Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A methodical selection process for the development of ketones and esters as bio-based replacements for traditional hydrocarbon solvents†
Fergal P.
Byrne
 a,
Bart
Forier
b,
Greet
Bossaert
b,
Charly
Hoebers
b,
Thomas J.
Farmer
a,
Bart
Forier
b,
Greet
Bossaert
b,
Charly
Hoebers
b,
Thomas J.
Farmer
 *a and
Andrew J.
Hunt
*a and
Andrew J.
Hunt
 *c
*c
aGreen Chemistry Centre of Excellence, Department of Chemistry, The University of York, Heslington, York YO10 5DD, UK. E-mail: thomas.farmer@york.ac.uk
bNitto Belgium NV, Eikelaarstraat 22, Belgium
cMaterials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen, 40002, Thailand. E-mail: andrew@KKU.ac.th
First published on 6th August 2018
Abstract
A “top down” approach to the development of sustainable, greener, low-polarity solvents is presented. Methyl butyrate, ethyl isobutyrate, methyl pivalate and pinacolone were identified as potential target solvents from trends in Hansen solubility parameters and known physical properties. Solubility, flammability and physical properties were determined which showed their potential to replace traditional, hazardous, volatile, non-polar solvents such as toluene. Each new candidate then demonstrated their suitability to replace these traditional solvents in solubility tests, despite being esters and ketones, each candidate demonstrated their similarity to traditional volatile non-polar solvents in terms of their solubility properties by their ability to dissolve natural rubber, a particularly low-polarity solute. This was reinforced by their performance in a model Menschutkin reaction and a radical-initiated polymerisation for the production of pressure-sensitive adhesives, where their performance was found to be similar to that of toluene. Importantly, a preliminary toxicity test (Ames test) suggested non-mutagenicity in all candidates. Each of the four candidates can be synthesised via a catalytic route from potentially renewable resources, thus enhancing their green credentials.
Introduction
Volatile, non-polar (VNP) solvents are essential throughout the chemical industry, with applications in synthetic chemistry, coatings industry and liquid–liquid extraction, where facile removal by evaporation is required. Many traditional VNP solvents such as the aliphatic hydrocarbon solvents hexane or cyclohexane and the aromatic solvents toluene or benzene suffer from high persistence, bioaccumulation and toxicity (PBT).1–4 More specifically, hexane is suspected of damaging fertility,1 toluene is suspected of damaging the unborn child,3 and benzene is a known carcinogen.4 As such, replacements for this class of solvent which adhere to the principles of green chemistry are needed.5 The challenge with the development of non-polar solvents from biomass is that biomass is highly functionalised with electronegative O atoms,6 which results in high polarity.7 In contrast, traditional non-polar solvents tend to be either hydrocarbons (e.g. hexane, cyclohexane, toluene) or chlorinated hydrocarbons (e.g. carbon tetrachloride, chloroform).8,9 Removing functionality from biomass to reduce polarity is possible, but what is left often closely resembles the traditional target solvents for replacement e.g. removing all functionality from glucose could yield hexane but this process would have a low atom economy.6 Chlorination is an option but the same toxicity and end-of-life issues that exist with traditional chlorinated solvents are likely to persist in chlorinated bio-based alternatives.10–13A number of alternatives to traditional VNP solvents have been recently proposed but many have issues of their own. The hydrocarbons D-limonene and para-cymene have a suitable polarity and are bio-based but are much less volatile than traditional hydrocarbon solvents (Tb = 177 °C in both cases).14–17 2-Methyltetrahydrofuran has suitably low polarity and high volatility (Tb = 80 °C) but forms hazardous peroxides in ambient conditions.18 Recently, the unusual ether 2,2,5,5-tetramethyltetrahydrofuran (2,2,5,5-tetramethyloxolane (TMO, previously reported as TMTHF)) has shown great promise to replace toluene.7 It shares a very similar boiling point to toluene (Tb = 112 °C compared to 111 °C for toluene), is of very low polarity and does not form peroxides.7 However, applications where TMO is not the optimal solvent will inevitably be identified and as such, more alternatives to traditional hydrocarbons are required. Esters and ketones tend to score well in solvent selection guides due to their low-toxicity and often facile production from biomass.6,8,9 However, commonly-used esters and ketones do not possess a combination of low-polarity and high volatility (Fig. 1). For example, ethyl acetate is volatile but of medium polarity, whereas n-butyl acetate is of medium-to-low polarity but its boiling point is significantly above that of toluene (Tb = 126 °C compared to 111 °C for toluene).19–21
 | ||
| Fig. 1 HSP solvent polarity map showing all permutations of esters with Tb <111 °C (green) and >111 °C (orange). Natural rubber is represented by a blue circle. | ||
