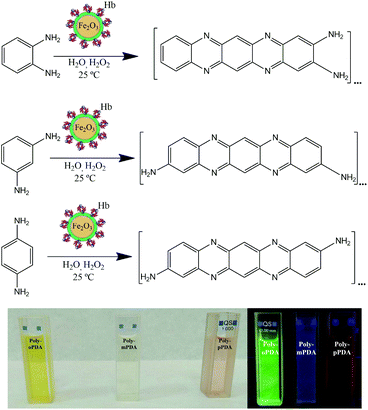
Synthesis of carbon-based fluorescent polymers driven by catalytically active magnetic bioconjugates†
Daily
Rodríguez-Padrón
 a,
Alexander D.
Jodlowski
a,
Alexander D.
Jodlowski
 b,
Gustavo
de Miguel
b,
Gustavo
de Miguel
 b,
Alain R.
Puente-Santiago
b,
Alain R.
Puente-Santiago
 *a,
Alina M.
Balu
*a,
Alina M.
Balu
 a and
Rafael
Luque
a and
Rafael
Luque
 *a
*a
aDepartamento de Química Orgánica, Grupo FQM-383, Instituto Universitario de Investigación de Quimica Fina y Nanoquimica (IUIQFN), Universidad de Cordoba, Campus de Rabanales, Edificio Marie Curie (C-3), Ctra Nnal IV-A, Km 396, E14014, Cordoba, Spain. E-mail: q62alsor@uco.es; apuentesantiago@gmail.com
bDepartamento de Química Física, Instituto Universitario de Investigación de Quimica Fina y Nanoquimica (IUIQFN)Universidad de Cordoba, Campus de Rabanales, Edificio Marie Curie (C-3), Ctra Nnal IV-A, Km 396, E14014, Cordoba, Spain
First published on 29th November 2017
Abstract
Bioconjugates based on a redox protein and iron oxide magnetic nanoparticles were employed in the catalytic polymerization of ortho-, meta- and para-substituted phenylenediamines at room temperature for the synthesis of carbon-based fluorescent polymers. UV-Vis absorption measurements of the three obtained products showed a red shift compared to the starting materials. These results together with the FT-IR and XPS analyses confirm the successful formation of the polymers. In particular, the component quantification in the C 1s XPS spectra revealed the high proportion of C–N bonds, associated with the oxidative polymerization of the precursors. MALDI-TOF MS analysis was performed in order to determine the molecular weights of the products. The synthesized poly-oPDA, poly-mPDA and poly-pPDA resulted to have a highly green, blue and red fluorescence, respectively. The reusability of the biocatalyst and the effect of the pH were investigated in the reaction for the ortho isomer. The biocatalytic system showed optimum results when the pH was below the enzyme isoelectric point (pI).
Introduction
The development of nanotechnology-inspired biocatalytic systems has gained a lot of interest in the last few years due to their importance in the design of biosensors,1,2 bioelectronic devices3,4 or biofuel cells.5,6 Particularly, the use of magnetic nanoparticles (MNPs) as versatile nanoscaffolds to immobilize enzymes has been widely used to design novel bioreactors which show high catalytic yields in different types of processes.7–9 Advantageously, they allow the use of an external magnetic field to retrieve the catalysts and subsequently reuse them in multiple reactions.10,11Among the enzyme-catalyzed reactions reported in the literature, the oxidative polymerization of aromatic diamines has been scarcely studied. A pioneering work reported a horseradish peroxidase (HRP) catalyzing, in the presence of hydrogen peroxide, the oxidation of aromatic diamines by an oxidative free-radical coupling mechanism.12,13 The attempts using other heme-containing proteins have also been successful. Tsutomu and coworkers developed a nanostructural environment catalytically activated system based on cytochrome C solubilized in reverse micelles for the oxidative catalysis of the o-phenylenediamine (o-PDA).14 In addition, Yimin Zhu et al. have synthesized a trimer from the oxidative polymerization of the o-PDA catalyzed by cytochrome C and H2O2.15 The mechanism of these metalloenzyme-catalyzed oxidative polymerizations is based on the oxidation of the metalloenzymes by H2O2 to obtain a metalloprotein–oxygen complex, which subsequently reacts with an aromatic diamine monomer to form a monomer radical and a Hb–OH compound (complex II). The monomer radical is also formed by the reaction between Hb–OH and the monomer, allowing the regeneration of the enzyme (Scheme S1, ESI†).16
The facile and environmentally friendly synthesis of carbon-based fluorescent nanoparticles still remains a significant challenge.17 The unique optical and low/non-toxic properties of these nanomaterials can be further applied to construct optoelectronic devices.18,19 Herein, our research group has designed for the first time a one-step synthesis of highly luminescent carbon based polymers at room temperature via the biocatalytic activity of magnetic nanobioconjugates (Scheme 1). As a consequence, novel fluorescent polymers have been synthesized by the oxidative polymerization of o-PDA, m-PDA and p-PDA precursors using a hybrid redox protein–MNP assembly based on hemoglobin immobilized on dopamine (DA) coated Fe2O3 nanoparticles (Hb-DA-Fe2O3).
