Phenylethynyl-substituted heterocycles inhibit cyclin D1 and induce the expression of cyclin-dependent kinase inhibitor p21Wif1/Cip1 in colorectal cancer cells
Vitaliy M.
Sviripa†
 *ab,
Liliia M.
Kril†
*ab,
Liliia M.
Kril†
 bd,
Wen
Zhang†
de,
Yanqi
Xie†
bd,
Wen
Zhang†
de,
Yanqi
Xie†
 de,
Przemyslaw
Wyrebek
de,
Przemyslaw
Wyrebek
 bd,
Larissa
Ponomareva
bd,
Larissa
Ponomareva
 ab,
Xifu
Liu
c,
Yaxia
Yuan
ab,
Xifu
Liu
c,
Yaxia
Yuan
 abf,
Chang-Guo
Zhan
abf,
David S.
Watt
abf,
Chang-Guo
Zhan
abf,
David S.
Watt
 *abcde and
Chunming
Liu
*abcde and
Chunming
Liu
 *cde
*cde
aDepartment of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY 40536-0596, USA. E-mail: vitaliy.sviripa@uky.edu
bCenter for Pharmaceutical Research and Innovation, College of Pharmacy, University of Kentucky, Lexington, KY 40536-0596, USA
cEpionc, Inc., P.O. Box 23436, Lexington, KY 40523, USA
dDepartment of Molecular and Cellular Biochemistry, College of Medicine, University of Kentucky, Lexington, KY 40536-0509, USA. E-mail: chunming.liu@uky.edu
eLucille Parker Markey Cancer Center, University of Kentucky, Lexington, KY 40536-0093, USA
fMolecular Modeling and Biopharmaceutical Center, College of Pharmacy, University of Kentucky, 40536-0596, USA
First published on 3rd November 2017
Abstract
Fluorinated phenylethynyl-substituted heterocycles that possessed either an N-methylamino or N,N-dimethylamino group attached to heterocycles including pyridines, indoles, 1H-indazoles, quinolines, and isoquinolines inhibited the proliferation of LS174T colon cancer cells in which the inhibition of cyclin D1 and induction of the cyclin-dependent kinase inhibitor-1 (i.e., p21Wif1/Cip1) served as readouts for antineoplastic activity at a cellular level. At a molecular level, these agents, particularly 4-((2,6-difluorophenyl)ethynyl)-N-methylisoquinolin-1-amine and 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylisoquinolin-1-amine, bound and inhibited the catalytic subunit of methionine S-adenosyltransferase-2 (MAT2A).
Introduction
In the course of studies focused on the mechanisms of colorectal cancer1–3 development, we employed chemical biology4–7 to probe cell signaling events8,9 that regulate gene expression. Selective targeting of key steps in this process offered the potential for developing therapeutic agents to treat these cancers.9 We reported, for example, that 2′,6′-dihalostyrylanilines 1 (Fig. 1) inhibited the expression of Wnt target genes, such as c-myc,10 and repressed colon cancer LS174T cell growth in vitro and in vivo.11–13 We established that this agent exclusively targeted the catalytic subunit of the oligomeric enzyme, methionine S-adenosyltransferase-2 (MAT2), which furnishes S-adenosylmethionine (SAM) to regulatory methyltransferases. We further established that heterocyclic variants13 of these 2′,6′-dihalostyrylanilines 1 possessed minimal gross toxicity, no human ether-à-go-go-related protein (hERG)14,15 activation, and reasonable bioavailability and pharmacokinetics. As desired, these stilbene-based agents did not affect methionine S-adenosyltransferase-1 (MAT1)16–19 that served as the principal source of SAM necessary for other cellular needs. Unlike a recent report20 on other MAT2A inhibitors, we also demonstrated in vivo potency in a xenograft model using colorectal cancer cells.12Concerns regarding thermal or photochemical E/Z-isomerizations21,22 in stilbenes 1 that could potentially complicate future pharmacodynamic studies led to synthesis and evaluation of the analogous diarylacetylenes232 (Fig. 1). In accord with the SAR study of the stilbenes 1, we found that the diarylacetylenes 2 bearing an N,N-dimethylamino group on one phenyl ring and either one or preferably two ortho-oriented fluoro or chloro groups on the other phenyl ring retained the in vitro potencies in a colorectal cancer LS174T cell proliferation assay. The diarylacetylenes 2 also possessed the desired property of having minimal effects on the cardiac potassium hERG channel.14,15 Molecular docking studies suggested that diphenylacetylenes, like their stilbene counterparts,12 targeted the catalytic subunit (MAT2A) of MAT2.23
Results and discussion
We performed additional structure–activity studies of the diarylacetylene pharmacophore with a view to obtaining improved potency, solubility and bioavailability relative to the sparingly water-soluble 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylaniline (2). Initial efforts focused on replacing the N,N-dimethylamino group in 2 with a heterocyclic ring as in the heterocyclic-substituted diphenylacetylenes 4 (Fig. 1). The Sonogashira coupling23–25 of 2,6-difluorophenylacetylene (3) with 4-iodophenyl-substituted heterocycles provided access to the oxopiperidinyl, morpholino, N-methylpiperazinyl, N-pyrrolyl, and 2,5-dimethyl-N-pyrrolyl analogs 4a–4e (Table 1). The N-pyrrolyl analog 4d showed activity in a cell proliferation assay comparable to 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylaniline (2) at 1 μM concentration but considerably diminished activity at 100 nM.| Compound | C-4′ substituent | 10 μM | 1 μM | 100 nM |
|---|---|---|---|---|
| a Ref. 23. | ||||
| 2 | N,N-Dimethylamino | 99.5 ± 0.1 | 85.2 ± 0.5 | |
| 4a | 1-Piperidinyl-2-one | 68.1 ± 6.9 | 6.4 ± 11.0 | |
| 4b | N-Morpholino | 32.1 ± 9.8 | 13.0 ± 2.5 | |
| 4c | N′-Methyl-N-piperazinyl | 99.6 ± 0.2 | 19.9 ± 18.2 | |
| 4d | N-Pyrrolyl | 96.7 ± 1.9 | 77.5 ± 9.2 | 11.0 ± 18.1 |
| 4e | 2,5-Dimethyl-N-pyrrolyl | 78.8 ± 4.8 | 20.9 ± 10.0 | |
We also examined other halogenation patterns (e.g., 2-fluoro, 2-chloro, 2-trimethylfluoro, 2-chloro-6-fluoro and 2,6-dichloro) in other heterocyclic-substituted diphenylacetylenes 4, but these studies failed to produce a compound with an in vitro potency that exceeded that of 2 (data not shown). In summary, we were unable to identify a heterocyclic variant of 4 with potency equal to that of the parent compound, 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylaniline23 (2).
Because heterocyclic variants in the stilbene series13 in which the N,N-dimethylaniline ring in 1 was replaced by either an N,N-dimethylaminopyridine or an N,N-dimethylaminopyrimidine ring exhibited improved solubility and minimal hERG activation, we next focused on replacing the N,N-dimethylaniline ring in the diarylacetylene series with a heterocyclic ring as in the phenylethynyl-substituted heterocycles 5 (Fig. 1). The Sonogashira coupling24,25 of 2,6-difluorophenylacetylene with iodo-substituted heterocycles provided access to most of these analogs (Table 2). In some cases, such as the synthesis of 5mm–5oo, it was preferable to couple 2,6-difluorophenylacetylene to 1-chloro-4-iodoisoquinoline and subsequently utilize a nucleophilic aromatic substitution reaction to replace the chloro group with a primary or a secondary amine. Yet another approach involved the coupling of 2,6-difluorophenylacetylene to 1-amino-4-iodoisoquinoline to afford 4-((2,6-difluorophenyl)ethynyl)isoquinolin-1-amine (5ll) and the subsequent N,N-dimethylation of the amino group using 1,2-bis[(dimethylamino)methylene]hydrazine via an intermediate 1,2,4-triazole,26 but this latter process proceeded in a lower overall yield than the yield in the nucleophilic aromatic substitution reaction. As summarized in Table 2, using these approaches, we synthesized 2,6-difluorophenylethynyl-substituted heterocycles including pyridines 5a–5c, pyrazine 5d, indoles 5e and 5f, 1H-indazoles 5g–5k, 1H-pyrrolo[2,3-b]pyridine 5l, quinolines 5m–5ee, isoquinolines 5ff–5oo, 1,6-naphthyridine 5pp, and quinazoline 5qq. The corresponding monofluorinated and monochlorinated analogs possessed comparable or diminished potency in cell proliferation assays at low concentrations relative to their difluorinated counterparts (data not shown).
| Compound | Heterocycle or aryl ring | Positiona | 10 μM | 1 μM | 100 nM | 30 nM |
|---|---|---|---|---|---|---|
| a Position of attachment of the 2,6-difluorophenylethynyl group. b Ref. 23. c Tested as the hydrochloride salt. | ||||||
| 2 | N,N-Dimethylaniline | C-4 | 99.5 ± 0.1 | 85.2 ± 0.5 | 57 ± 1.2 | |
| 5a | N,N-Dimethylpyridin-3-amine | C-6 | 85.2 ± 3.8 | 68.9 ± 1.3 | 15.1 ± 7.4 | |
| 5b | N,N-Dimethylpyridin-2-amine | C-5 | 83.6 ± 7.8 | 77.9 ± 7.0 | 0 ± 23.7 | |
| 5c | 2-(1H-Pyrrol-1-yl)pyridine | C-5 | 95.3 ± 0.8 | 80.5 ± 6.6 | 0 ± 27.7 | |
| 5d | N,N-Dimethylpyrazin-2-amine | C-5 | 91.6 ± 3.0 | 22.9 ± 9.8 | ||
| 5e | 1H-Indole | C-5 | 91.5 ± 3.5 | 52.9 ± 5.9 | ||
| 5f | N-Methylindole | C-5 | 95.2 ± 1.0 | 77.7 ± 13.6 | 3.0 ± 5.6 | |
| 5g | 1H-Indazole | C-4 | 96.2 ± 2.0 | 19.8 ± 13.6 | ||
| 5h | 1H-Indazole | C-5 | 82.9 ± 9.8 | 17.6 ± 7.9 | ||
| 5i | 1H-Indazole | C-6 | 97.0 ± 1.1 | 81.5 ± 2.9 | ||
| 5j | N-Methylindazole | C-5 | 94.6 ± 3.5 | 58.4 ± 9.6 | 0 ± 24.5 | |
| 5k | N-Methylindazole | C-6 | 98.1 ± 0.4 | 85.9 ± 5.4 | 0 ± 22.7 | |
| 5l | 1H-Pyrrolo[2,3-b]pyridine | C-5 | 77.9 ± 8.9 | 17.3 ± 12.0 | ||
| 5m | Quinoline | C-2 | 93.8 ± 1.7 | 67.3 ± 13.6 | 6.1 ± 27.0 | |
| 5n | Quinoline | C-3 | 90.4 ± 1.0 | 41.7 ± 6.5 | 0 ± 5.1 | |
| 5o | Quinoline | C-4 | 59.0 ± 7.5 | 15.4 ± 9.1 | ||
| 5p | 2-Chloroquinoline | C-3 | 85.1 ± 6.2 | 28.4 ± 10.5 | ||
| 5q | 4-Chloroquinoline | C-3 | 80.4 ± 13.7 | 51.5 ± 20.4 | 0 ± 26.3 | |
| 5r | Quinolin-6-amine | C-3 | 9.2 ± 15.7 | |||
| 5s | 7-Fluoroquinoline | C-3 | 95.1 ± 1.6 | 69.4 ± 4.5 | 14.8 ± 5.5 | |
| 5t | 8-Fluoroquinoline | C-3 | 89.1 ± 8.2 | 63.6 ± 8.4 | 8.8 ± 5.1 | |
| 5u | 7-Chloroquinoline | C-4 | 74.3 ± 0.2 | 36.0 ± 0.4 | ||
| 5v | Quinoline | C-5 | 94.7 ± 2.9 | 43.1 ± 11.2 | ||
| 5w | Quinoline | C-6 | 80.1 ± 1.0 | 11 ± 30.4 | ||
| 5x | Quinoline | C-7 | 99.4 ± 0.3 | 90.7 ± 0.6 | 2.9 ± 14.0 | |
| 5y | Quinoline | C-8 | 92.7 ± 2.9 | 47.6 ± 3.5 | ||
| 5z | 2-Chloroquinoline | C-6 | 63.3 ± 2.3 | 21.2 ± 11.4 | ||
| 5aa | 4-Chloroquinoline | C-6 | 89.7 ± 4.3 | 43.8 ± 10.8 | ||
| 5bb | 4-Chloroquinoline | C-7 | 70.8 ± 4.8 | 23.1 ± 8.3 | ||
| 5cc | Quinolin-4-amine | C-6 | 93.9 ± 0.4 | 66.2 ± 3.1 | 0 ± 5.4 | |
| 5dd | Quinolin-4-amine | C-7 | 97.7 ± 2.2 | 23.6 ± 2.5 | ||
| 5ee | N,N-Dimethylquinolin-2-amine | C-6 | 38.7 ± 4.3 | 2.0 ± 11.9 | ||
| 5ff | Isoquinoline | C-1 | 96.7 ± 0.2 | 35.4 ± 15.0 | ||
| 5gg | Isoquinoline | C-4 | 87.9 ± 1.0 | 16.5 ± 2.1 | ||
| 5hh | Isoquinoline | C-5 | 93.6 ± 0.5 | 64.2 ± 6.1 | ||
| 5ii | Isoquinoline | C-6 | 82.4 ± 10.2 | 11.6 ± 21.3 | ||
| 5jj | 2-Chloroisoquinoline | C-4 | 94.0 ± 0.5 | 48.1 ± 18.1 | 0.8 ± 36.1 | |
| 5kk | 7-Fluoroisoquinoline | C-1 | 96.2 ± 0.1 | 71.1 ± 3.5 | 0 ± 5.2 | |
| 5ll | Isoquinolin-2-amine | C-4 | 93.1 ± 1.6 | 86.2 ± 1.6 | 35.5 ± 1.7 | |
| 5mm | N,N-Dimethylisoquinolin-2-amine | C-4 | 97.6 ± 1.5 | 99.5 ± 0.1 | 81.7 ± 2.1 | 86.0 ± 2.0 |
| 5nn | N-Methylisoquinolin-2-amine | C-4 | 98.9 ± 1.0 | 99.8 ± 0.2 | 99.5 ± 0.5 | 94.5 ± 1.5 |
| 5oo | 1-(4-Methylpiperazin-1-yl)isoquinoline | C-4 | 100 ± 0c | 23.2 ± 9.4c | ||
| 5pp | N,N-Dimethyl-1,6-naphthyridin-5-amine | C-8 | 85.2 ± 1.0 | 87.9 ± 0.8 | 59.5 ± 3.5 | |
| 5qq | Quinazoline | C-7 | 96.3 ± 0.8 | 33.8 ± 4.8 | ||
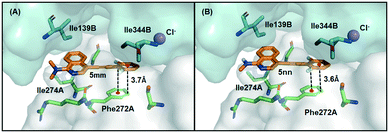
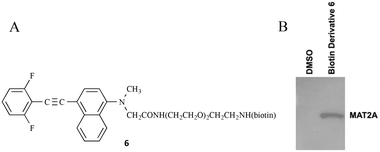
Molecular docking and molecular dynamics (MD) studies furnished a binding model for the phenylethynyl-substituted heterocycles 5 with the dimeric catalytic structure of human methionine S-adenosyltransferase-2 (pdb code: 2P02). A pocket formed at the interface between two α2 subunits of the MAT2A complex contained the SAM-binding site. The MD-simulated MAT2A–5mm or MAT2A–5nn complexes displayed a stable binding mode for these ligands in this pocket (Fig. 2 in which residues within 4 Å of the ligand are highlighted). A serine residue (Ser269) that is also part of this pocket within the 4 Å radius lies in front of the ligands in Fig. 2 and was removed for the sake of clarity. The ligands 5mm and 5nn exhibit hydrophobic van der Waals interactions with portions of the backbone and in particular with the side chains of phenylalanine-272 (F272) and isoleucine-274 (Ile274). Computational modeling studies provided guidance with respect to synthetic design but required experimental confirmation. Prior modeling work and pull-down assays confirmed MAT2A as the sole target of stilbene analogs,12 but it was important to confirm experimentally that the acetylene analogs, such as phenylethynyl-substituted heterocycles 5, shared the same target as these stilbene analogs. In support of the binding of phenylethynyl-substituted heterocycles 5 to MAT2A, we synthesized and utilized a biologically active D-(+)-biotin derivative 6 (Fig. 3A) of isoquinoline 5mm in a pull-down assay. The synthesis of biotin derivative 6 followed a similar pattern to that reported previously12 in which the biotin moiety was attached to one of the N-methyl groups in 5mm through a polyethylene glycol (PEG) spacer (Fig. 3A). We selected isoquinoline 5mm for biotinylation based on its potency in inhibiting LS174T cell proliferation (vide infra).
