiltration Technology, School of Materials Science and Engineering, Xi'an University of Technology, Xi'an 710048, Shaanxi, People's Republic of China. E-mail: sdsun@xaut.edu.cn; yxj@xaut.edu.cn; liangsh@xaut.edu.cn
bSchool of Science, State Key Laboratory for Mechanical Behavior of Materials, MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, Xi'an Jiaotong University, Xi'an 710049, Shaanxi, People's Republic of China
First published on 17th September 2018
Abstract
Mesocrystals are a new class of superstructures that are generally made of crystallographically highly ordered nanoparticles and could function as intermediates in a non-classical particle-mediated aggregation process. In the past decades, extensive research interest has been focused on the structural and morphogenetic aspects, as well as the growth mechanisms, of mesocrystals. Unique physicochemical properties including high surface area and ordered porosity provide new opportunities for potential applications. In particular, the oriented interfaces in mesocrystals are considered to be beneficial for effective photogenerated charge transfer, which is a promising photocatalytic candidate for promoting charge carrier separation. Only recently, remarkable advances have been reported with a special focus on TiO2 mesocrystal photocatalysts. However, there is still no comprehensive overview on various mesocrystal photocatalysts and their functional modifications. In this review, different kinds of mesocrystal photocatalysts, such as TiO2 (anatase), TiO2 (rutile), ZnO, CuO, Ta2O5, BiVO4, BaZrO3, SrTiO3, NaTaO3, Nb3O7(OH), In2O3−x(OH)y, and AgIn(WO4)2, are highlighted based on the synthesis engineering, functional modifications (including hybridization and doping), and typical structure-related photocatalytic mechanisms. Several current challenges and crucial issues of mesocrystal-based photocatalysts that need to be addressed in future studies are also given.
1. Introduction
Photocatalysis is a promising pathway to resolve the problems of energy depletion and environmental pollution via the photocatalytic conversion of solar light into chemical energy.1–4 Currently, the low utilization of visible light and short lifetimes of photogenerated charge carriers are two bottlenecks that limit the practical applications of photocatalysts.5–7 Therefore, improvements in the visible-light response and effective separation of photogenerated charge carriers for photocatalysts have garnered increased attention. Generally, the enhanced performances for single-component photocatalytic materials can be achieved by morphology-control or facet-control strategies. For example, a surface heterojunction can be constructed within a single anatase TiO2 polyhedron with coexposed (001) and (101) facets, which facilitates the transfer of photogenerated electrons and holes on the (101) and (001) facets, respectively, yielding better activity through the optimal ratio of the exposed (101) and (001) facets.8 However, the recombination of photogenerated charge carriers cannot be effectively restrained due to the lack of an energy barrier for charge separation, and mass migration is only occurring on the surfaces.1 Therefore, it is imperative to reasonably design and synthesize novel architectures for efficient interparticle or interfacial charge transfer, which can provide a significant breakthrough in optimizing single-component photocatalytic materials.Mesocrystals are a new class of superstructures, which are generally made of crystallographically highly ordered nanoparticles and could function as intermediates in a nonclassical particle-mediated aggregation process.9–13 It should be noted that a mesocrystal is the definition of a superstructure and not a formation mechanism, which displays a selected area electron diffraction (SAED) pattern similar to a single crystal or a quasi-single crystal, resulting in the enhancement of charge migration than that in traditional polycrystalline materials. Moreover, as compared to conventional single crystals, mesocrystals possess nanoscale subunits, anisotropic shapes, rough surfaces, or order porosity, which exhibit high specific surface areas and offer more active sites for photocatalytic reactions. Consequently, these features provide new opportunities for potential photocatalytic applications.
In the past decades, extensive research interest has been focused on the structural and morphogenetic aspects, as well as the growth mechanisms of mesocrystals. A number of reviews on the formation mechanisms have been published, mainly including nanoparticle-oriented aggregation along an ordered organic matrix by spatial constraints and the assistance of external physical fields (such as electronic, magnetic, and optical fields).14–25 In 2016, remarkable advances were reported, particularly focusing on the use of TiO2 mesocrystal photocatalysts.26 However, there is still no comprehensive overview on the photocatalytic applications of various metal oxide mesocrystals and their functional modification forms so far.
In this review, we mainly summarize the important progresses made in the development of photocatalysis-oriented mesocrystals in various species, including single-metallic oxides (such as TiO2 (anatase), TiO2 (rutile), ZnO, CuO, Ta2O5) and multimetallic oxides (such as BiVO4, BaZrO3, SrTiO3, NaTaO3, Nb3O7(OH), In2O3−x(OH)y, and AgIn(WO4)2) (Scheme 1). We start with the systematic review of the synthesis strategies and principles for enhanced performances of different mesocrystal photocatalysts. Next, the functional modifications (including hybridization and doping) for the construction of mesocrystal-based photocatalysts and structure-related photocatalytic mechanisms are presented. Finally, the current challenges and the crucial issues of mesocrystal-based photocatalysts that need to be addressed in future studies are mentioned.
2. Mesocrystal photocatalysts
Although a series of semiconductors with good structure–performance relationships have been investigated for improving the low quantum efficiency in photocatalysis, the existence of charge recombinations at the surface or inside the volume cannot be effectively avoided. With the development of a new class of porous metal oxide materials with uses ranging from catalysis to energy storage and conversion, highly ordered mesocrystal superstructures could limit the recombination of electron–hole pairs due to the anisotropic electron flow along the interparticles; therefore, the fast and efficient collection of photogenerated charge carriers on catalytic sites can be achieved by the dominant surface of mesocrystals. Therefore, understanding the synthesis strategies and corresponding formation mechanisms of typical single-component mesocrystal photocatalysts is significant for the controllable design of novel versatile mesocrystal-based composites. In this section, we mainly introduce the synthesis methods and photocatalytic mechanisms involving mesocrystal photocatalysts (Table 1), including TiO2 (anatase), TiO2 (rutile), ZnO, CuO, Ta2O5, BiVO4, BaZrO3, SrTiO3, NaTaO3, Nb3O7(OH), In2O3−x(OH)y, and AgIn(WO4)2. Initially, certain concepts involving the growth and formation mechanism of mesocrystals will be listed and explained. Topotactic transformation is a kind of structural transition in solid materials that enables structural transitions without losing the crystalline symmetry of the parental phase.27,28 Kirkendall effect is a kind of manifestation that induces void structures; due to the different diffusion rates between two species at an elevated temperature, movement can be induced at the interface between a diffusion couple.29,30 Oriented attachment is a nonclassical crystal growth model, where the nanocrystals align along a certain crystallographic direction in order to minimize their interfacial energy.31,32 Ostwald ripening process is a direct matter-relocation approach, which is defined as the “dissolution of small crystals or sol particles and the redeposition of the dissolved species on the surfaces of larger crystals or sol particles.”33,34| Photocatalyst | Morphology | Synthesis process | Formation mechanism | Application | Mean reasons of enhanced property | Ref. |
|---|---|---|---|---|---|---|
| Anatase TiO2 | Polyhedral mainly with exposed (001) facets | Prepared NH4TiOF3 mesocrystals and sintered at 700–900 °C | Topotactic transformation | Photodegradation of methylene blue | Special facets lead to a relatively large crystallite size | 35 |
| Anatase TiO2 | Polyhedral mainly with exposed (001) facets from top view and (101) facets from side view | Prepared NH4TiOF3 mesocrystals and calcined at 500 °C | Topotactic transformation | Photocatalytic degradation of 4-chlorophenol and Cr6+ and photocatalytic hydrogen evolution | (1) (001) facets have strong ability to form hydroxyl radicals | 37 |
| (2) Well-aligned nanocrystals can efficiently promote photoactive efficiency due to the facilitation of charge separation | ||||||
| Anatase TiO2 | Polyhedral mainly with exposed (001) facets | Sintered NH4TiOF3 precursors at 700–900 °C | Topotactic transformation | Photodegradation of methylene blue | Largely exposed (001) facets improved photodegradation | 38 |
| Anatase TiO2 | Truncated tetragonal bipyramidal Wulff shape | Solvothermal synthesis: TiCl4 added into octyl alcohol and heated at 100 °C | Oriented attachment mechanism | Photodegradation of rhodamine B | A higher number of activity sites in this photocatalytic reaction improves photodegradation | 39 |
| Anatase TiO2 | Sheet-like TiO2 mesocrystals with controllable nanothorns on the (101) facet | Intermediate NH4TiOF3 sheets treated with H3BO3 and NaOH, and then annealed at 500 °C | Topotactic transformation | Photocatalytic hydrogen evolution | Facet-induced charge separation and anisotropic electron flow improve photocatalytic property | 40 |
| Anatase TiO2 | Layered nanosheets with exposed (001) facets | Hydrothermal method: using (NH4)2TiF6, H3BO3, and 2-propanol, followed by calcination treatment | Topotactic transformation | Photodegradation of rhodamine B | (001) facets provide highly active sites and layered structures facilitate the transport of reactants and degradation products | 41 |
| Anatase TiO2 | Submicron-sized anatase TiO2 mesocrystals with exposed (001) surfaces | Solvothermal synthesis: NH4F and Ti(OC4H9)4 added into glacial acetic acid and then heated at 210 °C | Slow hydrolysis reaction controlled by the reaction between glacial acetic acid and Ti(OC4H9)4 | Photodegradation of gaseous styrene | Large surface area, good anatase crystallinity, high percentage of (001) facets, wider band energy, and unique mesoporous structure combined together to improve photocatalytic property | 42 |
| Anatase TiO2 | Regular-shaped TiO2 MCs enclosed with different proportions of (001) and (101) facets | Solvothermal synthesis: formic acid (FA) and titanium isopropoxide (TTIP) | Assembly of the subunits in ∼30–50 nm: FA molecules should be preferentially attached onto the specific (101) surfaces of the nanosheets, and therefore, lead to strongly anisotropic mutual interactions between formed small subunits | Photo-oxidation of nitrosobenzene | The synergistic effect of Ti3+, the higher proportion of (101) facets, and structural integrity of crystal are responsible for the higher photocatalytic activity | 43 |
| Anatase TiO2 | Nanoporous spindles | Solvothermal synthesis: tetrabutyl titanate (TBT) was added dropwise to acetic acid (HAc) and maintained at 200 °C for 24 h | Hydrolysis reaction, controlled by the reaction between acetic acid and TBT, leading to the nanoporous structure and nano-size of TiO2 | Photodegradation of gaseous benzene | The active anatase crystal phase, small crystallite size, high surface area, and narrow pore size distribution are important for yielding the best catalytic activity | 46 |
| Anatase TiO2 | Spindles | Hydrothermal method + calcination: TBOT added into HAc solution with some water and then calcinated at different temperatures for 1 h | Hydrolysis reaction formed TiO2 and calcination temperature determined the structure of TiO2 | Photodegradation of methylene blue | The TiO2 calcinated at a suitable temperature had increased the crystallinity and surface area, which were mainly responsible for the improvement in the photocatalytic properties | 47 |
| Anatase TiO2 | Spindles | Solvothermal synthesis: TiCl4 aqueous solution was mixed with 40 mL CH3COOH and heated at 200 °C | Nanoparticle-oriented assembly | Photodegradation of methylene blue | The high orientation of primary nanocrystals in mesoporous structure accelerated efficient charge separation | 48 |
| Anatase TiO2 | 3D olive shape with subunits in spindles structure | After preferential evaporation of tetrahydrofuran (THF) solvent and TiO2 NPs assembled by PEO-PPO-PEO/titania oligomer spherical micelles are formed at the liquid−liquid interface | Evaporation-driven oriented assembly | Photocatalytic decomposition of methylene blue | Large number of oxygen vacancies located on the surface and high percentage of reactive (001) facets | 49 |
| Anatase TiO2 | Hierarchical hollow microspheres | Ultrasound-assisted aerosol-spray method to prepare NH4TiOF3, and then, calcination for 2 h | Self-assembly and topotactic transformations | Photodegradation of 4-chlorophenol | Assembled nanosheets are favorable for multiple reflections, which greatly improve the utilization of UV light | 52 |
| Anatase TiO2 | Porous microspheres | Solvothermal synthesis: TiOSO4 mixed with tert-butyl alcohol (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165), heated at 110 °C and annealed at 700 °C 165), heated at 110 °C and annealed at 700 °C |
Annealed temperature determined the orientation of mesoporous TiO2 | Photodegradation of phenol and hydrogen evolution | Orderly arrangement of TiO2 nanocrystals can be more effective in the migration of photogenerated charges to have a higher photocurrent | 53 |
| Anatase TiO2 | Nano-spherical assemblies (<100 nm) | Microemulsion method | Self-assembly and slow hydrolysis process | Photodegradation of methylene blue | Not mentioned | 54 |
| Anatase TiO2 | Nano-spherical assemblies (<25 nm) | Microemulsion method | Using soft templates to assemble | Photodegradation of 2,4-dichlorophenol | High surface area and good crystallinity are effective for photodegradation | 55 |
| Rutile TiO2 | Hierarchical assemblies | Microwave-assisted hydrothermal method | Self-assembly of small rutile TiO2 | Photocatalytic oxidation of NO gas | A large effective surface area enabled the diffusive transport of photogenerated holes to oxidizable species | 56 |
| Rutile TiO2 | Hollow microspheres | K2TiO2(C2O4)2 and HNO3 were added to H2O2 aqueous solution and then maintained at 80 °C | Hydrolysis-dissolution-precipitation | Photodegradation of rhodamine B | Mesopores in the mesocrystals contributed to absorb molecular dyes | 57 |
| Anatase TiO2 | Sheets with exposed (001) facets | Microwave-assisted sonochemical method | Synergistic effect of microwave and sonication influence the hydrolysis process of TiO2 to induce oriented aggregation of TiO2 along the crystallographic axes | Photodegradation of rhodamine B | (1) Anatase mesocrystals have high energy facets, crystallinity | 58 |
| (2) Mesocrystal structures are conducive to reduce electron–hole recombination rates | ||||||
| Rutile TiO2 | Nanosheets | Substrates were left to stand in TiCl3 aqueous solution in a polypropylene vessel and maintained at 25 °C for several days | Oxidative deposition | Photodegradation of methylene blue | Exposed crystal facets are beneficial toward electron transfer | 59 |
| Rutile TiO2 | Porous sheets | Silica template hydrothermal method | Hydrolysis; HCl acted as etching agent | Photodegradation of methyl orange and hydrogen evolution | Effective reduction sites provided by the abundantly exposed (110) facets of rutile TiO2 improved the properties | 61 |
| Anatase TiO2 | Porous sheets | Silica template hydrothermal method | Hydrolysis; HCl and HF acted as etching agents | Photodegradation of methyl orange and hydrogen evolution | The preferentially exposed (001) facets of anatase TiO2 are responsible for the high oxidative photocatalytic activity | 61 |