Materials and methods
The synthesis of the biocatalyst was carried out by mechanochemical milling processes using horse hemoglobin (Hb) and dopamine hydrochloride (DA-HCl), together with pre-synthesized Fe2O3 magnetic nanoparticles (MNPs). A nanobiocatalyst has been broadly characterized by XRD, XPS, DLS, UV-Vis, IR spectroscopy and TEM (Fig. S1–S5, ESI†). Additionally, magnetic susceptibility measurements were performed, confirming the interesting magnetic properties of the nanobioconjugates (Table S1, ESI†). This synthetic protocol has been previously reported by our group for the successful preparation of nanobioconjugates.20 Subsequently, a simple protocol has been developed for the synthesis of fluorescent nanomaterials based on o-PDA, m-PDA and p-PDA. The polymerization reaction was carried out at room temperature for 24 h using 544 mg of each isomer and 60 μL of H2O2, 50 mg of the catalyst and 8 mL of H2O as the solvent, respectively. The samples were purified by silica gel column chromatography with gradient elution using ethyl acetate and cyclohexane as eluents. Finally, the products were dried via rotaevaporation. The three poly-oPDA, poly-mPDA and poly-pPDA were obtained in 26, 10 and 11 wt% yields, respectively. A full characterization of the materials was performed by TEM, MALDI-TOF MS, FT-IR, XPS, 13C-NMR, UV-Vis absorption and fluorescence spectroscopy.Results and discussion
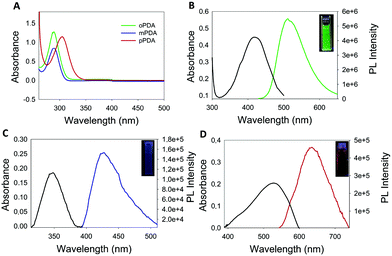
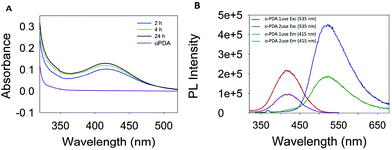
The morphology of the synthesized polymers was determined by TEM analysis. The micrographs (Fig. S6, ESI†) depict a homogeneous distribution with a mean radius of around 12 nm. Fig. 1 shows the UV-Vis absorption spectra of the three obtained products with characteristic absorption peaks at 422, 346 and 525 nm for the poly-oPDA, poly-mPDA and poly-pPDA, respectively.These absorption signals are significantly different from those of the corresponding starting materials (Fig. 1A), indicating the formation of π-conjugated systems in the polymerization process. The absorption bands can be ascribed to n → π* electronic transitions suggesting the formation of carbon based nanomaterials with smaller electronic bandgaps than the phenylenediamines.21 The fluorescence spectra of the obtained polymers were recorded to study in detail their photoluminescence (PL) properties (Fig. 1). Interestingly, the products showed different emission maxima at 520, 425, and 638 nm for the poly-oPDA, poly-mPDA and poly-pPDA, respectively (under a single excitation wavelength of 365 nm). Additionally, the emission spectra of the Hb-DA-Fe2O3 nanobiocatalyst were recorded for comparison (Fig. S7, ESI†). The PL lifetimes of the poly-oPDA, poly-mPDA and poly-pPDA were measured showing mono-exponential profiles with lifetimes of 1.78 ± 0.1 (λem = 402), 2.04 ± 0.1 (λem = 372) and 0.6 ± 0.1 ns (λem = 440), respectively. Additionally, the fluorescence quantum yields (QY) of 5, 2 and 0.01% were obtained for poly-oPDA, poly-mPDA and poly-pPDA, respectively (Table S2, ESI†). In particular, the poly-oPDA resulted to be a potential candidate for its use in the future development of optoelectronic devices, with a quantum yield in solution similar to others reported in the literature for these types of compounds synthesized using environmentally unfriendly approaches.22
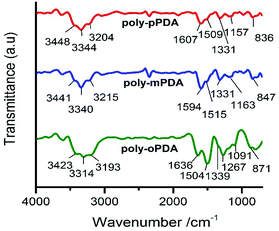
The infrared spectra of the poly-oPDA, poly-mPDA and poly-pPDA showed characteristic bands between 3200 and 3450 cm−1, which can be attributed to the N–H stretching vibrations of the –NH2 groups (Fig. 2). The signal at 1500–1510 cm−1 is associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. Significantly, peaks appearing at about 1330–1339 cm−1 can be attributed to the aliphatic C–N
C stretching vibration. Significantly, peaks appearing at about 1330–1339 cm−1 can be attributed to the aliphatic C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) stretching vibrations, which imply the intermolecular cyclization of the o-PDA, m-PDA and p-PDA precursors. Additionally, bands in the range of 830–870 cm−1 can be linked to a ring hydrogen deformation vibration isolation.23,24
stretching vibrations, which imply the intermolecular cyclization of the o-PDA, m-PDA and p-PDA precursors. Additionally, bands in the range of 830–870 cm−1 can be linked to a ring hydrogen deformation vibration isolation.23,24
XPS analysis indicated the presence of C, N and O in the three obtained polymers (Fig. S8, ESI†). The elemental quantification was performed and it is reported in Table 1, displaying similar percentages for each element in the three products.