The utilization of biotin-labeled molecules for the isolation and identification of protein targets is well established.27,28 Incubation of recombinant MAT2A with or without 6 and the use of streptavidin beads facilitated the pull-down of MAT2A bound to the biotinylated 6 attached to the beads. Elution of the MAT2A bound to these beads with 2.5 mM D-(+)-biotin, separation on a 10% SDS-PAGE gel, and analysis of the western blot using an anti-MAT2A Ab, as described previously,12 identified the MAT2A protein. In summary, the biotin derivative 6 functioned as a biologically competent analog of isoquinoline 5mm and bound MAT2A (Fig. 3B).
At 10 μM concentration, most of the 2,6-difluorophenylethynyl-substituted heterocycles 5 in Table 2 displayed potent effects on LS174T colon cancer cell proliferation assays, and at 1 μM concentration, several pyridines 5b and 5c, one indole 5f, two 1H-indazoles 5i and 5k, one quinoline 5x, three isoquinolines 5ll–5nn, and one naphthyridine 5pp showed greater than 75% inhibition. Two candidates that stood out with respect to potency among the 2,6-difluorophenylethynyl-substituted heterocycles 5 were 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylisoquinolin-1-amine (5mm) and 4-((2,6-difluorophenyl)ethynyl)-N-methylisoquinolin-1-amine (5nn). We tested the isoquinolines 5mm and 5nn against other colon cancer cell lines (i.e., HT-29 and Caco2) (Table 3) and against normal cell lines (i.e., lung epithelial BEAS-2B and fibroblast HEL 299 cells) (Table 4). Isoquinolines 5mm and 5nn showed better selectivity than 5-fluorouracil (5-FU) and cisplatin against colon cancer cells and in particular against colon LS174T cells where MAT2A levels are upregulated (Tables 3 and 4). In addition, several of the difluorinated phenylethynyl-substituted heterocycles 5, particularly 5oo and 5pp, met another of our developmental goals and furnished completely water-soluble hydrochloride salts. In summary, we identified phenylethynyl-substituted heterocycles 5 with potency and/or water solubility surpassing that of the parent compound, 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylaniline23 (2).
| Cell line | 5mm | 5nn | 5 -FU | Cisplatin | ||
|---|---|---|---|---|---|---|
| 1 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | |
| LS174T | 99.5 ± 0.1 | 99.8 ± 0.2 | 82.3 ± 2.5 | 6.5 ± 8.2 | 82.1 ± 1.4 | 24.2 ± 5.6 |
| HT-29 | 35.9 ± 3.8 | 76.8 ± 0.8 | 82.9 ± 1.8 | 11.6 ± 17.7 | 72.7 ± 3.5 | 4.4 ± 5.9 |
| Caco-2 | 54.5 ± 2.7 | 58.3 ± 1.7 | 60.2 ± 11.5 | 37.7 ± 25.9 | 69.8 ± 0.8 | 49 ± 9.6 |
| Cell line | 5mm | 5nn | 5 -FU | Cisplatin | ||||
|---|---|---|---|---|---|---|---|---|
| 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | |
| HEL 299 | 11.7 ± 3.1 | 13.9 ± 0.1 | 9.5 ± 6.3 | 6.3 ± 8.5 | 64.6 ± 3.2 | 12.1 ± 84.1 | 57.8 ± 0.3 | 37.5 ± 4.1 |
| BEAS-2B | 16.2 ± 7.2 | 1.5 ± 22.7 | 19.2 ± 12.4 | 5.8 ± 17.3 | 57.5 ± 9 | 4.5 ± 16 | 82.4 ± 0.2 | 39.2 ± 14.9 |
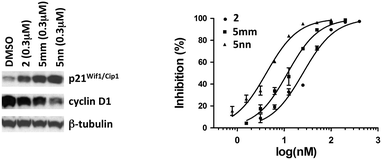
Variability in a MAT2A inhibition assay using a molybdate colorimetric assay for phosphate12 made the measurement of cell-cycle inhibition a preferred analytical tool for assessing the potency of diarylacetylenes. We tested the effect of these heterocyclic-substituted diphenylacetylenes 4 (Table 1) and fluorinated phenylethynyl-substituted heterocycles 5 (Table 2) on the proliferation of LS174T colon cancer cells. As shown in the western blot (Fig. 4), the most active compounds (i.e., 2, 5mm and 5nn) inhibited cyclin D1 at 300 nM concentration and, as expected for a cell-cycle inhibitor, induced cyclin-dependent kinase inhibitor p21Wif1/Cip1 at the same time. Consistent with results in prior studies,13,23 the phenylethynyl-substituted heterocycles 5 lacking fluorine substituents or possessing only one fluorine substituent at the ortho-position relative to the acetylenic linkage had lower potency than the 2,6-difluorinated counterparts (data not shown). Unlike the diarylacetylene series in which the N-methylanilino and N,N-dimethylanilino analogs of 2 showed comparable activity in inhibiting LS174T colon cancer cell proliferation (i.e., 2, IC50 = 25.1 ± 1.3 nM), the N-methylation pattern in the phenylethynyl-substituted heterocycles indicated that the N-methylaminoisoquinoline analog 5nn (IC50 = 4.2 ± 0.2 nM) was more potent than the N,N-dimethylaminoisoquinoline analog 5mm (IC50 = 11.8 ± 1.5 nM) (Fig. 4).
Conclusions
Phenylethynyl-substituted heterocycles 5 inhibited the proliferation of LS174T colon cancer cells in which the inhibition of cyclin D1 and induction of p21Wif1/Cip1 served as readouts for antineoplastic activity. At a molecular level, these agents act directly on the catalytic subunit of methionine S-adenosyltransferase-2 (MAT2A), consistent with prior findings using halogenated stilbenes.11–13 The absence of isomerization in 5 relative to stilbenes and the improved potency, and physical properties suggest that phenylethynyl-substituted heterocycles repress colon cancer proliferation through an epigenetic mechanism involving an alteration in histone methylation patterns. Future efforts will focus on the identification of the genes regulated by MAT2A inhibitors and the detailed molecular mechanisms that regulate the selectivity of these MAT2A inhibitors against different cell lines.Experimental
Chemicals were purchased from Sigma Aldrich or Fisher Scientific or were synthesized according to literature procedures. Solvents were acquired from commercial vendors and used without further purification unless otherwise noted. Nuclear magnetic resonance spectra were determined on Varian (1H, 400 MHz; 13C, 101 MHz) and Bruker (1H, 700 MHz; 13C, 176 MHz) instruments. High-resolution electrospray ionization (ESI) mass spectra were recorded on an LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The FT resolution was set at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (at 400 m/z). Samples were introduced through direct infusion using a syringe pump with a flow rate of 5 μL min−1. MALDI mass spectra were obtained on a Bruker ultrafleXtreme time-of-flight mass spectrometer (Billerica, MA), using a DHB (2,5-dihydroxybenzoic acid) matrix. The purity of compounds was established by combustion analyses by Atlantic Microlabs, Inc., Norcross, GA. The compounds were chromatographed on preparative layer Merck silica gel F254 unless otherwise indicated.
000 (at 400 m/z). Samples were introduced through direct infusion using a syringe pump with a flow rate of 5 μL min−1. MALDI mass spectra were obtained on a Bruker ultrafleXtreme time-of-flight mass spectrometer (Billerica, MA), using a DHB (2,5-dihydroxybenzoic acid) matrix. The purity of compounds was established by combustion analyses by Atlantic Microlabs, Inc., Norcross, GA. The compounds were chromatographed on preparative layer Merck silica gel F254 unless otherwise indicated.
General procedure for the Sonogashira coupling of 2,6-difluorophenylacetylene with aryl iodides or heteroaryl iodides
To a mixture of 2.1 mmol of an aryl iodide or heteroaryl iodide, 3 mmol of diisopropylethylamine, 0.02 mmol of Pd(PPh3)4, and 0.02 mmol of CuI in 7 mL of water was added 2 mmol of 2,6-difluorophenylacetylene. The mixture was stirred for 1–2 h at 75 °C. The mixture was cooled, and the product was collected by filtration or extraction using dichloromethane. The products 4 or 5 were purified by recrystallization and/or chromatography as noted below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane (Rf = 0.52); mp 148–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.58 (d, J = 8.5 Hz, 2H), 7.56–7.47 (m, 1H), 7.40 (d, J = 8.5 Hz, 2H), 7.26 (t, J = 8 Hz, 2H), 3.64 (t, J = 5.6 Hz, 2H), 2.42 (t, J = 6.3 Hz, 2H), 1.94–1.77 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 169.01, 162.01 (dd, J = 5.2, 251.5 Hz, 2C), 144.53, 131.78 (2C), 131.39 (t, J = 10 Hz), 126.19 (2C), 118.35, 111.94 (dd, J = 5.3, 18.2 Hz, 2C), 100.77 (t, J = 19.8 Hz), 98.63 (t, J = 3.1 Hz), 75.77, 50.29, 32.70, 22.91, 20.79. HRMS (ESI) calcd for C19H16F2NO [MH+]: 312.1194. Found: 312.1195. Anal. calcd for C19H15F2NO: C, 73.30; H, 4.86; N, 4.50. Found: C, 73.04; H, 4.85; N, 4.52.
10 methanol–dichloromethane (Rf = 0.52); mp 148–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.58 (d, J = 8.5 Hz, 2H), 7.56–7.47 (m, 1H), 7.40 (d, J = 8.5 Hz, 2H), 7.26 (t, J = 8 Hz, 2H), 3.64 (t, J = 5.6 Hz, 2H), 2.42 (t, J = 6.3 Hz, 2H), 1.94–1.77 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 169.01, 162.01 (dd, J = 5.2, 251.5 Hz, 2C), 144.53, 131.78 (2C), 131.39 (t, J = 10 Hz), 126.19 (2C), 118.35, 111.94 (dd, J = 5.3, 18.2 Hz, 2C), 100.77 (t, J = 19.8 Hz), 98.63 (t, J = 3.1 Hz), 75.77, 50.29, 32.70, 22.91, 20.79. HRMS (ESI) calcd for C19H16F2NO [MH+]: 312.1194. Found: 312.1195. Anal. calcd for C19H15F2NO: C, 73.30; H, 4.86; N, 4.50. Found: C, 73.04; H, 4.85; N, 4.52.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane and recrystallized from hexane; mp 144–145 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53–7.44 (m, 1H), 7.42 (d, J = 8.9 Hz, 2H), 7.22 (t, J = 7.9 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 3.73 (t, J = 4.8 Hz, 4H), 3.2 (t, J = 4.9 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 161.85 (dd, J = 5.3, 250.6 Hz, 2C), 151.36, 132.57 (2C), 130.51 (t, J = 10.1 Hz), 114.35 (2C), 111.89 (dd, J = 5.4, 250.6 Hz, 2C), 110.38, 101.55 (t, J = 19.9 Hz), 100.09 (t, J = 3 Hz), 74.28, 65.91 (2C), 47.21 (2C). HRMS (ESI) calcd for C18H16F2NO [MH+]: 300.1194. Found: 300.1195. Anal. calcd for C18H15F2NO: C, 72.23; H, 5.05; N, 4.68. Found: C, 71.98; H, 5.09; N, 4.72.
5 ethyl acetate–hexane and recrystallized from hexane; mp 144–145 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53–7.44 (m, 1H), 7.42 (d, J = 8.9 Hz, 2H), 7.22 (t, J = 7.9 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 3.73 (t, J = 4.8 Hz, 4H), 3.2 (t, J = 4.9 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 161.85 (dd, J = 5.3, 250.6 Hz, 2C), 151.36, 132.57 (2C), 130.51 (t, J = 10.1 Hz), 114.35 (2C), 111.89 (dd, J = 5.4, 250.6 Hz, 2C), 110.38, 101.55 (t, J = 19.9 Hz), 100.09 (t, J = 3 Hz), 74.28, 65.91 (2C), 47.21 (2C). HRMS (ESI) calcd for C18H16F2NO [MH+]: 300.1194. Found: 300.1195. Anal. calcd for C18H15F2NO: C, 72.23; H, 5.05; N, 4.68. Found: C, 71.98; H, 5.09; N, 4.72.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane (Rf = 0.43); mp 126–128 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53–7.42 (m, 1H), 7.39 (d, J = 8.8 Hz, 2H), 7.22 (t, J = 7.9 Hz, 2H), 6.96 (d, J = 8.9 Hz, 2H), 3.24 (t, J = 4.9 Hz, 4H), 2.43 (t, J = 5.1 Hz, 4H), 2.21 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82 (dd, J = 5.4, 250.6 Hz, 2C), 151.21, 132.56 (2C), 130.43 (t, J = 10.1 Hz), 114.49 (2C), 111.8 (dd, J = 5.3, 18.2 Hz, 2C), 109.85, 101.59 (t, J = 19.9 Hz), 100.22 (t, J = 3 Hz), 74.17, 54.35 (2C), 46.87 (2C), 45.73. HRMS (ESI) calcd for C19H19F2N2 [MH+]: 313.1511. Found: 313.1489. Anal. calcd for C19H18F2N2: C, 73.06; H, 5.81; N, 8.97. Found: C, 72.84; H, 5.75; N, 8.89.
10 methanol–dichloromethane (Rf = 0.43); mp 126–128 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53–7.42 (m, 1H), 7.39 (d, J = 8.8 Hz, 2H), 7.22 (t, J = 7.9 Hz, 2H), 6.96 (d, J = 8.9 Hz, 2H), 3.24 (t, J = 4.9 Hz, 4H), 2.43 (t, J = 5.1 Hz, 4H), 2.21 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82 (dd, J = 5.4, 250.6 Hz, 2C), 151.21, 132.56 (2C), 130.43 (t, J = 10.1 Hz), 114.49 (2C), 111.8 (dd, J = 5.3, 18.2 Hz, 2C), 109.85, 101.59 (t, J = 19.9 Hz), 100.22 (t, J = 3 Hz), 74.17, 54.35 (2C), 46.87 (2C), 45.73. HRMS (ESI) calcd for C19H19F2N2 [MH+]: 313.1511. Found: 313.1489. Anal. calcd for C19H18F2N2: C, 73.06; H, 5.81; N, 8.97. Found: C, 72.84; H, 5.75; N, 8.89.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.68); mp 122–124 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.76–7.61 (m, 4H), 7.60–7.49 (m, 1H), 7.48 (t, J = 2.2 Hz, 2H), 7.32–7.17 (m, 2H), 6.31 (t, J = 2.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.01 (dd, J = 5, 251.5 Hz, two C), 140.3, 132.98 (2C), 131.36 (t, J = 10.1 Hz), 119.15 (2C), 118.92 (2C), 117.61, 111.93 (dd, J = 5.4, 18.3 Hz, 2C), 111.19 (2C), 100.91 (t, J = 19.7 Hz), 98.45 (t, J = 3 Hz), 76.09. HRMS (ESI) calcd for C18H12F2N [MH+]: 280.0932. Found: 280.0924. Anal. calcd for C18H11F2N: C, 77.41; H, 3.97; N, 5.02. Found: C, 77.20; H, 4.21; N, 4.89.
5 ethyl acetate–hexane (Rf = 0.68); mp 122–124 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.76–7.61 (m, 4H), 7.60–7.49 (m, 1H), 7.48 (t, J = 2.2 Hz, 2H), 7.32–7.17 (m, 2H), 6.31 (t, J = 2.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.01 (dd, J = 5, 251.5 Hz, two C), 140.3, 132.98 (2C), 131.36 (t, J = 10.1 Hz), 119.15 (2C), 118.92 (2C), 117.61, 111.93 (dd, J = 5.4, 18.3 Hz, 2C), 111.19 (2C), 100.91 (t, J = 19.7 Hz), 98.45 (t, J = 3 Hz), 76.09. HRMS (ESI) calcd for C18H12F2N [MH+]: 280.0932. Found: 280.0924. Anal. calcd for C18H11F2N: C, 77.41; H, 3.97; N, 5.02. Found: C, 77.20; H, 4.21; N, 4.89.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate–hexane; mp 124–126 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.71 (d, J = 8.4 Hz, 2H), 7.62–7.51 (m, 1H), 7.36 (d, J = 8.5 Hz, 2H), 7.30–7.25 (m, 2H), 5.83 (s, 2H), 2.00 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 162.07 (dd, J = 5.2, 251.8 Hz, 2C), 139.2, 132.43 (2C), 131.66 (t, J = 10.2 Hz), 128.49 (2C), 127.55 (2C), 120.42, 111.96 (dd, J = 5.3, 19 Hz, 2C), 106.48 (2C), 100.69 (t, J = 19.7 Hz), 98.05 (t, J = 3 Hz), 76.71, 12.86 (2C). HRMS (ESI) calcd for C20H16F2N [MH+]: 308.1245. Found: 308.1229. Anal. calcd for C20H15F2N: C, 78.16; H, 4.92; N, 4.56. Found: C, 77.91; H, 5.03; N, 4.42.
10 ethyl acetate–hexane; mp 124–126 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.71 (d, J = 8.4 Hz, 2H), 7.62–7.51 (m, 1H), 7.36 (d, J = 8.5 Hz, 2H), 7.30–7.25 (m, 2H), 5.83 (s, 2H), 2.00 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 162.07 (dd, J = 5.2, 251.8 Hz, 2C), 139.2, 132.43 (2C), 131.66 (t, J = 10.2 Hz), 128.49 (2C), 127.55 (2C), 120.42, 111.96 (dd, J = 5.3, 19 Hz, 2C), 106.48 (2C), 100.69 (t, J = 19.7 Hz), 98.05 (t, J = 3 Hz), 76.71, 12.86 (2C). HRMS (ESI) calcd for C20H16F2N [MH+]: 308.1245. Found: 308.1229. Anal. calcd for C20H15F2N: C, 78.16; H, 4.92; N, 4.56. Found: C, 77.91; H, 5.03; N, 4.42.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane (Rf = 0.53); mp 120–122 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 3 Hz, 1H), 7.55–7.46 (m, 1H), 7.44 (d, J = 8.7 Hz, 1H), 7.29–7.2 (m, 2H), 7.06 (dd, J = 3.1, 8.8 Hz, 1H), 3 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 162.01 (dd, J = 5.3, 251.2 Hz, 2C), 145.52, 134.97, 130.95 (t, J = 10.1 Hz), 127.79, 127.47, 117.6, 111.96 (dd, J = 5.3, 18.2 Hz, 2C), 101.11 (t, J = 19.8 Hz), 99.79 (t, J = 3.1 Hz), 72.88, 39.33 (2C). HRMS (ESI) calcd for C15H13F2N2 [MH+]: 259.1041. Found: 259.1023. Anal. calcd for C15H12F2N2: C, 69.76; H, 4.68; N, 10.85. Found: C, 69.61; H, 4.85; N, 10.83.
2 ethyl acetate–hexane (Rf = 0.53); mp 120–122 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 3 Hz, 1H), 7.55–7.46 (m, 1H), 7.44 (d, J = 8.7 Hz, 1H), 7.29–7.2 (m, 2H), 7.06 (dd, J = 3.1, 8.8 Hz, 1H), 3 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 162.01 (dd, J = 5.3, 251.2 Hz, 2C), 145.52, 134.97, 130.95 (t, J = 10.1 Hz), 127.79, 127.47, 117.6, 111.96 (dd, J = 5.3, 18.2 Hz, 2C), 101.11 (t, J = 19.8 Hz), 99.79 (t, J = 3.1 Hz), 72.88, 39.33 (2C). HRMS (ESI) calcd for C15H13F2N2 [MH+]: 259.1041. Found: 259.1023. Anal. calcd for C15H12F2N2: C, 69.76; H, 4.68; N, 10.85. Found: C, 69.61; H, 4.85; N, 10.83.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.41); mp 110–112 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.29 (d, J = 2.1 Hz, 1H), 7.64 (dd, J = 2.4, 8.9 Hz, 1H), 7.53–7.43 (m, 1H), 7.22 (t, J = 7.9 Hz, 2H), 6.68 (d, J = 8.9 Hz, 1H), 3.08 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.74 (dd, J = 5.2, 250.7 Hz, 2C), 158.12, 150.93, 139.51, 130.56 (t, J = 10.1 Hz), 111.81 (dd, J = 3.9, 20.2 Hz, 2C), 105.54, 104.57, 101.48 (t, J = 19.8 Hz), 98.02 (t, J = 3 Hz), 76.11, 37.52 (2C). HRMS (ESI) calcd for C15H13F2N2 [MH+]: 259.1041. Found: 259.1023. Anal. calcd for C15H12F2N2: C, 69.76; H, 4.68; N, 10.85. Found: C, 69.49; H, 4.81; N, 10.76.
5 ethyl acetate–hexane (Rf = 0.41); mp 110–112 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.29 (d, J = 2.1 Hz, 1H), 7.64 (dd, J = 2.4, 8.9 Hz, 1H), 7.53–7.43 (m, 1H), 7.22 (t, J = 7.9 Hz, 2H), 6.68 (d, J = 8.9 Hz, 1H), 3.08 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.74 (dd, J = 5.2, 250.7 Hz, 2C), 158.12, 150.93, 139.51, 130.56 (t, J = 10.1 Hz), 111.81 (dd, J = 3.9, 20.2 Hz, 2C), 105.54, 104.57, 101.48 (t, J = 19.8 Hz), 98.02 (t, J = 3 Hz), 76.11, 37.52 (2C). HRMS (ESI) calcd for C15H13F2N2 [MH+]: 259.1041. Found: 259.1023. Anal. calcd for C15H12F2N2: C, 69.76; H, 4.68; N, 10.85. Found: C, 69.49; H, 4.81; N, 10.76.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.47) and recrystallization from abs. ethanol; mp 121–123 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.63 (d, J = 2.2 Hz, 1H), 8.12 (dd, J = 2.3, 8.6 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.73 (t, J = 2.3 Hz, 2H), 7.62–7.51 (m, 1H), 7.28 (t, J = 8 Hz, 2H), 6.34 (t, J = 2.3 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.02 (dd, J = 5, 252 Hz, 2C), 151.04, 150.19, 141.74, 131.8 (t, J = 10.1 Hz), 118.35 (2C), 114.63, 112.07 (m, 4C), 111.27, 100.54 (t, J = 19.7 Hz), 95.5 (t, J = 2.9 Hz), 78.6. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 280.0885. Found: 280.0886. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.74; H, 3.69; N, 9.84.
5 ethyl acetate–hexane (Rf = 0.47) and recrystallization from abs. ethanol; mp 121–123 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.63 (d, J = 2.2 Hz, 1H), 8.12 (dd, J = 2.3, 8.6 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.73 (t, J = 2.3 Hz, 2H), 7.62–7.51 (m, 1H), 7.28 (t, J = 8 Hz, 2H), 6.34 (t, J = 2.3 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.02 (dd, J = 5, 252 Hz, 2C), 151.04, 150.19, 141.74, 131.8 (t, J = 10.1 Hz), 118.35 (2C), 114.63, 112.07 (m, 4C), 111.27, 100.54 (t, J = 19.7 Hz), 95.5 (t, J = 2.9 Hz), 78.6. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 280.0885. Found: 280.0886. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.74; H, 3.69; N, 9.84.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.3 (d, J = 1.4 Hz, 1H), 8.19 (d, J = 1.4 Hz, 1H), 7.52 (m, 1H), 7.25 (t, J = 8 Hz, 2H), 3.13 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 161.93 (dd, J = 251.5, 5.2 Hz, 2C), 152.96, 145.35, 131.25 (t, J = 10.1 Hz), 130.64, 123.25, 111.93 (dd, J = 5.2, 18.6 Hz, 2C), 100.85 (t, J = 19.8 Hz), 97.31 (t, J = 3 Hz), 75.74, 37.2 (2C). HRMS (ESI) calcd for C14H12F2N3 [MH+]: 260.0994. Found: 260.0994. Anal. calcd for C14H11F2N3: C, 64.86; H, 4.28; N, 16.21. Found: C, 64.63; H, 4.25; N, 16.39.
10 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.3 (d, J = 1.4 Hz, 1H), 8.19 (d, J = 1.4 Hz, 1H), 7.52 (m, 1H), 7.25 (t, J = 8 Hz, 2H), 3.13 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 161.93 (dd, J = 251.5, 5.2 Hz, 2C), 152.96, 145.35, 131.25 (t, J = 10.1 Hz), 130.64, 123.25, 111.93 (dd, J = 5.2, 18.6 Hz, 2C), 100.85 (t, J = 19.8 Hz), 97.31 (t, J = 3 Hz), 75.74, 37.2 (2C). HRMS (ESI) calcd for C14H12F2N3 [MH+]: 260.0994. Found: 260.0994. Anal. calcd for C14H11F2N3: C, 64.86; H, 4.28; N, 16.21. Found: C, 64.63; H, 4.25; N, 16.39.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.44); mp 131–133 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.4 (s, 1H), 7.82 (s, 1H), 7.52–7.44 (m, 3H), 7.27 (dd, J = 1.6, 8.5 Hz, 1H), 7.23 (t, J = 8 Hz, 2H), 6.51–6.49 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 161.94 (dd, J = 5.4, 250.5 Hz, 2C), 136.08, 130.44 (t, J = 10.1 Hz), 127.63, 126.95, 124.24, 124.18, 112.06, 111.81 (dd, J = 5.4, 18.3 Hz, 2C), 111.35, 101.69 (t, J = 19.7 Hz), 101.59, 101.49 (t, J = 3 Hz), 73.26. HRMS (ESI) calcd for C16H10F2N [MH+]: 254.0776. Found: 254.0760. Anal. calcd for C16H9F2N: C, 75.88; H, 3.58; N, 5.53. Found: C, 76.07; H, 3.63; N, 5.41.
5 ethyl acetate–hexane (Rf = 0.44); mp 131–133 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.4 (s, 1H), 7.82 (s, 1H), 7.52–7.44 (m, 3H), 7.27 (dd, J = 1.6, 8.5 Hz, 1H), 7.23 (t, J = 8 Hz, 2H), 6.51–6.49 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 161.94 (dd, J = 5.4, 250.5 Hz, 2C), 136.08, 130.44 (t, J = 10.1 Hz), 127.63, 126.95, 124.24, 124.18, 112.06, 111.81 (dd, J = 5.4, 18.3 Hz, 2C), 111.35, 101.69 (t, J = 19.7 Hz), 101.59, 101.49 (t, J = 3 Hz), 73.26. HRMS (ESI) calcd for C16H10F2N [MH+]: 254.0776. Found: 254.0760. Anal. calcd for C16H9F2N: C, 75.88; H, 3.58; N, 5.53. Found: C, 76.07; H, 3.63; N, 5.41.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane; mp 100–102 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 1.6 Hz, 1H), 7.55–7.45 (m, 2H), 7.43 (d, J = 3 Hz, 1H), 7.33 (dd, J = 1.6, 8.5 Hz, 1H), 7.24 (t, J = 7.9 Hz, 2H), 6.5 (d, J = 2.6 Hz, 1H), 3.82 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.94 (dd, J = 5.4, 250.6 Hz, 2C), 136.45, 131.28, 130.51 (t, J = 10.1 Hz), 127.96, 124.41, 124.24, 111.83 (dd, J = 5.3, 19.6 Hz, 2C), 111.45, 110.42, 101.63 (t, J = 20.0 Hz), 101.28 (t, J = 3 Hz), 100.92, 73.52, 32.63. HRMS (ESI) calcd for C17H12F2N [MH+]: 268.0932. Found: 268.0917. Anal. calcd for C17H11F2N: C, 76.39; H, 4.15; N, 5.24. Found: C, 76.17; H, 4.31; N, 5.04.
2 ethyl acetate–hexane; mp 100–102 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 1.6 Hz, 1H), 7.55–7.45 (m, 2H), 7.43 (d, J = 3 Hz, 1H), 7.33 (dd, J = 1.6, 8.5 Hz, 1H), 7.24 (t, J = 7.9 Hz, 2H), 6.5 (d, J = 2.6 Hz, 1H), 3.82 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.94 (dd, J = 5.4, 250.6 Hz, 2C), 136.45, 131.28, 130.51 (t, J = 10.1 Hz), 127.96, 124.41, 124.24, 111.83 (dd, J = 5.3, 19.6 Hz, 2C), 111.45, 110.42, 101.63 (t, J = 20.0 Hz), 101.28 (t, J = 3 Hz), 100.92, 73.52, 32.63. HRMS (ESI) calcd for C17H12F2N [MH+]: 268.0932. Found: 268.0917. Anal. calcd for C17H11F2N: C, 76.39; H, 4.15; N, 5.24. Found: C, 76.17; H, 4.31; N, 5.04.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane; mp 178–179 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 8.11 (s, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.63–7.52 (m, 1H), 7.47–7.37 (m, 2H), 7.3 (t, J = 8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.05 (dd, J = 5.2, 251.8 Hz, 2C), 139.64, 132.2, 131.67 (t, J = 10.2 Hz), 126.16, 124.5, 123.35, 112.98, 112.22, 112.05 (dd, J = 5.3, 18.2 Hz, 2C), 100.9 (t, J = 19.7 Hz), 96.74 (t, J = 3 Hz), 79.12. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0712. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.60; H, 3.25; N, 10.92.