| Anatase TiO2 | Rods | Solvothermal synthesis: Ti(OC4H9)4, CH3COOH, C6H5COOH, and CH3CH2OH were mixed and treated at 180 °C for 12 h | Hydrolysis; benzoic acid helped to form rod-like structures; alcohol and acetic acid helped to form mesoporous structures | Photodegradation of methyl orange | High crystallinity and higher specific surface area of the sample lead to more active sites and better adsorptive capacity | 65 |
| ZnO | Nanosheet assemblies | Zn(NO3)2 mixed with NH4F and NaOH and stirred at room temperature | The small units were prepared by chemical precipitation; then, the units formed mesoporous structures through epitaxial self-assembly | Photodegradation of methylene blue | Exposed nonpolar (10-10) facets of ZnO have higher photocatalytic activity | 71 |
| ZnO | Hollow assemblies | Hydrothermal synthesis: Zn(AC)2, HF, and hexamethylenetetramine mixture maintained at 160 °C for 6 h and calcined in air at 500/800 °C for 2 h | The Zn(OH)F precursor formed during the hydrothermal process and helped to increase the oxygen vacancies of ZnO | Photodegradation of methylene blue | Abundant oxygen vacancies play a key role in narrowing the bandgap instead of the formation of active centers or trap centers | 73 |
| ZnO | Nanosheet assemblies | Hydrothermal synthesis: PVP, Zn(NO3)2, and urea mixture maintained at 150 °C and calcined at 300–700 °C | PVP worked as structure-directing reagent to assist ZnO self-assembly | Photodegradation of methylene blue | The high-ordered MC structure can promote the separation and transfer of photoinduced electrons and holes and provided larger specific surface area | 74 |
| ZnO | Nanosheet assemblies | Hydrothermal synthesis: Zinc acetate solution and sodium citrate mixture heated to 150 °C for 24 h | Ostwald ripening process and nonclassical mesocrystal growth mechanism | Photodegradation of methylene blue and 2,4,6-trichlorophenol | (1) The ordered alignment of nanoparticles facilitated the transfer of photoinduced carriers | 75 |
| (2) The defects located at the interfaces among the nanocrystals can act as active sites for photoreaction | ||||||
| ZnO | Nanosheets | Electrodeposition: used ZnCl2, H2O2, and NaNO3 as the electrolyte composition, Al substrate as the working electrode and Pt foil as the counter electrode. The system worked under supercritical CO2 (SC–CO2) atmosphere | Oriented attachment describes a spontaneous self-assembly process to form mesocrystals. Meanwhile, Cl− adsorption help to form 2D platelet structures. SC-CO2 helped to generate isotropical shape | Photoelectrochemical application | Highly oriented crystallinity and substantially long exciton lifetime of prepared ZnO improved photoelectrochemical properties | 76 |
| ZnO | Bundles | Precipitation-annealing: simply mixing the aqueous solutions of zinc acetate, sodium hydroxide, and tartaric acid and annealed at certain temperatures | Tartaric acid leads to oriented attachment of Zn(OH)2 and helps to assemble Zn (OH)2 bundles | Photodegradation of methyl orange and photoreduction of Cr6+ in water | The prepared catalysts had proper ZnO particle size and suitable porous structure | 79 |
| ZnO | Spindles | Ionic-liquid-based antisolvent method: ZnO-containing deep eutectic solvents was injected into (HOCH2)3CNH2 solution for 5 min at 70 °C | Tris molecules and deep eutectic solvents lead to oriented attachment and assembly of ZnO NPs | Photodegradation of methylene blue | (1) Mesostructure provided specific surface area and pore volume to improve photodegradation | 81 |
| (2) C-planes of ZnO, worked as a superior facet for photodegradation, exposed on the surface of the mesocrystals | ||||||
| CuO | Spindles | Additive-free complex-precursor solution method: Cu(NO3)2 solution mixed with NaOH solution stirred at 80 °C for 30 min | Hydrolysis–dissolution–precipitation and bottom-up assembly | Photodegradation of rhodamine B | These 3D mesostructural spindles exhibited more pores to absorb molecules and had the ability to reduce electron–hole recombinations | 86 |
| Ta2O5 | Nanosheets | (NH4)2Ta2O3F6 nanorods were prepared by vapor hydrolysis reaction method. Then, as-prepared mesocrystalline nanorods were annealed at ∼700–900 °C for 3 h | Self-assembly of (NH4)2Ta2O3F6 and topotactic transformation | Photocatalytic hydrogen evolution | Mesocrystalline Ta2O5 superstructures contributed to the generation of long lifetime photoinduced carriers and effective conductive pathways for photocatalytic hydrogen production | 88 |
| BiVO4 | Nanoparticle assemblies | Hydrothermal method: silica solution template filled with acidified BiVO4 precursor solution. Then, NaOH solution was used to form mesostructured BiVO4 | BiVO4 nuclei grew by Ostwald ripening mechanism. Vacuum atmosphere is necessary to ensure sufficient infiltration into silica template. | Photocatalytic oxygen evolution | (1) Inner pores can scatter more light | 95 |
| (2) High crystallinity and single coherent atomic configuration are good for the transfer of charge carriers | ||||||
| (3) Mesoporous structure can decrease the transfer distance | ||||||
| (4) Increase in surface area can also increase the active sites | ||||||
| NaTaO3 | Cubic assemblies | Surfactant-free solvothermal synthesis: TaCl5 in ethanol mixed with sodium ethoxide, heated at 240 °C for 4 h | Acidic alkoxide hydrolyzation yields particles with small size and high surface area | Photocatalytic hydrogen and oxygen evolution | Small particle size and high surface area improved the charge separation, migration of photogenerated carriers, and benefited the surface chemical reaction of catalysts | 101 |
| SrTiO3 | Cubic assemblies | Hydrothermal treatment: TiO2 mesocrystals in ethanol were added into Sr(OH)2 solution. Then, they were mixed with NaOH, polyethylene glycol solution, and water, and heated at 200 °C for ∼12–60 h | Topotactic transformation | Photocatalytic hydrogen and oxygen evolution | Well-defined superstructure can deliver photo-charges more efficiently | 105 |
| SrTiO3 | Porous spheres with wormhole-like structure | Solvothermal synthesis: Ti(C4H9O)4 and ammonia mixed to obtain a precipitated Ti(OH)4 and then added in Sr(NO3)2, KOH, and PVA solution and heated at 200 °C for ∼0.5–2 h | PVA leads to oriented aggregation and assembly of SrTiO3 | Photodegradation of rhodamine B | High-crystalline SrTiO3 mesoporous spheres with large pores and primary nanoparticles of optimum size are good for photocatalytic reaction | 107 |
| In2O3−x(OH)y | Porous rods | Precipitation + calcination: InCl3, H2O, and urea are used to prepare In(OH)3 nanorods. Then, calcinated at 250 °C for 6 h | Nanorods are preferentially oriented such that their body lengths are aligned parallel to the substrate surface | Photoreduction of CO2 | (1) Rod structure catalysts are more effective for inter-nanocrystal charge transfer | 108 |
| (2) charge transfer may occur between neighboring nanocrystals in In2O3−x(OH)y nanorods, resulting in prolonged lifetimes, thereby improving the photocatalytic activity | ||||||
| (3) In2O3−x(OH)y nanorods populated with surface hydroxyl groups and oxygen vacancies to improve photocatalytic properties | ||||||
| Nb3O7(OH) | Cubes with nanorods subunits | One-step hydrothermal method: cubes NbCl4-THF complex was mixed with HCl and then heated at 200 °C | Ostwald ripening process happened during the wire formation. Self-assembly via oriented aggregation | Photodegradation of methylene blue, rhodamine B, and indigo carmine | The mesocrystals benefit from their large surface area, high crystallinity, and direct electron transport path | 109 |
| AgIn(WO4)2 | Hierarchical rods | Microwave-assisted approach: AgNO3 and In(NO3)3 mixed with Na2WO4 heated to 180 °C by microwave irradiation for 20 min | Oriented-attachment process accompanying the Ostwald ripening process | Photodegradation of eosin Y, rhodamine B, and methyl orange | Not mentioned | 110 |
2.1 Binary oxide mesocrystals
Polyhedral TiO2 mesocrystals. The typical synthesis strategy for TiO2 mesocrystals comprising nanocrystals with exposed (001) facets involves the topotactic transformation of NH4TiOF3 mesocrystals at high annealing temperatures, which was first discovered by O'Brien's group in 2008.36 However, the experimental procedures were relatively complicated and could not be scaled up for practical applications. Therefore, it is necessary to develop a one-pot facile synthesis method to prepare TiO2 mesocrystals exposed with high active facets. Majima and coworkers have obtained plate-like TiO2 mesocrystals by annealing a thin layer of precursor solution containing TiF4, NH4F, and NH4NO3 on a silicon wafer. Evidently, the nonporous intermediate NH4TiOF3 precursor was first formed following the combination reactions from the mixtures of Ti4+, F−, NH4+, and H2O at low temperatures (<200 °C). As the annealing process required higher temperatures (>300 °C), the as-synthesized NH4TiOF3 precursor would be topotactically transformed into porous anatase TiO2 mesocrystals with dominant (001) facets. It should be noted that the gaps or holes are generated owing to nanocrystal diffusion and recrystallization during the removal of large amounts of N, H, and F atoms from the crystal lattice structure; therefore, the amount of NH4F is significant for the morphology-controlled synthesis of TiO2 mesocrystals (Fig. 1).26
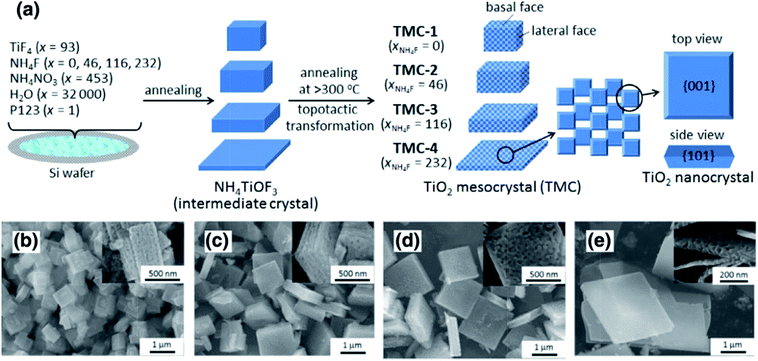 | ||
| Fig. 1 (a) Schematic illustration of the synthesis of differently shaped TiO2 mesocrystals. SEM images of different TiO2 mesocrystals prepared with x (molar ratio of NH4F) = 0 (b), 46 (c), 116 (d), and 232 (e). Insets display the corresponding high-magnification SEM images. It can be found that the thickness size of the as-synthesized particles gradually reduced along with an increase in the amount of NH4F. Adapted with permission from ref. 26. Copyright 2016 Elsevier. | ||
The TiO2 mesocrystals assembled with highly ordered alignment of anatase nanocrystals (size: 40 nm) can display porous structures with pore diameters of several nanometers (surface area: >90 m2 g−1), resulting in remarkably long-lifetime charges, higher photoconductivity and photocatalytic hydrogen evolution, as well as degradation of 4-chlorophenol and Cr6+ in the aqueous phase.37 In this case, it was observed that TiO2 mesocrystals exposed with different facet ratios exhibited different reactivity orders during photooxidation, i.e., (001) > (101), and photoreduction, i.e., (101) > (001), under UV-light irradiation. Interestingly, the authors have confirmed that the (001) facets were preferable during molecular adsorption and photogenerated electron injection from the photoexcited dye sensitizers (eosin Y and Ruthenizer 470) to the conduction band (CB) of TiO2 under visible-light irradiation, whereas the (101) facets were beneficial for the collection of photogenerated electrons because of the directional electron flow. These findings emphasized that the concept of crystal-facet-dependent photocatalytic reactions can be extended to mesocrystal systems.
Based on the above synthesis route, Zhou and coworkers have developed TiO2 mesocrystals largely exposed with (001) facets through the topotactic annealing of NH4TiOF3 mesocrystals at 800 °C synthesized by (NH4)2TiF6 and NH4OH without the need for extremely toxic HF treatments.35 In this case, the specific structure of the TiO2 mesocrystal with a large crystallite size, high specific surface area, and additional numbers of active (001) facets can greatly enhance the photocatalytic activity, whereas lower or higher processing temperatures (e.g., 700 °C and 900 °C, respectively) would damage the microstructure, and therefore, deteriorate the photocatalytic activity. In addition, these particles have good photochemical stability and much larger size than commercial P25, which suggests that they can be easily removed from the liquid-phase system by centrifugation and reused.38
Moreover, Leite and coworkers have proposed a kinetically controlled crystallization process by a nonaqueous sol–gel synthesis method to prepare anatase TiO2 recrystallized mesocrystals, a chemical process based on the reaction of titanium(IV) chloride with n-octanol. This is attributed to the oriented-attachment mechanism. During the oriented-attachment process, individual nanoparticles acting as primary building blocks could directly assemble with adjacent nanoparticles to yield a mesostructure with similar crystallographic orientation to minimize interfacial energy. The high-resolution transmission electron microscopy (HRTEM) analysis clearly revealed that the synthesized recrystallized anatase mesocrystals exhibited a truncated bipyramidal Wulff shape, indicating that its surface is dominated by (101) facets, which exhibited superior photoreactivity for rhodamine B degradation under visible-light irradiation as compared to commercial P25 as a benchmarking material.39
Furthermore, nanothorn TiO2 mesocrystals with dominant (101) facets displayed approximately 1.5 and 6 times higher photocatalytic hydrogen evolution activities than those of (001) facets and disordered nanocrystals, respectively, which can be attributed to the specific facet-induced charge separation and anisotropic electron flow. According to the single-particle photoluminescence measurements, Mott–Schottky analyses, and transient absorption measurements, efficient interfacial band alignment and charge separation were obtained, suggesting the contribution of the dominant (101) facet. This facet-induced anisotropic flow in the photocatalysis resulted in the realization of a photocatalyst that exhibited efficient charge separation and enhanced photocatalytic activity.40
In addition, layered TiO2 mesocrystals composed of nanosheets with exposed (001) facets were also successfully fabricated with the combination of hydrothermal treatments in the presence of (NH4)2TiF6, H3BO3, and PrOH, followed by calcination treatments. A schematic illustration of the formation of layered TiO2 is shown in Fig. 2.41 Initially, PrOH started to dissociate into PrO− under weak acidic conditions; then, both PrO− and BF4− would preferentially bind to unsaturated Ti4+ cations on the (001) facets of NH4TiOF3 nuclei to reduce their surface energies, leading to the formation of single-crystal NH4TiOF3 nanosheets with (001) facets. Subsequently, these NH4TiOF3 nanosheets with (001) facets capped with PrO− and BF4− orderly assemble to block the tabular grains. Finally, NH4TiOF3 nanosheets were transformed into layered TiO2 nanosheets after calcination treatment because of the removal of PrOH and the reduced volume from NH4TiOF3 to TiO2. With regard to the photocatalytic activity, the as-prepared layered TiO2 with (001) facet nanosheets exhibited excellent performance for the degradation of rhodamine B when compared with commercial P25, which can be ascribed to the synergetic effects between the layered structures and (001) facets. Similarly, anatase TiO2 mesocrystals with exposed (001) facets have been successfully synthesized by a facile one-step solvothermal method using NH4F as the structure regulator in a glacial acetic acid environment, which can be used in the photocatalytic decomposition of gaseous styrene.42
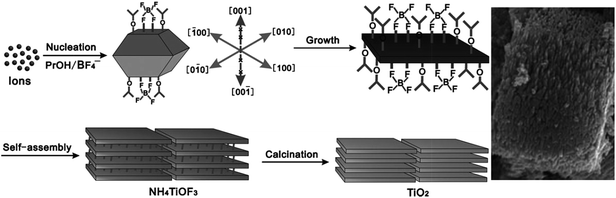 | ||
| Fig. 2 Schematic illustration of the formation of layered TiO2 mesocrystals. Adapted with permission from ref. 41. Copyright 2011 Wiley-VCH Verlag GmbH & Co. | ||
It should be noted that the photocatalytic activity of polyhedral TiO2 mesocrystals with controllable proportions of (101) and (001) facets is significant, so it is highly desirable to investigate the photocatalytic activities of TiO2 mesocrystals with different (101)/(001) facet ratios. Fortunately, Zhao and coworkers have synthesized regular-shaped TiO2 mesocrystals enclosed with different proportions of (001) and (101) facets by a simple approach in the presence of formic acid and titanium isopropoxide as the original reactants without any other additives and surfactants at 160 °C. Further, the control of the (101)/(001) ratio of TiO2 mesocrystals was achieved by altering the solvothermal treatment periods.43 The TiO2 mesocrystals enclosed by a high proportion of (101) facets showed higher photocatalytic activity for benching nitrosobenzene than those with a lower proportion, which was attributed to the synergistic effect of Ti3+ and the proportion of (101) facets. In addition, the normalized photocatalytic activity of TiO2 mesocrystals was better than that of nanocrystals as the proportion of (101) facets was equal, suggesting that the structural integrity played a key role in the photocatalytic activity.
Spindle-shaped TiO2 mesocrystals. The formation of a spindle shape is common for metal oxide mesocrystals due to the oriented attachment of the nanoparticles. Based on earlier literature,44–48 it is found that spindle-shaped anatase TiO2 mesocrystals can be facilely synthesized on a large scale through mesoscale assembly in titanium salt/acetic acid systems without any additives under solvothermal conditions. The acetic acid and solvothermal conditions are the key factors for the synthesis of spindle-shaped TiO2 mesocrystals, whereas the type of titanium salt is adjustable, including tetrabutyl titanate,44,45 butyl titanate,46 titanium butoxide,47 and titanium tetrachloride.48
For example, Qi and coworkers have demonstrated the first additive-free synthesis of porous anatase TiO2 mesocrystals with a single-crystal-like SAED pattern by using tetrabutyl titanate as the titanium source and acetic acid as the solvent.44 A complex nanoparticle-assembly process was obtained, involving the slow release of soluble species from metastable solid tetrabutyl titanate precursors for the continuous formation of anatase building blocks, followed by the oriented aggregation of tiny anatase nanocrystals under the capping of the as-produced butyl acetate, finally leading to the formation of porous spindle-shaped mesocrystals after the removal of organic residuals by calcination treatment. A schematic illustration of a tentative mechanism for the formation of porous anatase TiO2 mesocrystals without additives is shown in Fig. 3a. Fig. 3b shows a typical transmission electron microscopy (TEM) image of a single spindle particle, indicating that the particle consists of nanosized subunits. Its SAED pattern exhibits diffraction spots corresponding to the (010) zone axis of the anatase-phase TiO2, suggesting the possession of a “single-crystal-like” structure. Notably, it can be seen that the diffraction spots are slightly elongated, indicating that there is a small lattice mismatch between the boundaries of the nanoparticles, which is a typical characteristic of mesocrystals. The internal porosity of the particle is revealed by the TEM image taken on a microtomed sample (Fig. 3c). Lim and coworkers have synthesized spindle TiO2 mesocrystals using a hydrothermal method in the presence of titanium butoxide/acetic acid/water system and investigated the effect of calcination temperature (100–800 °C) on their morphology, crystallinity, and photocatalytic activity.47 In this work, the authors have found that controlling the calcination temperature is an effective pathway to control the morphology, crystallinity, and photocatalytic activity of TiO2 mesocrystals. The shape, dimension, and crystal structure of the TiO2 mesocrystals have no appreciable changes as the calcination temperature increased to 300 °C, and the crystallinity can be improved by increasing the temperature. However, the mesocrystal characteristic began to disappear at 400 °C, and the specific surface area decreased with increasing temperature due to the reduced boundaries. Hence, the photocatalytic degradation of methylene blue for TiO2 improved when the temperature increased to 300 °C owing to the enhanced crystallinity and elimination of byproducts; however, it became poor above 400 °C because of the decreased surface area.47 In addition, mesoporous anatase TiO2 prepared by using butyl titanate as the Ti source and acetic acid as the solvent can be used toward inducing photocatalytic activity in the degradation of gaseous benzene.46
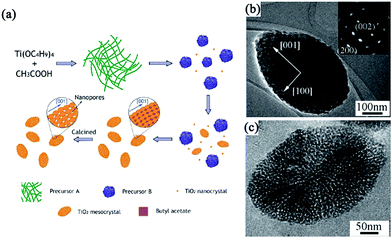 | ||
| Fig. 3 (a) Schematic illustration of a tentative mechanism for the additive-free synthesis of porous anatase TiO2 mesocrystals. (b) A low-magnification TEM image of a single spindle. Inset shows the corresponding SAED pattern. (c) A high-magnification TEM image of a porous particle. Adapted with permission from ref. 44. Copyright 2011 American Chemical Society. | ||
Apart from the above solvothermal methods, the hard-template strategy has also been developed for the fabrication of porous TiO2 mesocrystals. Zhao and coworkers have reported a facile evaporation-driven oriented assembly strategy to prepare olive-shaped mesoporous TiO2 mesocrystals in an acidic tetrahydrofuran (THF)/pluronic F127/water/HCl/acetic acid/titanium tetrabutoxide mixed solution,49 which started with the liquid–liquid phase separation as the preferential evaporation of THF at 60 °C. Then, spindle-shaped TiO2 particles assembled by pluronic F127/titania oligomer spherical micelles were generated at the liquid–liquid interface. Finally, 3D-open anisotropic spindle-like mesoporous TiO2 mesocrystals were obtained by the continuous evaporation of residual THF and hydrolyzed solvents, which could drive the oriented attachment of both mesopore channels and flake-like nanocrystals from the initial spherical composite micelles along the free radial and restricted tangential directions. Dye-sensitized solar cells based on the above samples showed ultrahigh photoconversion efficiencies (beyond 11%), which were attributed to the intrinsic mesocrystal nature as well as high porosity.
Mesocrystalline TiO2 assemblies. Although the studies on TiO2 mesocrystal photocatalysts have been mainly concentrated on the structure/morphology and porosity control by the abovementioned different synthesis techniques, the deep understanding the relationship between the oriented-attachment fashion and photocatalytic efficiency has been rarely investigated. Bian and coworkers have observed that TiO2 mesocrystals showed largely enhanced photoconductivity and photocatalytic activities than those of polycrystalline materials because of the remarkably increased long-lifetime charges under illumination for TiO2 mesocrystals.50,51 They have also synthesized hierarchical hollow microspheres with TiO2 mesocrystal nanosheets as building blocks by an ultrasound-assisted aerosol-spray method followed by topotactic transformations. TiO2 mesocrystal hollow microspheres can largely enhance photocatalytic performances.52 Furthermore, they found, for the first time, that the presence of small misorientations had an obvious harmful effect on the charge transfer, and hence, largely suppressed the photocatalytic efficiencies, as shown in Fig. 4.53 The involved two typical TiO2 spherical mesocrystals, one with oriented and the other with misoriented alignment, of secondary nanocrystals were prepared from a precursor containing TiOSO4 and tert-butyl alcohol by thermal annealing and hydrothermal recrystallization processes, respectively. Therefore, this finding should be taken into account and avoided during the design and synthesis of semiconductor mesocrystal photocatalysts.53
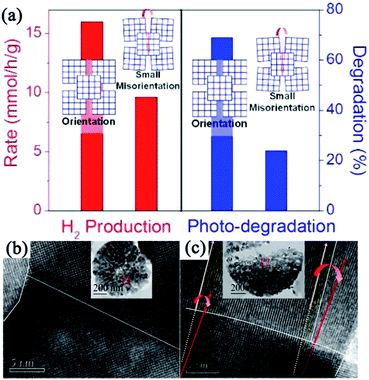 | ||
| Fig. 4 (a) Schematic illustration of disordered and ordered aggregations of TiO2 nanoparticles and their corresponding photocatalytic activities. (b) HRTEM image of TiO2 mesocrystal with ordered orientation. (c) HRTEM image of TiO2 mesocrystal with misorientation (crystal lattice mismatch). Adapted with permission from ref. 53. Copyright 2015 American Chemical Society. | ||
It is well known that the size effect is also significant for photocatalytic activity; therefore, the preparation of sub-100 nm mesocrystal-like porous assemblies is imperative. Tartaj and coworkers have developed an inverse microemulsion method for the synthesis of TiO2 mesocrystalline assemblies with mesopores, and all the sizes ranged from 25 to 70 nm. These 25 nm nanostructures exhibited good electrochemical performance and good capability for photocatalytic degradation.54,55
From the abovementioned results, it can be found that anatase TiO2 mesocrystals can be synthesized by a facile process, whereas rutile TiO2 mesocrystals for photocatalysis have been rarely reported. Jimmy C. Yu and coworkers have reported a simple and environmentally friendly approach for preparing photocatalytically active rutile TiO2 mesocrystals by a microwave-assisted hydrothermal method involving titanium(III) chloride as the only reactant. The as-synthesized one-dimensional (1D) rutile nanowires can easily assemble into three-dimensional (3D) hierarchical architectures without the help of surfactants or additives.56 Similarly, Wu and coworkers synthesized hollow TiO2 microspheres assembled with rutile mesocrystal nanorods directly from a mixed aqueous solution of K2TiO(C2O4)2, H2O2, and HNO3 at a low temperature of 80 °C, which displayed remarkable photocatalytic activity in photodegrading rhodamine B solution under UV-light illumination.57
Using a hard template is an effective alternate strategy for the synthesis of porous TiO2 nanosheets, which involved heterogeneous crystal nucleation and oriented growth within the templates.60 Hence, a series of mesoporous single-crystal-like structures, including anatase mesoporous TiO2 nanosheets with dominant (001) facets and rutile mesoporous TiO2 nanorods with tunable sizes, have been obtained in the presence of silica, titanium tetrachloride, titanium butoxide, hydrochloric acid, and hydrofluoric acid (Fig. 5).61 The resultant mesoporous TiO2 single-like crystals displayed enhanced photocatalytic performances on hydrogen evolution and degradation of methyl orange owing to their enlarged surface area, single-crystal nature, and exposure of reactive crystal facets coupled with a 3D connected mesoporous architecture. It was observed that the (110) facets of rutile mesoporous TiO2 can be essentially considered as reductive sites in the photoreduction reaction, while the (001) facets of anatase mesoporous TiO2 exhibited oxidation sites in the oxidative process.61 However, the use of a strong acid is not ecofriendly, so it is still a challenge to develop a new hard-template technology that can be used to fabricate mesoporous TiO2 mesocrystals with tunable facets and crystalline phase.
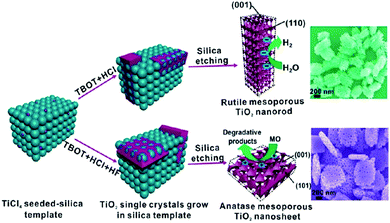 | ||
| Fig. 5 Schematic illustration of the synthesis pathways of rutile TiO2 mesocrystals and anatase TiO2 mesocrystals using silica templates by a hydrothermal method. Adapted with permission from ref. 61. Copyright 2013 American Chemical Society. | ||
1D architectures. 1D photocatalysts could trap solar light along their long axial direction and the simultaneous efficient carrier separation and collection in the nanometer-scale radial direction, resulting in the enhancement in the photocatalytic activity. Therefore, the synthesis of 1D TiO2 mesocrystals has attracted considerable attention.62,63 Qi and coworkers reported excellent broadband and quasi-omnidirectional antireflective structures based on highly stable, self-cleaning, mesocrystalline rutile TiO2 nanorod arrays (Fig. 6),64 which were prepared by a simple hydrothermal treatment of Ti foils in the presence of tetrabutyl titanate and hydrochloric acid. In this case, the nanorod building block is a single-crystal-like rutile TiO2 mesocrystal comprising many (001)-oriented nanotips (diameter: approximately 10–30 nm) grown on the top of a (001)-oriented stem nanorod (diameter: about 100–400 nm). The hierarchical TiO2 nanorod arrays showed the efficient suppression of reflection toward wavelengths ranging from visible to near-infrared (NIR) region, which was attributable to an optimized graded refractive index profile resulting from the multi-tips-on-rod structures.64 Liu and coworkers have synthesized rod-like TiO2 anatase mesocrystals with high specific surface area and excellent photocatalytic activity by a mild solvothermal route,65 in which the reagents were Ti(OC4H9)4, CH3COOH, C6H5COOH, and CH3CH2OH. It can be proposed that the oriented attachment of TiO2 nanoparticles was carried out under the synergism of hydrophobic bonds, π–π interactions, and mixed-ester templates. Further, the growth of the crystal facet of anatase was also affected by the π–π interactions. This study not only opens up new avenues for rationally designing TiO2 mesocrystal materials with ideal hierarchy and controllable sizes, but also provides a perspective toward uncovering the formation process of porous-crystalline superstructures.
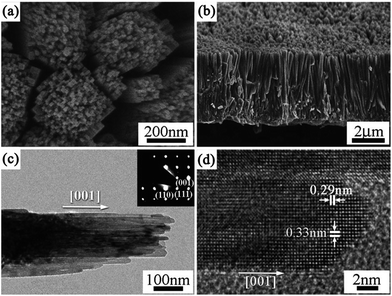 | ||
| Fig. 6 (a and b) SEM, (c) TEM, and (d) HRTEM images of rutile TiO2 nanorod arrays prepared at 150 °C on Ti foil for 20 h. The inset in (c) is the corresponding SAED pattern. Adapted with permission from ref. 64. Copyright 2012 Royal Society of Chemistry. | ||
Based on the abovementioned synthesis strategies, it can be observed that the species of precursors, reaction temperature, etching agent, and ratio of controlling agent play a significant role in the synthesis of novel TiO2 mesocrystals for photocatalysis. The improvement in the photocatalytic activity for TiO2 mesocrystals can be attributed to the synergistic effect of mesostructure (including size, morphology, and crystalline phase), (101)/(001) facet ratio, and Ti3+ vacancy. However, the integration process of the above parameters in a mesocrystal photocatalyst is still in its infancy. Therefore, it is necessary to develop a new strategy in this field.
Mesocrystalline ZnO assemblies. Thus far, the most common morphology of a ZnO mesocrystal is 3D hierarchical architecture with mesocrystalline ZnO building blocks. The ZnO lattice has both polar surfaces, (0001) as well as (000
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), and a nonpolar surface (10
), and a nonpolar surface (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), which differently interact with the surface-protecting surfactants or polymers. Generally, it is facile to obtain ZnO nanoplates instead of nanorods because of the oriented attachment of ZnO nanoparticles with their nonpolar surfaces by protecting the polar surfaces, and mesocrystalline ZnO assembly with stacked nanoplate building blocks would be generated through the capping agent or intrinsic electrostatic field.69 Mou and coworkers have prepared a nacre-like hierarchical mesocrystal structure of ZnO in the presence of a mixture of gelatin/Zn(NO3)2·6H2O/hexamethylenetetramine, where biopolymer gelatin containing many polar amino acids act as the surface-protecting agent for the polar surfaces of ZnO, finally resulting in the formation of micrometer-sized ZnO mesocrystals with hexagonal shapes resulting from the stacked nanoplates.69 Similarly, Lee and coworkers have reported a facile, low-temperature synthesis approach in an aqueous solution for the synthesis of various ZnO mesocrystals (including platelets, rings, and ellipsoids) due to the oriented attachment of ZnO nanoparticles, where the surfactant cetyltrimethylammonium bromide (CTAB) played two critical roles, namely, shape control and micelles for the aggregation of nanoparticles with temperature changes.70 Typically, the samples were prepared by injecting an aqueous solution of ammonia into a Zn(NO3)2 solution in the presence of CTAB. CTAB-mediated zinc hydroxy double salt (zinc-HDS) mesocrystal sheets were synthesized at room temperature, and these Zn-HDS mesocrystal sheets can be decomposed into ZnO superstructure with rigid hexagonal morphology as the reaction temperature increased. Significantly, Wang and coworkers have demonstrated the fast and spontaneous room-temperature formation of ZnO mesocrystals constructed with nanosheet building blocks by the edge-sharing lateral attachment of 1D nanorods for the first time,71 which involved the phase transformation from two intermediate compounds, namely, ZnF(OH) and Zn(OH)2. The epitaxial attachment of ZnO (10
0), which differently interact with the surface-protecting surfactants or polymers. Generally, it is facile to obtain ZnO nanoplates instead of nanorods because of the oriented attachment of ZnO nanoparticles with their nonpolar surfaces by protecting the polar surfaces, and mesocrystalline ZnO assembly with stacked nanoplate building blocks would be generated through the capping agent or intrinsic electrostatic field.69 Mou and coworkers have prepared a nacre-like hierarchical mesocrystal structure of ZnO in the presence of a mixture of gelatin/Zn(NO3)2·6H2O/hexamethylenetetramine, where biopolymer gelatin containing many polar amino acids act as the surface-protecting agent for the polar surfaces of ZnO, finally resulting in the formation of micrometer-sized ZnO mesocrystals with hexagonal shapes resulting from the stacked nanoplates.69 Similarly, Lee and coworkers have reported a facile, low-temperature synthesis approach in an aqueous solution for the synthesis of various ZnO mesocrystals (including platelets, rings, and ellipsoids) due to the oriented attachment of ZnO nanoparticles, where the surfactant cetyltrimethylammonium bromide (CTAB) played two critical roles, namely, shape control and micelles for the aggregation of nanoparticles with temperature changes.70 Typically, the samples were prepared by injecting an aqueous solution of ammonia into a Zn(NO3)2 solution in the presence of CTAB. CTAB-mediated zinc hydroxy double salt (zinc-HDS) mesocrystal sheets were synthesized at room temperature, and these Zn-HDS mesocrystal sheets can be decomposed into ZnO superstructure with rigid hexagonal morphology as the reaction temperature increased. Significantly, Wang and coworkers have demonstrated the fast and spontaneous room-temperature formation of ZnO mesocrystals constructed with nanosheet building blocks by the edge-sharing lateral attachment of 1D nanorods for the first time,71 which involved the phase transformation from two intermediate compounds, namely, ZnF(OH) and Zn(OH)2. The epitaxial attachment of ZnO (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) nanosheets led to the assembly of hierarchical mesocrystals, which was confirmed to be a geometrically ideal photocatalyst that was easily separable and recyclable. The superior efficiency of the UV and visible photocatalytic degradation of methylene blue can be ascribed to the maximized exposure of the reactive (10
0) nanosheets led to the assembly of hierarchical mesocrystals, which was confirmed to be a geometrically ideal photocatalyst that was easily separable and recyclable. The superior efficiency of the UV and visible photocatalytic degradation of methylene blue can be ascribed to the maximized exposure of the reactive (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) facets in the epitaxially assembled superstructures.