Several intelligent approaches to greener solvent selection and development have been reported in recent years.11,22,23 One such approach, developed by Moity et al., involves designing solvents by performing chemical transformations on a chosen bio-based platform molecule to generate a list of candidate solvents.24 The properties of each candidate are then determined and their suitability as solvents is assessed either in silico or in practise.24 Applications for the best candidates can be identified once their properties are known. This approach has been applied to levoglucosenone,22 itaconic acid,25 glycerol26 and isoamyl alcohol.27
Another approach is to design solvents to fit a set of physical and solvent property criteria, for which computer-aided molecular design is often employed.11 This approach is applied when a greener solvent is required to dissolve a known target solute or when a traditional solvent has been identified for replacement. Such an approach has been reported before by Jin et al. to identify replacement solvents for chlorinated hydrocarbons such as dichloromethane and chloroform.11 Importantly, this approach highlighted the need for toxicity testing at an early stage in the solvent development process.
Moity et al. reported the use of a hybrid of the abovementioned approaches; nitrocellulose was chosen as a target solute and glycerol was chosen as a bio-based platform in a “top-down” methodology.23
Herein, a similar “top-down” approach was utilised in an attempt to identify replacements for traditional VNP solvents, such as hexane and toluene among the ester and ketone family. Trends within esters and ketones were observed using HSPiP, after which several candidate molecules with an optimal balance of polarity and volatility to replace hydrocarbons were discovered. The best candidates were characterised in terms of their solubility properties using the Kamlet–Abboud–Taft (KAT) parameters28–30 and a model Menschutkin reaction.31–33 Solubility tests using natural rubber as an industrially-relevant probe solute were also carried out. Physical, flammability and toxicity (Ames test) properties were also determined. Furthermore, the best candidates were tested in the production of a commercial pressure-sensitive adhesive (PSA) via the radical-initiated polymerisation of acrylate monomers and subsequently tested by coating of the adhesive polymer on a carrier film. Finally, a green assessment was made on the most promising candidates.
Results and discussion
Solvent selection process
The aim of this article is to find replacements for traditional hydrocarbon solvents. Traditional hydrocarbons tend to be volatile and of low-polarity. As such, boundaries were set as to what was acceptable in terms of polarity and boiling point for new replacement solvents. The boiling point of toluene (111 °C) was set as the upper limit, while natural rubber (20 wt% concentration) was chosen as a low-polarity probe solute to compare potential candidates in terms of polarity. Hydrocarbon solvents such as toluene and hexane are traditionally used for the dissolution of natural rubber and it is insoluble in medium- to high-polarity solvents.HSPs are a useful tool in characterising solvents and solutes with respect to their polarity.34,35 HSP divides the solvent properties into three parameters which describe a molecules dipolarity (δP), hydrogen-bonding ability (δH) and dispersion forces (δD) separately, and solutes tend to be dissolved by solvents with similar HSPs.34 By plotting δH against δP, solvent properties can be easily visualised on two-dimensional solvent polarity maps. As such, HSPs were employed to identify trends within the target solvent classes (esters and ketones).
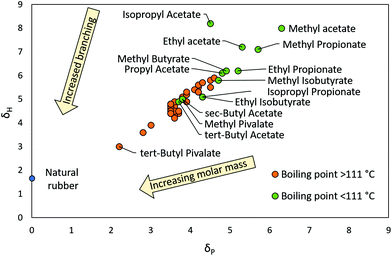
The relationship between structure, polarity and Tb in esters and ketones was examined by adding alkyl groups to fundamental ester and ketone structures (R1COOR2 and R3COR4 respectively) in both a linear and branched manner until all permutations of esters/ketones as far as C7 were generated. The search was stopped at C7 as this was the point at which Tb became higher than the boundaries set in this work. The total polarity of cyclic esters (lactones) and ketones is higher compared to their acyclic equivalents and therefore were not included. All permutations of ester and ketones were plotted on solvent maps of δH against δP, as shown in Fig. 1 and 2. Esters and ketones highlighted in green have Tb's below 111 °C while those in orange have Tb's above 111 °C (Tb's of each ester and ketone were obtained from the ChemSpider database). The molecules highlighted in green that are closest to natural rubber (Fig. 1 and 2) are likely to be able to dissolve natural rubber, whilst maintaining an acceptable Tb. The molecules highlighted in green that are furthest from the natural rubber have lower Tb's but are of higher polarity and thus, of greater disparity from hydrocarbon solvents.
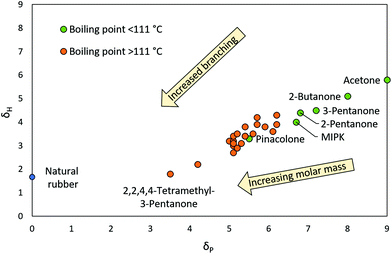 | ||
| Fig. 2 HSP solvent polarity map showing all permutations of ketones with Tb <111 °C (green) and >111 °C (orange). Natural rubber is represented by a blue circle. | ||
Several trends were observed within the ester and ketone classes. Firstly, the polarity of the target solvent decreased (HSPs approached 0) as alkyl groups were added to the fundamental ester and ketone structure. Secondly, increased branching in alkyl groups in esters and ketones resulted in lower Tb and lower polarity than their linear alkyl equivalents with the same number of carbon atoms. This is demonstrated by tert-butyl acetate and n-butyl acetate, whose Tb's are 96 °C and 126 °C respectively.20,36 In addition, tert-butyl acetate is less polar. Thirdly, in the case of esters, the carboxylate side of the ester tended to have more influence on polarity than the alcohol side, as demonstrated by solubility testing using natural rubber. For example, the C6 ester with a C3 carboxylate group, propyl propionate, was experimentally found to be unable to dissolve natural rubber whereas the C5 ester with a C4 carboxylate group, methyl butyrate, could. The cut-off for carboxylate groups able to dissolve the natural rubber appeared to be C4: butyrates and isobutyrates. Finally, the HSPs of esters and ketones showed that in general, esters have lower dipolarity (due to two O atoms competing against one another for electron density) but higher hydrogen-bonding ability (due to the presence of two O atoms with four hydrogen-bond accepting lone-pairs) than ketones.
Knowledge of these four trends led to the optimal polarity/Tb ratio in esters and ketones. Pivalate esters, particularly tert-butyl pivalate (shown in Fig. 3), were of noticeably lower polarity than the other esters due to their higher degree of branching (Fig. 1). Branching also maintained a relatively low Tb, as demonstrated by the two C9 esters, tert-butyl pivalate with a Tb of 134 °C,37 compared to pentyl butyrate with a Tb of 186 °C.38tert-Butyl pivalate provides an alternative ester option for applications where low polarity is more essential than volatility. Similarly, the highly branched 2,2,4,4-tetramethyl-3-pentanone (shown in Fig. 3) possesses the optimal polarity/Tb ratio in ketones (Fig. 2), and provides an alternative ketone option for such applications.39 However, both tert-butyl pivalate and 2,2,4,4-tetramethyl-3-pentanone fall outside the Tb limit set in this work and so were not tested any further.
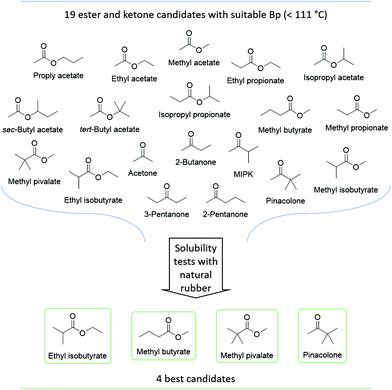
The thirteen esters and six ketones with Tb ≤111 °C (highlighted in green in Fig. 1 and 2) were tested for their ability to dissolve natural rubber and the results are shown in Table S1 (ESI†). Only methyl pivalate, methyl butyrate, ethyl isobutyrate (esters) and pinacolone (ketone) could dissolve the rubber and as such, were selected as the best candidates. The remaining esters and ketones were omitted. Thus, the 19 esters and ketones were reduced down to four, as shown in Fig. 4.
Characterisation of the best candidates
A range of solvent properties for ethyl isobutyrate, methyl butyrate, methyl pivalate and pinacolone are shown in Table 1 in comparison to the traditional hydrocarbon solvents, toluene and hexane.| Solvent property | Ester | Ketone | Aromatic hydrocarbon | Aliphatic hydrocarbon | ||
|---|---|---|---|---|---|---|
| Methyl pivalate | Methyl butyrate | Ethyl isobutyrate | Pinacolone | Toluene | Hexane | |
| a Pubchem. b Carried out by ITS testing services. c Carried out by Chilworth Technology. d This work. e Sangster.40 f Assumed value as molecule is aprotic. g This work, using N,N-diethyl-4-nitroaniline and 4-nitroaniline dyes. h This work, using N,N-diethyl-4-nitroaniline dye. i Predicted using HSPiP. | ||||||
| M w/g mol−1 | 116.16 | 102.13 | 116.16 | 100.16 | 92.14 | 86.18 |
| T b/°C | 100–101a | 100–102a | 108–110a | 105–106a | 111a | 69a |
| T m/°C | −70a | −85a | −88a | −53a | −93a | −95a |
| ρ/g ml−1 | 0.875a | 0.898a | 0.865a | 0.803a | 0.867a | 0.661a |
| AIT/°C | 443b | 428b | 451b | 428b | 522b | 225a |
| LEL/% | 1.3c | 1.3c | 0.9c | 1.3c | 1.1c | 1.1c |
| Ames test | Passd | Passd | Passd | Passd | Passd | Passd |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(o/w) P(o/w) |
1.74d | 1.20d | 1.54d | 1.21d | 2.73e | 4.00e |
| α | 0.00f | 0.00f | 0.00f | 0.00f | 0.00f | 0.00f |
| β | 0.45g | 0.48g | 0.48g | 0.58g | 0.10g | 0.00g |
| π* | 0.49h | 0.51h | 0.49h | 0.59h | 0.51h | 0.00h |
δ
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i/MPa0.5 i/MPa0.5 |
15.0 | 15.8 | 15.4 | 15.1 | 18.0 | 14.9 |
δ
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i/MPa0.5 i/MPa0.5 |
3.8 | 4.9 | 4.3 | 5.5 | 1.4 | 0.0 |
Δ
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i/MPa0.5 i/MPa0.5 |
5.0 | 6.2 | 5.1 | 3.3 | 2.0 | 0.0 |