2 ethyl acetate–hexane; mp 178–179 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (s, 1H), 8.11 (s, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.63–7.52 (m, 1H), 7.47–7.37 (m, 2H), 7.3 (t, J = 8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.05 (dd, J = 5.2, 251.8 Hz, 2C), 139.64, 132.2, 131.67 (t, J = 10.2 Hz), 126.16, 124.5, 123.35, 112.98, 112.22, 112.05 (dd, J = 5.3, 18.2 Hz, 2C), 100.9 (t, J = 19.7 Hz), 96.74 (t, J = 3 Hz), 79.12. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0712. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.60; H, 3.25; N, 10.92.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane (Rf = 0.63); mp 193–195 °C. 1H NMR (700 MHz, DMSO-d6) δ 13.34 (s, 1H), 8.15 (s, 1H), 8.08 (s, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.54–7.45 (m, 2H), 7.25 (t, J = 7.9 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.01 (dd, J = 5.2, 251 Hz, 2C), 139.52, 134.15, 130.94 (t, J = 10.1 Hz), 128.85, 124.98, 122.82, 112.96, 111.88 (dd, J = 3.8, 20.2 Hz, 2C), 110.94, 101.24 (t, J = 19.8 Hz), 100.03 (t, J = 3 Hz), 74.15. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0727. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.64; H, 3.22; N, 11.09.
2 ethyl acetate–hexane (Rf = 0.63); mp 193–195 °C. 1H NMR (700 MHz, DMSO-d6) δ 13.34 (s, 1H), 8.15 (s, 1H), 8.08 (s, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.54–7.45 (m, 2H), 7.25 (t, J = 7.9 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.01 (dd, J = 5.2, 251 Hz, 2C), 139.52, 134.15, 130.94 (t, J = 10.1 Hz), 128.85, 124.98, 122.82, 112.96, 111.88 (dd, J = 3.8, 20.2 Hz, 2C), 110.94, 101.24 (t, J = 19.8 Hz), 100.03 (t, J = 3 Hz), 74.15. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0727. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.64; H, 3.22; N, 11.09.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate–hexane; mp 192–194 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.31 (s, 1H), 8.15 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.77 (s, J = 1.2 Hz, 1H), 7.6–7.48 (m, 1H), 7.33–7.21 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.09 (dd, J = 5.2, 251.5 Hz, 2C), 139.31, 133.87, 131.44 (t, J = 10.2 Hz), 123.20, 123.04, 121.37, 118.43, 113.63, 111.99 (dd, J = 5.3, 19 Hz, 2C), 100.94 (t, J = 19.7 Hz), 99.67 (t, J = 3.1 Hz), 75.66. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0712. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.61; H, 3.06; N, 10.88.
1 ethyl acetate–hexane; mp 192–194 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.31 (s, 1H), 8.15 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.77 (s, J = 1.2 Hz, 1H), 7.6–7.48 (m, 1H), 7.33–7.21 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.09 (dd, J = 5.2, 251.5 Hz, 2C), 139.31, 133.87, 131.44 (t, J = 10.2 Hz), 123.20, 123.04, 121.37, 118.43, 113.63, 111.99 (dd, J = 5.3, 19 Hz, 2C), 100.94 (t, J = 19.7 Hz), 99.67 (t, J = 3.1 Hz), 75.66. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0712. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.61; H, 3.06; N, 10.88.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.31); mp 107–109 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.12 (d, J = 0.9 Hz, 1H), 8.06 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.55 (dd, J = 1.5, 8.7 Hz, 1H), 7.53–7.48 (m, 1H), 7.25 (t, J = 7.9 Hz, 2H), 4.08 (s, 3H). 13C NMR (176 MHz, DMSO-d6) δ 162.01 (dd, J = 5.2, 251.0 Hz, 2C), 139.19, 133.06, 130.98 (t, J = 10 Hz), 128.78, 125.17, 123.39, 113.04, 111.89 (dd, J = 3.8, 20.2 Hz, 2C), 110.54, 101.2 (t, J = 19.8 Hz), 99.87 (t, J = 3 Hz), 74.38, 35.53. HRMS (ESI) calcd for C16H11F2N2 [MH+]: 269.0885. Found: 269.0885. Anal. calcd for C16H10F2N2: C, 71.64; H, 3.76; N, 10.44. Found: C, 71.41; H, 3.75; N, 10.53.
5 ethyl acetate–hexane (Rf = 0.31); mp 107–109 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.12 (d, J = 0.9 Hz, 1H), 8.06 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.55 (dd, J = 1.5, 8.7 Hz, 1H), 7.53–7.48 (m, 1H), 7.25 (t, J = 7.9 Hz, 2H), 4.08 (s, 3H). 13C NMR (176 MHz, DMSO-d6) δ 162.01 (dd, J = 5.2, 251.0 Hz, 2C), 139.19, 133.06, 130.98 (t, J = 10 Hz), 128.78, 125.17, 123.39, 113.04, 111.89 (dd, J = 3.8, 20.2 Hz, 2C), 110.54, 101.2 (t, J = 19.8 Hz), 99.87 (t, J = 3 Hz), 74.38, 35.53. HRMS (ESI) calcd for C16H11F2N2 [MH+]: 269.0885. Found: 269.0885. Anal. calcd for C16H10F2N2: C, 71.64; H, 3.76; N, 10.44. Found: C, 71.41; H, 3.75; N, 10.53.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.35); mp 117–119 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.12 (s, 1H), 7.99 (s, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.63–7.47 (m, 1H), 7.32–7.2 (m, 3H), 4.09 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.09 (dd, J = 5.2, 251.6 Hz, 2C), 139.09, 132.68, 131.44 (t, J = 10.2 Hz), 123.56, 123.20, 121.49, 118.42, 113.46, 111.94 (dd, J = 5.2, 18.6 Hz, 2C), 100.88 (t, J = 19.7 Hz), 99.65 (t, J = 3 Hz), 75.81, 35.58. HRMS (ESI) calcd for C16H11F2N2 [MH+]: 269.0885. Found: 269.0886. Anal. calcd for C16H10F2N2: C, 71.64; H, 3.76; N, 10.44. Found: C, 71.48; H, 3.83; N, 10.39.
5 ethyl acetate–hexane (Rf = 0.35); mp 117–119 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.12 (s, 1H), 7.99 (s, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.63–7.47 (m, 1H), 7.32–7.2 (m, 3H), 4.09 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.09 (dd, J = 5.2, 251.6 Hz, 2C), 139.09, 132.68, 131.44 (t, J = 10.2 Hz), 123.56, 123.20, 121.49, 118.42, 113.46, 111.94 (dd, J = 5.2, 18.6 Hz, 2C), 100.88 (t, J = 19.7 Hz), 99.65 (t, J = 3 Hz), 75.81, 35.58. HRMS (ESI) calcd for C16H11F2N2 [MH+]: 269.0885. Found: 269.0886. Anal. calcd for C16H10F2N2: C, 71.64; H, 3.76; N, 10.44. Found: C, 71.48; H, 3.83; N, 10.39.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane; mp 192–194 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.99 (s, 1H), 8.41 (d, J = 2 Hz, 1H), 8.22 (d, J = 2 Hz, 1H), 7.59 (t, J = 3 Hz, 1H), 7.57–7.47 (m, 1H), 7.26 (t, J = 8 Hz, 2H), 6.52 (dd, J = 1.8, 3.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 161.95 (dd, J = 5.2, 251.2 Hz, 2C), 147.80, 145.04, 131.21, 131.02 (t, J = 10.3 Hz), 127.83, 119.20, 111.76 (dd, J = 5, 19 Hz, 2C), 109.53, 101.29 (t, J = 19.9 Hz), 100.42, 98.22 (t, J = 3 Hz), 75.88. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0710. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.57; H, 3.17; N, 10.88.
2 ethyl acetate–hexane; mp 192–194 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.99 (s, 1H), 8.41 (d, J = 2 Hz, 1H), 8.22 (d, J = 2 Hz, 1H), 7.59 (t, J = 3 Hz, 1H), 7.57–7.47 (m, 1H), 7.26 (t, J = 8 Hz, 2H), 6.52 (dd, J = 1.8, 3.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 161.95 (dd, J = 5.2, 251.2 Hz, 2C), 147.80, 145.04, 131.21, 131.02 (t, J = 10.3 Hz), 127.83, 119.20, 111.76 (dd, J = 5, 19 Hz, 2C), 109.53, 101.29 (t, J = 19.9 Hz), 100.42, 98.22 (t, J = 3 Hz), 75.88. HRMS (ESI) calcd for C15H9F2N2 [MH+]: 255.0728. Found: 255.0710. Anal. calcd for C15H8F2N2: C, 70.86; H, 3.17; N, 11.02. Found: C, 70.57; H, 3.17; N, 10.88.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 127–129 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (d, J = 8.5 Hz, 1H), 8.09–8.02 (m, 2H), 7.89–7.82 (m, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.72–7.66 (m, 1H), 7.66–7.58 (m, 1H), 7.32 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.44 (dd, J = 5, 252.8 Hz, 2C), 147.66, 141.73, 137.07, 132.57 (t, J = 10.2 Hz), 130.62, 128.75, 128.05, 127.82, 127.15, 124.25, 112.16 (dd, J = 4.6, 19 Hz, 2C), 99.97 (t, J = 19.7 Hz), 98.51 (t, J = 3 Hz), 75.62. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0756. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.73; H, 3.38; N, 5.17.
5 ethyl acetate–hexane; mp 127–129 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (d, J = 8.5 Hz, 1H), 8.09–8.02 (m, 2H), 7.89–7.82 (m, 1H), 7.76 (d, J = 8.4 Hz, 1H), 7.72–7.66 (m, 1H), 7.66–7.58 (m, 1H), 7.32 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.44 (dd, J = 5, 252.8 Hz, 2C), 147.66, 141.73, 137.07, 132.57 (t, J = 10.2 Hz), 130.62, 128.75, 128.05, 127.82, 127.15, 124.25, 112.16 (dd, J = 4.6, 19 Hz, 2C), 99.97 (t, J = 19.7 Hz), 98.51 (t, J = 3 Hz), 75.62. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0756. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.73; H, 3.38; N, 5.17.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate–hexane (Rf = 0.42); mp 115–117 °C. 1H NMR (400 MHz, DMSO-d6) δ 9 (d, J = 2.1 Hz, 1H), 8.72 (d, J = 2.2 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.85 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.64–7.52 (m, 1H), 7.30 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.12 (dd, J = 5, 252.2 Hz, 2C), 151.13, 146.64, 139.13, 132.02 (t, J = 10.2 Hz), 131.04, 128.87, 128.29, 127.7, 126.73, 115.41, 112.06 (dd, J = 5, 18.7 Hz, 2C), 100.46 (t, J = 19.7 Hz), 96.21 (t, J = 3.1 Hz), 78.9. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.50; N, 5.11.
10 ethyl acetate–hexane (Rf = 0.42); mp 115–117 °C. 1H NMR (400 MHz, DMSO-d6) δ 9 (d, J = 2.1 Hz, 1H), 8.72 (d, J = 2.2 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.85 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.64–7.52 (m, 1H), 7.30 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.12 (dd, J = 5, 252.2 Hz, 2C), 151.13, 146.64, 139.13, 132.02 (t, J = 10.2 Hz), 131.04, 128.87, 128.29, 127.7, 126.73, 115.41, 112.06 (dd, J = 5, 18.7 Hz, 2C), 100.46 (t, J = 19.7 Hz), 96.21 (t, J = 3.1 Hz), 78.9. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.50; N, 5.11.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 101–102 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.97 (d, J = 4.4 Hz, 1H), 8.29 (d, J = 8.1 Hz, 1H), 8.12 (d, J = 8.3 Hz, 1H), 7.95–7.84 (m, 1H), 7.83–7.75 (m, 2H), 7.70–7.58 (m, 1H), 7.34 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.22 (dd, J = 4.9, 252.9 Hz, 2C), 150.26, 147.50, 132.8 (t, J = 10.2 Hz), 130.44, 129.78, 128.15, 127.1, 126.35, 124.97, 123.86, 112.21 (dd, J = 5.3, 19 Hz, 2C), 100.06 (t, J = 19.8 Hz), 94.17 (t, J = 3 Hz), 84.92. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.21; H, 3.43; N, 5.24.
5 ethyl acetate–hexane; mp 101–102 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.97 (d, J = 4.4 Hz, 1H), 8.29 (d, J = 8.1 Hz, 1H), 8.12 (d, J = 8.3 Hz, 1H), 7.95–7.84 (m, 1H), 7.83–7.75 (m, 2H), 7.70–7.58 (m, 1H), 7.34 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.22 (dd, J = 4.9, 252.9 Hz, 2C), 150.26, 147.50, 132.8 (t, J = 10.2 Hz), 130.44, 129.78, 128.15, 127.1, 126.35, 124.97, 123.86, 112.21 (dd, J = 5.3, 19 Hz, 2C), 100.06 (t, J = 19.8 Hz), 94.17 (t, J = 3 Hz), 84.92. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.21; H, 3.43; N, 5.24.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 1H), 8.1 (d, J = 8.2 Hz, 1H), 8 (d, J = 8.5 Hz, 1H), 7.93–7.84 (m, 1H), 7.77–7.69 (m, 1H), 7.68–7.56 (m, 1H), 7.31 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.2 (dd, J = 5, 252.8 Hz, 2C), 148.86, 146.09, 143, 132.47 (t, J = 10.1 Hz), 132.3, 128.28, 128.16, 127.84, 126.14, 115.72, 112.17 (dd, J = 4.5, 19 Hz, 2C), 100.22 (t, J = 19.7 Hz), 94.07 (t, J = 2.9 Hz), 82.23. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0368. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.16; H, 2.73; N, 4.38.
5 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 1H), 8.1 (d, J = 8.2 Hz, 1H), 8 (d, J = 8.5 Hz, 1H), 7.93–7.84 (m, 1H), 7.77–7.69 (m, 1H), 7.68–7.56 (m, 1H), 7.31 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.2 (dd, J = 5, 252.8 Hz, 2C), 148.86, 146.09, 143, 132.47 (t, J = 10.1 Hz), 132.3, 128.28, 128.16, 127.84, 126.14, 115.72, 112.17 (dd, J = 4.5, 19 Hz, 2C), 100.22 (t, J = 19.7 Hz), 94.07 (t, J = 2.9 Hz), 82.23. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0368. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.16; H, 2.73; N, 4.38.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate–hexane; mp 184–185 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.54 (d, J = 2.1 Hz, 1H), 8.26 (d, J = 2.1 Hz, 1H), 7.73 (d, J = 9 Hz, 1H), 7.63–7.51 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 7.22 (dd, J = 2.4, 9 Hz, 1H), 6.83 (d, J = 2.5 Hz, 1H), 5.82 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.10 (dd, J = 5.1, 251.9 Hz, 2C), 148.05, 145.57, 140.98, 135.88, 131.76 (t, J = 10.2 Hz), 129.62, 128.76, 123, 115.13, 112.07 (dd, J = 5, 18.7 Hz, 2C), 104.41, 100.8 (t, J = 19.7 Hz), 97.1 (t, J = 3.1 Hz), 78.02. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0864. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.57; H, 3.64; N, 9.84.
1 ethyl acetate–hexane; mp 184–185 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.54 (d, J = 2.1 Hz, 1H), 8.26 (d, J = 2.1 Hz, 1H), 7.73 (d, J = 9 Hz, 1H), 7.63–7.51 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 7.22 (dd, J = 2.4, 9 Hz, 1H), 6.83 (d, J = 2.5 Hz, 1H), 5.82 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.10 (dd, J = 5.1, 251.9 Hz, 2C), 148.05, 145.57, 140.98, 135.88, 131.76 (t, J = 10.2 Hz), 129.62, 128.76, 123, 115.13, 112.07 (dd, J = 5, 18.7 Hz, 2C), 104.41, 100.8 (t, J = 19.7 Hz), 97.1 (t, J = 3.1 Hz), 78.02. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0864. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.57; H, 3.64; N, 9.84.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 142–143 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.03 (d, J = 2 Hz, 1H), 8.77 (d, J = 2.1 Hz, 1H), 8.17 (dd, J = 6.3, 9.1 Hz, 1H), 7.83 (dd, J = 2.5, 10.3 Hz, 1H), 7.66 (td, J = 2.6, 8.9 Hz, 1H), 7.61–7.54 (m, 1H), 7.3 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 163.16 (d, J = 250.1 Hz), 162.12 (dd, J = 5.1, 252.3 Hz, 2C), 152.29, 147.61 (d, J = 13 Hz), 139.24 (d, J = 1.3 Hz), 132.07 (t, J = 10.2 Hz), 131.14 (d, J = 10.3 Hz), 124.09, 118.13 (d, J = 25.5 Hz), 114.95 (d, J = 2.7 Hz), 112.58 (d, J = 20.6 Hz), 112.10 (dd, J = 5.3, 18.9 Hz, 2C), 100.4 (t, J = 19.7 Hz), 95.94 (t, J = 2.9 Hz), 78.94. HRMS (ESI) calcd for C17H9F3N [MH+]: 284.0682. Found: 284.0661. Anal. calcd for C17H8F3N: C, 72.09; H, 2.85; N, 4.95. Found: C, 71.83; H, 2.94; N, 4.70.
5 ethyl acetate–hexane; mp 142–143 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.03 (d, J = 2 Hz, 1H), 8.77 (d, J = 2.1 Hz, 1H), 8.17 (dd, J = 6.3, 9.1 Hz, 1H), 7.83 (dd, J = 2.5, 10.3 Hz, 1H), 7.66 (td, J = 2.6, 8.9 Hz, 1H), 7.61–7.54 (m, 1H), 7.3 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 163.16 (d, J = 250.1 Hz), 162.12 (dd, J = 5.1, 252.3 Hz, 2C), 152.29, 147.61 (d, J = 13 Hz), 139.24 (d, J = 1.3 Hz), 132.07 (t, J = 10.2 Hz), 131.14 (d, J = 10.3 Hz), 124.09, 118.13 (d, J = 25.5 Hz), 114.95 (d, J = 2.7 Hz), 112.58 (d, J = 20.6 Hz), 112.10 (dd, J = 5.3, 18.9 Hz, 2C), 100.4 (t, J = 19.7 Hz), 95.94 (t, J = 2.9 Hz), 78.94. HRMS (ESI) calcd for C17H9F3N [MH+]: 284.0682. Found: 284.0661. Anal. calcd for C17H8F3N: C, 72.09; H, 2.85; N, 4.95. Found: C, 71.83; H, 2.94; N, 4.70.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 methanol–dichloromethane; mp 130–132 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.99 (d, J = 4.4 Hz, 1H), 8.25 (d, J = 8.9 Hz, 1H), 8.17 (d, J = 2.1 Hz, 1H), 7.83 (dd, J = 2.1, 8.9 Hz, 1H), 7.80 (d, J = 4.5 Hz, 1H), 7.7–7.59 (m, 1H), 7.34 (t, J = 8.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.24 (dd, J = 4.9, 253.1 Hz, 2C), 151.62, 147.83, 135.06, 133.04 (t, J = 10.3 Hz), 128.78, 128.42, 127.32, 127.01, 125.05, 124.22, 112.24 (dd, J = 4.5, 19 Hz, 2C), 99.89 (t, J = 19.6 Hz), 93.66 (t, J = 3 Hz), 85.43. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0366. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.96; H, 2.68; N, 4.68.
150 methanol–dichloromethane; mp 130–132 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.99 (d, J = 4.4 Hz, 1H), 8.25 (d, J = 8.9 Hz, 1H), 8.17 (d, J = 2.1 Hz, 1H), 7.83 (dd, J = 2.1, 8.9 Hz, 1H), 7.80 (d, J = 4.5 Hz, 1H), 7.7–7.59 (m, 1H), 7.34 (t, J = 8.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.24 (dd, J = 4.9, 253.1 Hz, 2C), 151.62, 147.83, 135.06, 133.04 (t, J = 10.3 Hz), 128.78, 128.42, 127.32, 127.01, 125.05, 124.22, 112.24 (dd, J = 4.5, 19 Hz, 2C), 99.89 (t, J = 19.6 Hz), 93.66 (t, J = 3 Hz), 85.43. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0366. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.96; H, 2.68; N, 4.68.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate–hexane (Rf = 0.45); mp 98–100 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04–9 (m, 1H), 8.64 (d, J = 8.2 Hz, 1H), 8.15 (dd, J = 2.5, 8.5 Hz, 1H), 7.94 (dd, J = 2.8, 7.2 Hz, 1H), 7.9–7.78 (m, 1H), 7.78–7.69 (m, 1H), 7.66–7.52 (m, 1H), 7.40–7.23 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.06 (dd, J = 5.1, 251.9 Hz, 2C), 151.47, 147.34, 133.17, 131.9 (t, J = 10.2 Hz), 131.13, 131.06, 129.35, 127.62, 122.86, 119.2, 112.06 (dd, J = 5.3, 18.3 Hz, 2C), 100.56 (t), 95.63 (t, J = 3.1 Hz), 81.48. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.80; H, 3.47; N, 5.15.
10 ethyl acetate–hexane (Rf = 0.45); mp 98–100 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04–9 (m, 1H), 8.64 (d, J = 8.2 Hz, 1H), 8.15 (dd, J = 2.5, 8.5 Hz, 1H), 7.94 (dd, J = 2.8, 7.2 Hz, 1H), 7.9–7.78 (m, 1H), 7.78–7.69 (m, 1H), 7.66–7.52 (m, 1H), 7.40–7.23 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.06 (dd, J = 5.1, 251.9 Hz, 2C), 151.47, 147.34, 133.17, 131.9 (t, J = 10.2 Hz), 131.13, 131.06, 129.35, 127.62, 122.86, 119.2, 112.06 (dd, J = 5.3, 18.3 Hz, 2C), 100.56 (t), 95.63 (t, J = 3.1 Hz), 81.48. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.80; H, 3.47; N, 5.15.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane; mp 91–93 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.96 (dd, J = 1.8, 4.2 Hz, 1H), 8.44 (d, J = 8.1 Hz, 1H), 8.33 (d, J = 1.9 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.86 (dd, J = 1.9, 8.7 Hz, 1H), 7.61 (dd, J = 4.2, 8.3 Hz, 1H), 7.59–7.52 (m, 1H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.12 (dd, J = 5.1, 251.9 Hz, 2C), 151.86, 147.35, 136.13, 132.05, 131.74 (t, J = 10.2 Hz), 131.38, 129.7, 127.75, 122.44, 119.23, 112.01 (dd, J = 4.9, 18.7 Hz, 2C), 100.67 (t, J = 19.7 Hz), 98.47 (t, J = 3 Hz), 76.91. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.97; H, 3.33; N, 5.29.
2 ethyl acetate–hexane; mp 91–93 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.96 (dd, J = 1.8, 4.2 Hz, 1H), 8.44 (d, J = 8.1 Hz, 1H), 8.33 (d, J = 1.9 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.86 (dd, J = 1.9, 8.7 Hz, 1H), 7.61 (dd, J = 4.2, 8.3 Hz, 1H), 7.59–7.52 (m, 1H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.12 (dd, J = 5.1, 251.9 Hz, 2C), 151.86, 147.35, 136.13, 132.05, 131.74 (t, J = 10.2 Hz), 131.38, 129.7, 127.75, 122.44, 119.23, 112.01 (dd, J = 4.9, 18.7 Hz, 2C), 100.67 (t, J = 19.7 Hz), 98.47 (t, J = 3 Hz), 76.91. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.97; H, 3.33; N, 5.29.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate–hexane (Rf = 0.49); mp 123–125 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.98 (dd, J = 1.7, 4.2 Hz, 1H), 8.43 (d, J = 8.2 Hz, 1H), 8.21 (d, J = 1.1 Hz, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.73 (dd, J = 1.6, 8.4 Hz, 1H), 7.66–7.5 (m, 2H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.13 (dd, J = 5, 252 Hz, 2C), 151.81, 147.12, 136.01, 132.15, 131.86 (t, J = 10.2 Hz), 129.08, 128.46, 128.24, 122.56, 122.21, 112.7–111.37 (dd, J = 5, 18.6 Hz, 2C), 100.62 (t, J = 19.8 Hz), 98.35 (t, J = 3.1 Hz), 77.65. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.15; H, 3.28; N, 5.31.
10 ethyl acetate–hexane (Rf = 0.49); mp 123–125 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.98 (dd, J = 1.7, 4.2 Hz, 1H), 8.43 (d, J = 8.2 Hz, 1H), 8.21 (d, J = 1.1 Hz, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.73 (dd, J = 1.6, 8.4 Hz, 1H), 7.66–7.5 (m, 2H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.13 (dd, J = 5, 252 Hz, 2C), 151.81, 147.12, 136.01, 132.15, 131.86 (t, J = 10.2 Hz), 129.08, 128.46, 128.24, 122.56, 122.21, 112.7–111.37 (dd, J = 5, 18.6 Hz, 2C), 100.62 (t, J = 19.8 Hz), 98.35 (t, J = 3.1 Hz), 77.65. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.15; H, 3.28; N, 5.31.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 108–110 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04 (dd, J = 1.8, 4.2 Hz, 1H), 8.47 (dd, J = 1.8, 8.3 Hz, 1H), 8.11 (d, J = 8.2 Hz, 1H), 8.08 (dd, J = 1.4, 7.2 Hz, 1H), 7.7–7.62 (m, 2H), 7.6–7.5 (m, 1H), 7.28 (t, J = 8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.07 (dd, J = 5.2, 251.6 Hz, 2C), 151.47, 147.17, 136.71, 134.39, 131.37 (t, J = 10.2 Hz), 130, 128.02, 126.3, 122.36, 121.25, 111.94 (dd, J = 5.3, 19 Hz, 2C), 101.33 (t, J = 19.6 Hz), 97.3 (t, J = 3 Hz), 80.76. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.30; N, 5.29.
5 ethyl acetate–hexane; mp 108–110 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04 (dd, J = 1.8, 4.2 Hz, 1H), 8.47 (dd, J = 1.8, 8.3 Hz, 1H), 8.11 (d, J = 8.2 Hz, 1H), 8.08 (dd, J = 1.4, 7.2 Hz, 1H), 7.7–7.62 (m, 2H), 7.6–7.5 (m, 1H), 7.28 (t, J = 8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.07 (dd, J = 5.2, 251.6 Hz, 2C), 151.47, 147.17, 136.71, 134.39, 131.37 (t, J = 10.2 Hz), 130, 128.02, 126.3, 122.36, 121.25, 111.94 (dd, J = 5.3, 19 Hz, 2C), 101.33 (t, J = 19.6 Hz), 97.3 (t, J = 3 Hz), 80.76. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.30; N, 5.29.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 8.7 Hz, 1H), 8.39 (s, 1H), 8 (d, J = 8.7 Hz, 1H), 7.92 (dd, J = 8.7, 1.6 Hz, 1H), 7.69 (d, J = 8.6 Hz, 1H), 7.63–7.53 (m, 1H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.14 (dd, J = 252, 5.1 Hz, 2C), 151.17, 146.89, 140.01, 132.81, 131.97, 131.93 (t, J = 10.2 Hz), 128.64, 126.69, 123.53, 119.89, 112.06 (dd, J = 5.3, 19 Hz, 2C), 100.54 (t, J = 19.8 Hz), 98.05 (t, J = 3 Hz), 77.38. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0366. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.93; H, 2.70; N, 4.49.
5 ethyl acetate–hexane; mp 137–138 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 8.7 Hz, 1H), 8.39 (s, 1H), 8 (d, J = 8.7 Hz, 1H), 7.92 (dd, J = 8.7, 1.6 Hz, 1H), 7.69 (d, J = 8.6 Hz, 1H), 7.63–7.53 (m, 1H), 7.29 (t, J = 8.1 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.14 (dd, J = 252, 5.1 Hz, 2C), 151.17, 146.89, 140.01, 132.81, 131.97, 131.93 (t, J = 10.2 Hz), 128.64, 126.69, 123.53, 119.89, 112.06 (dd, J = 5.3, 19 Hz, 2C), 100.54 (t, J = 19.8 Hz), 98.05 (t, J = 3 Hz), 77.38. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0366. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.93; H, 2.70; N, 4.49.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.33) and recrystallization from hexane; mp 120–122 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.89 (d, J = 4.7 Hz, 1H), 8.33 (d, J = 1.8 Hz, 1H), 8.14 (d, J = 8.6 Hz, 1H), 7.96 (dd, J = 1.8, 8.6 Hz, 1H), 7.84 (d, J = 4.7 Hz, 1H), 7.67–7.5 (m, 1H), 7.29 (t, J = 8 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.14 (dd, J = 4.9, 252.3 Hz, 2C), 151.75, 148.13, 140.97, 132.55, 132 (t, J = 10.1 Hz), 130.46, 127.06, 125.49, 122.61, 120.88, 112.00 (dd, J = 3.8, 20.1 Hz, 2C), 100.41 (t, J = 19.7 Hz), 97.85 (t, J = 3 Hz), 77.94. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0389. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.85; H, 2.79; N, 4.53.
5 ethyl acetate–hexane (Rf = 0.33) and recrystallization from hexane; mp 120–122 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.89 (d, J = 4.7 Hz, 1H), 8.33 (d, J = 1.8 Hz, 1H), 8.14 (d, J = 8.6 Hz, 1H), 7.96 (dd, J = 1.8, 8.6 Hz, 1H), 7.84 (d, J = 4.7 Hz, 1H), 7.67–7.5 (m, 1H), 7.29 (t, J = 8 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.14 (dd, J = 4.9, 252.3 Hz, 2C), 151.75, 148.13, 140.97, 132.55, 132 (t, J = 10.1 Hz), 130.46, 127.06, 125.49, 122.61, 120.88, 112.00 (dd, J = 3.8, 20.1 Hz, 2C), 100.41 (t, J = 19.7 Hz), 97.85 (t, J = 3 Hz), 77.94. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0389. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 67.85; H, 2.79; N, 4.53.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane and recrystallized from acetonitrile; mp 148–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.92 (d, J = 4.7 Hz, 1H), 8.28 (d, J = 1.7 Hz, 1H), 8.25 (d, J = 8.7 Hz, 1H), 7.88 (dd, J = 1.7, 8.6 Hz, 1H), 7.84 (d, J = 4.7 Hz, 1H), 7.64–7.52 (m, 1H), 7.30 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.16 (dd, J = 5, 252.3 Hz, 2C), 151.83, 148.07, 141.2, 132.55, 132.16 (t, J = 10.1 Hz), 130.06, 125.83, 124.76, 123.5, 122.66, 112.08 (dd, J = 5.3, 19 Hz, 2C), 100.4 (t, J = 19.7 Hz), 97.62 (t, J = 3 Hz), 78.63. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0365. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.41; H, 2.97; N, 4.42.
5 ethyl acetate–hexane and recrystallized from acetonitrile; mp 148–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.92 (d, J = 4.7 Hz, 1H), 8.28 (d, J = 1.7 Hz, 1H), 8.25 (d, J = 8.7 Hz, 1H), 7.88 (dd, J = 1.7, 8.6 Hz, 1H), 7.84 (d, J = 4.7 Hz, 1H), 7.64–7.52 (m, 1H), 7.30 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.16 (dd, J = 5, 252.3 Hz, 2C), 151.83, 148.07, 141.2, 132.55, 132.16 (t, J = 10.1 Hz), 130.06, 125.83, 124.76, 123.5, 122.66, 112.08 (dd, J = 5.3, 19 Hz, 2C), 100.4 (t, J = 19.7 Hz), 97.62 (t, J = 3 Hz), 78.63. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0365. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.41; H, 2.97; N, 4.42.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane; mp 220–222 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.35 (br s, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.73 (dd, J = 1.7, 8.6 Hz, 1H), 7.61–7.51 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 7.27 (s, 2H), 6.62 (d, J = 5.3 Hz, 1H). 13C NMR (176 MHz, DMSO-d6) δ 162.07 (dd, J = 5, 251.6 Hz, 2C), 152.28, 150.46, 147.61, 131.44, 131.41 (t, J = 10.1 Hz), 128.67, 126.82, 118.06, 116.27, 111.98 (dd, J = 5, 18.6 Hz, 2C), 103.1, 100.91 (t, J = 19.8 Hz), 99.23 (t, J = 3 Hz), 76.01. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0887. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.84; H, 3.52; N, 10.06.
10 methanol–dichloromethane; mp 220–222 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.35 (br s, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.73 (dd, J = 1.7, 8.6 Hz, 1H), 7.61–7.51 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 7.27 (s, 2H), 6.62 (d, J = 5.3 Hz, 1H). 13C NMR (176 MHz, DMSO-d6) δ 162.07 (dd, J = 5, 251.6 Hz, 2C), 152.28, 150.46, 147.61, 131.44, 131.41 (t, J = 10.1 Hz), 128.67, 126.82, 118.06, 116.27, 111.98 (dd, J = 5, 18.6 Hz, 2C), 103.1, 100.91 (t, J = 19.8 Hz), 99.23 (t, J = 3 Hz), 76.01. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0887. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 72.84; H, 3.52; N, 10.06.
General procedure for the synthesis of N,N-dimethylamino-substituted quinolines by nucleophilic aromatic substitution
A mixture of 130 mg (0.43 mmol) of an appropriate chloro-substituted quinoline and 2.2 mL (4.3 mmol, 10 eq.) of 2 M methylamine or dimethylamine in THF was stirred at 130 °C for 2–3 h in a pressure tube. The mixture was poured into water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, concentrated and purified by chromatography as noted for individual compounds (5ee, 5mm, 5nn, 5oo, and 5pp) described below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.53) and recrystallization from abs. ethanol; mp 142–144 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.05 (d, J = 9.1 Hz, 1H), 7.98 (d, J = 1.9 Hz, 1H), 7.6 (dd, J = 2.0, 8.6 Hz, 1H), 7.54 (d, J = 8.6 Hz, 1H), 7.53–7.48 (m, 1H), 7.29–7.23 (m, 2H), 7.14 (d, J = 9.2 Hz, 1H), 3.19 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.97 (dd, J = 5.2, 251.1 Hz, 2C), 157.82, 147.85, 136.93, 131.54, 131.42, 130.93 (t, J = 10 Hz), 126.25, 121.85, 113.31, 111.88 (dd, J = 3.9, 20.2 Hz, 2C), 110.48, 101.25 (t, J = 19.8 Hz), 99.78 (t, J = 2.9 Hz), 75.07, 37.61 (2C). HRMS (ESI) calcd for C19H15F2N2 [MH+]: 309.1198. Found: 309.1198. Anal. calcd for C19H14F2N2: C, 74.01; H, 4.58; N, 9.09. Found: C, 74.04; H, 4.59; N, 9.09.
5 ethyl acetate–hexane (Rf = 0.53) and recrystallization from abs. ethanol; mp 142–144 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.05 (d, J = 9.1 Hz, 1H), 7.98 (d, J = 1.9 Hz, 1H), 7.6 (dd, J = 2.0, 8.6 Hz, 1H), 7.54 (d, J = 8.6 Hz, 1H), 7.53–7.48 (m, 1H), 7.29–7.23 (m, 2H), 7.14 (d, J = 9.2 Hz, 1H), 3.19 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.97 (dd, J = 5.2, 251.1 Hz, 2C), 157.82, 147.85, 136.93, 131.54, 131.42, 130.93 (t, J = 10 Hz), 126.25, 121.85, 113.31, 111.88 (dd, J = 3.9, 20.2 Hz, 2C), 110.48, 101.25 (t, J = 19.8 Hz), 99.78 (t, J = 2.9 Hz), 75.07, 37.61 (2C). HRMS (ESI) calcd for C19H15F2N2 [MH+]: 309.1198. Found: 309.1198. Anal. calcd for C19H14F2N2: C, 74.01; H, 4.58; N, 9.09. Found: C, 74.04; H, 4.59; N, 9.09.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 120–122 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.6 (d, J = 5.6 Hz, 1H), 8.45–8.37 (m, 1H), 8.13–8.05 (m, 1H), 7.97 (d, J = 5.6 Hz, 1H), 7.92–7.81 (m, 2H), 7.74–7.57 (m, 1H), 7.35 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.44 (dd, J = 5, 252.7 Hz, 2C), 143.08, 142.14, 135.38, 132.71 (t, J = 10.2 Hz), 131.22, 129.13, 128.6, 127.47, 125.45, 121.74, 112.21 (dd, J = 5.4, 18.3 Hz, 2C), 100.04 (t, J = 19.7 Hz), 96.00 (t, J = 3.1 Hz), 80. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.92; H, 3.50; N, 5.20.
5 ethyl acetate–hexane; mp 120–122 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.6 (d, J = 5.6 Hz, 1H), 8.45–8.37 (m, 1H), 8.13–8.05 (m, 1H), 7.97 (d, J = 5.6 Hz, 1H), 7.92–7.81 (m, 2H), 7.74–7.57 (m, 1H), 7.35 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.44 (dd, J = 5, 252.7 Hz, 2C), 143.08, 142.14, 135.38, 132.71 (t, J = 10.2 Hz), 131.22, 129.13, 128.6, 127.47, 125.45, 121.74, 112.21 (dd, J = 5.4, 18.3 Hz, 2C), 100.04 (t, J = 19.7 Hz), 96.00 (t, J = 3.1 Hz), 80. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.92; H, 3.50; N, 5.20.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane; mp 102–104 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.79 (s, 1H), 8.25 (t, J = 9.1 Hz, 2H), 7.99 (t, J = 7.6 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.68–7.53 (m, 1H), 7.32 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.04 (dd, J = 5, 252.1 Hz, 2C), 153.34, 146.2, 134.33, 132.32, 132.06 (t, J = 10.2 Hz), 128.63, 128.57, 127.34, 123.75, 113.84, 112.09 (dd, J = 4.9, 18.6 Hz, 2C), 100.58 (t, J = 19.6 Hz), 94.01 (t, J = 3 Hz), 83.24. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.49; N, 5.10.
5 ethyl acetate–hexane; mp 102–104 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.79 (s, 1H), 8.25 (t, J = 9.1 Hz, 2H), 7.99 (t, J = 7.6 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.68–7.53 (m, 1H), 7.32 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.04 (dd, J = 5, 252.1 Hz, 2C), 153.34, 146.2, 134.33, 132.32, 132.06 (t, J = 10.2 Hz), 128.63, 128.57, 127.34, 123.75, 113.84, 112.09 (dd, J = 4.9, 18.6 Hz, 2C), 100.58 (t, J = 19.6 Hz), 94.01 (t, J = 3 Hz), 83.24. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0758. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.68; H, 3.49; N, 5.10.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate–hexane; mp 115–117 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 8.7 (d, J = 5.8 Hz, 1H), 8.27 (d, J = 8.3 Hz, 1H), 8.11 (dd, J = 1.1, 7.2 Hz, 1H), 8.08 (d, J = 5.9 Hz, 1H), 7.81–7.72 (m, 1H), 7.66–7.55 (m, 1H), 7.33 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.06 (dd, J = 5.1, 252 Hz, 2C), 153.14, 144.54, 134.88, 134.71, 131.97 (t, J = 10.1 Hz), 129.62, 127.91, 127.36, 117.86, 117.53, 112.1 (dd, J = 5.3, 19 Hz, 2C), 100.63 (t, J = 19.6 Hz), 95.41 (t, J = 3 Hz), 81.86. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0756. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.75; H, 3.47; N, 5.23.
1 ethyl acetate–hexane; mp 115–117 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.44 (s, 1H), 8.7 (d, J = 5.8 Hz, 1H), 8.27 (d, J = 8.3 Hz, 1H), 8.11 (dd, J = 1.1, 7.2 Hz, 1H), 8.08 (d, J = 5.9 Hz, 1H), 7.81–7.72 (m, 1H), 7.66–7.55 (m, 1H), 7.33 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.06 (dd, J = 5.1, 252 Hz, 2C), 153.14, 144.54, 134.88, 134.71, 131.97 (t, J = 10.1 Hz), 129.62, 127.91, 127.36, 117.86, 117.53, 112.1 (dd, J = 5.3, 19 Hz, 2C), 100.63 (t, J = 19.6 Hz), 95.41 (t, J = 3 Hz), 81.86. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0756. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 76.75; H, 3.47; N, 5.23.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate–hexane; mp 97–99 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.58 (d, J = 6.1 Hz, 1H), 8.3 (s, 1H), 8.2 (d, J = 8.5 Hz, 1H), 7.9 (d, J = 5.7 Hz, 1H), 7.79 (dd, J = 1.6, 8.5 Hz, 1H), 7.68–7.5 (m, 1H), 7.3 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.18 (dd, J = 5, 252.1 Hz, 2C), 152.35, 143.91, 134.84, 132.1 (t, J = 10.2 Hz), 130.25, 129.29, 128.44, 123.12, 120.19, 112.11 (dd, J = 5.0, 19 Hz, 2C), 100.37 (t, J = 19.7 Hz), 98.29 (t, J = 3.2 Hz), 78.06. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.16; H, 3.39; N, 5.21.
1 ethyl acetate–hexane; mp 97–99 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.58 (d, J = 6.1 Hz, 1H), 8.3 (s, 1H), 8.2 (d, J = 8.5 Hz, 1H), 7.9 (d, J = 5.7 Hz, 1H), 7.79 (dd, J = 1.6, 8.5 Hz, 1H), 7.68–7.5 (m, 1H), 7.3 (t, J = 8.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.18 (dd, J = 5, 252.1 Hz, 2C), 152.35, 143.91, 134.84, 132.1 (t, J = 10.2 Hz), 130.25, 129.29, 128.44, 123.12, 120.19, 112.11 (dd, J = 5.0, 19 Hz, 2C), 100.37 (t, J = 19.7 Hz), 98.29 (t, J = 3.2 Hz), 78.06. HRMS (ESI) calcd for C17H10F2N [MH+]: 266.0776. Found: 266.0757. Anal. calcd for C17H9F2N: C, 76.98; H, 3.42; N, 5.28. Found: C, 77.16; H, 3.39; N, 5.21.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.62) and recrystallization from abs. ethanol; mp 145–146 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.63 (s, 1H), 8.39 (d, J = 8.2 Hz, 1H), 8.3 (d, J = 8.3 Hz, 1H), 8.1 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.95 (ddd, J = 1.1, 6.9, 8.3 Hz, 1H), 7.66–7.58 (m, 1H), 7.33 (t, J = 8.1 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.06 (dd, J = 4.9, 252.5 Hz, 2C), 151.11, 144.73, 136.29, 133.31, 132.36 (t, J = 10.1 Hz), 130.35, 126.5, 125.37, 124.82, 114.66, 112.13 (dd, J = 3.7, 20.1 Hz, 2C), 100.31 (t, J = 19.7 Hz), 93 (t, J = 3.0 Hz), 84.26. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0388. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.06; H, 2.78; N, 4.61.
5 ethyl acetate–hexane (Rf = 0.62) and recrystallization from abs. ethanol; mp 145–146 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.63 (s, 1H), 8.39 (d, J = 8.2 Hz, 1H), 8.3 (d, J = 8.3 Hz, 1H), 8.1 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.95 (ddd, J = 1.1, 6.9, 8.3 Hz, 1H), 7.66–7.58 (m, 1H), 7.33 (t, J = 8.1 Hz, 2H). 13C NMR (176 MHz, DMSO-d6) δ 162.06 (dd, J = 4.9, 252.5 Hz, 2C), 151.11, 144.73, 136.29, 133.31, 132.36 (t, J = 10.1 Hz), 130.35, 126.5, 125.37, 124.82, 114.66, 112.13 (dd, J = 3.7, 20.1 Hz, 2C), 100.31 (t, J = 19.7 Hz), 93 (t, J = 3.0 Hz), 84.26. HRMS (ESI) calcd for C17H9ClF2N [MH+]: 300.0386. Found: 300.0388. Anal. calcd for C17H8ClF2N: C, 68.13; H, 2.69; N, 4.67. Found: C, 68.06; H, 2.78; N, 4.61.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 methanol–dichloromethane; mp 177–178 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (d, J = 5.5 Hz, 1H), 8.23 (dd, J = 5.5, 9.1 Hz, 1H), 8.07–7.96 (m, 2H), 7.83 (td, J = 2.6, 8.9 Hz, 1H), 7.71–7.57 (m, 1H), 7.35 (t, J = 8.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.43 (dd, J = 5, 252.8 Hz, 2C), 161.17 (d, J = 249.3 Hz), 142.77 (d, J = 2.4 Hz), 141.58 (d, J = 6 Hz), 132.93, 132.79 (d, J = 8.6 Hz), 131.15 (d, J = 8.9 Hz), 129.49 (d, J = 8.8 Hz), 121.79 (d, J = 25.6 Hz), 121.56 (d, J = 1.6 Hz), 112.23 (dd, J = 5, 18.7 Hz, 2C), 108.72 (d, J = 22.2 Hz), 99.91 (t, J = 19.7 Hz), 95.49 (t, J = 2.7 Hz), 80.54. HRMS (ESI) calcd for C17H9F3N [MH+]: 284.0682. Found: 284.0662. Anal. calcd for C17H8F3N: C, 72.09; H, 2.85; N, 4.95. Found: C, 71.98; H, 2.69; N, 4.91.
200 methanol–dichloromethane; mp 177–178 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (d, J = 5.5 Hz, 1H), 8.23 (dd, J = 5.5, 9.1 Hz, 1H), 8.07–7.96 (m, 2H), 7.83 (td, J = 2.6, 8.9 Hz, 1H), 7.71–7.57 (m, 1H), 7.35 (t, J = 8.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.43 (dd, J = 5, 252.8 Hz, 2C), 161.17 (d, J = 249.3 Hz), 142.77 (d, J = 2.4 Hz), 141.58 (d, J = 6 Hz), 132.93, 132.79 (d, J = 8.6 Hz), 131.15 (d, J = 8.9 Hz), 129.49 (d, J = 8.8 Hz), 121.79 (d, J = 25.6 Hz), 121.56 (d, J = 1.6 Hz), 112.23 (dd, J = 5, 18.7 Hz, 2C), 108.72 (d, J = 22.2 Hz), 99.91 (t, J = 19.7 Hz), 95.49 (t, J = 2.7 Hz), 80.54. HRMS (ESI) calcd for C17H9F3N [MH+]: 284.0682. Found: 284.0662. Anal. calcd for C17H8F3N: C, 72.09; H, 2.85; N, 4.95. Found: C, 71.98; H, 2.69; N, 4.91.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane and recrystallization from methanol; mp 200–201 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.31 (d, J = 8.3 Hz, 1H), 8.17 (s, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.55–7.43 (m, 3H), 7.26 (t, J = 7.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.04 (dd, J = 5.4, 250.5 Hz, 2C), 158.49, 147.85, 136.25, 131.71, 130.77 (t, J = 10 Hz), 126.86, 125, 124.33, 116.64, 111.85 (dd, J = 5.4, 18.3 Hz, 2C), 102.27, 102.23 (t, J = 19.9 Hz), 97.26 (t, J = 2.9 Hz), 80.27. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0866. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 73.13; H, 3.69; N, 9.93.
10 methanol–dichloromethane and recrystallization from methanol; mp 200–201 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.31 (d, J = 8.3 Hz, 1H), 8.17 (s, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.55–7.43 (m, 3H), 7.26 (t, J = 7.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 162.04 (dd, J = 5.4, 250.5 Hz, 2C), 158.49, 147.85, 136.25, 131.71, 130.77 (t, J = 10 Hz), 126.86, 125, 124.33, 116.64, 111.85 (dd, J = 5.4, 18.3 Hz, 2C), 102.27, 102.23 (t, J = 19.9 Hz), 97.26 (t, J = 2.9 Hz), 80.27. HRMS (ESI) calcd for C17H11F2N2 [MH+]: 281.0885. Found: 281.0866. Anal. calcd for C17H10F2N2: C, 72.85; H, 3.60; N, 10.00. Found: C, 73.13; H, 3.69; N, 9.93.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.47); mp 122–124 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.31 (s, 1H), 8.2 (d, J = 8.4 Hz, 1H), 8.13 (d, J = 8.1 Hz, 1H), 7.84 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.62 (ddd, J = 1.3, 6.8, 8.3 Hz, 1H), 7.56–7.48 (m, 1H), 7.28 (t, J = 7.9 Hz, 2H), 3.19 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.72 (dd, J = 5.3, 250.9 Hz, 2C), 160.63, 145.03, 137.01, 130.96, 130.82 (t, J = 10.1 Hz), 126.8, 126.2, 124.21, 118.3, 111.91 (dd, J = 3.8, 20.1 Hz, 2C), 105.07, 101.45 (t, J = 19.8 Hz), 95.96 (t, J = 2.9 Hz), 81, 42.45 (2C). HRMS (ESI) calcd for C19H15F2N2 [MH+]: 309.1198. Found: 309.1200. Anal. calcd for C19H14F2N2: C, 74.01; H, 4.58; N, 9.09. Found: C, 73.75; H, 4.60; N, 9.00.
5 ethyl acetate–hexane (Rf = 0.47); mp 122–124 °C. 1H NMR (700 MHz, DMSO-d6) δ 8.31 (s, 1H), 8.2 (d, J = 8.4 Hz, 1H), 8.13 (d, J = 8.1 Hz, 1H), 7.84 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.62 (ddd, J = 1.3, 6.8, 8.3 Hz, 1H), 7.56–7.48 (m, 1H), 7.28 (t, J = 7.9 Hz, 2H), 3.19 (s, 6H). 13C NMR (176 MHz, DMSO-d6) δ 161.72 (dd, J = 5.3, 250.9 Hz, 2C), 160.63, 145.03, 137.01, 130.96, 130.82 (t, J = 10.1 Hz), 126.8, 126.2, 124.21, 118.3, 111.91 (dd, J = 3.8, 20.1 Hz, 2C), 105.07, 101.45 (t, J = 19.8 Hz), 95.96 (t, J = 2.9 Hz), 81, 42.45 (2C). HRMS (ESI) calcd for C19H15F2N2 [MH+]: 309.1198. Found: 309.1200. Anal. calcd for C19H14F2N2: C, 74.01; H, 4.58; N, 9.09. Found: C, 73.75; H, 4.60; N, 9.00.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane), mp 118–120 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 8.6 Hz, 1H), 8.25 (s, 1H), 8.08 (t, J = 4.5 Hz, 1H), 8.05 (d, J = 9.1 Hz, 1H), 7.81 (t, J = 7.5 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.55–7.44 (m, 1H), 7.26 (t, J = 7.9 Hz, 2H), 3.03 (d, J = 4.5 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.61 (dd, J = 5.4, 250.4 Hz, 2C), 156.29, 147.12, 135.26, 130.99, 130.33 (t, J = 10.1 Hz), 126.6, 124.05, 123.44, 117.01, 112.01 (dd, J = 3.8, 20.1 Hz, 2C), 101.78 (t, J = 19.9 Hz), 101.45, 96.88 (t, J = 2.9 Hz), 79.90, 28.29. HRMS (ESI) calcd for C18H13F2N2 [MH+]: 295.1041. Found: 295.1035. Anal. calcd for C18H12F2N2: C, 73.46; H, 4.11; N, 9.52. Found: C, 73.49; H, 4.23; N, 9.61.
2 ethyl acetate–hexane), mp 118–120 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 8.6 Hz, 1H), 8.25 (s, 1H), 8.08 (t, J = 4.5 Hz, 1H), 8.05 (d, J = 9.1 Hz, 1H), 7.81 (t, J = 7.5 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.55–7.44 (m, 1H), 7.26 (t, J = 7.9 Hz, 2H), 3.03 (d, J = 4.5 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.61 (dd, J = 5.4, 250.4 Hz, 2C), 156.29, 147.12, 135.26, 130.99, 130.33 (t, J = 10.1 Hz), 126.6, 124.05, 123.44, 117.01, 112.01 (dd, J = 3.8, 20.1 Hz, 2C), 101.78 (t, J = 19.9 Hz), 101.45, 96.88 (t, J = 2.9 Hz), 79.90, 28.29. HRMS (ESI) calcd for C18H13F2N2 [MH+]: 295.1041. Found: 295.1035. Anal. calcd for C18H12F2N2: C, 73.46; H, 4.11; N, 9.52. Found: C, 73.49; H, 4.23; N, 9.61.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane), mp 70–72 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.38 (s, 1H), 8.16 (d, J = 8.1 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.88 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.68 (ddd, J = 1.3, 6.9, 8.3 Hz, 1H), 7.61–7.5 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 3.47 (t, J = 5 Hz, 4H), 2.57 (t, J = 4.7 Hz, 4H), 2.27 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82 (dd, J = 5.3, 251.1 Hz, 2C), 160.77, 144.84, 136.70, 131.31, 131.15 (t, J = 9.2 Hz), 127.19, 126.14, 124.54, 119.27, 112.12 (dd, J = 3.7, 20.1 Hz, 2C), 107.32, 101.20 (t, J = 19.7 Hz), 95.34 (t, J = 3 Hz), 81.52, 54.56 (2C), 50.65 (2C), 45.78. HRMS (ESI) calcd for C22H20F2N3 [MH+]: 364.1620. Found: 364.1614. Anal. calcd for C22H19F2N3: C, 72.71; H, 5.27; N, 11.56. Found: C, 72.91; H, 5.12; N, 11.67.
10 methanol–dichloromethane), mp 70–72 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.38 (s, 1H), 8.16 (d, J = 8.1 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.88 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.68 (ddd, J = 1.3, 6.9, 8.3 Hz, 1H), 7.61–7.5 (m, 1H), 7.29 (t, J = 8 Hz, 2H), 3.47 (t, J = 5 Hz, 4H), 2.57 (t, J = 4.7 Hz, 4H), 2.27 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 161.82 (dd, J = 5.3, 251.1 Hz, 2C), 160.77, 144.84, 136.70, 131.31, 131.15 (t, J = 9.2 Hz), 127.19, 126.14, 124.54, 119.27, 112.12 (dd, J = 3.7, 20.1 Hz, 2C), 107.32, 101.20 (t, J = 19.7 Hz), 95.34 (t, J = 3 Hz), 81.52, 54.56 (2C), 50.65 (2C), 45.78. HRMS (ESI) calcd for C22H20F2N3 [MH+]: 364.1620. Found: 364.1614. Anal. calcd for C22H19F2N3: C, 72.71; H, 5.27; N, 11.56. Found: C, 72.91; H, 5.12; N, 11.67.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate–hexane), mp 149–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04 (dd, J = 1.7, 4.2 Hz, 1H), 8.59 (dd, J = 1.7, 8.5 Hz, 1H), 8.45 (s, 1H), 7.57 (dd, J = 4.2, 8.5 Hz, 1H), 7.53–7.46 (m, 1H), 7.25 (t, J = 8 Hz, 2H), 3.25 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 161.9 (dd, J = 5.3, 250.9 Hz, 2C), 160.35, 153.88, 152.18, 148.92, 135.3, 130.67 (t, J = 10.1 Hz), 120.82, 113.48, 111.84 (dd, J = 3.8, 20.1 Hz, 2C), 106.58, 101.86 (t, J = 19.8 Hz), 96.37 (t, J = 3.0 Hz), 79.73, 42.31 (2C). HRMS (ESI) calcd for C18H14F2N3 [MH+]: 310.1150. Found: 310.1147. Anal. calcd for C18H13F2N3: C, 69.89; H, 4.24; N, 13.58. Found: C, 70.01; H, 4.44; N, 13.53.
2 ethyl acetate–hexane), mp 149–150 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.04 (dd, J = 1.7, 4.2 Hz, 1H), 8.59 (dd, J = 1.7, 8.5 Hz, 1H), 8.45 (s, 1H), 7.57 (dd, J = 4.2, 8.5 Hz, 1H), 7.53–7.46 (m, 1H), 7.25 (t, J = 8 Hz, 2H), 3.25 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 161.9 (dd, J = 5.3, 250.9 Hz, 2C), 160.35, 153.88, 152.18, 148.92, 135.3, 130.67 (t, J = 10.1 Hz), 120.82, 113.48, 111.84 (dd, J = 3.8, 20.1 Hz, 2C), 106.58, 101.86 (t, J = 19.8 Hz), 96.37 (t, J = 3.0 Hz), 79.73, 42.31 (2C). HRMS (ESI) calcd for C18H14F2N3 [MH+]: 310.1150. Found: 310.1147. Anal. calcd for C18H13F2N3: C, 69.89; H, 4.24; N, 13.58. Found: C, 70.01; H, 4.44; N, 13.53.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate–hexane (Rf = 0.4) to provide 220 mg (81%) of tert-butyl N-(4-((2,6-difluorophenyl)ethynyl)isoquinolin-1-yl)-N-methylglycinate as an oil with a pale greenish hue: 1H NMR (400 MHz, DMSO-d6) δ 8.25 (s, 1H), 8.22 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 8.3 Hz, 1H), 7.86 (t, J = 7.3 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.58–7.47 (m, 1H), 7.28 (t, J = 8 Hz, 2H), 4.2 (s, 2H), 3.37 (s, 3H), 1.41 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 169.07, 161.74 (dd, J = 5.3, 251 Hz, 2C), 159.24, 144.56, 137.13, 131.11, 130.93 (t, J = 10.2 Hz), 126.49, 126.31, 124.28, 117.77, 111.92 (dd, J = 3.8, 20.1 Hz, 2C), 105.36, 101.39 (t, J = 19.7 Hz), 95.76 (t, J = 3 Hz), 81.12, 80.53, 55.11, 42.44, 27.75 (3C). HRMS (ESI) calcd for C24H23F2N2O2 [MH+]: 409.1722. Found: 409.1719. A mixture of 100 mg (0.24 mmol) of tert-butyl N-(4-((2,6-difluorophenyl)ethynyl)isoquinolin-1-yl)-N-methylglycinate and 92 mg (0.24 mmol) of (+)-biotinyl-3,6-dioxaoctanediamine29,30 in 1 mL of 1,4-dioxane was refluxed for 6 h. After cooling, the mixture was poured into water, extracted with dichloromethane, and dried over anhydrous magnesium sulfate. The solution was filtered, concentrated, and chromatographed with 1
5 ethyl acetate–hexane (Rf = 0.4) to provide 220 mg (81%) of tert-butyl N-(4-((2,6-difluorophenyl)ethynyl)isoquinolin-1-yl)-N-methylglycinate as an oil with a pale greenish hue: 1H NMR (400 MHz, DMSO-d6) δ 8.25 (s, 1H), 8.22 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 8.3 Hz, 1H), 7.86 (t, J = 7.3 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.58–7.47 (m, 1H), 7.28 (t, J = 8 Hz, 2H), 4.2 (s, 2H), 3.37 (s, 3H), 1.41 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 169.07, 161.74 (dd, J = 5.3, 251 Hz, 2C), 159.24, 144.56, 137.13, 131.11, 130.93 (t, J = 10.2 Hz), 126.49, 126.31, 124.28, 117.77, 111.92 (dd, J = 3.8, 20.1 Hz, 2C), 105.36, 101.39 (t, J = 19.7 Hz), 95.76 (t, J = 3 Hz), 81.12, 80.53, 55.11, 42.44, 27.75 (3C). HRMS (ESI) calcd for C24H23F2N2O2 [MH+]: 409.1722. Found: 409.1719. A mixture of 100 mg (0.24 mmol) of tert-butyl N-(4-((2,6-difluorophenyl)ethynyl)isoquinolin-1-yl)-N-methylglycinate and 92 mg (0.24 mmol) of (+)-biotinyl-3,6-dioxaoctanediamine29,30 in 1 mL of 1,4-dioxane was refluxed for 6 h. After cooling, the mixture was poured into water, extracted with dichloromethane, and dried over anhydrous magnesium sulfate. The solution was filtered, concentrated, and chromatographed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol–dichloromethane (Rf = 0.31) to provide 19 mg (11%) of 6 as a viscous oil: 1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 8.31 (d, J = 7.8 Hz, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.84 (t, J = 5.4 Hz, 1H), 7.75 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.56 (ddd, J = 1.3, 6.9, 8.3 Hz, 1H), 7.35–7.27 (m, 1H), 6.98 (dd, J = 7, 8.4 Hz, 2H), 6.51 (t, J = 5.6 Hz, 1H), 6.36 (s, 1H), 5.42 (s, 1H), 4.45–4.39 (m, 1H), 4.23–4.2 (m, 1H), 4.19 (s, 2H), 3.64–3.52 (m, 8H), 3.5 (t, J = 5.1 Hz, 2H), 3.4–3.33 (m, 2H), 3.27 (s, 3H), 3.11–3.01 (m, 1H), 2.84 (dd, J = 4.9, 12.8 Hz, 1H), 2.68 (d, J = 12.8 Hz, 1H), 2.15 (t, J = 7.5 Hz, 2H), 1.72–1.52 (m, 4H), 1.42–1.31 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 173.37, 170.67, 163.97, 162.88 (dd, J = 5.3, 253.5 Hz, 2C), 160.12, 144.64, 138.09, 131.02, 129.78 (t, J = 9.9 Hz), 126.79, 126.01, 125.85, 119.58, 111.45 (dd, J = 6, 18.5 Hz, 2C), 109.20, 102.69 (t, J = 19.7 Hz), 95.41 (t, J = 3.1 Hz), 82.16, 70.31, 70.19, 70.04, 70, 61.86, 60.28, 58.49, 55.64, 41.93, 40.6, 39.24, 39.17, 36.04, 28.28, 28.18, 25.68. HRMS (ESI) calcd for C36H43F2N6O5S [MH+]: 709.2978. Found: 709.2983.
10 methanol–dichloromethane (Rf = 0.31) to provide 19 mg (11%) of 6 as a viscous oil: 1H NMR (400 MHz, CDCl3) δ 8.36 (s, 1H), 8.31 (d, J = 7.8 Hz, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.84 (t, J = 5.4 Hz, 1H), 7.75 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.56 (ddd, J = 1.3, 6.9, 8.3 Hz, 1H), 7.35–7.27 (m, 1H), 6.98 (dd, J = 7, 8.4 Hz, 2H), 6.51 (t, J = 5.6 Hz, 1H), 6.36 (s, 1H), 5.42 (s, 1H), 4.45–4.39 (m, 1H), 4.23–4.2 (m, 1H), 4.19 (s, 2H), 3.64–3.52 (m, 8H), 3.5 (t, J = 5.1 Hz, 2H), 3.4–3.33 (m, 2H), 3.27 (s, 3H), 3.11–3.01 (m, 1H), 2.84 (dd, J = 4.9, 12.8 Hz, 1H), 2.68 (d, J = 12.8 Hz, 1H), 2.15 (t, J = 7.5 Hz, 2H), 1.72–1.52 (m, 4H), 1.42–1.31 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 173.37, 170.67, 163.97, 162.88 (dd, J = 5.3, 253.5 Hz, 2C), 160.12, 144.64, 138.09, 131.02, 129.78 (t, J = 9.9 Hz), 126.79, 126.01, 125.85, 119.58, 111.45 (dd, J = 6, 18.5 Hz, 2C), 109.20, 102.69 (t, J = 19.7 Hz), 95.41 (t, J = 3.1 Hz), 82.16, 70.31, 70.19, 70.04, 70, 61.86, 60.28, 58.49, 55.64, 41.93, 40.6, 39.24, 39.17, 36.04, 28.28, 28.18, 25.68. HRMS (ESI) calcd for C36H43F2N6O5S [MH+]: 709.2978. Found: 709.2983.
Cell proliferation assay
Colon cancer cells LS174T, HT-29 and Caco2 were obtained from Dr. B. Mark Evers, and BEAS-2B and HEL 299 cells were obtained from Dr. Jon Thorson at the University of Kentucky. These cells were grown in RPMI or DMEM medium (Mediatech) supplemented with 5% or 10% fetal bovine serum and 1% penicillin/streptomycin. For cell proliferation assays, 3 × 104 cells per well grown in 12-well plates were treated with DMSO or inhibitors. The cell numbers and viability were analyzed using a Vi-Cell cell viability analyzer after 4 days. The IC50 values were calculated with GraphPad Prism 5 (GraphPad Software).Western blotting and streptavidin pulldown
Western blotting was performed as described previously.23 The following antibodies were used: MAT2A (GTX112535), anti-cyclin D1 (Cell Signaling, 2922), anti-p21Wif1/Cip1 (Cell Signaling, 2947), and anti-β-tubulin (Developmental Studies Hybridoma Bank, E7). Streptavidin pulldown was performed using recombinant MAT2A protein as described previously.12Molecular modeling
The ligands binding to MAT2A were modeled by using a previously described open-state structure12 of the MAT2A homodimer. Each ligand was docked into the active site of the enzyme using the AutoDock 4.2 program,31 as described previously for other enzyme–ligand binding structures.32–34 During the docking process, the Solis and Wets local search method35 was used for the conformational search, and the Lamarckian genetic algorithm (LGA)36 was employed to deal with the enzyme–ligand interactions. The grid size was set to be 120 × 120 × 120. The final enzyme–ligand binding structures possessed the lowest binding free energies.Author contributions
V. M. S. contributed to the design, synthesis, NMR characterization, and mass spectral characterization of some of the fluorinated phenylethynyl-substituted heterocycles of formula 5 (Table 2), design of the biotinylated analog 6 in Fig. 3, and writing the paper;L. M. K. contributed to the synthesis of all of the phenylethynyl-substituted heterocycles of formula 4 (Table 1) and some of the heterocycles of formula 5 (Table 2);
W. Z. contributed to in vitro experimental biological studies (i.e., cell proliferation studies in Tables 1 and 2; pull-down experiment in Fig. 3);
Y. X. contributed to the synthesis of several phenylethynyl-substituted heterocycles and performed in vitro experimental biological studies (i.e., some of the cell proliferation studies in Tables 1 and 2);
P. W. contributed to the synthesis of the biotinylated analog 6 in Fig. 3;
L. P. contributed to in vitro testing of phenylethynyl-substituted heterocycles 5mm and 5nn and other antineoplastic agents in normal and in various cancer cell lines (Tables 3 and 4);
X. L. contributed to the design of biological studies;
Y. Y. performed the computational studies (Fig. 2);
C.-G. Z. designed and wrote the programs for the computational studies and contributed to writing the paper;
D. S. W. contributed to the design of fluorinated phenylethynyl-substituted heterocycles, the development of synthetic routes to these compounds, and writing the paper;
C. L. contributed to the design of the biological studies of biotinylated phenylethynyl-substituted heterocycles and writing the paper.
Conflicts of interest
The authors declare no competing interest.Acknowledgements
CL and DSW were supported by CA172379 from the NIH. CGZ was supported by NSF grant CHE-1111761. CL and DSW have partial ownership of a new-start company, Epionc, Inc., that seeks to develop these compounds as commercial agents. CL and DSW disclosed this information and complied with requirements to mitigate any potential conflicts of interest in accord with University of Kentucky policy. DSW was also supported by the Office of the Dean of the College of Medicine, by the Center for Pharmaceutical Research and Innovation in the College of Pharmacy, and by NIH grant number P30 GM110787 from the National Institute of General Medical Sciences to L. Hersh, PI. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS. DSW was also supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Prostate Cancer Research Program under Award No. W81XWH-16-1-0635. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.Notes and references
- P. Polakis, Genes Dev., 2000, 14, 1837–1851 Search PubMed.
- M. Bienz and H. Clevers, Cell, 2000, 103, 311–320 CrossRef PubMed.
- H. Suzuki, D. N. Watkins, K. W. Jair, K. E. Schuebel, S. D. Markowitz, W. D. Chen, T. P. Pretlow, B. Yang, Y. Akiyama, M. Van Engeland, M. Toyota, T. Tokino, Y. Hinoda, K. Imai, J. G. Herman and S. B. Baylin, Nat. Genet., 2004, 36, 417–422 CrossRef PubMed.
- D. P. Walsh and Y. T. Chang, Chem. Rev., 2006, 106, 2476–2530 CrossRef PubMed.
- D. R. Spring, Chem. Soc. Rev., 2005, 34, 472–482 RSC.
- A. C. Runcie, K. H. Chan, M. Zengerle and A. Ciulli, Curr. Opin. Chem. Biol., 2016, 33, 186–194 CrossRef PubMed.
- S. L. Schreiber, Nat. Chem. Biol., 2005, 1, 64–66 CrossRef PubMed.
- A. Huston, C. H. Arrowsmith, S. Knapp and M. Schapira, Nat. Chem. Biol., 2015, 11, 542–545 CrossRef PubMed.
- C. H. Arrowsmith, C. Bountra, P. V. Fish, K. Lee and M. Schapira, Nat. Rev. Drug Discovery, 2012, 11, 384–400 CrossRef PubMed.
- D. M. Miller, S. D. Thomas, A. Islam, D. Muench and K. Sedoris, Clin. Cancer Res., 2012, 18, 5546–5553 CrossRef PubMed.
- W. Zhang, V. Sviripa, L. M. Kril, X. Chen, T. Yu, J. Shi, P. Rychahou, B. M. Evers, D. S. Watt and C. Liu, J. Med. Chem., 2011, 54, 1288–1297 CrossRef PubMed.
- W. Zhang, V. Sviripa, X. Chen, J. Shi, T. Yu, A. Hamza, N. D. Ward, L. M. Kril, C. W. Vander Kooi, C. G. Zhan, B. M. Evers, D. S. Watt and C. Liu, ACS Chem. Biol., 2013, 8, 796–803 CrossRef PubMed.
- V. M. Sviripa, W. Zhang, A. G. Balia, O. V. Tsodikov, J. R. Nickell, F. Gizard, T. Yu, E. Y. Lee, L. P. Dwoskin, C. Liu and D. S. Watt, J. Med. Chem., 2014, 57, 6083–6091 CrossRef PubMed.
- W. S. Redfern, L. Carlsson, A. S. Davis, W. G. Lynch, I. MacKenzie, S. Palethorpe, P. K. Siegl, I. Strang, A. T. Sullivan, R. Wallis, A. J. Camm and T. G. Hammond, Cardiovasc. Res., 2003, 58, 32–45 CrossRef PubMed.
- B. D. Guth and G. Rast, Br. J. Pharmacol., 2010, 159, 22–24 CrossRef PubMed.
- J. Cai, W. M. Sun, J. J. Hwang, S. C. Stain and S. C. Lu, Hepatology, 1996, 24, 1090–1097 CrossRef PubMed.
- H. Chen, M. Xia, M. Lin, H. Yang, J. Kuhlenkamp, T. Li, N. M. Sodir, Y. H. Chen, H. Josef-Lenz, P. W. Laird, S. Clarke, J. M. Mato and S. C. Lu, Gastroenterology, 2007, 133, 207–218 CrossRef PubMed.
- K. Ito, S. Ikeda, N. Kojima, M. Miura, K. Shimizu-Saito, I. Yamaguchi, I. Katsuyama, K. Sanada, T. Iwai, H. Senoo and S. Horikawa, Surg. Today, 2000, 30, 706–710 CrossRef PubMed.
- Q. Liu, J. Chen, L. Liu, J. Zhang, D. Wang, L. Ma, Y. He, Y. Liu, Z. Liu and J. Wu, J. Biol. Chem., 2011, 286, 17168–17180 CrossRef PubMed.
- C. L. Quinlan, S. E. Kaiser, B. Bolanos, D. Nowlin, R. Grantner, S. Karlicek-Bryant, J. L. Feng, S. Jenkinson, K. Freeman-Cook, S. G. Dann, X. Wang, P. A. Wells, V. R. Fantin, A. E. Stewart and S. K. Grant, Nat. Chem. Biol., 2017, 13, 785–792 CrossRef PubMed.
- S. P. Kwasniewski, L. Claes, J.-P. Francois and M. S. Deleuze, J. Chem. Phys., 2003, 118, 7823–7836 CrossRef.
- D. H. Waldeck, Chem. Rev., 1991, 415–436 CrossRef.
- V. M. Sviripa, W. Zhang, L. M. Kril, A. X. Liu, Y. Yuan, C. G. Zhan, C. Liu and D. S. Watt, Bioorg. Med. Chem. Lett., 2014, 24, 3638–3640 CrossRef PubMed.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46–49 CrossRef.
- R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC.
- R. W. Miles, V. Samano and M. J. Robins, J. Am. Chem. Soc., 1995, 117, 5951–5957 CrossRef.
- B. Chen, M. E. Dodge, W. Tang, J. Lu, Z. Ma, C. W. Fan, S. Wei, W. Hao, J. Kilgore, N. S. Williams, M. G. Roth, J. F. Amatruda, C. Chen and L. Lum, Nat. Chem. Biol., 2009, 5, 100–107 CrossRef PubMed.
- S. M. Huang, Y. M. Mishina, S. Liu, A. Cheung, F. Stegmeier, G. A. Michaud, O. Charlat, E. Wiellette, Y. Zhang, S. Wiessner, M. Hild, X. Shi, C. J. Wilson, C. Mickanin, V. Myer, A. Fazal, R. Tomlinson, F. Serluca, W. Shao, H. Cheng, M. Shultz, C. Rau, M. Schirle, J. Schlegl, S. Ghidelli, S. Fawell, C. Lu, D. Curtis, M. W. Kirschner, C. Lengauer, P. M. Finan, J. A. Tallarico, T. Bouwmeester, J. A. Porter, A. Bauer and F. Cong, Nature, 2009, 461, 614–620 CrossRef PubMed.
- K. Chiba, M. Asanuma, M. Ishikawa, Y. Hashimoto, K. Dodo, M. Sodeoka and T. Yamaguchi, Chem. Commun., 2017, 53, 8751–8754 RSC.
- X. Ning, J. Guo, M. A. Wolfert and G. J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253–2255 CrossRef PubMed.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785–2791 CrossRef CAS PubMed.
- X. Huang, F. Zheng, P. A. Crooks, L. P. Dwoskin and C.-G. Zhan, J. Am. Chem. Soc., 2005, 127, 14401–14414 CrossRef CAS PubMed.
- X. Huang, F. Zheng and C.-G. Zhan, J. Am. Chem. Soc., 2008, 130, 16691–16696 CrossRef CAS PubMed.
- M. Zhan, S. Hou, C.-G. Zhan and F. Zheng, Biochem. J., 2014, 457, 197–206 CrossRef CAS PubMed.
- F. J. Solis and R. J.-B. Wets, Math. Oper. Res., 1981, 6, 19–30 CrossRef.
- G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, R. K. Belew and A. J. Olson, J. Comput. Chem., 1998, 19, 1639–1662 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |