Mitigating oil spills in the water column†
Edward
Barry
ab,
Joseph A.
Libera
b,
Anil U.
Mane
b,
Jason R.
Avila
b,
David
DeVitis
c,
Keith
Van Dyke
c,
Jeffrey W.
Elam
 *b and
Seth B.
Darling
*b and
Seth B.
Darling
 *ad
*ad
aNanoscience & Technology Division, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: darling@anl.gov
bEnergy Systems Division, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: jelam@anl.gov
cNational Oil Spill Response Research & Renewable Energy Test Facility (Ohmsett), Leonardo, NJ 07737, USA
dInstitute for Molecular Engineering, Argonne National Laboratory, Argonne, IL 60439, USA
First published on 5th October 2017
Abstract
The scale and scope of uncontrolled oil spills can be devastating. Diverse marine environments and fragile ecologies are some of the most susceptible to the many ill effects, while the economic costs can be crippling. A notoriously difficult challenge with no known technological solution is the successful removal of oil dispersed in the water column. Here, we address this problem through cheap and reusable oil sorbents based on the chemical modification of polymer foams. Interfacial chemistry was optimized and subsequently tested in a simulated marine environment at the National Oil Spill Response Research & Renewable Energy Test Facility, Ohmsett. We find favorable performance for surface oil mitigation and, for the first time, demonstrate the advanced sorbent's efficiency and efficacy at pilot scale in extraction of crude oil and refined petroleum products dispersed in the water column. This is a potentially disruptive technology, opening a new field of environmental science focused on sub-surface pollutant sequestration.
Water impactOil spill countermeasures, techniques, and equipment have been deployed worldwide, but these traditional methods have limited effectiveness for surface slicks and virtually no impact on hydrocarbons in the water column itself. The advanced, reusable sorbents employed in this study demonstrate that such dispersed clouds of oil droplets can be effectively recovered, potentially revolutionizing oil spill response. |
Introduction
Beyond the extensive and easily identifiable surface slick in an oil spill such as the Exxon Valdez,1 the effects of oil dispersed in the water column can rival or exceed surface contamination in ecological devastation. In the aftermath of the Deepwater Horizon disaster, for example, toxic hydrocarbon concentrations in the abyssal, bathypelagic, and mesopelagic waters of the Gulf of Mexico were many times their pre-spill levels.2 Invisible to the eye, many of these ill effects are often left to run their course because the methods used to address surface slicks, which are often inefficient and carry their own ecological impacts,3,4 do not work below the water's surface. Microbial bioremediation of oil spills, whether purely natural or enhanced by nutrient additives, has proven successful in some environments,5,6 including coastal regions.7 Nonetheless, complete degradation can take years in more challenging environments,8 and even when biological processes are comparatively rapid, substantial ecological damage is inevitable without additional remediation strategies.A feasible solution entails the design of materials capable of selectively adsorbing one component (oil) over another (water), while meeting stringent practical considerations for scalability and deployment. Significant progress has been made in the design of advanced technologies and chemistries, but laboratory scale demonstrations rarely rely on scalable processes, and most sorption technologies are single-use. Aiming to overcome these issues, we turned to low-cost and reusable foam that could act as an effective sponge.
While many sorbents have been developed to address oil floating on the surface of water, capturing clouds of microscopic droplets of oil from within the water column is a fundamentally different process. Surface tension of the dispersed droplets must first be disrupted, and then the oil has to physically displace water that already fills the submerged sorbent's free volume. Here we achieve this goal by manipulating the interfacial chemistry of polymer foams to impart superoleophilicity.
Surface treatment of commercially available polymeric foam was described in a recent report from our group.9 Based upon this work, we utilized a two-step chemical modification scheme to retain the favorable characteristics of foam and introduce a suitable oleophilic chemistry: (i) sequential infiltration synthesis10–12 and (ii) the subsequent covalent binding of a superoleophilic compound. Here, we focused our attention on two types of open cell foam, polyurethane and polyimide. Sequential infiltration synthesis (SIS) was achieved through alternating exposures to vapors of an organometallic precursor (trimethylaluminum) and deionized H2O acting as the oxygen source in a homemade travelling-wave reactor. SIS treatment imparted the foams with a robust thin layer (∼10 nm) of aluminum oxide. The oxide coating subsequently served as a suitable binding site for a silicon-based oleophilic agent, (3-aminopropyl)triethoxysilane.