0) facets in the epitaxially assembled superstructures.
Furthermore, a hydrothermal strategy is an effective alternative for the synthesis of mesocrystalline ZnO assembly.72 For example, Xu and coworkers have prepared stable yellow ZnO microring mesocrystals with a relatively narrow bandgap (Eg = 3.09 eV) and visible-light response by the hydrothermal route in the presence of hexamethylenetetramine/HF/zinc acetate dihydrate at 160 °C for 6 h.73 Raman and X-ray photoelectron spectroscopy spectra revealed that a large amount of oxygen vacancies existed in the yellow ZnO mesocrystals, resulting in the narrowing of the bandgap and an increase in the visible-light response of yellow ZnO. Further, the concentration of oxygen defects decreased with an increase in the annealing temperature in air. In addition, the electron paramagnetic resonance spectra confirmed that the yellow ZnO mesocrystals possessed abundant surface defects, leading to strong photoluminescence emission. Therefore, the yellow ZnO mesocrystals with highly ordered porous structures were found to be efficient for the photodecomposition of methyl blue under visible-light irradiation, which were favorable for directional transport and efficient charge carrier separation. It should be noted that these yellow ZnO microrings were very stable for at least one year. Moreover, various shaped ZnO architectures were synthesized through a simple hydrothermal route in the presence of a soft template as a structure-directing reagent.74 The flower-like hierarchical assembly was constructed with leaf-shaped mesocrystals that were composed of nanocrystals aligned along the (111) orientation, which displayed the highest photocatalytic activity when compared with the counterpart of nanocrystal ZnO, pencil-shaped mesocrystal ZnO, and plate-like mesocrystal ZnO. The improved photocatalytic activity could be attributed to not only the hierarchical structure, large specific surface area, and high crystallinity, but also the highly ordered mesostructured architecture.
Apart from the morphological architectures, defects engineering of photocatalysts is significant in the determining the photocatalytic activity. Wang and coworkers have reported that the interface-defect-mediated photocatalytic activity of pompon-like ZnO mesocrystal photocatalyst could be synthesized via a hydrothermal approach in the presence of sodium citrate without any other organic templates.75 The as-prepared pompon-like ZnO assemblies were composed of mesocrystal nanosheets with exposed high energy (002) facets having high crystallinity. Here the defects were located at the interfaces among the nanocrystals in the ZnO mesocrystals, playing a key role in the photocatalytic degradation of organic pollutants (such as methylene blue and 2,4,6-trichlorophenol) than that of interstitial zinc vacancies in bulk.
Electrodeposition can also be used to synthesize ZnO mesocrystals. Lin and coworkers first developed an effective supercritical CO2 (sc-CO2) emulsion-assisted electrochemical strategy for the cathodic deposition of ZnO mesocrystals.76 The deposition process involved the formation of primary nanocrystals possessing high surface energy, followed by the oriented attachment of primary nanocrystals along an energetically favorable orientation to generate ZnO mesocrystals. Because of the highly oriented crystallinity of mesocrystals, the as-deposited ZnO mesocrystals exhibited remarkable near-band-edge emissions at room temperature and substantially long exciton lifetime, which can limit the nonradiative charge recombination to extend the exciton decay dynamics. Hence, these ZnO mesocrystals exhibited largely enhanced photoactivity toward photoelectrochemical water oxidation, which was attributed to the advantageous structural characteristics of mesocrystals, including high crystallinity and abundant porosity.76
Mesocrystalline ZnO microspheres. As mentioned above, the dipole-induced electrostatic interactions between the building units can act as the aligning force for the formation of ZnO mesocrystals. However, the dipole-field-induced assembly along the c-axis forming anisotropic ZnO superstructures has been less investigated. Liu and coworkers have demonstrated the direct evidences for a unique core–shell-structured ZnO mesocrystal microsphere constructed by densely packed nanoplatelets by a low-temperature, polymer-mediated, one-pot hydrothermal route in the presence of a water-soluble polymer poly(sodium-4-styrenesulfonate).77 These nanoplatelet-based core–shell mesocrystal ZnO microsphere formed via a nonclassical crystallization process, which involved the synergistic effects of the electric fields of the core and the dipole–dipole interaction between the nanoplatelets on the shell. The calculation based on a dipole model confirms the dipole-field-driven mechanism forming apple-like structures and mesocrystals, as shown in Fig. 7.77 Significantly, green light can stimulate terahertz emissions from these core–shell mesocrystal ZnO microspheres.78
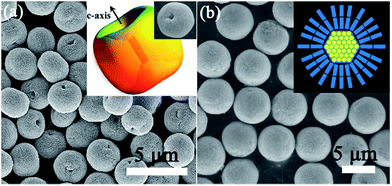 | ||
| Fig. 7 (a) SEM image of ZnO apple-like structures. Adapted with permission from ref. 78. Copyright 2011 Nature Publishing Group. Inset shows the corresponding schematic illustration and a typical particle. Adapted with permission from ref. 77. Copyright 2009 American Chemical Society. (b) SEM images of the ZnO mesocrystal microspheres. Inset is the corresponding schematic illustration. Adapted with permission from ref. 78. Copyright 2011 Nature Publishing Group. | ||
Mesocrystalline ZnO bundles. Similar to the formation of TiO2 mesocrystals, the topotactic transformation of precursor mesocrystals at high annealing temperatures is also suitable for the synthesis of ZnO mesocrystals. For example, Guo and coworkers have reported a simple and scalable wet-chemical route combined with a facile post-annealing process to produce rod-like ZnO mesocrystals with L(+)-tartaric acid (TA) as the orientation inducer.79 In this synthesis process, the authors proposed that the mild acidic characteristics and unique molecular structure of TA is important in assembling Zn(OH)2–TA into having unique mesostructural morphology. Then, the mesocrystals composed of ZnO nanoparticles were generated after being annealed in air at certain temperatures, which inherited the rod-like morphology of Zn(OH)2–TA composites. A schematic representation of the formation mechanism of rod-like ZnO mesocrystals is shown in Fig. 8a. The annealing temperature played a crucial role in the photocatalytic performance, as shown in Fig. 8b. In comparison with individual ZnO nanoparticles, ZnO mesocrystals exhibited decent photocatalytic activities with respect to the photodegradation of methyl orange and photoreduction of Cr6+.
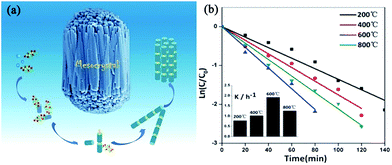 | ||
| Fig. 8 (a) Schematic illustration of the growth pathways of bundle-like ZnO mesocrystals. (b) Photocatalytic dynamics curves of methyl orange with ZnO mesocrystals synthesized at 200, 400, 600, and 800 °C as catalysts. Adapted with permission from ref. 79. Copyright 2013 Royal Society of Chemistry. | ||
Mesocrystalline ZnO spindles. Although the shape-controlled synthesis of ZnO mesocrystals has been achieved by the various abovementioned synthesis methods, the invariable residual organic additives attached to the surfaces of the building blocks resulted in unfortunate problems in their practical applications. Furthermore, the additive-assisted preparation approach not only increased the cost, but also made it more difficult for large-scale synthesis. Hence, it is still a challenge for us to develop new strategies to prepare well-defined ZnO mesocrystals with building blocks as surfactant free as possible. In our previous work, unusual designated tailoring on the zone-axis preferential construction of surfactant-free ZnO mesocrystals with different shapes and sizes was successfully achieved by an additive-free complex-precursor solution method.80 The controllable synthesis of ZnO mesocrystals was essentially determined by the characteristic of [Zn(OH)4]2− precursors, and an oriented nanoparticle aggregation with tailored sizes and shapes can be generated with different concentrations of reactants at high reaction temperatures. For example, spindle-like ZnO mesocrystals with controllable sizes (along the c-axis direction) were prepared by adjusting the concentration of hydroxyl ions, and peanut-like ZnO mesocrystals with tunable sizes (along the c-axis direction) and shapes (perpendicular c-axis direction) were synthesized by tailoring the concentration of zinc ions (Fig. 9).80 The investigation assumes significance in the bottom-up assembly of controllable ordering structures, and it offers a new opportunity to understand the growth mechanism and fundamental significance of zone-axis preferential construction of ZnO mesocrystals. Further, it might provide a green approach to design novel surfactant-free metal oxide mesocrystals with well-defined shapes. Dong and coworkers have developed an ultrafast antisolvent method for the synthesis of spindle-like ZnO mesocrystals.81 A deep eutectic solvent, generated by simply mixing and heating urea and choline chloride at 70 °C, can act as the anti-solvent to trigger the ultrafast formation of ZnO mesocrystals. The as-prepared spindle-like ZnO mesocrystals possessed mesoporous and near-single-crystalline characteristic with high specific surface areas, leading to excellent photocatalytic activity toward the photodegradation of methylene blue.
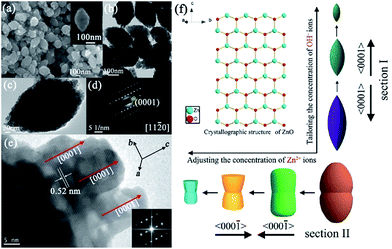 | ||
| Fig. 9 (a) SEM image of the spindle-like ZnO crystals. Inset shows an individual particle. (b) TEM image of the spindle-like ZnO crystals. (c) An individual spindle-like ZnO particle. (d) SAED pattern of the product, as shown in panel (c). (e) HRTEM image of the particle as shown in panel (c); inset shows the corresponding fast Fourier transform (FFT) image. (f) A schematic illustration of the zone-axis preferential growth and reaction pathways of controllable ZnO mesocrystals for different reactant concentrations. Adapted with permission from ref. 80. Copyright 2012 American Chemical Society. | ||
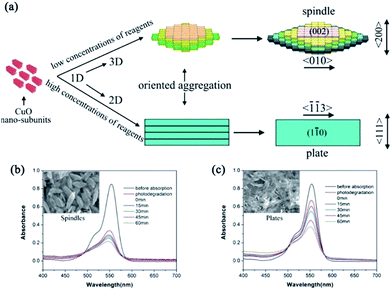 | ||
| Fig. 10 (a) Schematic illustration of the reaction pathway and the ordered-aggregation-driven growth from surfactant-free 1D CuO nanocrystals into dimension-controlled mesostructure (3D mesospindles and 2D mesoplates). (b) and (c) Absorption spectra of the photodegradation of rhodamine B by 3D CuO mesospindles and 2D mesoplates, respectively. Adapted with permission from ref. 86. Copyright 2013 Royal Society of Chemistry. | ||
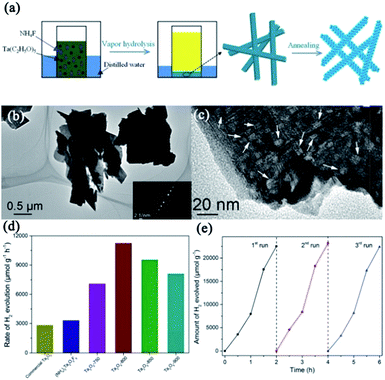 | ||
| Fig. 11 (a) Schematic illustration for the preparation of mesocrystalline Ta2O5 nanosheets. (b) and (c) TEM and HRTEM images of mesocrystalline Ta2O5-800 nanosheets (annealed at 800 °C), respectively. (d) Photocatalytic hydrogen evolution rates of commercial Ta2O5, mesocrystalline (NH4)2Ta2O3F6 nanorods, and mesocrystalline Ta2O5 nanosheets. (e) Recyclable photocatalytic performance of mesocrystalline Ta2O5 nanosheets. Adapted with permission from ref. 88. Copyright 2018 Royal Society of Chemistry. | ||
2.2 Ternary oxide mesocrystals
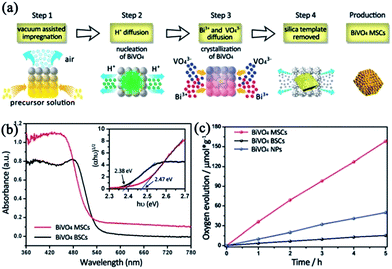 | ||
| Fig. 12 (a) Schematic illustration of the formation mechanism of BiVO4 mesoporous single crystals (MSCs). (b) UV-vis diffuse reflectance spectra of BiVO4 bulk single crystals (BSCs) (black line) and BiVO4 MSCs (red line). The inset shows the plots of (αhν)1/2versus photon energy (hν) of the two samples. (c) Photocatalytic oxygen evolution of BiVO4 MSCs, BSCs, and nanoparticles. The transient photocurrent and photocatalytic oxygen evolution were conducted using a 300 W Xe lamp (420 nm cut-off filter) as the light source. Adapted with permission from ref. 95. Copyright 2016 Royal Society of Chemistry. | ||
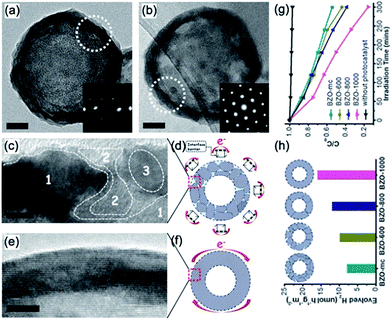 | ||
| Fig. 13 Typical TEM images of individual hollow nanospheres (a) BZO-mc and (b) BZO-1000. Insets (a and b): corresponding SAED patterns of the white dotted cycles; (a and b) scale bars: 20 nm. HRTEM images and corresponding schematic models of the (c and d) BZO-mc and (e and f) BZO-1000 shells. In (d and f), the e− and red arrows represent the photogenerated electrons that were transferred around the outer surface of the hollow nanospheres. Inset (c): area 1 denotes the host lattice and areas 2 and 3 denote the disordered domains. Inset (d): the “hurdle frames” represent the interface barrier among the outer surface grain boundaries. (c and e) Scale bars: 5 nm. (g) Typical photocatalytic activities for hydrogen evolution, and (h) methyl orange degradation curves of BZO-mc, BZO-600, BZO-800, and BZO-1000, respectively. Adapted with permission from ref. 98. Copyright 2014 Royal Society of Chemistry. | ||
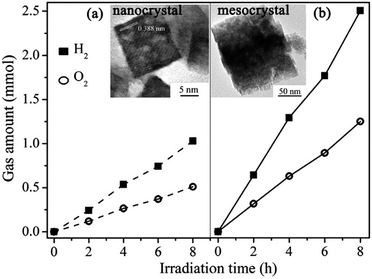 | ||
| Fig. 14 Photocatalytic water splitting for hydrogen and oxygen generation. (a) Nanocrystals (dashed line) and (b) NaTaO3 mesocrystals (solid line). Adapted with permission from ref. 101. Copyright 2013 Elsevier. | ||
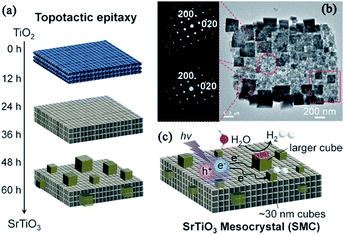 | ||
| Fig. 15 (a) Schematic illustration of the topotactic epitaxy of SrTiO3 mesocrystals from TiO2 mesocrystals. (b) TEM image of SrTiO3 mesocrystals (reaction time: 48 h) with SAED from near the center and at the edge (red circle). (c) Anisotropic electron transport from the inside to the outside of SrTiO3 mesocrystals comprising aligned nanocubes with dominant (100) facets. The symbols e− and h+ indicate photogenerated electrons and holes, respectively. Adapted with permission from ref. 105. Copyright 2017 Wiley-VCH Verlag GmbH & Co. | ||
In addition, single-crystal-like mesoporous SrTiO3 sub-micrometer spheres with large surface area and high crystallization were successfully produced by a facile hydrothermal approach in the presence of tetrabutyl titanate/strontium nitrate/potassium hydroxide/polyvinyl alcohol (PVA) system.107 The oriented aggregation of nanoparticles was proposed to be the dominant formation mechanism, which was accompanied by the ripening process. Typically, the pore density of the as-prepared SrTiO3 spheres obviously increased as the PVA concentration increased, and the average pore size ranged from 4.5 to 16.1 nm. The photocatalytic degradation of rhodamine B with the as-produced mesocrystalline SrTiO3 spheres was a function of PVA concentration and reaction time. The highest photocatalytic activity has been achieved in mesocrystalline SrTiO3 synthesized at 200 °C for 6 h with a higher PVA concentration.