| Product | C (%) | N (%) | O (%) |
|---|---|---|---|
| Poly-oPDA | 74.90 | 11.93 | 13.17 |
| Poly-mPDA | 78.72 | 12.73 | 8.55 |
| Poly-pPDA | 74.49 | 8.24 | 17.26 |
The deconvoluted C 1s XPS spectra exhibited three different contributions associated with the presence of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic carbon), C–N and C–O (carboxylate). Noticeably, the component quantification in this region reveals a high percentage of C–N bonds (Table S3, ESI†). This result strongly supports the successful polymerization of the precursor materials and corroborates the IR results. In addition, the N 1s spectra can be deconvoluted in two peaks, corresponding to the pyrimidinic and amino groups (Fig. 3). These results are in good agreement with others reported in the literature for similar materials, taking into account the different synthetic metodologies.2113C-NMR characterization was additionally performed for the three solid products, showing a signal above 140 ppm, that can be ascribed to C
C (aromatic carbon), C–N and C–O (carboxylate). Noticeably, the component quantification in this region reveals a high percentage of C–N bonds (Table S3, ESI†). This result strongly supports the successful polymerization of the precursor materials and corroborates the IR results. In addition, the N 1s spectra can be deconvoluted in two peaks, corresponding to the pyrimidinic and amino groups (Fig. 3). These results are in good agreement with others reported in the literature for similar materials, taking into account the different synthetic metodologies.2113C-NMR characterization was additionally performed for the three solid products, showing a signal above 140 ppm, that can be ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the quinoid units of the polymers.25 The peaks at ≈125–128 ppm clearly correspond to carbons in C–H bonds while peaks between 110 and 119 ppm can be assigned to the nitrogen-bonded carbon (C–NH) in the benzoic units (Fig. S9, ESI†).
N in the quinoid units of the polymers.25 The peaks at ≈125–128 ppm clearly correspond to carbons in C–H bonds while peaks between 110 and 119 ppm can be assigned to the nitrogen-bonded carbon (C–NH) in the benzoic units (Fig. S9, ESI†).
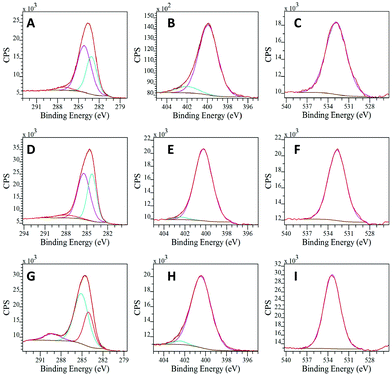 | ||
| Fig. 3 Deconvoluted XPS spectra of poly-oPDA for (A) C 1s, (B) N 1s and (C) O 1s, poly-mPDA for (D) C 1s, (E) N 1s and (F) O 1s and poly-pPDA for (G) C 1s, (H) N 1s and (I) O 1s. | ||
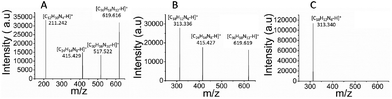
The three products of the polymerization of ortho-, meta- and para-substituted phenylenediamines were analyzed by MALDI-TOF MS in positive ion mode. The poly-oPDA spectrum revealed the presence of four oligomers: C12H10N4, C24H14N8, C30H16N10, and C36H18N12. Furthermore, poly-mPDA showed a similar polymer weight distribution in the presence of C18H12N6, C24H14N8, and C36H18N12 as can be observed in the respective mass spectra (Fig. 4). In particular, the poly-pPDA spectrum exclusively showed the presence of the corresponding trimer C18H12N6.
During the polymerization reaction, several samples were taken every 2 hours and characterized by UV-Vis absorption spectroscopy. As can be observed in Fig. 5A, the absorbance and therefore the product concentration did not change considerably after 4 h.
Moreover, the reusability of the catalyst was studied for the oxidative polymerization of the ortho isomer. After the first use, the catalyst was recovered by using a magnet, washed several times with H2O, and allowed to dry at 30 °C for 48 h. Once the catalyst was dry, the reaction was repeated under the same conditions. A decrease in the intensity of the emission and excitation spectra was observed, which can be attributed to the adsorption of organic species on the surface of the biocatalytic system (Fig. 5B). This assumption was further supported by FT-IR spectroscopy, which revealed a spectrum similar to that of poly-oPDA (Fig. S10, ESI†). The XPS quantification of Hb-DA-MNP after the reaction showed likewise a N/Fe ratio of 98 which is 3 times higher than that obtained for Hb-DA-MNP before the reaction (Fig. S11, Table S4, ESI†). In addition, UV-Vis analysis also confirmed the presence of oligomeric species adsorbed on the catalyst (Fig. S11, ESI†). Although, the intensity of the emission and excitation spectra decreases after the second use, the Hb-DA-Fe2O3 can be reused in the polymerization of o-PDA. These results together with the magnetic properties of this material, which allow its easy separation and manipulation, make it a good option as a catalyst for the polymerization of phenylenediamines. Remarkably, this work could represent a breakthrough in the scientific community and the beginning of novel environmentally friendly protocols for the synthesis of carbon based fluorescent nanomaterials.
All enzyme-coated NPs generally show striking pH-dependent colloidal stability properties.26–29 As a consequence, Z-potential measurements at different pH values have been performed in order to determine the pI of the Hb-DA-Fe2O3 (Fig. 6A). At low pH values, the Z-potential exhibits positive values between 7 and 9 mV and slowly changes to negative values at higher pH values, which give rise to pI = 7.3. Accordingly, in the pH range around the pI, the nanobioconjugates become unstable and start agglomerating and maintain good colloidal stability properties at pH > pI and pH < pI. UV measurements (Fig. 6B) reveal a significant enhancement of the absorbance in the poly-oPDA synthesized at pH = 4, while the absorbance when the reaction was carried out at pH = 7 and pH = 8, which are close to the pI, decreases significantly. Consequently, the catalytic yields in the polymerization are directly influenced by the colloidal stability of the nanobioconjugates and in turn by the pH environment. Also, it is worth noting that a red shift occurs in the maximum absorption at pH = 4 indicating that the conjugation in the poly-oPDA structure is extended at low polymerization pH values.30
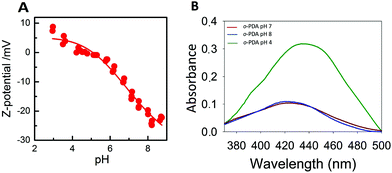 | ||
| Fig. 6 (A) Z-potential titration curve of the nanobioconjugates. (B) UV-Vis spectra of the polymerization of oPDA at different pH values. | ||
Conclusions
In summary, the Hb-DA-Fe2O3 biocatalyst turned out to be a potential candidate for its use in the oxidative polymerization of phenylenediamines. The combination of the redox properties of the protein together with the magnetic characteristics of the nanoparticles, which facilitate its reusability, could lead to a wide range of applications in catalyzed oxidation reactions. Remarkably, the reaction was performed at room temperature, being advantageous from an environmental point of view. The obtained polymers possess an interesting fluorescence behaviour, which could be further employed for the development of optoelectronic devices, which is currently under investigation in our research group.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. Luque gratefully acknowledges MINECO for funding under the project CTQ2016-78289-P.References
- M. Filice and J. M. Palomo, ACS Catal., 2014, 4, 1588 CrossRef CAS.
- I. Willner and B. Willner, Nano Lett., 2010, 10, 3805 CrossRef CAS PubMed.
- Y. Xiao, F. Patolsky, E. Katz, J. F. Hainfeld and I. Willner, Science, 2003, 299, 1877 CrossRef CAS PubMed.
- M. Dagys, A. Laurynenas, D. Ratautas, J. Kulys, R. Vidziunaite, M. Talaikis, G. Niaura, L. Marcinkeviciene, R. Meskys and S. Shleev, Energy Environ. Sci., 2017, 10, 498 CAS.
- C. Zhao, P. Gai, R. Song, Y. Chen, J. Zhang and J. J. Zhu, Chem. Soc. Rev., 2017, 46, 1545 RSC.
- J. Cheng, Y. Han, L. Deng and S. Guo, Anal. Chem., 2014, 86, 11782 CrossRef CAS PubMed.
- A. K. Vahidi, Y. Yang, T. P. N. Ngo and Z. Li, ACS Catal., 2015, 5, 3157 CrossRef CAS.
- S. C. Corgie, P. Kahawong, X. Duan, D. Bowser, J. B. Edward, L. P. Walker and E. P. Giannelis, Adv. Funct. Mater., 2012, 22, 1940 CrossRef CAS.
- J. Garcia, Y. Zhang, H. Taylor, O. Cespedes, M. E. Webb and D. Zhou, Nanoscale, 2011, 3, 3721 RSC.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Bassett, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- Y. Pan, X. W. Du, F. Zhao and B. Xu, Chem. Soc. Rev., 2012, 41, 2912 RSC.
- X. G. Li, M. R. Huang, W. Duan and Y. L. Yang, Chem. Rev., 2002, 102, 2925 CrossRef CAS PubMed.
- H. P. Dai, Q. H. Wu, S. G. Sun and K. K. Shiu, J. Electroanal. Chem., 1998, 456, 47 CrossRef CAS.
- T. Ono, K. Kawakami, M. Goto and S. Furusaki, J. Mol. Catal. B: Enzym., 2001, 11, 955 CrossRef CAS.
- Y. M. Zhu, J. H. Li, Z. M. Liu, G. J. Cheng, S. J. Dong and E. K. Wang, J. Mol. Catal. B: Enzym., 2001, 4, 33 CrossRef.
- D. Ichinohe, T. Muranaka, T. Sasaki, M. Kobayashi and H. Kise, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2593 CrossRef CAS.
- G. E. LeCroy, S. T. Yang, F. Yang, Y. Liu, K. A. S. Fernando, C. E. Bunker, Y. Hu, P. G. Luo and Y. P. Sun, Coord. Chem. Rev., 2016, 320, 66 CrossRef.
- F. L. Yuan, Z. B. Wang, X. H. Li, Y. C. Li, Z. A. Tan, L. Z. Fan and S. H. Yang, Adv. Mater., 2017, 29, 516 Search PubMed.
- H. Choi, S. J. Ko, Y. Choi, P. Joo, T. Kim, B. R. Lee, J. W. Jung, H. J. Choi, M. Cha, J. R. Jeong, I. W. Hwang, M. H. Song, B. S. Kim and J. Y. Kim, Nat. Photonics, 2013, 7, 732 CrossRef CAS.
- D. Rodriguez-Padron, A. R. Puente-Santiago, A. M. Balu, A. A. Romero and R. Luque, Chem. Commun., 2017, 53, 7635 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2015, 54, 5360 CrossRef CAS PubMed.
- C. Gu, N. Huang, Y. Wu, H. Xu and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 11540 CrossRef CAS PubMed.
- N. Kannapiran, A. Muthusamy, P. Chitra, S. Anand and R. Jayaprakash, J. Magn. Magn. Mater., 2017, 423, 208 CrossRef CAS.
- K. R. Das and M. J. Antony, Polymer, 2016, 87, 215 CrossRef.
- I. Amer and D. A. Young, Polymer, 2013, 54, 505 CrossRef CAS.
- M. Chanana, M. A. Correa-Duarte and L. M. Liz-Marzan, Small, 2011, 7, 2650 CrossRef CAS PubMed.
- M. S. Strozyk, M. Chanana, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Adv. Funct. Mater., 2012, 22, 1436 CrossRef CAS.
- I. Dewald, O. Isakin, J. Schubert, T. Kraus and M. Chanana, J. Phys. Chem. C, 2015, 119, 25482 CAS.
- J. Y. Zhang, A. R. Zhu, T. Zhao, L. Wu, P. Wu and X. D. Hou, J. Mater. Chem. B, 2015, 3, 5942 RSC.
- I. Losito, E. De Giglio, N. Cioffi and C. Malitesta, J. Mater. Chem., 2001, 11, 1812 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc03295a |
| This journal is © The Royal Society of Chemistry 2018 |