The Tb's of each candidate are lower than toluene meaning facile removal by evaporation is possible. Low melting points indicate that all candidates are liquids at room temperature, while all are less dense than water, and similar to toluene. Importantly, the autoignition temperatures (AIT) of all candidates are >200 °C (the threshold set in the CHEM21 solvent guide9) which is vital for the safe use of volatile organic liquids and all are comparable to toluene and far superior to hexane. In addition, methyl pivalate, methyl butyrate and pinacolone have a superior (i.e. higher, all 1.3%) lower explosion limit (LEL) to toluene (1.1%) and hexane (1.1%). Only ethyl isobutyrate had a lower LEL (0.9%). However, when the density and molecular weight (Mw) is considered, all candidates are superior to toluene in terms of explosion limits (calculations shown in ESI†).
An Ames test showed that none of the four candidates were mutagenic (Fig. S2†).41,42 While the Ames test is a useful preliminary assessment, extended in vitro and in silico investigations are currently underway to further explore the safety profile of these candidate compounds. The octanol/water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w) shows that all four candidates are immiscible with water (log
Po/w) shows that all four candidates are immiscible with water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w > 1), allowing aqueous extraction and washing during work-up. However, none have log
Po/w > 1), allowing aqueous extraction and washing during work-up. However, none have log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w values large enough to suggest that they are likely to bioaccumulate (in all cases log
Po/w values large enough to suggest that they are likely to bioaccumulate (in all cases log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w < 4.5).43,44
Po/w < 4.5).43,44
The Kamlet–Abboud–Taft (KAT) parameters are an empirical measurement of polarity based on solvatochromism. The absorbances of probe dyes are used to determine values for three parameters α,28β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and π*,30 which represent hydrogen-bond donating and accepting abilities and dipolarity/polarisability respectively. The KAT parameters are useful in generating linear solvation-energy relationships (LSERs) for model reactions such as Menschutkin,32,33 amidation14 and esterification14 reactions.
29 and π*,30 which represent hydrogen-bond donating and accepting abilities and dipolarity/polarisability respectively. The KAT parameters are useful in generating linear solvation-energy relationships (LSERs) for model reactions such as Menschutkin,32,33 amidation14 and esterification14 reactions.
While toluene possesses a lower permanent dipole than the four candidates (shown by δP), it is more polarisable due to the delocalised electrons in the aromatic ring (shown by δD), resulting in very similar π* values (KAT measurement of dipolarity and polarisability combined) between the four candidates and toluene. Like toluene, none of the candidates are protic (α = 0.00). However, the key difference between the four candidates and toluene is in their hydrogen-bond accepting ability. The presence of lone-pairs on the O atoms in the four candidates mean that a degree of basicity is present (β = 0.45–0.58), whereas the lack of lone-pairs on toluene mean its basicity is far weaker (β = 0.11). The slight basicity is due to the slight interaction between the electron-rich aromatic ring with protic substrates.
However, such a low polarity, like traditional hydrocarbon solvents, is not required for most applications. This is exemplified in the solubility tests where very low-polarity natural rubber could successfully be dissolved by solvents of higher polarity. In the past many traditional non-polar hydrocarbon solvents were chosen due to their volatility and low cost. Although each candidate must be assessed for their suitability to replace hydrocarbon solvents in a case-by-case basis, each of the four candidates has demonstrated an ability to dissolve the very low-polarity natural rubber, suggesting that issues with their slightly higher polarity are unlikely.
Odour is another issue which is important to consider in industrial processes, especially those in open systems. Methyl pivalate and ethyl isobutyrate have pleasant apple and strawberry odours respectively, while pinacolone has a characteristic ketone odour, much like acetone or 2-butanone (MEK). However, the smell of methyl butyrate is similar to butyric acid and is very unpleasant, which may hinder its use in open systems.
Synthetic routes to the four best candidates from biomass
Each of the four candidates (methyl butyrate, ethyl isobutyrate, methyl pivalate and pinacolone) can be produced from renewable resources. The synthesis of butyric acid and isobutyric acid from glycerol in excellent selectivities has been reported previously by Coskun et al.45 A potential flow diagram for large-scale production, analogous to that of the Cativa process, is shown in Fig. S4 (ESI†). Glycerol can be sourced renewably from used cooking oil and has previously been used for solvent production.26,46–48The precursor to methyl pivalate and pinacolone is pivalic acid, which can be produced by the carbonylation of isobutene with carbon monoxide in a Koch reaction. Isobutene can be produced by fermentation of sugars, a process that has recently been commercialised by Global Bioenergies,49 ketonisation of pivalic acid using acetone50 or acetic acid51 to produce pinacolone in good yields has previously been reported.
Esterification of butyric, isobutyric and pivalic acids with the corresponding alcohols to yield methyl butyrate, ethyl isobutyrate and methyl pivalate respectively was carried out as part of this work (Fig. S3†). Almost full conversions (99%) of butyric and isobutyric acids using the corresponding alcohols were obtained after 45 minutes using methanesulfonic acid as the catalyst in a reactive distillation apparatus. The rate of pivalic acid conversion was slightly slower due to the bulky tert-butyl group on the acid, with 88% conversion of the acid observed after 45 minutes. The heterogeneous acid catalyst, Amberlyst 15, also achieved high conversions of butyric acid (>95%) and pivalic acid (71%) with methanol in the same reactive distillation apparatus after 45 minutes. Despite the rate of carboxylic acid conversion being slightly slower than methanesulfonic acid, Amberlyst 15 has the advantage of being non-corrosive and reusable, adding to its greenness and meaning acid resistant steel is not required in large-scale production.
Application testing: Menschutkin reaction
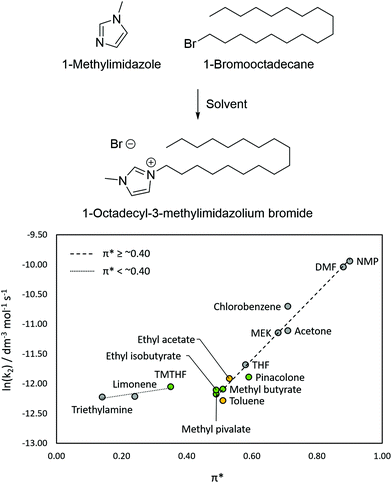
The Menshutkin reaction was the first model reaction to be used to correlate solvent polarity with reaction rate.31–33 The reaction of triethylamine with iodethane to form the quaternary ammonium salt was the original probe reaction and it was found that high solvent dipolarity increased the reaction rate due to transition state stabilisation.31 More recently, a similar reaction between 1-methylimidazole with 1-bromooctane (C8) was exploited for the characterisation of the bio-based dipolar aprotic solvents, cyrene32 and N-butylpyrrolidinone (NBP),33 in comparison with a selection of traditional dipolar protic solvents.Although not dipolar aprotic solvents, the four candidates can be compared to toluene in terms of their π* using a similar Menschutkin reaction. The Menschutkin reaction between 1-methylimidazole and the longer chained 1-bromooctadecane (C18) was used in this work to compare the esters and ketone with toluene and ethyl acetate due to the insolubility of the C8 product, 1-octyl-3-methylimidazolium bromide, in the lower polarity candidate solvents (Fig. 5). The integrated second-order rate equation was used to calculate the reaction rate based on peak integration using 1H NMR spectroscopy.
A selection of other solvents was also used to generate the LSER: NMP, DMF, MEK, acetone, chlorobenzene, THF, triethylamine, TMO and limonene. The LSER in Fig. 5 shows a correlation between rate and π* among the solvents with π* ≥ ∼0.4, which plateaus for solvents with π* < ∼0.4 (triethylamine, limonene and TMO). The cause of the plateau in low polarity solvents is likely to be due to micelle formation due to the highly charged “head” and lipophilic “tail” of the product.52 The charged centre of the micelle could host reactions between trapped 1-methylimidazole and 1-bromooctadecane at a rate greater than what would be expected in low π* solvents. The reaction rates in each of the esters was very similar and all were slightly higher than that of toluene. The reaction rate in toluene was slightly slower than what would be expected in a solvent with π* = 0.51 according the LSER in Fig. 5. This can be explained by toluene's high polarisability concealing its low dipolarity when measured using solvatochromic dyes, but which is revealed when measured in the Menschutkin reaction. The rate of reaction in pinacolone was slightly quicker than the candidate esters, consistent with its higher π* (0.59), although this rate of reaction was still slightly slower than what would be expected according to the LSER; the LESR would suggest a rate similar to that of THF. This could potentially be explained by the presence of a small amount of the enol form of pinacolone (Fig. S5, ESI†). Protic solvents are known to inhibit the reaction rate severely due to stabilising interactions with 1-methylimidazole.53 1-Methylimidazole could in turn stabilise the enol form of pinacolone just enough to cause the slight reduction in reaction rate observed in Fig. 5.
Overall, despite limitations in the use of the Menschutkin reaction to assess the polarity of lower polarity solvents (π* < 0.4), it was useful in comparing the esters and pinacolone to toluene in terms of their π* and highlighted their similarities. In spite of pinacolones higher π*, it was shown to behave more like a lower polarity solvent such as ethyl acetate.
Application testing: pressure sensitive adhesive production
A radical-initiated polymerisation for the production of an industrial relevant commercial available pressure sensitive adhesive (PSA) was carried out using each of the four candidate solvents in comparison to toluene. A selection of acrylic monomers, which cannot be disclosed, were used in the polymerisation reaction. In general, PSA production requires high Mw polymers (>30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 but often far higher) and therefore solvent choice is important.54 VNP solvents such as toluene and hexane are commonly used for this application at present, but due to REACH restrictions on the use of toluene in adhesives and spraypaints3 and a CoRAP on hexane, greener alternatives are required.1
000 g mol−1 but often far higher) and therefore solvent choice is important.54 VNP solvents such as toluene and hexane are commonly used for this application at present, but due to REACH restrictions on the use of toluene in adhesives and spraypaints3 and a CoRAP on hexane, greener alternatives are required.1
The polymerisation results can be seen in Table 2. The target Mw of the polymer was 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, which all four candidates achieved. In addition, adhesion, cohesion and tack were found to be acceptable for use in PSAs. However, when ethyl isobutyrate was used as the solvent the conditions had to be altered as sufficiently high Mw's could not be obtained. It has previously been shown that chain transfer occurred when 2-methyltetrahydrofuran was used as the solvent in the radical-initiated polymerisation of butyl acrylate (100 g) and methyl acrylate (5 g), resulting in low Mw polymers.7 Therefore, chain transfer was suspected of being the cause of the lower Mw's in ethyl isobutyrate.
000 g mol−1, which all four candidates achieved. In addition, adhesion, cohesion and tack were found to be acceptable for use in PSAs. However, when ethyl isobutyrate was used as the solvent the conditions had to be altered as sufficiently high Mw's could not be obtained. It has previously been shown that chain transfer occurred when 2-methyltetrahydrofuran was used as the solvent in the radical-initiated polymerisation of butyl acrylate (100 g) and methyl acrylate (5 g), resulting in low Mw polymers.7 Therefore, chain transfer was suspected of being the cause of the lower Mw's in ethyl isobutyrate.
| Solvent |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/g mol−1 a/g mol−1 |
Conversionb/% | Adhesionc/cN 20 mm−1 | Cohesion/daysd | Tack/ge |
|---|---|---|---|---|---|
| a Measured by GPC at Nitto Europe. b Percent of solid polymer dissolved in solvent. c Measured by the breaking load test at Nitto Europe. d Measured by the shear adhesion test at Nitto Europe, values shown are the time of failure in days. e Measured by the rolling ball test at Nitto Europe. | |||||
| Methyl butyrate | 601![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
95.5 | 1506 | >10 | 655 |
| Ethyl isobutyrate | 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
97.6 | 1641 | >10 | 681 |
| Methyl pivalate | 581![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
95.2 | 1532 | >10 | 727 |
| Pinacolone | 589![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
96.3 | 1646 | >10 | 856 |
| Toluene | 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
94.6 | 1587 | >10 | 900 |
A proposed mechanism of radical formation resulting in chain termination in ethyl isobutyrate is shown in Fig. S6 (ESI†). A hydrogen atom can be abstracted from the solvent molecule, terminating the chain.55 The newly formed radical on the solvent molecule can also react with a radical on the polymer, again, terminating the chain.55 For chain transfer to the solvent to occur, stable radicals must be able to form on the solvent molecules.55 It was thought that if ethyl isobutyrate was undergoing chain transfer with the polymer, it may also undergo autoxidation to form peroxides, like ethers such as 2-MeTHF, and that this peroxide formation could be directly proportional to its radical formation.
To test this hypothesis, the four candidates were exposed to ultra-violet light and bubbling air, conditions which are known to promote peroxide formation, and the results can be seen in Table 3. Significant amounts of peroxide were formed in ethyl isobutyrate in three hours under the peroxide formation accelerating conditions (0 ppm → 10–30 ppm). Peroxides were also formed in the other candidates, but at a slower rate than ethyl isobutyrate. The rate of peroxide formation in methyl pivalate was 0 ppm → 3–10 ppm, methyl butyrate was 0 ppm → 3 ppm in three hours, and in pinacolone was 3 ppm → 10–30 ppm (3 ppm peroxide was present in the bottle of pinacolone before use). The rate of peroxide formation reflects the Mw's obtained in the polymerisation tests. Ethyl isobutyrate was subject to the greatest chain transfer, resulting in the lowest Mw's, and quickest peroxide formation. As such, ethyl isobutyrate may not be ideal for the production of this type of polymer, however, it could be useful for the production of lower Mw polymers or non-radical-initiated polymers and remains an excellent candidate to replace toluene in many applications.
| Solvent | Experiment | T = 0 hours (ppm) | T = 3 hours (ppm) | |||
|---|---|---|---|---|---|---|
| a Tested using QUANTOFIX® Peroxide 100 test strips. | ||||||

|
Ethyl isobutyrate | Control |

|
0 |

|
0 |
| Test |

|
0 |

|
10–30 | ||
| Methyl butyrate | Control |

|
0 |

|
0 | |
| Test |

|
0 |

|
3 | ||
| Methyl pivalate | Control |

|
0 |

|
0 | |
| Test |

|
0 |

|
3–10 | ||
| Pinacolone | Control |

|
3 |

|
3 | |
| Test |

|
3 |

|
10–30 | ||
| Ethyl acetate | Control |

|
0 |

|
0 | |
| Test |

|
0 |

|
1–3 | ||
| MEK | Control |

|
0 |

|
0 | |
| Test |

|
0 |

|
3–10 | ||
It is important to note that while peroxide formation in solvents is undesirable, peroxides were only observed under extreme conditions (air and UV light) for each of the esters. As a comparison, ethyl acetate and MEK, two commonly used solvents, were exposed to the same conditions where they too were found to form peroxides (0 ppm → 1–3 ppm and 0 ppm → 3–10 ppm respectively, Table 3). No peroxide formation was observed in any of the esters after one year of storage in a glass bottle in ambient conditions. Some peroxide formation was observed in pinacolone in the same storage conditions, so it is recommended to take necessary precautions in its use (use of peroxide formation inhibitor).
Green credentials
Limited toxicity data is publicly available in the literature for the candidate solvents but, what is available can be seen in Table S2 (ESI†). The LD50 for rats (administered orally) is only available for pinacolone and is 611 mg kg−1, which is very similar to that of toluene, 636 mg kg−1. All candidates passed an Ames mutagenicity test (Fig. S2†). The IGC50 for Tetrahymena pyriformis (48 h) for each of the candidates are similar (1800–2772 mg L−1) and are far superior to that of toluene (52 mg L−1). The LC50 for the fathead minnow (96 h) is only available for pinacolone (87 mL L−1) and is better than that of toluene (34 mL L−1). Further in vitro and in silico investigations are currently underway to verify their beneficial safety profile. Eventually, a final testing proposal will be established to meet REACH requirements.56The four candidates can be produced via a catalytic route (principle 7) from renewable resources (principle 9): glycerol from used cooking oil, isobutene and ethanol from fermentation and CO and methanol from syngas. The atom economies of each of the proposed synthetic routes are high (>80% in all cases), with water and carbon dioxide as benign by-products (principle 2). The low Tb's of each candidate allows facile removal by evaporation, reducing the energy demand where products are isolated by such means (principle 6).
Conclusions
In conclusion, a “top-down” approach, focussing on esters and ketones was utilised to identify greener solvents with the potential to replace hazardous VNP solvents such as toluene and hexane. Four top candidates – methyl butyrate, ethyl isobutyrate, methyl pivalate and pinacolone – were identified as the best candidates. Each candidate was characterised in terms of its solubility (HSP and KAT parameters), physical (Tb, Tm, density), flammability (AIT and LEL), and toxicity (Ames test) properties, where they were all shown to have excellent potential to replace traditional VNP solvents. Despite being esters and ketones, each demonstrated their similarity to traditional VNP solvents in terms of their solubility properties by their ability to dissolve natural rubber, a particularly low-polarity solute. This was reinforced by their performance in a model Menschutkin reaction, where their performance was found to be similar to that of toluene.In a radical-initiated polymerisation of acrylic monomers for the production of PSAs, each of the four candidates were found to produce PSAs of comparable quality to toluene. Some interesting effects were noticed in ethyl isobutyrate which were explained using peroxide tests. It was found that ethyl isobutyrate formed peroxides at a faster rate than other commonly-used esters and ketones and this is suspected of being linked to its poorer performance in the radical-initiated polymerisation. As such, the use of antioxidant additives is recommended for ethyl isobutyrate. Peroxide formation was also observed in the other candidates, but this is common in many traditional solvents in harsh conditions, demonstrated by a comparison with ethyl acetate and MEK. Importantly, each of the four candidates can be synthesised via a catalytic route from potentially renewable resources, thus enhancing their green credentials. Overall, these esters and ketones of low polarity and volatility provide several options for industry for the replacement of hazardous VNP solvents for a range of processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Nitto Belgium NV for funding the project. This publication also includes results from the ReSolve project. This project has received funding from the Bio-Based Industries Joint Undertaking under the European Union‘s Horizon2020 research and innovation programme under agreement No 745450. The publication reflects only the authors’ view and the JU is not responsible for any use that may be made of the information it contains. The authors would also like to thank Dr James Sherwood and Dr Con Robert McElroy for discussions throughout this work; the Biorenewable Development Centre (BDC) for the use of their lab where Ames testing was carried out and Emma Chotin for work carried out during a summer internship.Notes and references
- n-Hexane – substance information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.003.435, (accessed 12 September 2016).
- Cyclohexane – substance information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.003.461?_disssubsinfo_WAR_disssubsinfoportlet_backURL=https%3A%2F%2Fecha.europa.eu%2Fsearch-for-chemicals%3Fp_p_id%3Ddisssimplesearch_WAR_disssearchportlet%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn-1%26p_p_col_count%3D1%26_disssimplesearch_WAR_disssearchportlet_sessionCriteriaId%3DdissSimpleSearchSessionParam101401493666964251, (accessed 1 May 2017).
- Toluene – substance information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.003.297, (accessed 2 March 2017).
- Benzene – substance information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.000.685, (accessed 4 April 2017).
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- T. J. Farmer and M. Mascal, in Platform Molecules, Chichester, UK, 2nd edn, 2015 Search PubMed.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer, J. H. Clark and A. J. Hunt, Green Chem., 2017, 19, 3671–3678 RSC.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18, 288–296 RSC.
- M. J. Molina and F. S. Rowland, Nature, 1974, 249, 810–812 CrossRef.
- S. Jin, F. Byrne, C. R. McElroy, J. Sherwood, J. H. Clark and A. J. Hunt, Faraday Discuss., 2017, 202, 157–173 RSC.
- Dichloromethane – substance Information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.000.763, (accessed 7 March 2017).
- Chloroform – substance information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.000.603, (accessed 7 March 2017).
- J. H. Clark, D. J. Macquarrie and J. Sherwood, Green Chem., 2012, 14, 90–93 RSC.
- A. Medvedovici, S. Udrescu and V. David, Biomed. Chromatogr., 2013, 27, 48–57 CrossRef PubMed.
- M. A. Martín-Luengo, M. Yates, M. J. Martínez Domingo, B. Casal, M. Iglesias, M. Esteban and E. Ruiz-Hitzky, Appl. Catal., B, 2008, 81, 218–224 CrossRef.
- T. H. M. Petchey, J. W. Comerford, T. J. Farmer, D. J. Macquarrie, J. Sherwood and J. H. Clark, ACS Sustainable Chem. Eng., 2018, 6, 1550–1554 CrossRef.
- D. F. Aycock, Org. Process Res. Dev., 2006, 11, 156–159 CrossRef.
- Ethyl acetate – Pubchem, https://pubchem.ncbi.nlm.nih.gov/compound/ethyl_acetate, (accessed 13 June 2017).
- Pubchem, butyl acetate, https://pubchem.ncbi.nlm.nih.gov/compound/31272, (accessed 20 September 2017).
- Toluene – Pubchem, https://pubchem.ncbi.nlm.nih.gov/compound/toluene, (accessed 10 February 2017).
- A. Alves Costa Pacheco, J. Sherwood, A. Zhenova, C. R. McElroy, A. J. Hunt, H. L. Parker, T. J. Farmer, A. Constantinou, M. De bruyn, A. C. Whitwood, W. Raverty and J. H. Clark, ChemSusChem, 2016, 9, 3503–3512 CrossRef PubMed.
- L. Moity, V. Molinier, A. Benazzouz, B. Joossen, V. Gerbaud and J.-M. Aubry, Green Chem., 2016, 18, 3239–3249 RSC.
- L. Moity, M. Durand, A. Benazzouz, C. Pierlot, V. Molinier and J.-M. Aubry, Green Chem., 2012, 14, 1132–1145 RSC.
- L. Moity, V. Molinier, A. Benazzouz, R. Barone, P. Marion and J.-M. Aubry, Green Chem., 2014, 16, 146–160 RSC.
- L. Moity, A. Benazzouz, V. Molinier, V. Nardello-Rataj, M. K. Elmkaddem, P. de Caro, S. Thiébaud-Roux, V. Gerbaud, P. Marion and J.-M. Aubry, Green Chem., 2015, 17, 1779–1792 RSC.
- M. Bandres, P. de Caro, S. Thiebaud-Roux and M.-E. Borredon, C. R. Chim., 2011, 14, 636–646 CrossRef.
- R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef.
- M. J. Kamlet and R. W. Taft, J. Am. Chem. Soc., 1976, 98, 377–383 CrossRef.
- M. J. Kamlet, J. L. Abboud and R. W. Taft, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef.
- N. Menschutkin, Z. Phys. Chem., 1890, 6U, 41–57 Search PubMed.
- J. Sherwood, M. D. Bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, Green Chem., 2016, 18, 3990–3996 RSC.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press, 2nd edn, 2012 Search PubMed.
- C. M. Hansen and S. Abbott, Hansen Solubility Parameters in Practice, Hansen-Solubility, 2008 Search PubMed.
- Pubchem, tert-butyl acetate, https://pubchem.ncbi.nlm.nih.gov/compound/10908, (accessed 20 September 2017).
- Pubchem, tert-butyl pivalate, https://pubchem.ncbi.nlm.nih.gov/compound/519272, (accessed 20 September 2017).
- Pubchem, pentyl valerate, https://pubchem.ncbi.nlm.nih.gov/compound/62433, (accessed 20 September 2017).
- Pubchem, hexamethylacetone, https://pubchem.ncbi.nlm.nih.gov/compound/13152, (accessed 20 September 2017).
- J. Sangster, J. Phys. Chem. Ref. Data, 1989, 18, 1111–1229 CrossRef.
- B. N. Ames, F. D. Lee and W. E. Durston, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 782–786 CrossRef.
- B. N. Ames, W. E. Durston, E. Yamasaki and F. D. Lee, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 2281–2285 CrossRef.
- W. B. Neely, D. R. Branson and G. E. Blau, Environ. Sci. Technol., 1974, 8, 1113–1115 CrossRef.
- ECHA, Guidance on Information Requirements and Chemical Safety Assessment – Chapter R.11: PBT/vPvB assessment, https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf/a8cce23f-a65a-46d2-ac68-92fee1f9e54f, (accessed 16 May 2017).
- T. Coskun, C. M. Conifer, L. C. Stevenson and G. J. P. Britovsek, Chem. – Eur. J., 2013, 19, 6840–6844 CrossRef PubMed.
- A. E. Díaz-Álvarez, J. Francos, B. Lastra-Barreira, P. Crochet and V. Cadierno, Chem. Commun., 2011, 47, 6208–6227 RSC.
- J. R. Dodson, T. D. C. M. Leite, N. S. Pontes, B. Peres Pinto and C. J. A. Mota, ChemSusChem, 2014, 7, 2728–2734 CrossRef PubMed.
- J. I. García, H. García-Marín, J. A. Mayoral and P. Pérez, Green Chem., 2010, 12, 426–434 RSC.
- Global Bioenergies, Glob. Bioenergies, 2015 Search PubMed.
- R. L. Cryberg and R. M. Bimber, SDS Biotech Corporation, US4570021A, 1986 Search PubMed.
- A. V. Ignatchenko, M. M. King and Z. Liu, US20070088181A1, 2007 Search PubMed.
- J. H. Fendler, Acc. Chem. Res., 1976, 9, 153–161 CrossRef.
- J. Sherwood, PhD Thesis, University of York, 2013.
- G. Bossaert, C. Hoebers, B. Forier, F. Byrne, A. J. Hunt, T. J. Farmer and J. H. Clark, WO2018033634 (A1), 2018.
- S. D. Tommaso, P. Rotureau, O. Crescenzi and C. Adamo, Phys. Chem. Chem. Phys., 2011, 13, 14636–14645 RSC.
- Understanding REACH – ECHA, https://echa.europa.eu/regulations/reach/understanding-reach, (accessed 12 February 2017).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc01132j |
| This journal is © The Royal Society of Chemistry 2018 |