Results and discussion
Pure oil adsorption capacities of treated foams were measured in canola/olive oil, diesel fuel, and crude oil (Alaskan Northern Slope (ANS) and Hoover Offshore Oil Pipeline System (HOOPS)). Surface treatment was optimized for sorbent performance in pure solvent conditions, yielding adsorption capacities on the order of 30 g g−1 and 70 g g−1 for treated polyurethane (PU-T) and polyimide (PI-T) foams, respectively. As described previously, the greater adsorption capacity for this particular polyimide foam can be attributed to its greater native porosity.9,13In comparison with the untreated foams, SIS/oleophilic treatment introduced a 10× increase in adsorption capacity, increased the foam's oil-to-water selectivity by 8 fold (Fig. S1†), and greatly enhanced sorption kinetics (rate constants of 0.11 grams per s and 0.8 grams per s for untreated and treated, respectively).9 Oil adsorption capacities for treated foams remained fixed (>90%) when repeatedly tested for at least 10 adsorption/desorption cycles indicating no loss in performance over relevant testing scales (Fig. S2†).
As described below, reusability effectively normalizes a sorbent's sorption capacity, and serves as an important, yet often overlooked, parameter when describing performance metrics of advanced oil sorbents. In particular, the integrated performance of a reusable sorbent can often exceed single-use technologies with higher coefficients14–23 after only a few cycles, enhancing its practical real-world value.
Similarly, selectivity and the ability for the sorbent to retain performance in multi-component mixtures is essential. In laboratory scales, we compared adsorption capacities measured in pure solvents with those obtained in multi-component mixtures of oil and water. Using cubical 1′′ pieces of treated foam, adsorption and selectivity were investigated in relevant limits extending from over-saturated to under-saturated limits where the amount of oil deposited onto the water surface was more (and less) than could be adsorbed by a single piece of foam. Data are shown in the ESI† (Fig. S3).
Use of sorbents for mitigating sub-surface oil necessarily entails dragging the oil-soaked sorbent through nominally clean water en route to collection. To explore the ability of the sorbent to retain captured oil, we translated saturated foams at increasing speeds in a water bath (Fig. S4†). Here, our data demonstrated that oil remains effectively fixed within the foam's pores, unaffected by perturbations until reaching speeds of ∼20 knots.
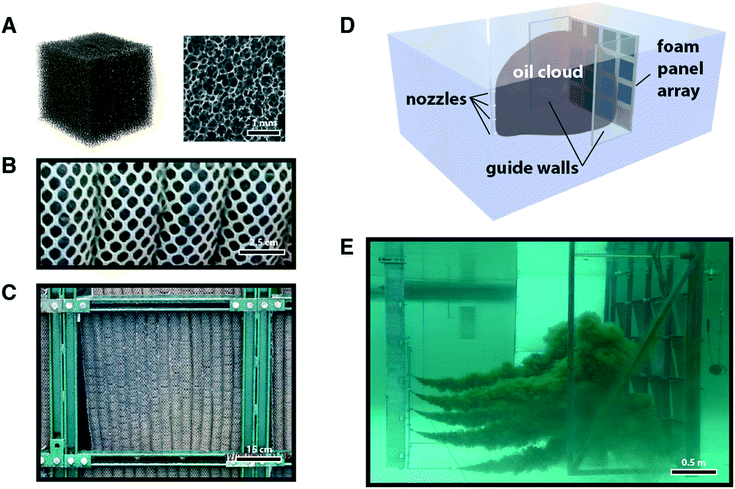
Scaled-up prototypes of the treated foam (see e.g.Fig. 1B and C) largely retained performance features in both pure solvent and surface adsorption testing, where adsorption coefficients on the order of 20 g g−1 and 50 g g−1 were measured for PU-T and PI-T pads, respectively (Table S1†).
The core question, however, was how these features would translate into a sorbent's ability to pull a diffuse cloud of dispersed/emulsified oil droplets from the water column. Oil droplets can remain suspended in the water column without the introduction of chemical dispersants when they are below some critical size (typically in the range of tens of microns) as determined by the physical and chemical properties of the fluids as well as the current patterns within the water. Oil leaking from a burst subsurface pipe will exhibit droplet sizes governed, in part, by the orifice diameter and the exiting pressure. Such dispersed microscopic oil droplets represent a fundamentally different physical system compared with surface oil slicks due to effects such as surface tension. When an oil droplet in the water column reaches an oil-saturated surface, the droplet's surface tension must be broken to cohere the constituent fluid to the previously sorbed oil.
For these reasons, it is not intuitive that there exists any correspondence between lab-scale testing environments and those conditions encountered in the field. To answer these questions, we turned to the National Oil Spill Response Research & Renewable Energy Test Facility, Ohmsett. Mesh bags with sewn channels housing treated foam were produced for a prototype with enough material to fill a 64 ft2 frame, representing a ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× scale up from the lab-scale testing (see Fig. S5†). Mesh bags were anchored onto a frame, and subsequently submerged within the seawater tank with the bottom of the frame at a depth of approximately eight feet.
000× scale up from the lab-scale testing (see Fig. S5†). Mesh bags were anchored onto a frame, and subsequently submerged within the seawater tank with the bottom of the frame at a depth of approximately eight feet.
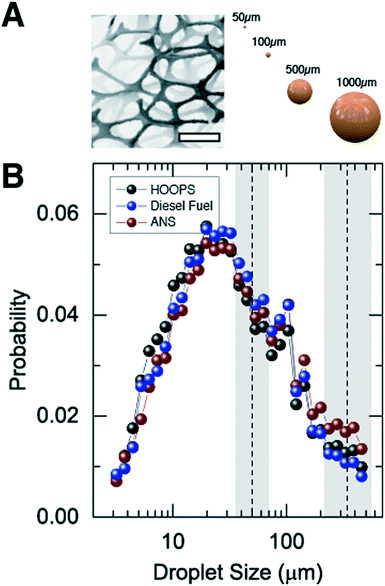
Clouds of microscopic droplets of crude oil and diesel fuel were delivered to the frame using a pressurized nozzle delivery system (see Fig. 1E). Testing conditions (pressure and nozzle size) were used to create a range of droplet sizes (see Fig. 2A), which remained neutrally buoyant in the water column for sufficient time to perform a sorption test. Individual sorption tests were carried out for a specified duration (typically on the order of ∼10 minutes) during which the foam pads were continuously exposed to a stream of oil or fuel dispersed vertically throughout the water column. We examined HOOPS and ANS, representative crude oils with different compositions and densities, as well as diesel fuel.
After completing each test, the foam pads were removed from the frame, and the adsorbed fluid was removed via mechanical wringing. Identical foam pads in individual rows were processed together in a single measurement. Extracted fluids were separated using centrifugation, after which the quantities of sorbed water and oil/fuel were determined. Complete data sets for individual tests are shown in the ESI† (Tables S2–S5).
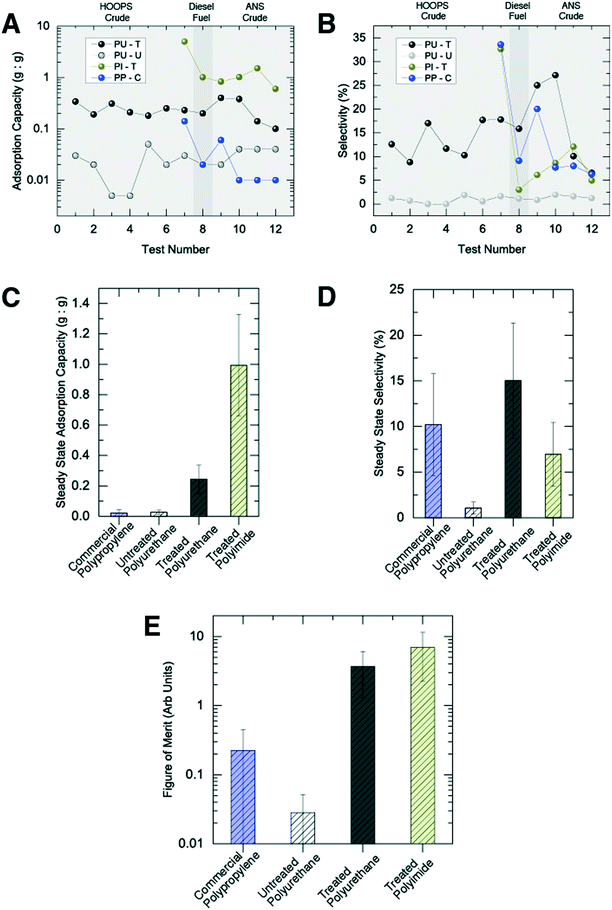
As no preexisting benchmarks exist for sorption of oil from the water column, we evaluated four different sorbent materials: untreated and treated polyurethane foams (PU-U and PU-T), treated polyimide foam (PI-T), and a commercial polypropylene sorbent (PP-C).
Performance was evaluated using three metrics: (i) adsorption capacity (the total mass of oil/fuel that can be adsorbed for a given mass of sorbent), (ii) selectivity (the ratio of extracted oil/fuel to total fluid volume) and (iii) reusability. Reusability was defined by the rate of change of the two distinct performance metrics, adsorption capacity and selectivity.
Similar to the behavior observed with surface oil sorption in laboratory testing, untreated polyurethane foam displayed poor adsorption characteristics in the water column (SPU-U ∼ 0.01 g g−1) and virtually no oil selectivity (φPU-U < 1%) (Fig. 3A and B). SIS/oleophilic treatment of polyurethane foams, however, increased the adsorption capacity by more than 10 fold (SPU-T ∼ 0.25 g g−1) and produced selectivity as high as 25% (φPU-T = 15 ± 6%) (Fig. S6†).
Not surprisingly, in comparison with values obtained in surface adsorption testing where the sorbents were exposed to substantially pure oil slicks, water-column adsorption coefficients and selectivity were significantly lower. We postulate that such behavior can be explained using observations from laboratory-scale testing in multi-component mixtures. Such tests identified that oil adsorption and selectivity were dependent on the amount of oil available to the foam. Based upon these data, we expect that, during water column testing, the quantity of oil exposure plays an important role in the ultimate performance. Oil dispersions in the Ohmsett tests represented relatively low concentrations (<1%). Support for such a conclusion can be found in the influence of operational testing conditions on the foam's sorption performance. For example, under a modest increase in bridge speed (0.1 to 0.2 knots), adsorption coefficients increased considerably (see Fig. S7†), with the larger speed translating to a greater rate of oil delivery to the foam and thereby yielding more adsorption.
Comparing treated polyurethane (PU-T) and polyimide foams (PI-T) (Fig. 3), the latter displayed a significantly enhanced adsorption coefficient in initial testing (SPI-T ∼ 5 g g−1), which dropped after subsequent runs (SPI-T ∼ 1 g g−1). The initially higher adsorption coefficient is associated primarily with the native porosity of the two foams, εPU ∼ 95% and εPI ∼ 98%, respectively,9,13 while the drop in performance is associated with mechanical properties of the polyimide foam. In particular, polyimide foam fails to recover to its initial volume following the first compression in the wringer, recovering only to ∼60% its original thickness.
Perhaps the most informative benchmark material is the commercial polypropylene (PP-C) sorbent. This is the industry standard material for oil-selective sorption. While the PP-C sorbent was able to retrieve some oil during its first test, it failed entirely with respect to reusability. In subsequent tests, the PP-C was only able to retrieve trace amounts of oil. It is effectively a single-use sorbent, which renders it impractical for large-scale spill mitigation.
In an effort to capture the concurrent needs for capacity, selectivity, and reusability, we herein establish a figure of merit for sorption of oil in the water column. For this, we consider only the “steady-state” capacity and selectivity for each sorbent, averaging the values for all tests following their initial use to reveal the reusability. While given operational situations might favor capacity over selectivity, or vice versa, here we simply use the product of these two parameters to provide a single figure of merit (Fig. 3E). It should be noted that the error bars on the oil adsorption capacity for PP-C and PU-U include a value of approximately zero—a scenario in which their figures of merit would also be effectively zero.
Fig. 4 provides a Venn diagram for a qualitative presentation of the placement of the various tested sorbents according to the three merit criteria. Untreated PU foam, while equally reusable as the treated PU foam, exhibited inadequate capacity and selectivity. Commercial PP sorbent displayed some oil sorption capacity and selectivity, but was almost entirely non-reusable. The two treated foams (PU-T and PI-T) both presented strong capacity, selectivity, and reusability, with the polyurethane-based material possessing superior mechanical properties suitable for repeated compression and reuse. While the capacity of the PU-T was lower than that of the PI-T, even in the steady state, its capacity was limited only by the original porosity of the starting material, which could readily be increased using widely available materials.
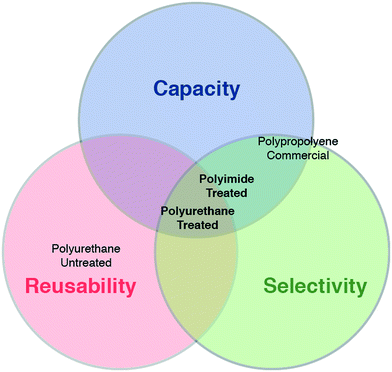 | ||
| Fig. 4 Evaluating sorbent performance using multiple metrics. Venn diagram summarizing water-column oil mitigation performance characteristics for the various sorbent materials. | ||
Experimental
Materials
Polyurethane (PU) and polyimide (PI) foam were obtained from a commercial supplier (McMaster-Carr, Elmhurst, IL) and used as received. PU was supplied as 1′′ × 1′′ × 24′′ strands. PI was cut into identically sized pieces using a knife. The commercial polypropylene sorbent was obtained from a commercial supplier (NewPig, Tipton, PA) in the form of a sorbent sock.SIS/oleophilic treatment
Foam was subjected to SIS/oleophilic treatment as described previously (5). For scaled-up production, we utilized a modified ALD/SIS travelling-wave reactor equipped with a 1.5 m-long deposition tube and an inside diameter of 3.75 inches. Four strands were loaded into the reactor and processed at the same time. SIS treatment was achieved using alternating exposures to an organometallic precursor, trimethylaluminum (SigmaAldrich, St. Louis, MO) and deionized H2O. Chemical vapors were swept through the travelling-wave reactor using a 300 sccm flow of N2 acting as the carrier gas. The base pressure of N2 without precursor vapor was 0.4 Torr. Precursor vapor was introduced for an exposure time of 10 s, bringing the total pressure (precursor pressure plus base pressure) to 0.7 Torr. Immediately after precursor exposure, pure N2 gas was used to purge the reactor for 45 s. To complete the SIS cycle, an identical exposure/purge process was used for distilled H2O, acting as the oxygen source for a 10 s exposure followed by a 120 s N2 purge. The process was repeated for a total of 70 cycles for PU foams and 30 cycles for PI foams.SIS-treated foams were subsequently functionalized by grafting an oleophilic compound, (3-aminopropyl)triethoxysilane (APTS, Sigma-Aldrich). APTS was dissolved to a final concentration of 1% in ethanol and foams were immersed overnight (8+ hours). Foams were then removed and washed in a pure ethanol bath. The washing procedure was repeated three more times using water.
System configuration for pilot-scale testing
In order to test performance in the Ohmsett large tow tank, treated foam was prepared to create a number of 24′′ square panels. Pieces of the foam with 1′′ cross section were secured within parallel sewn pockets of nylon mesh with grommets serving as attachment points on the top and bottom. The tops of the panels were secured to a steel lattice frame with sixteen positions available in a 4 × 4 array (Fig. 3). The bottoms of the panels were not attached to the frame, but were weighted using steel bars that allowed the individual panels to pivot outwards in a controlled fashion with increasing water flow while also counteracting the natural buoyancy of the foam. This array was mounted to one of the mobile bridges at the Ohmsett tank and held rigidly in place during testing.For PP-C, a foam pad was created using five strands of 3′′ diameter and 22′′ length socks, in which approximately 25% of the adsorbent material was removed to decrease the overall thickness and enable wringing using the same equipment as for the other panels.
Oil dispersion and testing conditions
Oil was introduced into the water column via an array of submerged nozzles. A container housed on the second tank bridge supplied the oil at high pressure (80 psi) into these nozzles to form a dispersed cloud of oil droplets spanning virtually the entire depth of the tank from surface to floor and with sufficient width to span the entire lattice of foam panels (∼8′). Two transparent plastic walls, one on each side, extended approximately four feet forward from the panel array to contain the oil cloud in the region proximal to the array. The two bridges moved together at a fixed distance of separation (10–13′) at controlled speeds of 0.1–0.2 knots, with the length of each test varied to explore effects related to oil concentration, oil volume, and potential leaching of adsorbed oil into the water.Pure oil sorption capacity
In order to quantify the maximum potential capacity for the panels of treated polyurethane foam, sorption measurements using pure oil were performed outside of the Ohmsett tank. Foam bags were fully immersed in a bucket, and left undisturbed for 5 minutes. The foam bag was then removed and the weight recorded. The foam bag was then allowed to drain for 1 minute and the weight recorded again. Table S1† provides the data obtained from these measurements.Mass corrections
In order to properly determine the oil sorption capacity of the various sorbents, it is imperative to correct for the fraction of the overall mass of each panel that is comprised of other materials (nylon straps, mesh bag, eyelets). Over the course of the testing at Ohmsett, four types of sorbent materials were investigated: untreated polyurethane (PU-U) foam, treated polyurethane foam (PU-T), treated polyimide (PI-T) foam, and commercial polypropylene (PP-C). Mass corrections for both PU and PI are straightforward to perform using known masses of the constituent pieces for each panel. In the case of the PP sorbent, values were estimated using vendor specifications.On average, a polyurethane (PU-U and PU-T) foam mesh bag weighed:
| MTOTAL = 0.9275 ± 0.043 lbs = 420.7 ± 19.5 grams |
The total weight of the bag contains contributions from nylon straps, the mesh bag, and eyelets, such that MTOTAL = MFoam + MMesh + MNylon + MEyelets.
The following values were used/measured for the nylon straps, mesh bag, and eyelets:
| MNylon = 4 × (1.4118 g in−1)(19 in) = 107.9 g |
| MMesh = = 80 g |
| MEyelets = 8 × 0.287 g = 2.3 g |
The weight of the foam is therefore:
| (MFoam)PU = 0.51 ± 0.037 lbs = 230.5 ± 17.0 grams |
For polyimide (PI-T), the weight of the foam was directly measured:
| (MFoam)PI = 0.126 ± 0.016 lbs = 57.2 ± 7.3 grams |
For commercial polypropylene (PP-C), the corresponding mass of sorbent material used was:
| (MFoam)PP ≈ 1.7 lbs = 760 grams |
These numbers were used to normalize sorption capacity data for direct comparison of performance among the various sorbent materials. For the capacity and selectivity calculations, we used the following approach:
(1) The volume of oil adsorbed per pad (mL) was converted to mass (moil) using the densities: 0.8461 g mL−1 (HOOPS), 0.8768 g mL−1 (ANS), and 0.8439 g mL−1 (diesel fuel)
(2) Oil adsorption capacity was calculated via:
| S(wt/wt) = moil/MFoam/Sock |
(3) Selectivity was calculated via:
| Selectivity(%) = mOil/(mOil + mWater) × 100 |
Sorption of oil from the water column
Twelve test runs were executed during the experimental campaign at Ohmsett, with each test varying one or more of the following parameters: type of oil, bridge speed, volume of oil, number of nozzles, spacing between bridges, types of sorbents in the array, and whether or not the array was dragged after oil dispensing was ceased. After each test, the pads were removed from the array and allowed to drain for varying periods of time, in the spirit of ASTM sorbent testing standards (see ASTM Standard F726: Test Method for Sorbent Performance of Adsorbents). During the draining period, no oil was observed to shed from the foam. In an operational setting, the water dripping from the foam as it is extracted from the water would simply return to the body of water, thereby reducing the need to store that additional volume of fluid.Once drained of this free water, the pads were run twice through a compression wringer to expunge sorbed fluid (both oil/fuel and water). Collected fluid was allowed to settle for a preliminary measurement of oil/fuel and water volumes. Subsequently, the fractions were centrifuged to separate out any residual oil/fuel in the water or residual water in the oil/fuel. The data tables listed in the ESI† (Tables S2–S5) describe the parameters used in each test as well as the final volumes of oil and water recovered.
Conclusions
In this report we present the first successful demonstration of a sorption technology capable of addressing the environmental scourge of oil in the water column. The results presented herein provide a set of benchmarks against which future materials and technologies for subsurface marine oil mitigation can be evaluated. One can imagine an operational implementation of reusable sorbents involving integration with a trawling system. Vessels towing trawling “nets” functionalized with PU-T or related sorbents would traverse dispersions of oil in the water column, subsequently winching the saturated sorbent nets onto the deck for compression and redeployment. Potential impact of dispersants on the performance of these sorbents is an important area to explore in future studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the United States Coast Guard and Bureau of Safety and Environmental Enforcement under Agreement #HSCG32-15-XR00006. The authors thank Alex Balsley and Kristi McKinney for helpful discussions throughout the course of this work. Use of the Center for Nanoscale Materials, an Office of Science user facility, was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.References
- L. Guterman, Science, 2009, 323, 1558–1559 CrossRef CAS PubMed.
- C. M. Reddy, J. S. Arey, J. S. Seewald, S. P. Sylva, K. L. Lemkau, R. K. Nelson, C. A. Carmichael, C. P. McIntyre, J. Fenwick, G. T. Ventura, B. A. S. Van Mooy and R. Camilli, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 20229–20234 CrossRef CAS PubMed.
- C. Teas, S. Kalligeros, F. Zanikos, S. Stournas, E. Lois and G. Anastopoulos, Desalination, 2001, 140, 259–264 CrossRef CAS.
- A. Bayat, S. F. Aghamiri, A. Moheb and G. R. Vakili-Nezhaad, Chem. Eng. Technol., 2005, 28, 1525–1528 CrossRef CAS.
- E. A. Dubinsky, M. E. Conrad, R. Chakraborty, M. Bill, S. E. Borglin, J. T. Hollibaugh, O. U. Mason, Y. M. Piceno, F. C. Reid, W. T. Stringfellow, L. M. Tom, T. C. Hazen and G. L. Andersen, Environ. Sci. Technol., 2013, 47, 10860–10867 CrossRef CAS PubMed.
- T. C. Hazen, E. A. Dubinsky, T. Z. DeSantis, G. L. Andersen, Y. M. Piceno, N. Singh, J. K. Jansson, A. Probst, S. E. Borglin, J. L. Fortney, W. T. Stringfellow, M. Bill, M. E. Conrad, L. M. Tom, K. L. Chavarria, T. R. Alusi, R. Lamendella, D. C. Joyner, C. Spier, J. Baelum, M. Auer, M. L. Zemla, R. Chakraborty, E. L. Sonnenthal, P. D'haeseleer, H.-Y. N. Holman, S. Osman, Z. Lu, J. D. Van Nostrand, Y. Deng, J. Zhou and O. U. Mason, Science, 2010, 330, 204–208 CrossRef CAS PubMed.
- W. F. M. Röling, M. G. Milner, D. M. Jones, K. Lee, F. Daniel, R. J. P. Swannell and I. M. Head, Appl. Environ. Microbiol., 2002, 68, 5537–5548 CrossRef.
- J. R. Bragg, R. C. Prince, E. J. Harner and R. M. Atlas, Nature, 1994, 368, 413–418 CrossRef CAS.
- E. Barry, A. U. Mane, J. A. Libera, J. W. Elam and S. B. Darling, J. Mater. Chem. A, 2017, 5, 2929–2935 CAS.
- Q. Peng, Y.-C. Tseng, S. B. Darling and J. W. Elam, Adv. Mater., 2010, 22, 5129–5133 CrossRef CAS PubMed.
- Q. Peng, Y.-C. Tseng, S. B. Darling and J. W. Elam, ACS Nano, 2011, 5, 4600–4606 CrossRef CAS PubMed.
- Y.-C. Tseng, Q. Peng, L. E. Ocola, J. W. Elam and S. B. Darling, J. Phys. Chem. C, 2011, 115, 17725–17729 CAS.
- J. Pinto, A. Athanassiou and D. Fragouli, J. Phys. D: Appl. Phys., 2016, 49, 145601 CrossRef.
- H. C. Bi, Z. Y. Yin, X. H. Cao, X. Xie, C. L. Tan, X. Huang, B. Chen, F. T. Chen, Q. L. Yang, X. Y. Bu, X. H. Lu, L. T. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- J. Y. Hong, E. H. Sohn, S. Park and H. S. Park, Chem. Eng. J., 2015, 269, 229–235 CrossRef CAS.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed.
- W. W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- L. Li, Z. Y. Liu, Q. Q. Zhang, C. H. Meng, T. R. Zhang and J. Zhai, J. Mater. Chem. A, 2015, 3, 1279–1286 CAS.
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770–4799 CrossRef CAS PubMed.
- Z. X. Xue, S. T. Wang, L. Lin, L. Chen, M. J. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS PubMed.
- J. K. Yuan, X. G. Liu, O. Akbulut, J. Q. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS PubMed.
- J. P. Zhao, W. C. Ren and H. M. Cheng, J. Mater. Chem., 2012, 22, 20197–20202 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data on sorbent performance, including videos showing underwater oil mitigation. See DOI: 10.1039/c7ew00265c |
| This journal is © The Royal Society of Chemistry 2018 |