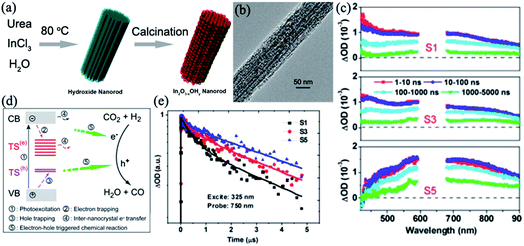 | ||
| Fig. 16 (a) Schematic illustration of the synthesis of rod-like In2O3−x(OH)y mesocrystals. (b) Typical TEM image of a mesocrystalline In2O3−x(OH)y rod. (c) Time-resolved absorption spectra (nanosecond to microsecond range) observed after 325 nm laser pulse excitation of different In2O3−x(OH)y samples in N2 gas. (d) Schematic illustrations of the photoexcited electron–hole dynamics and migration of a photogenerated electron between neighboring nanocrystals. Surface trapping states and interparticle charge transfer are in favor of the spatial separation of electron–hole pairs, which promotes the photo-redox reaction. (e) Normalized transient absorption traces observed at 750 nm for S1 (synthesis time = 2 h), S3 (synthesis time = 3 h), and S5 (synthesis time = 5 h). Adapted with permission from ref. 108. Copyright 2016 American Chemical Society. | ||
A typical nanorod exhibiting a nanoporous superstructure is shown in Fig. 16b. It has been demonstrated that interparticle charge transfer generated within the nanocrystal superstructure and the lifetime of photoinduced carriers was prolonged in In2O3−x(OH)y mesocrystals, which was in favor of the increase in the conversion rate of the gas-phase, light-assisted reverse water–gas shift reaction. Under solar-light illumination, photogenerated electrons from the VB would be excited into the CB of the semiconductor, leading to the formation of photogenerated holes in the VB. The photogenerated holes migrated into the surface hydroxide trap states, while the photogenerated electrons located in the CB might be captured in the oxygen vacancies. Notably, the mesocrystalline In2O3−x(OH)y nanorods were made up of close-contact nanocrystals, which would cause the spatial separation of the photoexcited carriers between the neighboring subunits, and the migration of holes between the neighboring nanocrystals has a lower probability than electron movement (Fig. 16c–e).
In addition, it is interesting to note the nanorod length dependence on the hydrogenation rate of carbon dioxide to carbon monoxide.
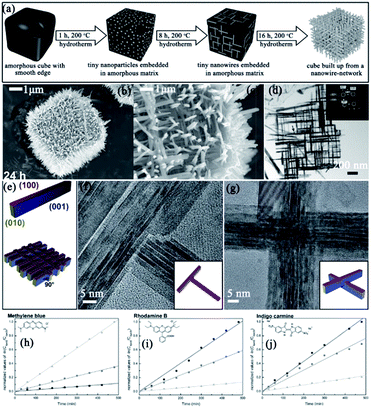 | ||
| Fig. 17 (a) Schematic illustration of the hydrothermal growth of Nb3O7(OH) mesocrystals. (b) and (c) Low- and high-magnification SEM images, respectively. (d) TEM image of a fragment of one cube wall; the inset shows the corresponding SAED pattern. (e) Schematic drawing illustrating the crystal shape of the nanowires and crystallographic arrangement of the nanowires in the network. (f) HRTEM image of a T-shaped nanowire junction and schematic illustration showing the arrangement of the nanowires at the junction (inset). (g) HRTEM image of a nanowire crossing and schematic drawing of the junction (inset). (h)–(j) Measurement of the photocatalytic degradation of three different dyes at three different pH values (pH 2 (■), pH 6 (●), and pH 10 (▲)). The kinetic rate constant can be determined from the curve obtained by plotting −ln(Cdye/C0) versus the irradiation time t. The corresponding curves are shown in (h) for methylene blue, in (i) for rhodamine B, and in (j) for indigo carmine. Adapted with permission from ref. 109. Copyright 2014 Royal Society of Chemistry. | ||
2.3 Quaternary mesocrystals
Thus far, quaternary metal oxide mesocrystals have been rarely reported. AgIn(WO4)2 is a typical example. Yu and coworkers have reported a facile and mild microwave-assisted route for synthesizing caterpillar-like AgIn(WO4)2 mesocrystals with tuned shapes in the presence of AgNO3, In(NO3)3, and Na2WO4.110 In this synthesis, microwaves might improve the nucleation and growth of AgIn(WO4)2 in the Ag/In/W/O system. Initially, small amorphous nanoparticles were generated and randomly assembled in an aggregative manner. Subsequently, these connected nanoparticles evolved into tiny olive-like core structures by the oriented-attachment process. Finally, outgrowths occurred on the surface of the core and produced caterpillar-like architectures under the oriented-attachment process accompanied by Ostwald ripening. It should be noted that the pH value played an important role in the phase formation and morphology evolution. These caterpillar-like AgIn(WO4)2 mesocrystals displayed selective photocatalytic properties for degrading organic dyes under UV- and visible-light irradiation. The rate constants for the degradation of eosin Y, rhodamine B, and methyl orange were 0.111, 0.044, and 0.0084 min−1, respectively, which clearly demonstrated that the photodegradation process of eosin Y was much faster than those of rhodamine B and methyl orange (Fig. 18).110 Moreover, the photoluminescence spectra of AgIn(WO4)2 mesocrystals with different morphologies have been investigated by the above authors. It can be found that all the products displayed white emission in the visible region when excited by visible light with a wavelength of 460 nm because of the surface nanostructures of the outgrowths.111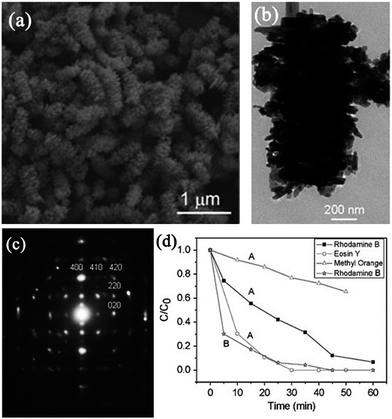 | ||
| Fig. 18 (a) SEM image of caterpillar-like AgIn(WO4)2 mesocrystals. (b) TEM image of an individual caterpillar-like particle. (c) Corresponding SAED pattern. (d) Photocatalytic degradation of different organic dyes under 300 W Xe lamp irradiation with AgIn(WO4)2 mesocrystals. Adapted with permission from ref. 110. Copyright 2010 Royal Society of Chemistry. | ||
Based on the above overview, the synthesis strategies of diversified photocatalyst mesocrystals are an exciting direction to fabricate compounds with high activity. Further, it provides an opportunity to investigate structure-related photocatalytic performance relationship. However, it should be noted that a series of hybrid mesocrystal-based heterogeneous photocatalysts with well-controlled compositions, shapes, and sizes have been demonstrated in the field of photocatalysis along with rapid progresses in nanomaterials science and nanotechnology.
3 Functionally modified mesocrystal photocatalysts
Effectively understanding the correlations between the modified interfacial/electronic structures and improved photocatalytic performances is crucial for developing novel mesocrystal-based photocatalysts. Generally, the modifications of mesocrystal photocatalysts can be divided into two strategies: hybridization and doping. Hybridization is a general strategy for inducing unexpected physicochemical characteristics to improve the potential application of a single material, which is attributed to the synergistic effect between the active component and support.112 Therefore, mesocrystals acting as host components would offer a good chance to tune the interfacial property of hybrid mesocrystal-based micro/nanostructures for improving practical applications. Furthermore, an alternative strategy for improving the physicochemical properties is to dope heteroatoms into the mesocrystal to modify its electronic structure. However, a systematic review of mesocrystal-based architectures has not been reported so far. In this section, we will firstly summarize the significant advances in the development of different types of hybrid mesocrystal-based photocatalysts, such as hybrid semiconductor–mesocrystals, hybrid mesocrystal–metal nanostructures, and hybrid mesocrystal–carbon nanostructures. Next, doped mesocrystal photocatalysts will be introduced based on some typical examples.3.1 Hybridization
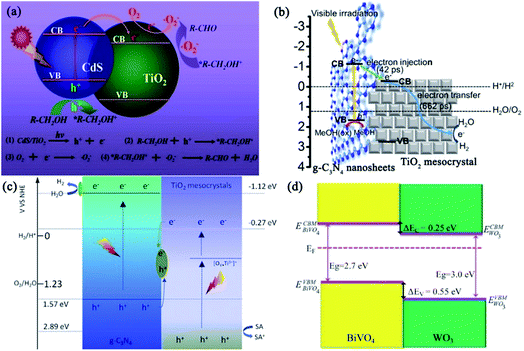 | ||
| Fig. 19 (a) Schematic illustration of the CdS photosensitizing effect, photogenerated electron transfer from CdS to TiO2 mesocrystal via the heterojunction, and mechanism of photocatalytic selective oxidation of alcohols into aldehydes. Adapted with permission from ref. 113. Copyright 2016 Elsevier. (b) Representative scheme of photogenerated electron injection and movement in g-C3N4 nanosheet (31 wt%)/TiO2 mesocrystals under visible-light irradiation. Adapted with permission from ref. 114. Copyright 2017 American Chemical Society. (c) Possible visible-light photocatalytic mechanism of Ti3+-doped mesocrystalline TiO2/g-C3N4 composites for hydrogen production. Adapted with permission from ref. 115. Copyright 2018 Elsevier. (d) Band alignment of BiVO4/WO3 heterojunction. EVBM is the VBMs, ECBM is the CB minima, and ΔEV and ΔEc are the VB and CB offsets, respectively. Adapted with permission from ref. 116. Copyright 2017 Nature Publishing Group. | ||
The photocatalytic activity of graphitic carbon nitride (g-C3N4) was inhibited to lower efficiencies because of the fast recombinations of the photogenerated charge carriers. Majima and coworkers have synthesized g-C3N4 nanosheets/TiO2 mesocrystals metal-free composites to improve the charge separation. The photocatalytic hydrogen evolution experiments demonstrated that coupling g-C3N4 nanosheets (31 wt%) with TiO2 mesocrystals exhibited the highest photocatalytic activity, which was 20 times larger than that of pristine g-C3N4 without any noble metal cocatalyst under visible-light irradiation (λ > 420 nm) and 7 times higher than that of g-C3N4/P25, suggesting the significance of the strong interface interactions between 2D g-C3N4 nanosheets and plate-like TiO2 mesocrystals. Significantly, femtosecond time-resolved diffuse reflectance (fs-TDR) spectra revealed that the migration of photogenerated charge carriers was promoted and their lifetimes were prolonged because of the tight interface between the g-C3N4 nanosheets and plate-like TiO2 mesocrystals. Consequently, the fs-TDR spectra indicated that TiO2 mesocrystals acted as an electron transfer channel to promote charge separation (Fig. 19b).114 Similarly, direct Z-scheme Ti3+ self-doped TiO2 mesocrystals/g-C3N4 composites were synthesized by a simple solvothermal method, which exhibited visible-light absorption and photocatalytic activity for hydrogen production. This direct Z-scheme photocatalytic mechanism is confirmed as follows (Fig. 19c).115 Under visible-light irradiation, the Ti3+-doped TiO2 mesocrystals and g-C3N4 nanosheets easily excited the photogenerated carriers, and the photogenerated electrons from the VB of g-C3N4 nanosheets had strong reduction potential when compared with the CB electrons of TiO2. They would react with water for hydrogen evolution, and the VB holes in Ti3+-doped TiO2 possessed strong oxidizing potential and could oxidize the sacrificial agents, but the VB holes of g-C3N4 could not react with the sacrificial agents due to a lower oxidizing potential. In addition, the CB electrons of Ti3+-doped TiO2 mesocrystals would react with the generated VB holes of g-C3N4 owing to the short charge-transfer distance, leading to an improvement in charge separation. In addition, Van and coworkers have demonstrated a self-assembled nanocomposite photoanode composed of an epitaxial BiVO4 matrix embedded with WO3 mesocrystals for visible-light-driven photoelectrochemical applications. In this system, the orientation of the crystal facets and interfaces offered a superior template to investigate the intimate contact between the two constituent phases, which revealed that the interfacial coupling of the mesocrystal and matrix enhanced the separation and migration of photogenerated carriers (Fig. 19d), leading to a largely improved photoelectrochemical activity when compared with their counterparts.116
As an efficient oxygen-evolution cocatalyst, cobalt phosphate (CoPi) has been decorated onto TiO2 mesocrystals for enhancing the photocatalytic water-splitting ability. For a CoPi/TiO2 mesocrystal/Pt photocatalytic hydrogen evolution system, active Co III/IV species in CoPi, as well as holes in the VB of TiO2, can oxidize the probe dye of 3′-p-aminophenyl fluorescein and 3′-p-hydroxyphenyl fluorescein to produce fluorescein as the fluorescent product through O-dearylation reaction. During this reaction, the photogenerated holes in the VB of TiO2 would transfer to the CoPi cocatalysts and get deposited onto the surface of the (001) facets upon UV-light irradiation. The photoexcited electrons in the CB of TiO2 preferentially transferred to the lamellar (101) facet to deposit Pt, finally limiting the recombination of charge carriers (Fig. 20).117 Moreover, Majima's group has demonstrated that polyhedral TiO2 mesocrystals packed with an exfoliated MoS2 shell can exhibit promising reactive efficiency and good stability in photocatalytic hydrogen evolution, which is attributable to the anisotropic electron flow to achieve enhanced photocatalytic performance.118
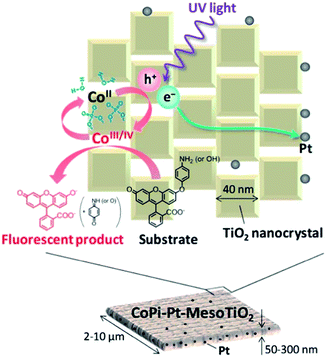 | ||
| Fig. 20 Schematic illustration of the photogenerated charge transfer on the surface of CoPi/Pt/TiO2 mesocrystal. Adapted with permission from ref. 117. Copyright 2014 Royal Society of Chemistry. | ||
It is well known that the Fermi level and electron-accepting states of noble metals are generally located at an energy level below the CB of TiO2 semiconductors.1 Accordingly, with regard to a noble metal–TiO2 system, the photogenerated electrons in the CB of TiO2 will effectively migrate to the deposited metal nanoparticles under UV-light irradiation, while the photoexcited holes stay in the VB of TiO2, finally resulting in the achievement of charge carrier separation. Moreover, the photoinduced electrons can transfer to TiO2 under visible-light irradiation due to the localized surface plasmon resonance.120 Notably, TiO2 mesocrystals provide good support for depositing noble metals to enhance the photocatalytic activity, because the highly ordered superstructure with high surface area can avoid the numerous interfacial defects, facilitate charge separation as well as transfer, and provide abundant reaction sites for photocatalytic reactions. Bian and coworkers have developed a facile photodeposition strategy to synthesize Au- or Pt-nanoparticle-loaded TiO2 mesocrystals, and the transport and reaction dynamics of the photogenerated charge carriers in individual composite materials are investigated.119 Based on the single-molecule fluorescence spectroscopy measurements on a single composite particle, it has been found that most of the photoexcited electrons could transfer from the dominant (001) facet to the edge of TiO2 mesocrystals in micrometer distances, and the photoreduction reactions mainly occurred at the lateral surfaces containing (101) facets. Therefore, this anisotropic electron flow in the superstructure considerably limited the electron recombinations with holes, leading to improved photocatalytic oxidation activity (Fig. 21a).119 More interestingly, TiO2 mesocrystals composited with Au nanorods can be used for highly efficient visible-NIR-photocatalytic hydrogen production (Fig. 21b).121 Yan and coworkers have deposited Au nanoparticles selectively anchored on the (101) facet of polyhedral TiO2 mesocrystals, which exhibited highly selective photocatalytic reduction of nitroarenes because of the plasmonic effect of Au nanoparticles, unique superstructure of TiO2 mesocrystals, and highly strong interaction between Au and TiO2 through the close Schottky heterointerface (Fig. 21c).122 In addition, the detailed electron–hole separation dynamics for visible-light- and UV-vis-induced catalytic mechanisms of Au/TiO2 mesocrystals were discussed in detail by Wang and coworkers (Fig. 21d).123 Similarly, Ag/hematite mesocrystal composites can exhibit high photo-Fenton activity in the oxidation of rhodamine B, methyl orange, and glyphosate under visible-light irradiation.124 Apart from the abovementioned synthesis strategy, wet chemical impregnation method and ion exchange–reduction approach might be useful for the synthesis of metal–mesocrystal composites, but it has not been extensively studied so far.
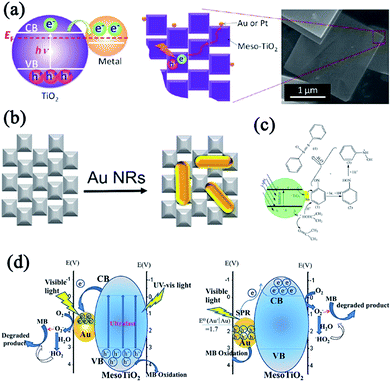 | ||
| Fig. 21 (a) Schematic illustration of electron transfer from TiO2 to noble metal (Au, Pt) nanoparticles upon irradiation of UV light, and electron transfer on Au/TiO2 mesocrystal or Pt/TiO2 mesocrystal. Adapted with permission from ref. 119. Copyright 2012 American Chemical Society. (b) Preparation of rod-like Au/TiO2 mesocrystals. Adapted with permission from ref. 121. Copyright 2017 Elsevier. (c) Proposed mechanism for the photocatalytic reduction of nitrosobenzene to azoxybenzene by Au/TiO2 mesocrystals. Adapted with permission from ref. 122. Copyright 2016 IOP Publishing. (d) Proposed mechanism for the photocatalytic activity of Au/TiO2 mesocrystals under UV-vis-light excitation (left) and visible-light excitation (right). Adapted with permission from ref. 123. Copyright 2016 Royal Society of Chemistry. | ||
CDs with a large number of active groups (including hydroxyl groups and carboxyl groups) have been widely applied in photocatalysis due to their excellent photoelectric properties, excellent water solubility, and suitable chemical reactivity. Hence, it is expected that the coupling of CDs with mesocrystals could inhibit photogenerated charge recombinations, leading to enriched photoelectrons. Bian and coworkers have demonstrated the enhanced photoreduction of Cr(VI) to Cr(III) by using CDs-coupled TiO2 mesocrystals, where CDs played the roles of electron collectors and active sites (Fig. 22a). Furthermore, the CDs coupled on TiO2 mesocrystals can facilitate photogenerated charge separation. In addition, the authors have found that the positive charges on the surface of CDs/TiO2 was in favor of the selective adsorption of Cr(VI) and rapid desorption of Cr(III), which obviously promotes the photocatalytic reduction of Cr(VI) and retention of photoreduction activity. Therefore, the photoreduction of Cr(VI) performance of the as-prepared CDs/TiO2 is about 5.4 times higher than that of pure TiO2 mesocrystals under UV-light illumination.117 Yan and coworkers have reported that CDs/TiO2 mesocrystal composites synthesized through a simple one-pot solvothermal method can exhibit effective photocatalytic activity in the degradation of methyl orange under visible-light irradiation.126
 | ||
| Fig. 22 (a) Proposed adsorption–photoreduction desorption mechanisms of photocatalytic reduction of Cr(VI) in the presence of CDs/TiO2 mesocrystal composite. Adapted with permission from ref. 125. Copyright 2018 Elsevier. (b) Proposed photo-Fenton synergistic mechanism of nitrogen-doped GO/Fe2O3 mesocrystal nanocomposites. Adapted with permission from ref. 130. Copyright 2017 Elsevier. (c) Possible photocatalytic mechanism of carbon-modified NaTaO3 mesocrystals. Adapted with permission from ref. 131. Copyright 2014 Royal Society of Chemistry. | ||
As an sp2-bonded carbon sheet with the thickness of a single atom, graphene has received much more attention owing to its large surface area, good flexibility, high electrical conductivity, and high chemical stability, which allows it to be an effective host support for the heterogeneous growth of the desired active guest materials because the surface functional groups, such as hydroxyl groups, act as favorable nucleation sites for guest materials.127 Moreover, graphene can decrease the recombinations of photogenerated electron–hole pairs, increasing the charge transfer rate of electrons and surface-adsorbed amount of chemical molecules through π–π interactions. Consequently, the integration of graphene and mesocrystal semiconductors is promising in the field of photocatalysis. For example, Yang and coworkers have employed a facile template-free solvothermal method for obtaining well-dispersed spindle-like anatase TiO2 mesocrystals anchored onto graphene nanosheets, which were synthesized by mixing GO with acetic acid under ultrasonication, followed by the dropwise addition of tetrabutyl titanate into the above suspension.128 The as-synthesized graphene/anatase TiO2 mesocrystals can considerably enhance the photocatalytic activity of TiO2 under visible-light irradiation.
As a graphene derivative with an edge-bearing oxygen functionality, GO provides the advantage of uniformly loading metal oxide nanoparticles on its surface through oxygen-containing groups as nucleation centers.129 Nonetheless, the poor electron migration ability of GO is undesirable in photocatalysis, which limits the charge transfer between the nanoparticles and GO. Decreasing the oxygen-containing groups was an effective approach to increase the charge transportation capacity of GO. Liu and coworkers have synthesized a pyrrolic nitrogen (N)-doped GO/Fe2O3 mesocrystal nanocomposite by a simple solvothermal route and optimizing the oxygen-containing groups on GO. The as-prepared N-doped GO/Fe2O3 mesocrystals can enhance the efficient separation of electron–hole pairs and promote the fast conversion of Fe(II)and Fe(III) in photo-Fenton synergistic reactions because of the excellent electroconductivity of pyrrolic-N-doped GO and a large specific surface area (Fig. 22b).130 The photodegradation rate of methyl blue increased by 1.5 times and the conversion rate of glyphosate increased by 2.3 times for the GO/Fe2O3 mesocrystals under visible-light irradiation as compared to bare Fe2O3 mesocrystals.
Besides the above CDs, GO, and graphene, amorphous carbon can be also used to improve the photocatalytic activity of mesocrystal photocatalysts. Wu and coworkers have found that carbon-modified NaTaO3 mesocrystal nanoparticles can be prepared by a one-step solvothermal method using TaCl5 and NaOH as the starting materials and distilled water/ethylene glycol mixed solution as the reaction solvent in the presence of appropriate amounts of glucose.131 These carbon-modified NaTaO3 mesocrystals exhibited excellent efficiency for continuous NO gas conversion under UV irradiation, short-wavelength visible light (>400 nm), and even long-wavelength visible light (>510 nm), which was considerably better than those of unmodified NaTaO3 and commercial P25 due to the large specific surface area, high crystallinity, and carbon-induced visible-light harvesting (Fig. 22c).131
The rapid emergence of novel carbon–mesocrystal heterogeneous nanostructures can provide a new opportunity to further understand the fundamental importance of mesocrystals as well as to improve their practical applicability. Notably, other carbon nanomaterials possessing unique physicochemical properties, such as carbon nanotubes (CNTs), C60, polymer polypyrrole (PPy), and metal–organic frameworks (MOFs), should be decorated with mesocrystals in the future.
3.2 Doping
It is well known that doping is a well-demonstrated strategy for effectively enhancing the physicochemical properties by changing the electronic structure of semiconductors; therefore, studies on doped mesocrystals have attracted increased attention. Herein, we will discuss structure-related photocatalytic mechanisms based on some typical doped-mesocrystal photocatalyst examples. Table 2 summarizes the metal- and non-metal-doped mesocrystal photocatalysts and their physiochemical properties, as well as their photocatalytic applications.| Doping element | Precursor | Synthesis method | Application | Ref. |
|---|---|---|---|---|
| Sr2+ | NaTaO3 mesocrystals | Hydrothermal method | Electrons exciting | 134 |
| Sr2+ | NaTaO3 mesocrystals | Molten salt method | Photocatalytic hydrogen generation | 135 |
| Ti3+ | TiO2 mesocrystals | Solvothermal synthesis | Photocatalytic removal of NO gas | 137 |
| Ti3+ | Au/Cl–TiO2 mesocrystals | Vapor hydrolysis + photoreduction | Photocatalytic hydrogen generation | 138 |
| Cr3+ and Sb5+ | TiO2 mesocrystals | Hydrothermal method | Photodegradation of methyl orange | 139 |
| Nb5+ and Sb5+ | TiO2 mesocrystals | Microwave-assisted approach | Photodegradation of methylene blue and rhodamine B | 140 |
| Zn2+ | Fe3O4 mesocrystals | Solvothermal synthesis | Photo-Fenton | 141 |
| F | TiO2 mesocrystals | Topotactic transformation | Photocatalytic hydrogen generation | 145 |
| N and F | TiO2 mesocrystals | Topotactic transformation | Photodegradation of methylene blue | 149 |
| N and F | TiO2 mesocrystals | Hydrothermal method | Photodegradation of 4-nitrophenol and rhodamine B | 150 |
An and coworkers have synthesized Sr2+-doped NaTaO3 photocatalysts by solid-state and hydrothermal routes.134 The as-synthesized NaTaO3–SrSr1/3Ta2/3O3 solid solution was a Sr-rich shell covered with a Sr-poor core. This heteroepitaxial core–shell interface can induce surface reconstruction with regularly separated steps due to lattice mismatch. Therefore, the recombination of electron–hole pairs was limited in solid-solution photocatalysts. The steady-state photogenerated electrons increased by up to 180 times in the solid-solution photocatalysts under Hg–Xe lamp irradiation, in which both A sites and B sites of the perovskite-structured lattice were doped with Sr2+ (Fig. 23a). Surface reconstruction and enhanced electron population were absent in the A-site-doped photocatalysts.134 In addition, a simple molten salt route without using any organic additives has been employed to prepare Sr2+-doped NaTaO3 mesocrystals with high crystallinity and orientation-aggregated morphology, which exhibited excellent photocatalytic hydrogen generation activity because of their nanosteps, high porosity, and preferred oriented direction.135
 | ||
| Fig. 23 (a) Crystal structure of NaTaO3–SrSr1/3Ta2/3O3 solid solutions. Adapted with permission from ref. 134. Copyright 2015 American Chemical Society. (b) Schematic of the formation mechanism of Sb-mesoNb/TiO2. Adapted with permission from ref. 140. Copyright 2017 American Chemical Society. (c) Schematic of the growth process for Zn-doped Fe3O4 hollow sub-microsphere mesocrystals and their photocatalytic activities. Adapted with permission from ref. 141. Copyright 2017 American Chemical Society. (d) Schematic illustration of a facile hydrothermal treatment synthesis process of N-doped TiO2 mesocrystals and (N,F)-doped TiO2 mesocrystals. Adapted with permission from ref. 150. Copyright 2016 Elsevier. | ||
As for TiO2 photocatalysts, Ti3+ self-doping can result in the visible-light response of TiO2 materials without introducing recombination centers for the photogenerated charges.136 Therefore, the achievement of Ti3+ self-doped anatase TiO2 mesocrystals would be significant for the construction of an ideal photocatalyst. For instance, Zhao and coworkers have prepared spindle-like Ti3+ self-doped anatase TiO2 mesocrystals through the calcination of pre-synthesized anatase TiO2 mesocrystals in N2 atmosphere.49 However, volatile organic solvents and an expensive surfactant additive were inevitably utilized in this work. Hence, it is highly desirable to develop a simple, green synthesis strategy for fabricating Ti3+-doped TiO2 mesocrystals. Recently, Tan and coworkers have reported a novel method for the growth of spindle-shaped anatase TiO2 mesocrystals through the one-step hydrolysis reaction of TiCl3 in the green and recyclable media, polyethylene glycol. Subsequently, Ti3+ sites can be easily generated in the anatase crystal lattice, leading to the formation of Ti3+ self-doped mesocrystals after being annealed in vacuum. These as-transformed Ti3+ self-doped anatase mesocrystals exhibited enhanced visible-light photocatalytic activity toward the removal of NO gas, which could be attributed to their intrinsic Ti3+ self-doping nature, as well as high crystallinity and high porosity of the mesocrystalline architecture.137 Moreover, Yu and coworkers have successfully prepared willow-leaf-like plasmonic Ti3+-doped Au/Cl–TiO2 mesocrystals by means of modified two-phase vapor hydrolysis and photoreduction methods. The as-prepared Ti3+-doped Au/Cl–TiO2 mesocrystals displayed higher visible-light harvesting and visible-light photocatalytic activity for hydrogen evolution, which was 208.70 times as high as that of P25 TiO2 and 1.59 times as high as that of Ti3+-doped TiO2 mesocrystals.138 Therefore, the co-doping of TiO2 mesocrystals might provide remarkably higher activity than that of single doping and bare TiO2. Mesocrystalline Cr- and Sb-co-doped anatase TiO2 nanoparticles with a solid solution characteristic were prepared by the hydrothermal aging of an aqueous solution containing titanium alkoxide, chromium acetylacetonate, antimony acetate, and triethanolamine as the stabilizer, which could improve the visible-light harvesting ability, thereby revealing high efficiency in the degradation of methyl orange dye under visible-light irradiation.139
Multicomponent Sb–Nb:TiO2 mesocrystals have been synthesized by a microwave-assisted nonaqueous sol–gel method, with size of 25–35 nm and composed of crystallographically aligned Nb:TiO2 subunits, embedded in a porous amorphous Sb-rich scaffold. The formation of Sb–Nb:TiO2 mesocrystals is responsible for a particle-based assembly mechanism. In this process, the Sb scaffold acted as a nucleation site for the construction of Nb:TiO2 subunits, which grew and rotated in a mutual crystallographic orientation. It then prevented the complete fusion of Nb:TiO2 subunits, leading to the porosity of Sb–Nb:TiO2 mesocrystals (Fig. 23b).140 When compared with undoped TiO2, Sb–Nb:TiO2 mesocrystals exhibited superior photocatalytic activity for the degradation of organic dyes under simulated solar or visible light, which can be attributed to the high crystallinity, abundant porosity, and additional exposed reactive surfaces.
In addition, magnetic recyclable mesocrystalline Zn-doped Fe3O4 hollow sub-microspheres were successfully prepared through a facile one-step solvothermal method and were used for fabricating efficient heterogeneous photo-Fenton catalysts.141 A possible growth mechanism of doped mesocrystalline hollow materials was proposed. Initially, Fe3O4 mesocrystals were assembled by oriented nanocrystals, and Zn-rich amorphous shells grew onto the surfaces. Subsequently, Zn element gradually diffused into Fe3O4 mesocrystals to generate Zn-doped Fe3O4 because of the Kirkendall effect by increasing the reaction time, in which a directional flow of zinc species at the Fe3O4 interfaces could result in the formation of voids in the products. Simultaneously, the inner solids would be dissolved, and the outer particles would grow larger due to the Ostwald ripening process, finally resulting in the formation of a hollow structure with a porous shell. The Zn-doped hollow Fe3O4 mesocrystals possessed high and stable photo-Fenton activity for the degradation of rhodamine B and cephalexin under visible-light irradiation in the presence of H2O2 (Fig. 23c),141 and it could be easily separated and reused by an external magnetic field.
In brief, metal ions were extensively employed as dopants for mesocrystal photocatalysts. Generally, the introduction of metal ions can form new energy levels in the bandgap, optimize the visible-light response, and accelerate the separation of the electron–hole charges. Although many studies on transition metal-doped mesocrystals have been reported, non-metal doping is still in its infancy.
It has been demonstrated that N-doping is one of the most efficient avenues to generate N 2p in the localized mid-gap state, resulting in an increase in the thermal stability and decrease in recombination centers.146–148 Therefore, it is necessary to integrate N- and F-doping into TiO2 mesocrystals, which could combine the advantages of these single dopants for further optimizing the photocatalytic activity. With regard to synthesizing co-doped mesocrystals, only TiO2 mesocrystals with N- and F-co-doping have been studied thus far. Topochemical transformation is an effective method for the synthesis of (N,F)-co-doped TiO2 (NFT) mesocrystals exposed with (001) facets. The distribution of two dopants in NFT strongly depended on the annealing temperature. It was found that NFT (500 °C) displayed the highest photocatalytic efficiency from Cr(VI) to Cr(III) because of the highest concentration of N with the surface modification from F coupling.149 Similarly, Majima and coworkers have found that N-doped TiO2 mesocrystals can be synthesized by a facile hydrothermal treatment with triethanolamine, while F-doped TiO2 mesocrystals were easily formed by a simple stirring of TiO2 mesocrystals with NaF at room temperature. Therefore, (N,F)-co-doped TiO2 mesocrystals were prepared by a two-step post-modification process. A schematic illustration of the preparation of N-doped and (N,F)-co-doped TiO2 mesocrystals is shown in Fig. 23d.150 In this system, it was seen that doping has no effect on the crystal structure of TiO2 mesocrystals, and the plate-like structure and high surface area can be retained. These (N,F)-co-doped TiO2 mesocrystals displayed high photocatalytic activities for the degradation of rhodamine B and 4-nitrophenol under visible-light irradiation, which was attributed to the synergistic effect of N- and F-doping. The introduction of heterogeneous N atoms enhanced visible-light absorption, resulting in a decrease in the bandgap energy by adjusting the concentration of N, while F increased the production of hydroxyl radicals, adsorption, and charge separation efficiency. Therefore, TiO2 mesocrystals with N- and F-co-doping provided a particular interest in promising visible-light-driven photocatalytic efficiency and allowed us to devote the effect on developing new materials as a photocatalyst.
4. Conclusions and perspective
Mesocrystal photocatalysts with tunable crystalline phases, sizes, and morphologies have been widely studied owing to crystallographically highly ordered superstructures for photogenerated charge transfer and separation, high surface area, and ordered porosity as compared to corresponding single crystal and polycrystalline nanomaterials. Herein, a comprehensive review on the synthesis strategies and growth mechanisms, morphological diversities, functional modifications including hybridization and doping, and current applications, as well as the corresponding structure-related photocatalytic mechanisms, have been summarized for different types of mesocrystal photocatalysts, such as TiO2 (anatase), TiO2 (rutile), ZnO, CuO, Ta2O5, BiVO4, BaZrO3, SrTiO3, NaTaO3, Nb3O7(OH), In2O3−x(OH)y, and AgIn(WO4)2. Recent advances in designated tailoring of various kinds of mesocrystals provides a promising approach for the rational design and construction of high-performance photocatalysts, as well as the enrichment of their surface atomic/or electronic and interfacial properties. Although extensive investigations and significant achievements made during the past decades, as discussed in this review article, there is still a long way ahead before mesocrystal photocatalysts can be comprehensively understood and satisfactorily used in industrialization. In order to achieve this, we have numerous tasks to particularly investigate in the future.With regard to the synthesis of novel mesocrystal photocatalysts, an important task is to promote the synthesis of mesostructural architecture with desired crystallographic facets, particularly high-index facets. The selection of suitable capping agents for constructing targeted mesostructures based on computer-assisted surface energy variations might considerably promote the synthesis of targeted mesocrystalline photocatalysts. With regard to popular TiO2 mesocrystals, the literature mainly focuses on the anatase phase with diverse architectures, whereas studies involving the rutile phase are still in their infancy. Moreover, the size-controlled synthesis of polyhedral TiO2, particularly ultrathin monodisperse nanoparticles (size < 100 nm), is an urgent task. Notably, the as-reported polyhedral TiO2 mesocrystal photocatalysts generally exhibited low-index (001) and (101) facets, which strongly restrained investigations on facet-dependent properties. Therefore, considerable efforts should be further expended to explore the synthesis of novel polyhedral TiO2 mesocrystals exposed with controllable-index facets for uncovering the relationship between surface structures and performances.
Apart from TiO2, it is urgently necessary to focus more attention on enriching the structural and morphogenetic aspects, as well as formation mechanisms, of other mesocrystals, which are beneficial toward tuning the size, morphology, crystalline phase, monodispersity, surface atomic arrangement, and intrinsic electronic structure. Significantly, the surfactant-free, large-scale controllable synthesis of low-dimensional mesocrystalline architectures is still a challenge for all the current mesocrystal species because the general growth mechanism is still uncertain.
In order to optimize the performances of mesocrystal photocatalysts, functional modifications, including doping and hybridization, should be thoroughly explored. For the doped mesocrystal photocatalysts, the current literature concentrates mainly on metal doping, studies have not focused on morphology- or sized-controlled syntheses of doped ones. Moreover, non-metal doping should also be taken into consideration because it is a promising research avenue. In addition, the co-doping and selective doping of heteroatoms for synergistically optimizing the electronic structures is still a formidable challenge. As a result, the development of doped precursors for annealing or transformation might be an effective strategy to achieve the controllable synthesis of doped mesocrystals. For mesocrystal-based hybridized photocatalysts, decorating active components with controllable structures on specific sites of mesocrystals should attract extensive attention, and revealing the relationship between facet-dependent interfacial and photocatalytic activities is still a primary target. Significantly, it is imperative to focus more efforts toward the synthesis of multicomponent mesocrystals (including heterojunction and hetero-phase junctions) for exploring novel photocatalysts. Notably, the theoretical calculations involving the electronic structures of doped mesocrystals and interfacial structures of hybridization architectures should be investigated for the actual cognition of photocatalytic mechanisms.
To summarize, the comprehensive and in-depth review on the synthesis engineering and functional modifications (including doping and hybridization), as well as their corresponding structure-related enhanced photocatalytic mechanisms, can facilitate the development of new mesocrystal-based photocatalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (NSFC No. U1502274, 51302213, 51471132 and 51272209), the National High Technology Research and Development Program of China (863 Program, No. 2015AA034304), the Pivot Innovation Team of Shaanxi Electric Materials and Infiltration Technique (2012KCT-25), the Hundred Talent Program of Shaanxi Province, the Shaanxi Province Science Fund for Distinguished Young Scholars (2018JC-027), the Science Foundation of Jiangsu Province of China (No. BK20161250), and the Zhejiang Public Welfare Technology Research Industry Project (No. 2016C31G4181807).Notes and references
- S. Bai, J. Jiang, Q. Zhang and Y. J. Xiong, Chem. Soc. Rev., 2015, 44, 2893–2939 RSC.
- Y. P. Yuan, L. W. Ruan, J. Barber, S. C. J. Loo and C. Xue, Energy Environ. Sci., 2014, 7, 3934–3951 RSC.
- S. S. Zhu and D. W. Wang, Adv. Energy Mater., 2017, 7, 1700841 CrossRef.
- D. Q. Zhang, G. S. Li, H. X. Li and Y. F. Lu, Chem.–Asian J., 2013, 8, 26–40 CrossRef CAS PubMed.
- J. X. Low, C. J. Jiang, B. Cheng, S. Wageh, A. A. Al-Ghamdi and J. G. Yu, Small Methods, 2017, 1700080 CrossRef.
- H. J. Li, W. G. Tu, Y. Zhou and Z. G. Zou, Adv. Sci., 2016, 3, 1500389 CrossRef PubMed.
- S. W. Cao, J. X. Low, J. G. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- J. G. Yu, J. X. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136, 8839–8842 CrossRef CAS PubMed.
- H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef PubMed.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576–5591 CrossRef PubMed.
- M. Niederberger and H. Cölfen, Phys. Chem. Chem. Phys., 2006, 8, 3271–3287 RSC.
- L. Zhou and P. O'Brien, Small, 2008, 4, 1566–1574 CrossRef CAS PubMed.
- V. M. Yuwono, N. D. Burrows, J. A. Soltis and R. L. Penn, J. Am. Chem. Soc., 2010, 132, 2163–2165 CrossRef CAS PubMed.
- R. Q. Song and H. Cölfen, Adv. Mater., 2010, 22, 1301–1330 CrossRef CAS PubMed.
- E. V. Sturm and H. Cölfen, Chem. Soc. Rev., 2016, 45, 5821–5833 RSC.
- E. V. Sturm and H. Cölfen, Crystals, 2017, 7, 207 CrossRef.
- H. Imai, Prog. Cryst. Growth Charact., 2016, 62, 212–226 CrossRef CAS.
- L. Zhou and P. O'Brien, J. Phys. Chem. Lett., 2012, 3, 620–628 CrossRef CAS PubMed.
- L. Bergström, E. V. Sturm, G. S. Alvarez and H. Cölfen, Acc. Chem. Res., 2015, 48, 1391–1402 CrossRef PubMed.
- E. Uchaker and G. Z. Cao, Nano Today, 2014, 9, 499–524 CrossRef CAS.
- Y. Q. Liu, Y. Zhang and J. Wang, CrystEngComm, 2014, 16, 5948–5967 RSC.
- M. G. Ma and H. Cölfen, Curr. Opin. Colloid Interface Sci., 2014, 19, 56–65 CrossRef CAS.
- L. D. Bahrig, S. G. Hickey and A. Eychmüller, CrystEngComm, 2014, 16, 9408–9424 RSC.
- Q. Zhang, S. J. Liu and S. H. Yu, J. Mater. Chem., 2009, 19, 191–207 RSC.
- P. Zhang, T. S. Tachikawa, M. Fujitsuka and T. Majima, Chem.–Eur. J., 2017, 23, 1–14 CrossRef.
- P. Zhang, M. Fujitsuka and T. Majima, J. Energy Chem., 2016, 25, 917–926 CrossRef.
- P. H. Wen, Y. Ishikawa, H. Itoh and Q. Feng, J. Phys. Chem. C, 2009, 113, 20275–20280 CrossRef CAS.
- A. Khare, D. Shin, T. S. Yoo, M. Kim, T. D. Kang, J. Lee, S. Roh, I. H. Jung, J. Hwang, S. W. Kim, T. W. Noh, H. Ohta and W. S. Choi, Adv. Mater., 2017, 29, 1606566–1606574 CrossRef PubMed.
- A. D. Smigelskas and E. O. Kirkendall, Transactions of the AIME, 1947, 171, 130–142 Search PubMed.
- B. D. Anderson and J. B. Tracy, Nanoscale, 2014, 6, 12195–12216 RSC.
- S. Yang and X. Luo, Nanoscale, 2014, 6, 4438–4457 RSC.
- R. L. Penn and J. F. Banfield, Science, 1998, 281, 969–971 CrossRef CAS PubMed.
- C. C. Yec and H. C. Zeng, J. Mater. Chem. A, 2014, 2, 4843–4851 RSC.
- J. Aleman, A. V. Chadwick, J. He, M. Hess, K. Horie, R. G. Jones, P. Kratochvil, I. Meisel, I. Mita, G. Moad, S. Penczek, R. F. T. Stepto and R. G. Jones, Pure Appl. Chem., 2007, 79, 1801–1829 CAS.
- Y. Guo, H. Li, J. Chen, X. J. Wu and L. Zhou, J. Mater. Chem. A, 2014, 2, 19589–19593 RSC.
- L. Zhou, D. S. Boyle and P. O'Brien, J. Am. Chem. Soc., 2008, 130, 1309–1320 CrossRef CAS PubMed.
- P. Zhang, T. S. Tachikawa, Z. F. Bian and T. Majima, Appl. Catal., B, 2015, 176−177, 678–686 CrossRef CAS.
- L. Zhou, J. Chen, C. Ji, L. Zhou and P. O'Brien, CrystEngComm, 2013, 15, 5012–5015 RSC.
- R. O. D. Silva, R. H. Gonçalves, D. G. Stroppa, A. J. Ramirez and E. R. Leite, Nanoscale, 2011, 3, 1910–1916 RSC.
- P. Zhang, S. Kim, M. Fujitsuka and T. Majima, Chem. Commun., 2017, 53, 5306–5309 RSC.
- H. Yu, B. Z. Tian and J. L. Zhang, Chem.–Eur. J., 2011, 17, 5499–5502 CrossRef CAS PubMed.
- J. Y. Chen, G. Y. Li, H. M. Zhang, P. Liu, H. J. Zhao and T. C. An, Catal. Today, 2014, 224, 216–224 CrossRef CAS.
- Q. F. Chen, W. H. Ma, C. C. Chen, H. W. Ji and J. C. Zhao, Chem.–Eur. J., 2012, 18, 12584–12589 CrossRef CAS PubMed.
- J. F. Ye, W. Liu, J. G. Cai, S. Chen, X. W. Zhao, H. H. Zhou and L. M. Qi, J. Am. Chem. Soc., 2011, 133, 933–940 CrossRef CAS PubMed.
- M. T. Di, Y. G. Li, H. Z. Wang, Y. C. Rui, W. Jia and Q. H. Zhang, Electrochim. Acta, 2018, 261, 365–374 CrossRef CAS.
- F. He, J. L. Li, T. Li and G. X. Li, Chem. Eng. J., 2014, 237, 312–321 CrossRef CAS.
- G. C. Park, T. Y. Seo, C. H. Park, J. H. Lim and J. Joo, Ind. Eng. Chem. Res., 2017, 56, 8235–8240 CrossRef CAS.
- X. X. Fu, Z. M. Ren, C. Y. Fan, C. X. Sun, L. Shi, S. Q. Yu, G. D. Qian and Z. Y. Wang, RSC Adv., 2015, 5, 41218–41223 RSC.
- Y. Liu, Y. f. Luo, A. A. Elzatahry, W. Luo, R. C. Che, J. W. Fan, K. Lan, A. M. Al-Enizi, Z. K. Sun, B. Li, Z. W. Liu, D. K. Shen, Y. Ling, C. Wang, J. X. Wang, W. J. Gao, C. Yao, K. P. Yuan, H. S. Peng, Y. Tang, Y. H. Deng, G. f. Zheng, G. Zhou and D. Y. Zhao, ACS Cent. Sci., 2015, 1, 400–408 CrossRef CAS PubMed.
- Z. Bian, T. Tachikawa and T. Majima, J. Phys. Chem. Lett., 2012, 3, 1422–1427 CrossRef CAS PubMed.
- Z. Bian, T. Tachikawa, W. Kim, W. Choi and T. Majima, J. Phys. Chem. C, 2012, 116, 25444–25453 CrossRef CAS.
- C. Tang, L. F. Liu, Y. L. Li and Z. F. Bian, Appl. Catal., B, 2017, 201, 41–47 CrossRef CAS.
- F. F. Chen, F. L. Cao, H. X. Li and Z. F. Bian, Langmuir, 2015, 31, 3494–3499 CrossRef CAS PubMed.
- P. Tartaj, Chem. Commun., 2011, 47, 256–258 RSC.
- P. Tartaj and J. M. Amarilla, Adv. Mater., 2011, 23, 4904–4907 CrossRef CAS PubMed.
- D. Q. Zhang, G. S. Li, F. Wang and J. C. Yu, CrystEngComm, 2010, 12, 1759–1763 RSC.
- L. L. Lai and J. M. Wu, Ceram. Int., 2015, 411, 2317–2322 Search PubMed.
- N. B. Sumina, S. N. Kumar, R. Achu, B. S. D. Kumar, A. K Ray, K. G. K. Warrier and S. Pillai, ChemistrySelect, 2016, 1, 6221–6229 CrossRef CAS.
- Y. K. Aoyama, Y. Y. Oaki, R. Ise and H. Imai, CrystEngComm, 2012, 14, 1405–1411 RSC.
- X. L. Zheng, Y. Y. Lv, Q. Kuang, Z. L. Zhu, X. Long and S. H. Yang, Chem. Mater., 2014, 26, 5700–5709 CrossRef CAS.
- X. L. Zheng, Q. Kuang, K. Y. Yan, Y. C. Qiu, J. H. Qiu and S. H. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 11249–11257 CrossRef CAS PubMed.
- F. Qian, G. M. Wang and Y. Li, Nano Lett., 2010, 10, 4686–4691 CrossRef CAS PubMed.
- M. J. Bierman and S. Jin, Energy Environ. Sci., 2009, 2, 1050–1059 RSC.
- J. G. Cai, J. F. Ye, S. Y. Chen, X. W. Zhao, D. Y. Zhang, S. Chen, Y. R. Ma, S. Jin and L. M. Qi, Energy Environ. Sci., 2012, 5, 7575–7581 RSC.
- L. Li and C. Y. Liu, CrystEngComm, 2010, 12, 2073–2078 RSC.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed.
- R. Kumar, G. Kumar and A. Umar, Nanosci. Nanotechnol. Lett., 2014, 6, 631–650 CrossRef CAS.
- S. M. Lam, J. C. Sin, A. Z. Abdullah and A. R. Mohamed, Desalin. Water Treat., 2012, 41, 131–169 CrossRef CAS.
- Y. H. Tseng, H. Y. Lin, M. H Liu, Y. F. Chen and C. Y. Mou, J. Phys. Chem. C, 2009, 113, 18053–18061 CrossRef CAS.
- H. Tang, J. C. Chang, Y. Y. Shan and S. T. Lee, J. Phys. Chem. B, 2008, 112, 4016–4021 CrossRef CAS PubMed.
- M. S. Wang, Y. P. Zhang, Y. J. Zhou, F. W. Yang, E. J. Kim, S. H. Hahn and S. G. Seong, CrystEngComm, 2013, 15, 754–763 RSC.
- M. S. Mo, S. H. Lim, Y. W. Mai, R. K. Zheng and S. P. Ringer, Adv. Mater., 2008, 20, 339–342 CrossRef CAS.
- Y. Peng, Y. Wang, Q. G. Chen, Q. Zhu and A. W. Xu, CrystEngComm, 2014, 16, 7906–7913 RSC.
- W. X. Ma, B. S. Ren, Z. Huang, Q. F. Chen, X. F. Cao and Y. C. Guo, Mater. Chem. Phys., 2017, 186, 341–352 CrossRef CAS.
- H. Wang, C. C. Wang, Q. F. Chen, B. S. Ren, R. F. Guan, X. F. Cao, X. P. Yang and R. Duan, Appl. Surf. Sci., 2017, 412, 517–528 CrossRef CAS.
- W. H. Lin, T. F. M. Chang, Y. H. Lu, T. Sato, M. Sone, K. H. Wei and Y. J. Hsu, J. Phys. Chem. C, 2013, 117, 25596–25603 CrossRef CAS.
- Z. Liu, X. D. Wen, X. L. Wu, Y. J. Gao, H. T. Chen, J. Zhu and P. K. Chu, J. Am. Chem. Soc., 2009, 131, 9405–9412 CrossRef CAS PubMed.
- X. L. Wu, S. J. Xiong, Z. Liu, J. Chen, J. C. Shen, T. H. Li, P. H. Wu and P. K. Chu, Nat. Nanotechnol., 2011, 6, 103–106 CrossRef CAS PubMed.
- Y. Q. Yang, H. X. Wu and S. W. Guo, CrystEngComm, 2013, 15, 2608–2615 RSC.
- S. D. Sun, X. Z. Zhang, J. Zhang, X. P. Song and Z. M. Yang, Cryst. Growth Des., 2012, 12, 2411–2418 CrossRef CAS.
- J. Y. Dong, W. H. Lin, Y. J. Hsu, D. S. H. Wong and S. Y. Lu, CrystEngComm, 2011, 13, 6218–6222 RSC.
- Q. B. Zhang, K. L. Zhang, D. G. Xu, G. C. Yang, H. Huang, F. Nie, C. M. Liu and S. H. Yang, Prog. Mater. Sci., 2014, 60, 208–337 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 8124–8125 CrossRef CAS PubMed.
- Z. M. Zhang, H. P. Sun, X. Q. Shao, D. F. Li, H. D. Yu and M. Y. Han, Adv. Mater., 2005, 17, 42–47 CrossRef CAS.
- A. Taubert, A. Uhlmann, A. Hedderich and K. Kirchhoff, Inorg. Chem., 2008, 47, 10758–10764 CrossRef CAS PubMed.
- S. D. Sun, X. Z. Zhang, J. Zhang, L. Q. Wang, X. P. Song and Z. M. Yang, CrystEngComm, 2013, 15, 867–877 RSC.
- P. Zhang, J. Zhang and J. Gong, Chem. Soc. Rev., 2014, 43, 4395–4422 RSC.
- X. Yu, W. Li, J. Huang, Z. H. Li, J. W. Liu and P. A. Hu, Dalton Trans., 2018, 47, 1948–1957 RSC.
- Z. F. Huang, L. Pan, J. J. Zou, X. W. Zhang and L. Wang, Nanoscale, 2014, 6, 14044–14063 RSC.
- Z. Min, B. W. Hao, B. Jian, L. Lin, W. L. Xiong and X. Yi, Angew. Chem., Int. Ed., 2013, 52, 8579–8583 CrossRef PubMed.
- R. G. Li, F. X. Zhang, D. E. Wang, J. X. Yang, M. R. Li, J. Zhu, X. Zhou, H. X. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- D. Wang, R. G. Li, J. Zhu, J. Y. Shi, J. F. Han, X. Zong and C. Li, J. Phys. Chem. C, 2012, 116, 5082–5089 CrossRef CAS.
- R. G. Li, H. X. Han, F. X. Zhang, D. E. Wang and C. Li, Energy Environ. Sci., 2014, 7, 1369–1376 RSC.
- J. X. Yang, D. E. Wang, X. Zhou and C. Li, Chem.–Eur. J., 2013, 19, 1320–1326 CrossRef CAS PubMed.
- Q. F. Wu, S. Y. Bao, B. Z. Tian, Y. F. Xiao and J. L. Zhang, Chem. Commun., 2016, 52, 7478–7481 RSC.
- Y. P. Yuan, X. L. Zhang, L. F. Liu, X. J. Jiang, J. Lv, Z. S. Li and Z. G. Zou, Int. J. Hydrogen Energy, 2008, 33, 5941–5946 CrossRef CAS.
- Y. P. Yuan, Z. Y. Zhao, J. Zheng, M. Yang, L. G. Qiu, Z. S. Li and Z. G. Zou, J. Mater. Chem., 2010, 20, 6772–6779 RSC.
- T. N. Ye, M. Xu, W. Fu, Y. Y. Cai, X. Wei, K. X. Wang, Y. N. Zhao, X. H. Li and J. S. Chen, Chem. Commun., 2014, 50, 3021–3023 RSC.
- S. Yan, Z. Wang, Z. Li and Z. Zou, Solid State Ionics, 2009, 180, 1539–1542 CrossRef CAS.
- H. Kato and A. Kudo, J. Phys. Chem. B, 2001, 105, 4285–4292 CrossRef CAS.
- W. Jiang, X. L. Jiao and D. R. Chen, Int. J. Hydrogen Energy, 2013, 38, 12739–12746 CrossRef CAS.
- M. S. Wrighton, A. B. Ellis, P. T. Wolczanski, D. L. Morse, H. B. Abrahamson and D. S. Ginley, J. Am. Chem. Soc., 1976, 98, 2774–2779 CrossRef CAS.
- S. Ahuja and T. R. N. Kutty, J. Photochem. Photobiol., A, 1996, 97, 99–107 CrossRef CAS.
- Y. F. Chang, H. Ning, J. Wu, S. T. Zhang, T. Q. Lü, B. Yang and W. W. Cao, Inorg. Chem., 2014, 53, 11060–11067 CrossRef CAS PubMed.
- P. Zhang, T. Ochi, M. Fujitsuka, Y. Kobori, T. Majima and T. Tachikawa, Angew. Chem., Int. Ed., 2017, 129, 5383–5387 CrossRef.
- V. Kalyani, B. D. D. S. Vasile, A. Ianculescu, M. T. Buscaglia, V. Buscaglia and P. Nanni, Cryst. Growth Des., 2012, 12, 4450–4456 CrossRef CAS.
- X. Wei, G. Xu, Z. H. Ren, C. X. Xu, W. J. Weng, G. Shen and G. R. Han, J. Am. Ceram. Soc., 2010, 93, 1297–1305 CrossRef CAS.
- L. He, T. E. Wood, B. Wu, Y. C. Dong, L. B. Hoch, L. M. Reyes, D. Wang, C. Kübel, C. X. Qian, J. Jia, K. Liao, P. G. O'Brien, A. Sandhel, J. Y. Y. Loh, P. Szymanski, N. P. Kherani, T. C. Sum, C. A. Mims and G. A. Ozin, ACS Nano, 2016, 10, 5578–5586 CrossRef CAS PubMed.
- S. B. Betzler, A. Wisnet, B. J. Breitbach, C. Mitterbauer, J. Weickert, L. S. Mende and C. Scheu, J. Mater. Chem. A, 2014, 2, 12005–12013 RSC.
- B. Hu, L. H. Wu, S. J. Liu, H. B. Yao, H. Y. Shi, G. P. Li and S. H. Yu, Chem. Commun., 2010, 46, 2277–2279 RSC.
- B. Hu, L. H. Wu, Z. Zhao, M. Zhang, S. F. Chen, S. J. Liu, H. Y. Shi, Z. J. Ding and S. H. Yu, Nano Res., 2010, 3, 395–403 CrossRef CAS.
- H. C. Zeng, Acc. Chem. Res., 2013, 46, 226–235 CrossRef CAS PubMed.
- X. R. Li, J. G. Wang, Y. Men and Z. F. Bian, Appl. Catal., B, 2016, 187, 115–121 CrossRef CAS.
- O. M. Elbanna, M. Fujitsuka and T. Majima, ACS Appl. Mater. Interfaces, 2017, 9, 34844–34854 CrossRef CAS PubMed.
- X. Yu, X. L. Fan, L. An, G. B. Liu, Z. H. Li, J. W. Liu and P. A. Hu, Carbon, 2018, 128, 21–30 CrossRef CAS.
- C. N. Van, T. H. Do, J. W. Chen, W. Y. Tzeng, K. A. Tsai, H. L. Song, H. J. Liu, Y. C. Lin, Y. C. Chen, C. L Wu, C. W. Luo, W. C. Chou, R. Huang, Y. J. Hsu and Y. H. Chu, NPG Asia Mater., 2017, 9, 357 CrossRef.
- T. Tachikawa, P. Zhang, Z. F. Bian and T. Majima, J. Mater. Chem. A, 2014, 2, 3381–3388 RSC.
- P. Zhang, T. Tachikawa, M. Fujitsuka and T. Majima, Chem. Commun., 2015, 51, 7187–7190 RSC.
- Z. F. Bian, T. S. Tachikawa, W. Kim, W. Y. Choi and T. Majima, J. Phys. Chem. C, 2012, 116, 25444–25453 CrossRef CAS.
- L. Q. Liu, X. H. Zhang, L. F. Yang, L. T. Ren, D. F. Wang and J. H. Ye, Natl. Sci. Rev., 2017, 0, 1–20 Search PubMed.
- O. Elbanna, S. Kim, M. Fujitsuka and T. Majima, Nano Energy, 2017, 35, 1–8 CrossRef CAS.
- Z. R. Yan, Q. F. Chen, P. Xia, W. X. Ma and B. S. Ren, Nanotechnology, 2016, 27, 035707 CrossRef PubMed.
- T. H. Han, H. G. Wang and X. M. Zheng, RSC Adv., 2016, 6, 7829–7837 RSC.
- X. J. Chen, F. G. Chen, F. L. Liu, X. D. Yan, W. Hu, G. B. Zhang, L. H. Tian, Q. H. Xia and X. B. Chen, Catal. Sci. Technol., 2016, 6, 4184–4191 RSC.
- Y. X. Zhang, M. J. Xu, H. Li, H. Ge and Z. F. Bian, Appl. Catal., B, 2018, 226, 213–219 CrossRef CAS.
- D. Yan, Y. Liu, C. Y. Liu, Z. Y. Zhang and S. D. Nie, RSC Adv., 2016, 6, 14306–14313 RSC.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- X. F. Yang, J. L. Qin, Y. Li, R. X. Zhang and H. Tang, J. Hazard. Mater., 2013, 261, 342–350 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- B. Liu, L. H. Tian, R. Wang, J. F. Yang, R. Guan and X. B. Chen, Appl. Surf. Sci., 2017, 422, 607–615 CrossRef CAS.
- X. Y. Wu, S. Yin, B. Liu, M. Kobayashi, M. Kakihana and T. Sato, J. Mater. Chem. A, 2014, 2, 20832–20840 RSC.
- L. B. Jiang, X. Z. Yuan, Y. Pan, J. Liang, G. M. Zeng, Z. B. Wu and H. Wang, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS.
- S. D. Sun, X. J. Zhang, Q. Yang, S. H. Liang, X. Z. Zhang and Z. M Yang, Prog. Mater. Sci., 2018, 96, 111–173 CrossRef CAS.
- L. J. An and H. Onishi, ACS Catal., 2015, 5, 3196–3206 CrossRef CAS.
- J. X. Sun, G. Chen, J. Pei, R. C. Jin, Q. Wang and X. Y. Guang, J. Mater. Chem., 2012, 22, 5609–5614 RSC.
- W. Zhou, W. Li, J. Q. Wang, Y. Qu, Y. Yang, Y. Xie, K. Zhang, L. Wang, H. Fu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 9280–9283 CrossRef CAS PubMed.
- B. Y. Tan, X. H. Zhang, Y. J. Li, H. Chen, X. Z. Ye, Y. Wang and J. F. Ye, Chem.–Eur. J., 2017, 23, 5478–5487 CrossRef CAS PubMed.
- X. Yu, X. L. Fan, Z. H. Li and J. W. Liu, Dalton Trans., 2017, 46, 11898–11904 RSC.
- J. M. Calatayud, J. Balbuena, M. C. Yusta, F. Martín, P. Pardo, L. Sánchez and J. Alarcón, Ceram. Int., 2018, 44, 7319–8726 CrossRef.
- D. K. Primc and M. Niederberger, Chem. Mater., 2017, 29, 10113–10121 CrossRef CAS.
- X. S. Nguyen, G. K. Zhang and X. F. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 8900–8909 CrossRef CAS PubMed.
- H. Seo, L. R. Baker, A. Hervier, J. Kim, J. L. Whitten and G. A. Somorjai, Nano Lett., 2011, 11, 751–756 CrossRef CAS PubMed.
- W. Q. Fang, X. L. Wang, H. Zhang, Y. Jia, Z. Huo, Z. Li, H. Zhao, H. G. Yang and X. Yao, J. Mater. Chem. A, 2014, 2, 3513–3520 RSC.
- X. Ma, Y. Dai, W. Wei, B. Huang and M. H. Whangbo, J. Phys. Chem. Lett., 2015, 6, 1876–1882 CrossRef CAS PubMed.
- P. Zhang, T. Tachikawa, M. Fujitsuka and T. Majima, ChemSusChem, 2016, 9, 617–623 CrossRef CAS PubMed.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O'Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- R. Asahi, T. Morikawa, H. Irie and T. Ohwaki, Chem. Rev., 2014, 114, 9824–9852 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, S. P. Chai, S. T. Yong and A. R. Mohamed, Nanoscale, 2014, 6, 1946–2008 RSC.
- P. Zhang, M. Fujitsuka and T. Majima, Appl. Catal., B, 2016, 185, 181–188 CrossRef CAS.
- O. Elbanna, P. Zhang, M. Fujitsuka and T. Majima, Appl. Catal., B, 2016, 192, 80–87 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |