Emerging investigator series: it's not all about the ion: support for particle-specific contributions to silver nanoparticle antimicrobial activity†
Lisa M.
Stabryla
 a,
Kathryn A.
Johnston
a,
Kathryn A.
Johnston
 b,
Jill E.
Millstone
b,
Jill E.
Millstone
 bcd and
Leanne M.
Gilbertson
bcd and
Leanne M.
Gilbertson
 *a
*a
aDepartment of Civil and Environmental Engineering, University of Pittsburgh, 3700 O'Hara Street, Pittsburgh, PA 15261, USA. E-mail: leanne.gilbertson@pitt.edu; Tel: +(412) 624 1683
bDepartment of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA
cDepartment of Chemical and Petroleum Engineering, University of Pittsburgh, 3700 O'Hara Street, Pittsburgh, PA 15261, USA
dDepartment of Mechanical Engineering and Materials Science, University of Pittsburgh, 3700 O'Hara Street, Pittsburgh, PA 15261, USA
First published on 5th July 2018
Abstract
Silver nanoparticles (AgNPs) and other ionizing engineered nanomaterials (ENMs) are candidates for the development of antimicrobial agents due to their efficacy, multiple modes of bacterial inactivation, and tunability with respect to both the magnitude and mechanisms of antimicrobial activity. Exploiting this versatility requires elucidating the bacterial inactivation pathway(s) of the ENM, and in particular, the link between material properties and the desired biological endpoint. The mechanisms of antimicrobial activity for macrosilver, Ag salts, and AgNPs have been widely studied, and largely attribute this activity to the release of Ag ions via oxidation and dissolution of the surface Ag atoms. However, it has also been established that Ag ion exposure alone does not elicit the same bacterial response as exposure to AgNPs, which suggests that the observed antimicrobial activity is induced not only by solubilized ions but also by the ENM itself. Resolving the role of the AgNP is critical to informing design of nano-enabled antimicrobials a priori. Herein, we present a systematic review of the AgNP antimicrobial activity literature and specifically focus on studies that scale Ag ion controls to the likely quantities of bioavailable Ag released from AgNPs. This literature selection criterion reveals the critical role of scaled ion controls in distinguishing ion and particle contributions to the observed antimicrobial activity. Overall, our analysis of this literature indicates that in most cases of bacteria exposure to AgNPs, particle-specific activity is observed and acts in concert with and/or independently from solubilized Ag ions alone. These results are exciting and suggest that more efficacious Ag- and ENM-enabled antimicrobials can be obtained through ENM design.
Environmental significanceWhile the antimicrobial activity of ionizing nanoparticles is largely attributed to the released ions, the role of the particle is not yet fully realized. This mechanistic ambiguity is not only due to complex exposure environments, but also inconsistencies in experimental designs, the most critical being the ion controls. We identify studies that scale Ag(I) ion controls to the concentration of Ag(I) released from AgNPs and find particle-specific effects acting jointly with and/or sometimes independently from the ions. This support for the particle suggests the ability to leverage manipulation of particle properties to further tune the mechanism and magnitude of AgNP antimicrobial activity. This strategic design of nano-enabled antimicrobial agents could positively impact global public health in this era of antimicrobial resistance. |
1. Introduction
Antimicrobial agents are crucial and ubiquitous in many industries, including health care,1–9 food and agriculture,6,7,10–12 water treatment,4,6–8,13 and drinking water distribution.4,6–8,13 An increasingly critical challenge associated with antimicrobial use is that the target microbe can build resistance over time.1,3,4,6,9,14–18 Silver nanoparticles (AgNPs) and other ionizing engineered nanomaterials (ENMs) are candidates for the development of superior antimicrobial agents due to their efficacy, multiple modes of organism inactivation, and tunability with respect to both the magnitude and mechanisms of antimicrobial activity. Exploiting the enhanced functionality of ENMs as well as ensuring that they combat rather than contribute to the global antimicrobial resistance challenge requires resolving their mechanisms of organism inactivation, particularly the link between material properties and the desired biological endpoint.Ag and AgNPs are among the oldest and most widely used antimicrobial agents,13,19,20 and their antimicrobial properties have been studied extensively.14,18 For example, dating back to 7000 years ago, Ag-lined vessels and Ag coins in containers of water or milk were used to preserve rations during military conflicts.9,21 Ag has also been used to treat ulcers and aid in wound healing.14,21 The antimicrobial mechanism(s) of Ag at the macroscale are attributed to the release of Ag(I) ions and their interactions with various aspects of the microbe.14,18,21 For example, Ag(I) ions can interact with sulfhydryl groups on the cell surface, where the subsequent formation of the Ag–S bonds blocks respiration and electron transfer, leading to the collapse of the proton motive force, the de-energizing of the membrane, and eventually cell death.4 The ionic radius of a Ag(I) ion is also sufficiently small (0.115 nm)22 to travel through transmembrane proteins such as porins (30–50 kDa; pore size, 1–3 nm).23 Once inside the cell, Ag(I) ions may react with thiol functional groups in proteins and nucleic acids, interfering with DNA replication and deactivating many enzymatic functions.4 In general, Ag(I) ions also increase the level of reactive oxygen species (ROS) inside the cell because thiol-containing anti-oxidative enzymes are deactivated by Ag, thus exacerbating the damage done to proteins, lipids, and nucleic acids.4
Compared to their macroscale counterparts, AgNPs exhibit enhanced ion release per unit mass mainly due to an increased surface area to volume ratio. Yet, the precise mechanism(s) of AgNP action remain unresolved, particularly the dynamic contributions of the NP and Ag(I) ion.14,18 There are three overarching possibilities for mechanisms of AgNP antimicrobial activity. The first is that AgNPs are simply a passive Ag(I) ion reservoir, and (as in the case of macroscopic Ag) released Ag(I) ions are responsible for any antimicrobial activity.14,24–30 The second possibility is that antimicrobial activity of AgNPs is a result of particle-only effects (e.g., physical disruption or alteration of the phospholipid cell membrane, production of ROS at the cell surface, or surface proteins that can bind to the NP but do not bind Ag(I) ions), putting into question the necessity of Ag(I) ions and their claim as the main agent of cellular impact.14,24,31,32 The third possibility is some combination of the first two mechanisms and/or some degree of synergistic effects between the released Ag(I) ions and the particle action. For example, there may be unique passive transport pathways in the bacterial cell (e.g., outer membrane porins, altered membrane permeability) accessible to particles at the nanoscale that facilitate the delivery of intracellular Ag(I) ions, a phenomenon known as the Trojan horse mechanism.14,28,33–36,37 Overall, the current literature contains support for all three possibilities. This ambiguity in the mechanisms of AgNP antimicrobial activity limits our ability to rationally design nanoparticle-enabled antimicrobials, and further, to leverage the material tunability of these ENMs.
While some of the existing mechanistic ambiguity is surely due to the complexity of the systems themselves, a non-trivial component arises from inconsistences in experimental designs across studies. Critical components of the experimental design include control of AgNP morphology (i.e. size, shape, surface chemistry), NP synthesis and washing steps, composition of the bacterial growth medium, bacterial strain used, measured toxicity endpoint and the methodology used to assess it,38 and the treatment of the pure Ag(I) ion control.
Of all of these parameters, the most critical for distinguishing particle-specific antimicrobial activity is arguably the measurement of and experimental controls for Ag(I) ion release. Ion controls are used in experiments analyzing the antimicrobial activity of ionizing ENMs in order to identify the biological effects that arise solely due to the ion portion of the ENM system and then compare to the effects of the ENM. Ion controls may be delivered to the experimental system as a Ag salt (e.g., AgNO3, AgC2H3O2, Ag2SO4) or as the isolated ions in the supernatant of a AgNP suspension. There are two key components to a pure Ag(I) ion control: the concentration of ions introduced to the bacteria and the kinetics of that introduction. The total concentration of Ag(I) ion used as a control should reflect, as closely as possible, the concentration of ions released from the NPs used in the study. Matching these concentrations requires the ability to measure released Ag(I) ions, which can be challenging (vide infra).
In order to accurately dose the bacteria with the established concentration of ions, ideally, the rate of Ag(I) ion release would also be known. Many studies deliver a single or pulse dose of Ag(I) ions that is equivalent to the total Ag concentration in the AgNP system, which mimics the complete and instantaneous dissolution of the AgNP rather than the release of Ag(I) ions from the AgNP over time. In addition to not capturing the kinetics of Ag(I) ion release, this approach to ion controls does not accurately represent the AgNP system, since only a fraction of the AgNP forms Ag(I) ions at any given time.39–41 As such, the resulting conclusions regarding ion-only, particle-specific, and combined ion-particle effects are confounded by the inaccurate representation of the Ag(I) ion component of the AgNP system.
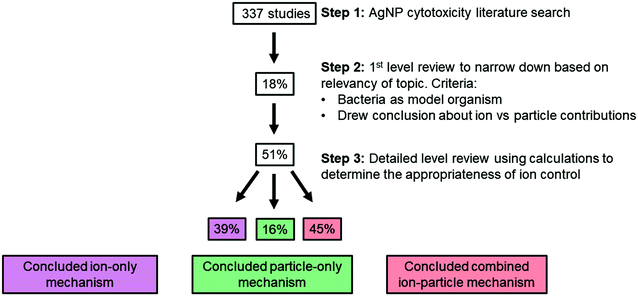
Here, we review more than 300 publications on the cytotoxicity of AgNPs, 59 of which specifically aimed to distinguish ion-only, particle-only, and combined ion-particle contributions to the observed antimicrobial activity using methodologies that included an ion control. In other words, we focus on studies that specifically draw conclusions about the contribution that the ion and particle play in the mechanism of antimicrobial activity and not studies that only evaluated the potency of AgNPs. We then critically analyze the conclusions from 30 of these 59 studies (51%), focusing on those studies that implemented scaled ion controls (vide infra), to identify trends in particle parameters and other experimental factors that indicate ion-only, particle-specific, and combined ion-particle mechanisms of antimicrobial activity. Interestingly, while the results of this analysis suggest the important role of Ag(I) ions, they also clearly highlight that the impact of the particle alone cannot be ignored. Further, the analysis reveals a critical opportunity to elucidate these particle-specific antimicrobial mechanisms as well as whether particle-specific parameters can be manipulated to influence these mechanisms.
2. State of the art: factors influencing ion release and current practices of scaling ion controls
It is critical to systematically quantify released Ag(I) ions from the AgNPs to inform conclusions regarding the ion and particle contributions on antimicrobial activity. In this section, we discuss the state of the art in measuring the quantity and kinetics of Ag(I) ion release from AgNPs to inform ion controls.2.1 Factors that influence the extent of AgNP oxidation and ion release can guide ion control selection
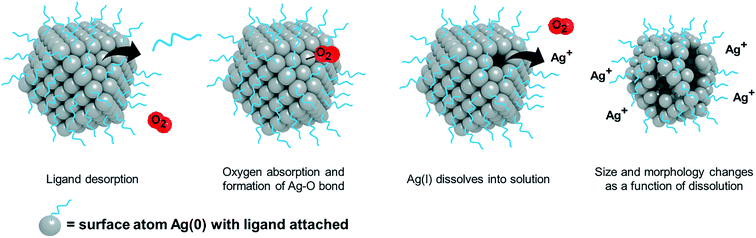
For AgNPs in aqueous systems, Ag(I) ion release typically begins with oxidation of the NP surface. Oxidation of the AgNP surface and release of Ag(I) ions involves several processes that occur simultaneously and are dependent on both particle (e.g., size, shape, and surface chemistry) and experimental factors (e.g., dissolved oxygen, time, and broth chemistry).27,28,31,38,39,42–47 Modulating these pathways can control both the quantity and kinetics of ion release.In general, Ag(I) ion release is initiated by the adsorption of oxygen onto the particle surface followed by subsequent electron transfer.47 Then, AgNPs evolve a surface bound Ag oxide (Ag2O) layer.39,48 The process of ion release begins as this layer is stripped and a new layer forms. However, once the initial Ag oxide surface layers are removed by dissolution, Ag(I) ion release is minimized.39 The amount and strength of oxidizers (e.g., H2O2versus O2) present in solution influence the extent of oxidation.40,49 The stronger the oxidizer (i.e., having greater redox potential), the faster the oxidation rate, where the process follows Arrhenius behavior.46,49 Protons are then required for the dissolution of the Ag oxide layer and thus, Ag(I) ion release is strongly pH dependent.39,46,48 In addition, suppression of oxidation can occur with the addition of organic matter and stabilizing ligands, a reduction in temperature, or an increase in pH.46 Given these differences in kinetics, the relevant extent of Ag(I) ion release also depends on the exposure time of AgNPs with the bacteria system of interest.46 This time-dependent ion release suggests the importance of monitoring ion release continuously in the AgNP system and using it to inform both the concentration of the ion control and the rate at which it should be delivered to the system in order to mimic the AgNP system as accurately as possible.
Particle properties can influence the oxidation and dissolution process and so the ion control needed for each particle type will be different. Generally, smaller particles have a greater radius of curvature and therefore oxidize at a faster rate, a phenomenon described by the Ostwald–Freundlich equation.45,50,51 Different exposed surface facets also have different reactivity towards oxygen,36,52–55 highlighting the effect of parameters such as particle shape on the oxidation process. Finally, surface chemistry also influences dissolution via capping ligands33 and insoluble passivation layers, which influence the dissolution behavior (total concentration and location).42–45,56 Ag oxide surface layers can be removed to varying degrees when AgNPs are washed post-synthesis (to remove residual impurities), synthesized anaerobically, or synthesized aerobically and reduced by hydrogen to zero-valent AgNPs before exposure to bacteria, which can all significantly reduce Ag(I) release.39 Biologically synthesized NPs from plant extracts can also induce different surface chemistries (number and packing distribution of ligands and biomolecules) as compared to chemically synthesized NPs.30,57,58 Thus, the AgNP synthesis and purification approach may affect the surface structure and as a result, Ag(I) ion release. These studies highlight the importance of performing and reporting the AgNP method of synthesis and purification procedures.31,39 Overall, particle type-specific dissolution is a determining factor guiding the selection of an appropriately scaled ion control for specific particle types.
It is critical that ion release is monitored in the exposure media/environment that the AgNPs are exposed to in experiments with bacteria because the type of environment can influence dissolution, and in turn, the ion control that should be used for that specific system. For example, there are multiple types of growth media used to provide vital nutrients that enable bacterial growth (e.g., buffers, sodium chloride, water, and commonly used broths such as Mueller Hinton (MH) and Luria-Bertani (LB)). Due to differences in pH and media constituents (e.g., dissolved ionic species, proteins, peptides, carbohydrates), the specific media used can impact dissolution, the magnitude of AgNP antimicrobial activity, and the dominant mechanism through which that activity occurs (i.e., the Ag(I) ion, the AgNP, or a dynamic synergism between the particle and its released Ag(I) ions). In one case, we have compared AgNP antibacterial activity in two commonly used bacterial growth media (LB and MH broth).38 Using controlled exposures to AgNPs and Ag(I) ion, we measured a differential impact (measured as a difference in the duration of the bacterial lag phase and maximum achieved bacterial growth in relation to the untreated bacteria) – in the two media.38 This difference suggests that there is a complex interplay between the particle and the surrounding environment, which can result in enhancement or inhibition of Ag(I) release from the AgNP surface42–46,59 as well as changes in surface charge and particle stability that could eliminate particle-specific effects.42–45,49,60,61 For example, dissolution can be influenced by the concentration of chloride present in the growth medium; low chloride concentrations form a AgCl(s) passivation layer on the AgNP surface that inhibits dissolution whereas high concentrations of chloride result in the formation of soluble AgCl complexes and promote dissolution.59 Overall, the confounding influence of media on AgNP behavior not only complicates comparison of antimicrobial activity across studies, but also underlines the importance of determining interactions of media constituents with themselves, the NP surface, and the released ions.
The presence of bacteria and their metabolic state additionally affect dissolution and thus will affect the concentration of the ion control needed to accurately model the AgNP system. Bacteria (i) play a role in altering the dissolved oxygen concentration and pH of the experimental system and (ii) introduce extracellular polymeric substances (EPS) that can non-specifically adsorb to AgNPs.27 For example, EPS (and other components of the growth media) can form a protein corona around the NP surface and either prevent dissolution or increase the dissolution gradient by binding the released Ag(I) ions upon direct association with the cell (via Le Chatelier's principle).27 In this regard, bulk dissolution should be monitored in the presence of bacteria and be used to inform accurate concentrations and kinetics needed for appropriately scaled ion controls.
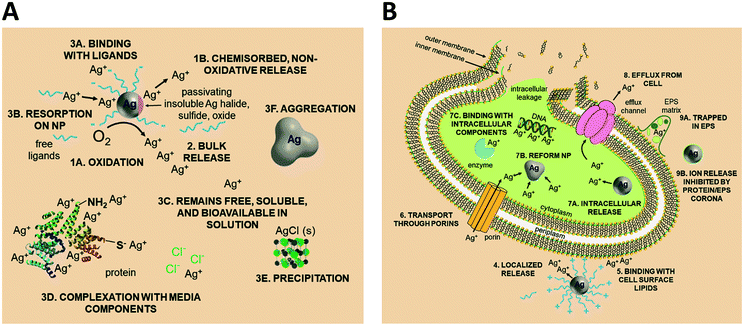
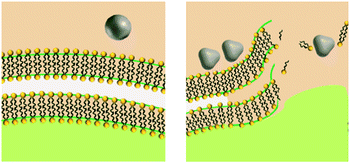
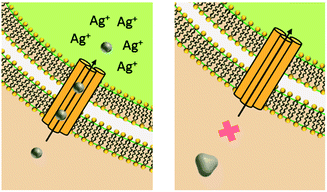
Upon release to the experimental system, there are numerous binding and partitioning events that Ag(I) ions can experience. Ag(I) ions can resorb onto the AgNP surface, remain free in solution, complex with media components, bind to the cell surface, or enter the cell where they can bind to intracellular components or be reduced by them to form new AgNPs.45,46 However, more work is needed to accurately capture the dynamics of the AgNP oxidation process by considering the complex interplay of all factors discussed.46 This complexity suggests the need to monitor intracellular ion release in addition to bulk ion release. Also, techniques should be used to resolve Ag bound to macromolecules in suspension. Combined, such experiments can inform the delivery of ion controls that accurately mimic the AgNP system and allow us to decouple true ion and particle contributions to antimicrobial activity. Fig. 1 presents a visual summary of potential Ag(I) ion release pathways, binding, and partitioning events.
2.2 Equating the concentration of the ion control to the total Ag concentration in the AgNP system
The challenge with many ion controls used in AgNP antimicrobial activity studies is that they are equivalent to the total Ag concentration in the AgNP system and therefore, do not accurately represent the portion of Ag(I) ions present in the test system. There are different forms and concentrations of Ag present in the AgNP system (i.e., the Ag(0) form of the nanoparticle and the dissolved Ag(I) form, Fig. S1A†), which influences the bioavailability of the Ag and the resulting biological impacts. The amount of Ag(I) present depends on the extent to which the AgNP oxidizes to release Ag(I) ions as well as the tendency for those ions to complex with components of the exposure media (e.g., chloride to form AgCl(s) or soluble AgCl complexes). Typically, only a portion of the AgNP oxidizes to form Ag(I) ions and as a result, the proportion of Ag present as Ag(I) is small relative to Ag(0). Therefore, using an ion control at the same mass concentration as the total Ag content of the AgNPs (hereafter referred to as an overestimated ion control) makes for an unequal comparison of the resulting biological activity and calls into question the mechanistic conclusions drawn from such studies (Fig. S1B and C†).2.3 Quantifying ion release in bulk solution to inform an ion control
An improved approach to selecting the concentration of the ion control involves the quantification of Ag(I) ion release in the AgNP system, which is commonly pursued using either a Ag(I) ion-selective electrode or ultraviolet-visible (UV-vis) spectroscopy, or by first separating the Ag(I) ions from the AgNPs by centrifugation or dialysis and then analyzing with inductively coupled plasma mass spectrometry or atomic emission spectroscopy (ICP-MS or AES) or graphite furnace atomic absorption spectroscopy (GF-AAS).24,27,28,33,38,57,62–71 It is important to note that when dialysis membranes are used to separate ions from particles,72–74 the osmotic pressure difference does not allow for complete isolation of Ag(I) ions, limiting the utility of this approach. Furthermore, Ag(I) ion concentration in the bulk suspension is most often measured at a single time point (typically at the culmination of the experiment),24,27,28,33,62,64,65,70,72,75 which excludes the kinetics of ion release. An alternative approach that aims to capture the dynamics of ion release will quantify Ag(I) ion concentration at multiple time points over the duration of the experiment.26,38,57,63,66,68,73,76 When this approach is used, Ag(I) ion controls can be employed in a way that closely mimics the AgNP exposure system. Finally, these techniques measure the free Ag(I) ions present in the bulk solution but do not capture the Ag(I) ions that are removed from solution via subsequent interactions with the surrounding environment (e.g., inside the cell or bound to macromolecules in the suspension). As a result of this limitation, the concentration of Ag(I) ions dosed in as a Ag salt control may be inconsistent with the “real” concentration released by the AgNP (both intracellularly and extracellularly).2.4 Quantifying intracellular ion release to inform an ion control
In an effort to circumvent the abovementioned confounding interactions, researchers measure intracellular Ag using a bioluminescent E. coli Ag-biosensor23,24,66,75,77 or the Ag content of the membrane and cytoplasm fractions of the cell,33 rationalizing that these fractions of Ag are impacting the bacteria. Yet similar to the methods above, intracellular ion release has only been monitored at a single time point.24,66,75,77 While intracellular Ag monitoring quantifies the concentration of Ag to which the cell is directly exposed, it has not typically been used to inform the concentrations and dosing of Ag salt ion controls. Doing so would allow for accurate modeling of the AgNP system, particularly if used in combination with bulk dissolution monitoring.2.5 Suggested best-practice for scaling ion controls
The complexity of the system and challenges faced when quantifying the presence of released ions in solution and in the microorganism suggest that a new approach to ion controls is necessary to obtain comprehensive ion release profiles within the AgNP exposure condition. The desired approach will account for the kinetics of Ag(I) ion release and model an accurate representation of the AgNP system. To ascertain and then implement these controls, there are three key experimental components: (i) continuous monitoring of ion release from AgNPs in the exposure media and in the presence of bacteria as well as intracellularly over the duration of the experiment,26,38,57,63,66,68,73,76 (ii) delivering these measured ion concentrations as a series of continuous doses that mirror their release from the ENM, and (iii) subsequently monitoring the bacterial endpoint of interest. This preferred comprehensive best practice approach to achieve an appropriately scaled ion control, although labor intensive, is necessary to robustly decouple the contributions of the ion and particle.3. Experimental
3.1 Literature review
A comprehensive literature survey to identify studies on the cytotoxicity of AgNPs resulted in more than 300 publications. Google Scholar, Scopus, Compendex, and Web of Science databases were queried using all combinations of the following search terms: “silver nanoparticle”, “silver ion”, “antimicrobial”, “(cyto)toxicity”, and “mechanism”. The SciFinder database was also queried using “silver nanoparticle and toxicity and mechanism” as the initial search term and then further refined to include “ion” and “bacteria”. The sheer size of this set of studies and the heterogeneity of experimental designs did not allow for meaningful discernment of the contributions of the AgNP and Ag(I) ions. Given the extensive use of AgNPs as antimicrobials in a wide range of products, we decided to limit the scope of our literature review to studies that use bacteria as their model organism. With the goal of resolving the ion and particle debate, we further narrowed the scope to those publications that specifically aimed to distinguish ion-only, particle-only, and combined ion-particle contributions to the observed antimicrobial activity. In other words, we focus on studies that specifically draw conclusions about the contribution that the ion and particle play in the mechanism of antimicrobial activity and not studies that only evaluated the potency of AgNPs (often culminating in the conclusion that the Ag(I) ions are more toxic than the AgNPs). This selective literature set included 59 studies. The literature was further refined to attain a final subset of studies that (i) included comprehensive characterization of AgNP size (by transmission electron microscopy, TEM, at a minimum) and shape, (ii) defined the AgNP dose(s) delivered under the exposure condition using ICP-MS, UV-vis, or GF-AAS, and (iii) included a scaled ion control (as determined through calculation, described in detail below, using results from criteria i and ii) that was delivered under the same exposure conditions as the AgNP (i.e., in the same growth medium and bacteria). This final subset of literature contained 30 studies and was used in our analysis. Given that we used scaled ion controls as a metric for determining inclusion and exclusion of studies (i.e., pre-specified eligibility criteria), we refer to our analysis as a systematic review.78 A descriptive summary of each study is compiled in Table S1.†A scaled ion control is defined here as having an equivalent concentration of Ag atoms to the bioavailable Ag that is released from the AgNPs as Ag(I) and is delivered to the experimental system as a Ag salt (e.g., AgNO3, AgC2H3O2, Ag2SO4) or as the isolated ions from the AgNP. This factor is a distinguishing criterion because scaled ion controls are crucial for valid comparison of ion-only, particle-only, and combined ion-particle contributions to AgNP antimicrobial activity. The calculations we use to determine a scaled ion control consider the extent to which a AgNP of a given particle size and shape can dissolve to its bioavailable Ag(I) form based on the surface atoms available for oxidation and will be discussed in detail below.
3.2 Scaled ion control calculations
The following calculations outline our approach to determine scaled ion controls in each experimental AgNP system. Briefly, the percentage of AgNP oxidation and subsequent ion release necessary to obtain Ag(I) ion concentrations equivalent to the ion controls used in a given study (i.e., the expected bioavailable Ag) was calculated by comparing the total concentration of Ag atoms (on a mol mL−1 basis) present in the reported ion control and in the delivered particle dose(s). The theoretical percentage of AgNP surface atoms available for oxidation was calculated by considering the size and shape of the AgNP studied (vide infra), which is why inclusion of particle characterization by TEM is critical and serves as the basis for defining a scaled ion control.Due to ambiguity in the concentrations of the ion controls and AgNPs reported in the literature, we carried out the calculations under two feasible assumptions: (i) the reported concentration indicates the total concentration of Ag salt or AgNPs (i.e. as concentration of NPs, not Ag atoms), and (ii) the reported concentration indicates the total Ag atom concentration of the Ag salt or AgNP. The difference in results for these two assumptions was insignificant for the Ag salt but significant for the AgNP. The assumption that the reported concentrations of AgNPs were as amount of AgNPs per unit volume resulted in unrealistic values of necessary AgNP oxidation (e.g., 1029%) and rejection of every study for having an overestimated ion control. Therefore, the calculations proceeded assuming the reported concentration was of the total Ag atom concentration unless otherwise specified in the study. This assumption is further rationalized by the fact that those studies including details on how they determined AgNP concentrations used characterization techniques (e.g., ICP-MS or AES, UV-vis, or GF-AAS) that measure total Ag atom concentrations.
The following example calculation demonstrates the approach used to determine the concentration of Ag(I) ions in the Ag salt control in units of moles per mL:
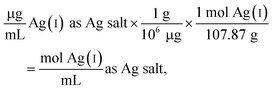 | (1) |
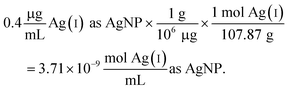 | (2) |
Since the Ag salt ion control is at the same mass concentration as the AgNP (0.4 μg mL−1), the values are the same, which is expected given the assumption that the concentration represents the total Ag atoms. This ion control assumes that the AgNPs completely dissolve, and thus delivers an ion concentration that exceeds the amount of Ag(I) ions that is realistically released from the AgNPs (vide infra). Example calculations using the alternative assumption are outlined in the ESI.† The percentage of AgNP oxidation necessary to release Ag(I) ion concentrations equivalent to the ion controls used in the respective study was calculated as follows:
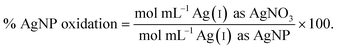 | (3) |
To determine whether the necessary extent of oxidation is reasonable, the theoretical extent of oxidation was calculated under the assumption that only a single monolayer of the NP is available for oxidation. This assumption is empirically supported. For example, Sotiriou et al. found that the equilibrium Ag(I) ion concentration released from the particle into solution corresponds with the dissolution of one to two monolayers and is dependent on the particle size.39 For particles greater than 8 nm, the mass fraction of released Ag(I) ions is equivalent to the mass of a single Ag oxide monolayer, whereas dissolution of particles less than 5 nm in diameter corresponds to oxidization of two Ag oxide surface layers.39 An intermediate extent of oxidation, i.e., in between one to two Ag oxide surface layers, appears for particles sizes between 5–8 nm. We proceeded with the assumption that theoretical dissolution was equivalent to the oxidation of the outermost surface monolayer because 95.5% (64/67) of the AgNPs in the identified literature are greater than 5 nm (the three studies that include AgNPs less than 5 nm also included AgNPs with diameters greater than 5 nm). To estimate this monolayer, the percentage of surface atoms on a given AgNP was calculated as follows:
 | (4) |
The method used to calculate the volume (needed for determination of the total number of atoms) and surface area (necessary for determination of the total number of surface atoms) takes into account the size and shape of the AgNP used in a given study (equations in ESI†). For the determination of surface atoms, the percentages of different surface facets present on AgNPs of different shapes were also considered, as this factor can influence the number and packing of the atoms on the surface, as well as influence surface reactivity and the propensity to oxidize. Pseudo-spherical particles were the predominant particle shape studied in the identified literature subset and were approximated as cuboctahedrons, having eight (111) faces and six (100) faces, containing 63.4% and 36.6% of the surface atoms, respectively.79 The monotonic relationship between nanoparticle size and the total number and percentage of surface atoms is well established; the total number of surface atoms increases with increasing particle size and the percentage of surface atoms increases with decreasing particle size (see Fig. 2 and Table 2 in ref. 80).80 Forty percent oxidation was determined as a conservative threshold for a scaled ion control based on (i) the available percentage of surface atoms (0.6–26% based on calculation of the NPs studied with sizes ranging from 5–200 nm) determined from the AgNP size and shape, and (ii) the potential for additional ionization due to known influences of experimental conditions (vide supra), which cannot uniformly or robustly be considered in these calculations. As a result, ion controls that require >40% of the AgNP to oxidize were considered overestimated. Following the example calculation above, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Ag(I) ions as Ag salt and AgNP would result in 100% of the AgNP needing to oxidize. This systematic review focuses solely on the results and conclusions presented in the subset of studies that included at least one scaled ion control. A schematic illustrating the approach to scaled ion control determination is presented in Fig. 2.
1 ratio of Ag(I) ions as Ag salt and AgNP would result in 100% of the AgNP needing to oxidize. This systematic review focuses solely on the results and conclusions presented in the subset of studies that included at least one scaled ion control. A schematic illustrating the approach to scaled ion control determination is presented in Fig. 2.
These calculations enabled isolation of those studies that incorporated a scaled ion control that is representative of the total possible Ag(I) ion released from the AgNP studied. This narrowed the focus to conclusions drawn from these isolated studies with the goal of gaining clarity in the ion versus particle antimicrobial activity debate. Still, the calculations are not without limitations. First, the solvent environment (e.g., growth media),28,38,42–44 the presence of bacteria,27,68 and surface chemistry (e.g., ligand identity, Ag oxide formation),24,31,33,38,45,56,57,62,67,70,81–83 among other factors, have all been shown to influence AgNP ionization (vide supra). Yet, the mechanisms remain unresolved and thus limit our ability to predict the influence of experimental conditions on Ag(I) ion release in the studies reviewed herein. Second, the calculations determine how much Ag can theoretically ionize from the AgNPs indicating the bulk concentration of Ag(I) ion in solution, not the concentration of Ag(I) ion internalized by the bacteria (intracellular Ag), which Ivask et al. reports as being a better indicator and comparison of antimicrobial activity.24 Once Ag(I) ion enters the bulk solution, there are many possible interactions that occur depending on the environment, including diverse binding events and equilibria that can inhibit or drive ion release (vide supra). These interactions influence the fate of Ag(I) ions in a given experimental system and as a result, it is impossible to predict how many Ag(I) ions can and will enter the cell. Third, the calculations do not consider kinetics of ion release (i.e., the result represents the total possible dose of Ag(I) ion released from the AgNP). This omission is not to say that the studies reviewed do not consider kinetics, but rather that the kinetics of ion release are not included to determine whether a study incorporated a scaled ion control. Finally, these calculations are applicable to only particle diameters greater than 5 nm because the shapes are well-defined with easily calculable volume and surface area, have face-centered cubic (FCC) packing, and allow us to include a correction for the surface facets. As the particle diameter decreases to below 5 nm, the number of competing structural factors increases and the propensity for it to be referred to as a ‘nanocluster’ emerges. Different atom packing densities and arrangements become competitive and influence the shape and geometry of nanoclusters (e.g., the cuboctahedral geometry becomes icosahedral) so that they can no longer be easily approximated as spheres49,84,85 (Note: only 3 studies included particles with diameters <5 nm and those studies also included particles >5 nm, so the calculations for the 5 nm NPs will not significantly influence the conclusions drawn in this study).
3.3 Using pivot tables to identify AgNP properties and experimental variables that discriminate ion-only, particle-only, and combined ion-particle contributions to observed antimicrobial activity
The pivot table feature in Microsoft Excel (Ver. 2016, Microsoft Corporation, Redmond, WA) was used to identify discriminating factors influencing ion-only, particle-only, and combined ion-particle contributions to AgNP antimicrobial activity. Pivot tables are particularly useful for summarizing and making sense of large, detailed data sets. Qualitative data (e.g., experimental method parameters, conclusion drawn from the study) and empirical data (e.g., particle size, zeta potential) was compiled for each study and organized into an Excel spreadsheet (Table S1†), from which several analyses using pivot tables were conducted.Conclusions from the identified subset of studies were categorized based on the study conclusions and accompanying data in support of an ion-only, particle-only, or combined ion-particle mechanism. Studies that attribute antimicrobial activity solely to Ag(I) ion release and concluded negligible particle-specific effects were categorized as ‘ion-only’. Studies that identified and demonstrated the particle influence on antimicrobial activity – independent or in concert with Ag(I) ion release – were categorized as having a ‘particle-only’ or ‘combined ion-particle’ effect, respectively. For example, enhanced localized dissolution at the NP–cell interface or intracellular dissolution is enabled by the NP (as in the case of appending cationic capping ligands to guide targeted delivery24), yet the increased concentration of ions released in close proximity to the bacteria is often claimed to be responsible for the inactivation. The resulting enhanced bioavailability increases the antimicrobial impact over the equivalent concentration of bulk dissolved Ag. The particle does not work independently from the ion, but the particle parameters can influence the magnitude of impact. In this paper, this mechanism is classified as ‘combined ion-particle’ since the ion and particle are both necessary to achieve this enhanced antimicrobial activity. However, because ion release occurs concurrently with particle-only effects, it is difficult to decouple ‘particle-only effects’ and ‘combined ion-particle’ effects, especially when multiple particle types and support for multiple mechanisms appear in one study. Some studies include support for multiple conclusions as a result of particle manipulations and different methodology used to assess antimicrobial activity and so were categorized under multiple mechanisms.24,28 For example, in one study, there is evidence for ‘combined ion-particle’ effects when using one particle type, but ‘particle-only’ effects are supported when using another particle type.24 The unknown mechanism through which the particle induces specific effects or enhances localized and/or intracellular dissolution adds to the complexity of decoupling these contributions, but further suggests the importance of the particle and the ability to shift mechanisms by manipulating particle parameters.
4. Results and discussion
4.1 Identification and evaluation of studies implementing scaled ion controls
From the 59 studies identified as investigating ion-only, particle-only, and combined ion-particle contributions to bacterial cytotoxicity or antimicrobial activity, 30 studies (51%) included a scaled ion control as determined by the calculations presented above. Results of these calculations are compiled in Table S2.† From these 30 studies, 22 studies (73%) measured Ag(I) ion release from the AgNP in the experimental system. The reported dissolution in these 22 studies strongly agreed with our calculated theoretical dissolution, which serves as further validation of the calculation method (see Table S2 and Fig. S2† for demonstrated agreement of values). Additionally, this validation establishes the calculation method as a robust approach that can be used to inform scaled Ag(I) ion controls, especially if coupled with an empirical kinetic law of oxidation to estimate the kinetics of ion release, similar to those developed by Liu and Hurt for low AgNP concentrations46 and Molleman and Hiemstra for pH-dependent and size-dependent ion release.48 Of those 22 studies, the majority (74%) did not monitor the kinetics of Ag(I) ion release but rather measured Ag(I) ion concentration at a single time point. While monitoring of kinetic release was not a mandatory criterion for inclusion in our systematic review, it is an important aspect to consider given the wide variability of Ag(I) ion release than can occur and challenges associated with capturing kinetics of Ag(I) ion release (vide supra).4.2 Support for ion-only, particle-only, and combined ion-particle contributions to AgNP antimicrobial activity
As stated earlier, the three possible contributions of the ion and particle to antimicrobial activity are supported by the literature and influenced by the physicochemical properties of the AgNP. Table 1 summarizes the influence of particle parameters (i.e., size, shape, and surface chemistry) on the ion and particle contributions outlined in each of the three possibilities.Isolating those studies that include a scaled ion control in their comparison of ion-only, particle-only, and combined ion-particle contributions to antimicrobial activity was intended to eliminate potentially confounding conclusions and to focus on those studies that offer robust discriminating conclusions. Of the 30 studies, 39% concluded that the observed antimicrobial activity results from an ion-only mechanism while 16% and 45% of studies concluded a particle-only and combined ion-particle mechanism, respectively (Fig. 3).
These results indicate that the impact of the particle cannot be ignored and that there remains an opportunity to elucidate both underlying ion-independent antimicrobial mechanisms and mechanisms that act in concert with the Ag(I) ions as well as whether particle-specific parameters can be manipulated to influence these mechanisms. While the contribution of Ag(I) ions to AgNP antimicrobial activity is largely indisputable, empirical support for ion-independent, particle-only effects suggests an opportunity to manipulate particle properties to further tune the mechanism and magnitude of impact. The precise mechanisms through which the particle induces antimicrobial activity are not resolved, yet particle size and surface chemistry are two critical factors that have been suggested and are further supported by the studies reviewed herein.24,31,57,65–67,70,71,73,86 The identified subset of literature included only one study75 investigating the effect of particle shape, limiting our ability to draw any meaningful conclusions about the influence of shape.
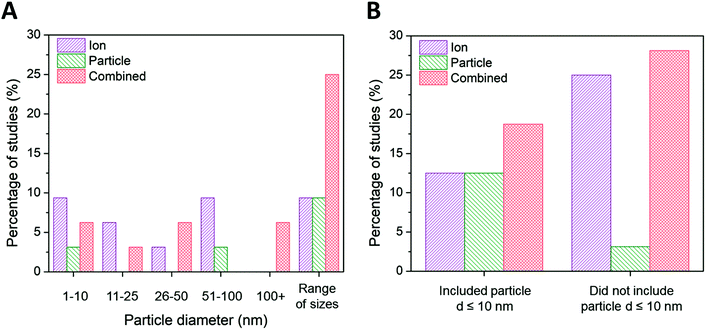 | ||
| Fig. 4 (A) Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that include AgNPs only within the size range of 1–10 nm,23,33,63,64,72,82 11–25 nm,38,62,68 26–50 nm,28,76 51–100 nm,75,86,90,91 greater than 100 nm,26,92 and AgNPs from more than one size range.24,27,31,57,65–67,69–71,73,77,89 (B) Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that include23,24,27,31,33,63–66,70–72,82 and exclude26,28,38,57,62,67–69,73,75–77,86,89–92 AgNPs with d ≤ 10 nm. Size is an influencing factor inducing particle-specific effects, in which the role of the particle emerges only when multiple particle sizes from different size categories are included. The role of the particle also emerges when particle sizes ≤10 nm are included in the study. Note: this trend is not reflected in the ‘1–10 nm’ size category of the main figure because studies including AgNPs ≤ 10 nm are split between the ‘1–10 nm’ and ‘range of sizes’ categories. | ||
Ion-only and combined ion-particle mechanisms emerge as nearly equivalent contributions to antimicrobial activity for particle diameters greater than 10 nm, with negligible particle-only contributions. Interestingly, 67% of the studies that conclude ion-only mechanisms of antimicrobial activity do not include ≤10 nm AgNPs (Fig. 4B).28,62,68,69,75,89–91 Those studies that included a wide range of particle sizes (including AgNPs from two or more of the size categories included in Fig. 4A) overwhelmingly (79%) concluded particle-only or combined ion-particle effects24,31,57,65–67,70,71,73,77 as opposed to studies that looked at a single size or narrow size range, of which 28% concluded particle-only or combined ion-particle effects.23,26,33,38,72,76,86,92 These results suggest that size is a factor inducing particle-specific effects that emerges only when multiple AgNPs are studied simultaneously. Including a range of sizes in a study will thus establish the relationship (e.g., monotonic, monotonic and linear, non-monotonic) between mechanism of antimicrobial activity and size as well as whether the relationship is preserved across a wide range of sizes. Adherence to or deviation from these relationships can provide support for either an ion-driven or, in this case, particle-specific or combined ion-particle mechanisms that would otherwise not be realized by studying a single particle size or narrow size range in isolation.
Due to the ability for surface charge to influence the aggregation state of AgNPs and the affinity for the bacterial cell, the subset of studies was organized around surface charge to determine potential influences on the conclusions drawn. The results reveal shifts in conclusions surrounding ion-only, particle-only, and combined ion-particle contributions to antimicrobial activity (Fig. 5). We used zeta potential as the indicator of surface charge, which was included in 53% (16/30) of the studies in our identified subset. A large portion of studies (43%) that do not specify the ligand bound to the particle and do not include characterization of surface charge conclude the antimicrobial activity of AgNPs is governed by Ag(I) ions.63,64,69,82,89,91 Studies that used negatively charged capping ligands supported, in almost equal proportion, mechanisms that are governed by the released ions alone27,28,62,68,75,90 as well as synergistic effects of the ion and particle,28,33,38,57,66,73,76 while only one study concluded a particle-only mechanism of activity.86 Some of those studies supporting ion-only mechanisms, however, simultaneously varied capping ligand and particle size, which precludes isolation of a definitive conclusion surrounding the effect of the capping ligand. These studies also noted that surface chemistry-dependent antimicrobial activity did not always correlate with ionization, suggesting an additional factor (particle-driven) may be at play.27,62 Depending on interactions with media components, Ag–Cl and Ag–S could have formed a passivation layer on the AgNP surface that influences ion release.42–45,56 Only when both cationic NPs and anionic NPs are included in a study do particle-only or combined ion-particle mechanisms truly emerge. Studies including multiple particle types (cationic NPs and anionic NPs) overwhelmingly (75%) supported a particle-only mechanism.24,31,70 These results suggest that surface chemistry may be an influencing factor in inducing particle-specific effects, which is strongly supported when multiple ligand charges are compared. Since the particle is necessary to serve as the host for the capping ligand, the role of the particle is critical for introducing charge- (magnitude and type) and surface chemistry-induced antimicrobial activity.
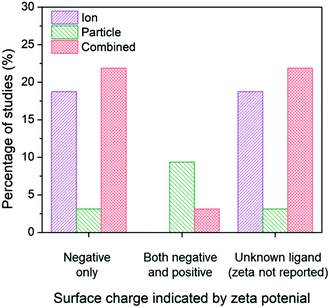 | ||
| Fig. 5 Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that include AgNPs with a single capping ligand type inducing a negative surface charge,27,28,33,38,57,62,66,68,73,75,76,86,90 two capping ligand types to compare a negative and positive terminal group,24,31,70 and unknown capping ligands.23,26,63–65,67,69,71,72,77,82,89,91,92 Surface chemistry is an influencing factor in inducing particle-specific effects, in which the role of the particle emerges only when both negatively-charged and positively-charged particles are compared. Note: no studies included a positively-charged or neutral capping ligand only. | ||
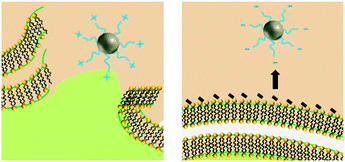
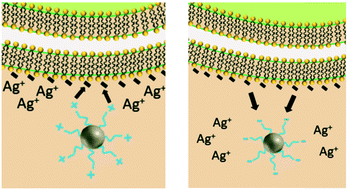
Similar to NP size, the exact role of positively-charged capping ligands remains unresolved but it is speculated to include a combination of indirect ROS formation, cell membrane disruption, and/or control of bioavailable intracellular Ag. AgNPs with positive charges due to positively-charged capping ligands (hereafter referred to as cationic AgNPs) demonstrate enhanced antimicrobial activity compared to their neutral and negative counterparts, which is attributed to their electrostatic attraction and subsequent adherence to the negatively-charged bacterial cell surface.24,31,70 These observed effects are not attributed to the Ag(I) ion, since less bulk dissolution occurred with the cationic AgNPs studied than for other particle types (e.g., AgNPs with negative charges) and the Ag(I) ions are present in smaller amounts than the Ag salt control.24,70 However, the affinity of the cationic AgNP for the negatively-charged cell allows for enhanced localized release of Ag(I) ions, internalization of the particle and/or ions, and ROS production at the cell membrane.24 Furthermore, cationic AgNPs induce physical damage to the cell membrane (i.e., pitting observed by TEM)31 that disrupts ion efflux systems, which in turn hinders the pumping efficiency of Ag(I) ions out of the cell, thus increasing intracellular ROS generation compared to exposure to the same amount of Ag(I) ions from the equivalent Ag salt control or other particle types.77 Additionally, cationic AgNPs increase the amount of intracellular Ag(I) ions that deactivate antioxidative enzymes, potentially allowing for the buildup of ROS to occur.4 The pathway through which the cell mitigates stress caused by cell membrane-associated ROS from cationic AgNPs was found to be similar to the pathway demonstrated by cationic polystyrene NPs, suggesting that a particle type-specific response is elicited for cationic particles independent of the particle core composition.24
In addition to the pH-dependent charge of the terminal moiety of the ligand, the molecular weight, ligand density (number of capping ligands per unit surface area), and chemical composition of the capping ligand may also influence antimicrobial activity and the mechanism through which it occurs. While one study in the identified subset33 supports the influence of all three regions of the capping ligand on bulk and intracellular dissolution and found antimicrobial activity to increase with this dissolution, the remaining studies lacked critical information (e.g., the absence of comprehensive capping ligand characterization, particularly those corresponding to polymer- and protein-based capping ligands) to be able to organize in a pivot table around binding moiety, intramolecular region, and terminal group. Still, the following discussion includes potential influences of the multiple capping ligand components.
First, the NP-binding moiety influences dissolution; atoms with weaker binding affinities (e.g., oxygen versus sulfur) enable adsorption exchange with ambient oxygen and allow increased Ag(I) ion release to occur.33 Second, the molecular weight of the intramolecular region influences the bulk density of organic matter surrounding the AgNP core, which can “shield” ion and particle effects (e.g., influence the release of Ag(I) ions to the bulk solution).33,99 Third, terminal groups of the capping ligands have different affinities to interact with Ag(I) ions, so the amount of Ag(I) ions remaining on the particle surface and being leached into solution can be different.33 For example, it may be possible that the positively-charged Ag(I) ions electrostatically interact with capping ligands having negatively-charged terminal groups, limiting the amount of bioavailable Ag(I) ions. Finally, ligand density93 influences the interaction between the NP surface and surrounding environment (e.g., dissolved O2), and consequently, should influence Ag(I) ion release. Ion release is hypothesized to scale inversely with initial ligand density (i.e., more ion release occurs with a lower initial ligand density because more of the AgNP surface is exposed for oxidation). No studies have directly measured how the initial ligand density affects ion release, but a recent study measured changes in ligand density after AgNPs were incubated in bacteria growth media, where the hypothesis was that AgNPs with a larger decrease in ligand density would also have greater Ag(I) ion release. However, no correlation was found between the change in ligand density and the degree of Ag(I) ion release.38 This complex relationship between ligand density and ion release illustrates a need for further research aimed at establishing ion release profiles of multiple ligand chemistries and densities and resolving the contribution of surface chemistry to antimicrobial activity. In doing so, opportunities to tune ion-driven antimicrobial activity through controlled manipulation of the AgNP surface will be revealed.
In the identified subset of literature, some studies consider aggregation early in the experimental design as demonstrated through the choice of low ionic strength growth media or in the ligand selection to enhance particle stability,26,82,92 but may not actually monitor aggregation under experimental conditions. Interestingly, the inclusion or absence of aggregation monitoring as well as the method used to monitor aggregation impacts the conclusions drawn in these studies (Fig. 6).
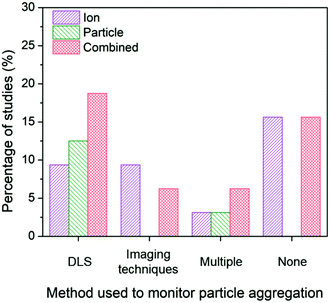 | ||
| Fig. 6 Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that monitor particle aggregation using hydrodynamic diameter and/or zeta potential from dynamic light scattering (DLS),24,31,33,57,66,68–70,73,76,86,90 size and morphology from imaging techniques (e.g., TEM, SEM),23,63,77,82,89 more than one technique (e.g., DLS, TEM, and UV-vis),38,62,65,72 or none at all.26–28,64,67,71,75,91,92 Aggregation is an important factor influencing the contributions, and as such, the role of the particle emerges when aggregation is monitored with DLS. | ||
When aggregation is not characterized, no conclusion regarding the mechanisms of antimicrobial activity can be discerned.26–28,64,67,71,75,91,92 The majority of these studies noticeably did not specify the ligand bound to the particle and did not include characterization of surface charge, providing no indication about the stability of the AgNPs, thus increasing the potential for elimination of particle-specific effects. Of those studies that characterize aggregation, dynamic light scattering (DLS) is the most common method used to monitor the change in hydrodynamic diameter and/or zeta potential of the AgNP suspension. The majority of studies (77%) employing DLS conclude particle-only or combined ion-particle effects,24,31,33,57,66,70,73,76,86 and whether aggregation was monitored throughout the duration of the experiment or pre- and post-experiment had no effect on the conclusion being drawn. When imaging techniques (e.g., TEM, scanning electron microscopy (SEM)) were employed, there was a slight predominance of ion-only conclusions. N.B. there are inherent challenges to characterizing particle aggregation state via electron microscopy because samples are prepared and subsequently imaged outside of their native solution, and the removal and subsequent sample preparation (most notably, sample drying) influences particle–particle interactions and may not reflect the native dispersed state. Finally, when multiple methods are employed and considered together to determine the influence of aggregation on the antimicrobial activity of AgNPs, the majority of studies conclude particle-only or combined ion-particle effects. Taken together, the analysis suggests that aggregation is an important factor influencing ion-only, particle-only, and combined ion-particle contributions to antimicrobial activity, and as such, is critically important to monitor and consider when drawing conclusions within a given experimental system.
4.3 Experimental methods used to distinguish ion-only, particle-only, and combined ion-particle mechanisms influence the conclusion drawn
While the scaling of the ion control is one experimental factor we used to isolate the subset of literature and eliminate confounding effects, we identified the method used to decouple ion and particle contributions as an additional factor influencing the conclusions with respect to those contributions. Other experimental factors that were considered and found to have a less significant independent influence on conclusions drawn (i.e., resulted in a nearly equal number of studies concluding ion-only, particle-only, and combined ion-particle) include the AgNP dose, purification and washing procedure, method of dissolution monitoring, time of dissolution monitoring, and the gram stain of the organism used (Fig. S3–S7†). There are numerous methods available to study interactions and the impact of ENMs on bacteria, which vary based on the type and resolution of information obtained. Within the subset of literature reviewed herein, the methods used to distinguish ion and particle contributions are grouped into the following categories: (i) Ag(I) ion release is quantified (either in the bulk solution or intracellularly) and compared to AgNP exposure scenarios,23,24,57,63,64,66,68,72,73,75,77 (ii) the experimental system is designed to create a particle-only exposure (e.g., anaerobic environments eliminate oxidation of Ag(0) to Ag(I)),27,28,33,62,82 and (iii) molecular or fluorescent indicators are used to resolve specific mechanisms of interaction.23,24,26,31,33,62,63,65,68,71,72,76,77,82,90–92Fig. 7A shows the breakdown of conclusions drawn using these three general methods, each discussed in detail below.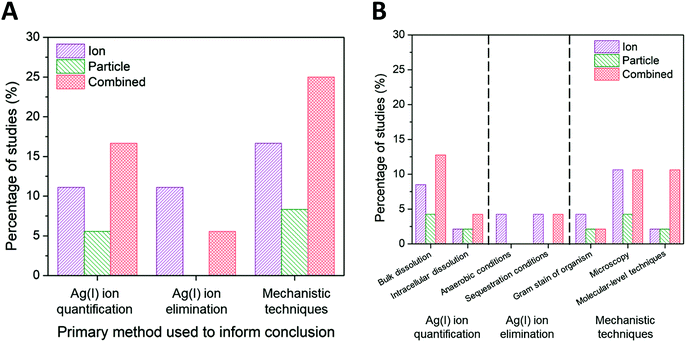 | ||
| Fig. 7 (A) Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that used three main methods: Ag(I) ion quantification techniques,23,24,57,63,64,66,68,72,73,75,77 Ag(I) ion elimination techniques,27,28,33,62,82 or mechanistic techniques.23,24,26,31,33,62,63,65,68,71,72,76,77,82,90–92 (B) A further break down of these primary methods into specific methods is presented. Note: some studies used multiple methods to aid in their conclusion drawn. Quantifying Ag(I) ion release and using molecular-level techniques provide support for particle-only and combined ion-particle mechanisms of antimicrobial activity while aerobic and sequestration conditions provide support for an ion-only mechanism. | ||
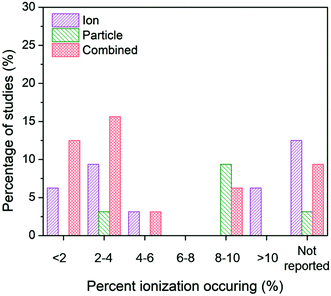 | ||
| Fig. 8 Ion-only, particle-only, and combined ion-particle conclusions drawn from studies that measured <2%,26,33,57,62,64,67 2–4%,28,38,63,66,72,73,75,77 4–6%,27,76 6–8%, 8–10%,24,31,38,62,65,70,73,77,91 >10%,68,69 or did not measure23,71,82,86,89–92 average ionization taking place in the AgNP system. The majority of studies across varying percentages of ionization occurring in the AgNP system concluded particle-only or combined ion-particle effects, suggesting that the mechanism of antimicrobial activity is not due to the particle releasing more Ag(I) ions. | ||
With the bulk dissolved Ag(I) ion concentration quantified, a specific approach to isolating ion-only, particle-only, and combined ion-particle contributions is to normalize the measured endpoints (e.g., the minimum inhibitory concentration (MIC) or the EC50) or dose–response curves obtained under AgNP exposure conditions to the quantified concentration of Ag(I) ion to then compare the normalized impact with the corresponding Ag salt control.23,24,57,63,64,66,68,72,73,75,77 The results are interpreted as particle-specific or combined ion-particle effects when the magnitude of impact of the AgNP exposure condition is greater than that of the equivalent released Ag(I) ion concentration dosed in as a Ag salt. When the measured endpoints or growth curves are similar or when the equivalent amount of released Ag(I) ions imparts greater impact on the bacteria growth curve, the results are interpreted as being governed by the ions. Studies using this normalization approach predominantly (67%) conclude that particle-specific or combined ion-particle effects govern the observed impact of AgNPs (Fig. 7B).23,24,57,66,72,73,77
Still, definitively isolating different forms of Ag in these complex systems remains an ongoing challenge in the field. The described current best-practice techniques capture free Ag(I) ions present in solution; they do not measure intracellular Ag content or resolve the fraction of Ag bound to the surrounding environment (i.e., ligands, media constituents, and components of the bacteria cell), as some Ag-complexes will partition/be captured in the supernatant and others will be pelleted, depending on the size of the complex and the separation technique being used. Additionally, studies that measure ionization at a single time point (oftentimes at the culmination of the experiment) exclude the kinetics of ion release. As mentioned above, oxidation of the AgNP surface is a dynamic process and is highly dependent on the surrounding aqueous chemistry, the specifics of which are not comprehensively resolved. As a result of our inability to capture the complexity of the system, we are currently unable to mimic the kinetics of ion release from AgNPs in ion-only exposures, limiting our ability to further distinguish ion-only, particle-only, and combined ion-particle mechanisms of antimicrobial activity.
In an effort to circumvent these limitations, an alternative method is employed in which intracellular Ag is measured using a genetically engineered Ag(I)-biosensor bacteria, Escherichia coli (E. coli) MC1061 (pSLcueR/pDNPcopAlux).24,66,75 This recombinant bacterial strain harbors two plasmids from the copper resistance system essential for sensor function: (i) pSLcueR, which contains genes for the regulatory protein cueR, and (ii) pDNPcopAlux, which contains the lux-cassette (a group of bioluminescence encoding genes) fused to the promoter pCopA.101 The protein cueR resides in the cytosol and tightly binds both cytoplasmic copper ions (Cu(I)) and Ag(I) ions due to their similar binding affinities, ionic radii, and charge densities.26 Upon binding, cueR activates expression of copA, a Cu(I)/Ag(I)-translocating P-type ATPase involved in Cu and Ag efflux, and the lux-cassette required for the production of bioluminescence.26,101 This method measures strictly intracellular Ag(I) ions because it is the Ag(I) ion binding to the cysteine residues on cueR that is essential to induce bioluminescence. The more binding events, the stronger the bioluminescent signal (in relative light units), thus serving as an indicator of intracellular Ag(I) ion concentration.66 Conclusions from studies employing this methodological approach predominantly (67%) support particle-only or combined ion-particle effects.24,66
4.3.2.1 Anaerobic conditions. Formation of Ag(I) ions from the AgNP surface is an oxidation process. In anaerobic environments, oxygen is removed from the system, thus eliminating oxidative Ag(I) ion release. To eliminate the potential for microbial differences to confound results acquired in anaerobic studies, it is important to use a bacterial strain that grows under both aerobic and anaerobic conditions and exhibits equal susceptibility to Ag(I) ions in both conditions.27,28 The AgNP concentrations that elicit complete inhibition vary among anaerobic studies, ranging from above 195 mg L−1 (ref. 27) to 5 mg L−1 (ref. 28 and 102) AgNP (compared to 75 mg L−1 (ref. 27) to 1 mg L−1 (ref. 28 and 102) AgNP under aerobic conditions). While this approach is aimed at eliminating the Ag(I) ions and marginal concentrations are observed after multiple days (e.g., less than 1 μg L−1 Ag(I)27), other studies report detectable Ag(I) ions under anaerobic conditions, reaching the same level as in the comparative aerobic conditions.102 Evidence for Ag(I) ion release in anaerobic conditions is one potential explanation for the significant discrepancy in AgNP concentrations eliciting complete inhibition in the above-mentioned studies. Additionally, Ag(I) ion release can occur through non-oxidative means. AgNPs can release chemisorbed Ag(I) ions, which result from the partially oxidized AgNP surface even in the absence of an oxidizer.45,102,103 The presence of chemisorbed and “free” Ag(I) ions will vary between studies based on (i) the exposure conditions (i.e., in the presence of bacteria whose metabolic state can change the pH to influence dissolution and extracellular polymeric substances released from bacteria that can sequester Ag(I) ions),27 and (ii) the AgNP synthesis and purification process.31,39,45,102 For example, when mild reducing agents (e.g., sodium citrate) are used during the synthesis, some of the Ag salt may not undergo reduction and thus remain as free ions in solution or bound to the AgNP surface.45 As such, the absence of free Ag(I) ions in solution does not preclude the presence of chemisorbed Ag(I) ions, which could be transported to the cell by the AgNP. Additionally, if particle internalization occurs and the cytosolic pH is lower than that of the growth medium, intracellular release may occur in anaerobic bacteria. Since nitrate compounds are used as electron acceptors in anaerobic bacteria, reactive nitrogen species (e.g., nitrogen dioxide, nitrous oxide) may also form intracellularly,104 acting as an oxidizing agent to induce Ag(I) ion release. To test this combined ion-particle hypothesis, intracellular Ag could be measured using an anaerobic Ag-biosensor.
Nonetheless, when designed to avoid these potential confounding experimental factors, anaerobic exposure environments are good model systems to probe ion and particle contributions independently. Current anaerobic studies (2 reviewed herein) provide convincing support for Ag(I) ion-only mechanisms but do not conclusively rule out particle-only contributions since particle parameters (e.g., size and surface chemistry) have not been comprehensively varied under anaerobic conditions. An inability to demonstrate particle-only effects does not prove an ion-only mechanism. Studying a comprehensive AgNP suite that systemically varies shape, size, and surface chemistry under anaerobic conditions is necessary to rule out particle-only effects. Finally, these studies have considered a single bacterial strain and so multiple bacterial strains, having different defense systems for ions and particles, should be evaluated under anaerobic conditions.
4.3.2.2 Sequestering Ag(I) ions. Another approach to eliminate the effect of Ag(I) ions without precluding their formation is to introduce a compound that sequesters free Ag(I) ions to the system. This approach reduces the bioavailability of the Ag(I) ion, thereby minimizing interaction with the bacteria. Commonly used sequestering compounds include cysteine, chloride, sulfide, thiosulfate, phosphate, and glutathione reductase,28,33,62,82 which demonstrate efficient binding with Ag(I) ions in both AgNP and Ag salt exposure conditions. While half of the studies employing this approach conclude an ion-only mechanism of antimicrobial activity (Fig. 7B),62,82 there is also evidence supporting the combined ion-particle possibility and specifically, particle-mediated intracellular ionization.28,33 One limitation of this approach is that the sequestration compounds also have a strong affinity for the AgNP and upon binding, could influence the NP surface chemistry and hinder the particle-only and combined ion-particle effects.32 Furthermore, the complexed Ag may still exhibit bioavailability and induce antimicrobial activity that may be different than the effect of free Ag(I) ions.105 Despite the fact that this approach successfully establishes an ion-free system to aid in decoupling ion and particle contributions, it alters the system in a way that does not allow for a direct comparison.
A suite of gram-negative and gram-positive bacteria can be used to study how differences in the cell wall – the first point of contact in the AgNP–bacteria interaction – influence the response to Ag(I) ions and AgNPs. Gram-negative bacteria have a thin peptidoglycan cell wall and an outer cell membrane, while gram-positive bacteria have a thicker peptidoglycan layer and no outer cell membrane.76 These differences in architecture introduce different susceptibilities and defense systems to AgNPs and Ag(I) ions in a way that reveals different mechanisms (Fig. 7B). It is critical, however, that both bacteria strains are able to grow in the same exposure media to eliminate confounding effects associated with differences in interactions with the surrounding media. Thirteen percent (4/30) of the studies reviewed herein include both gram-negative and gram-positive bacteria,72,76,82,90 half of which showed that Ag(I) ions and AgNPs induced different magnitudes of antimicrobial activity suggesting different modes of action of the two forms of Ag.72,76 One study shows AgNPs having greater antimicrobial activity in gram-positive bacteria compared to gram-negative bacteria while the Ag(I) ions induced approximately equivalent antimicrobial activity in both.76 On the contrary, the thick peptidoglycan wall of the gram-positive bacteria is proposed to obstruct ions,72,90 rendering the action of Ag(I) ions alone less effective in gram-positive bacteria and suggesting that particle attachment to the cell surface contributes to the antimicrobial activity in a particle-only or combined ion-particle manner. However, different AgNP surface coatings can prevent this attachment, which explains why an earlier study reported AgNPs as having less antimicrobial activity in gram-positive bacteria.72 Using multiple bacterial strains and particle types can differentially affect the antimicrobial activity of Ag(I) ions and AgNPs. This outcome underscores the importance of implementing well-defined and well-controlled materials and biological systems to comprehensively study that differential impact and isolate the contributions of the ion and particle. Using gram positive and negative bacteria in combination with the anaerobic and/or sequestration approaches would further resolve the influence of the bacterial cell wall on the susceptibility and dominant mechanisms of antimicrobial impact.
Capturing visual evidence of Ag(I) ion and AgNP interactions with bacterial cells as well as the resulting morphological changes can aid in determining the contributions of each to the observed antimicrobial activity. Common microscopy techniques used include TEM, SEM, atomic force microscopy (AFM), and phase contrast microscopy.24,26,31,33,62,63,68,71,76,82,91 In the studies reviewed, nearly 40% relied on a microscopy technique to image bacteria–NP interactions and cell morphology,24,26,31,33,62,63,68,71,76,82,91 of which 42% concluded an ion-only mechanism,62,63,68,82,91 17% concluded particle-only effects,24,31 and 42% concluded a combined ion-particle mechanism24,26,33,71,76 (Fig. 7B). Direct AgNP contact with and fusion to the membrane, shrinkage of cell size to reduce its surface area available for interaction, particle entry into the cytosol, resulting membrane damage (e.g., pitting, rupture), and subsequent leakage of intracellular components occurs in AgNP exposures exclusively, suggesting that these physical effects are the result of the AgNP itself and are not dependent on Ag(I) ions.24,31,71,76 Ag(I) ions, on the other hand, have also been visualized in cells as large deposits of reduced Ag(I) (AgNPs) that destroy the cell82,91 but may also induce another bacterial response, often culminating in the clumping of DNA in the cell center.106 While internalization and morphological changes may be visualized and used to isolate physical contributions of the particle from the chemical contributions of the Ag(I) ions, the mechanism by which each enters the cell remains unknown. The observed presence of ‘particles’ inside the cell could instead be Ag(I) ion agglomerates or particles formed by metabolite reduction of Ag(I) ions intracellularly.45,82 Finally, TEM imaging on its own is not a convincing technique for visualization of particle uptake by cells due to the potential for a variety of misleading artifacts.26
The use of molecular-level techniques (e.g., transcriptomics, gene deletion strains) to distinguish ion-only, particle-only, and combined ion-particle contributions is valuable due to the ability to concurrently elucidate the underlying mechanism(s) of antimicrobial activity. Given that these molecular approaches target specific mechanisms, the results of a given study might be limited in scope due to the focus on isolating a particular ion or particle interaction or impact. Still, the resulting conclusions from the 20% (6/30) of studies using molecular-level techniques are overwhelmingly (86%) in support of particle-only or combined ion-particle effects (Fig. 7B).23,24,26,71,92 The compiled findings from the 6 studies provides valuable mechanistic insight. For example, genome-wide approaches show differential gene regulation under AgNP and Ag(I) ion exposures and are used to highlight particle-only23,24,71 and particle type-specific mechanisms (e.g., cationic AgNPs).24 Specifically, particles induce antimicrobial activity by affecting cell surface activity through disruption of outer membrane lipopolysaccharide formation.24,92 These particle-only effects differ from the action caused by Ag(I) ions alone, which include disruption of copper homeostasis, inducement of additional redox stress responses, and regulation of outer membrane porin proteins.23,92 However, the interpretation of genetic responses can lead to different conclusions about ion and particle contributions. For example, McQuillan and Shaw credit the differential genetic response between AgNPs and Ag(I) ions to the delivery mode of Ag(I) ions to the cell that affects the magnitude, location, and kinetics of Ag(I) ion release rather than to particle-only effects, so this combination is considered a combined ion-particle mechanism.92 Despite the challenges associated with interpretation, molecular-level techniques provide resolution of the mechanism of antimicrobial activity and can highlight particle-specific stress responses that occur at small time scales,26,92 with longer time scales deserving attention to study how resistance can build up over time and through multiple generations.15
5. Conclusions and future outlook
Results from this systematic review on the AgNP antimicrobial activity literature provide supporting evidence for particle-specific effects that act both in concert with as well as independently from the Ag(I) ions. The discriminating pivot table analysis suggests the potential to create more effective, Ag-enabled antimicrobials using critical particle properties – size, shape, and surface chemistry. The following are suggestions for ongoing research to this end.Further research is necessary to elucidate the contributions of each NP property (both their independent and combined effects) and would be most productively pursued by using experimental conditions that have successfully demonstrated the ability to distinguish ion and particle contributions (e.g., anaerobic environments). Given that NP morphology is known to influence surface reactivity and physical interactions with the bacteria membrane, particle shape is a topic deserving investigation that could provide insightful contributions to resolving the ion and particle mechanisms. Additional resolution of the influence of environmental constituents on surface chemistry of AgNPs is needed since Ag(I) ion release and bacterial interactions are both surface phenomena and these constituents can influence ligand chemistry as well as promote or inhibit oxidation of the NP surface. As a result, more comprehensive characterization of surface chemistry will be necessary for future studies, including monitoring of changes in surface chemistry that result from exposure to the experimental system. Finally, complete resolution of the ion versus particle debate requires development of a robust approach to isolating and quantifying released Ag(I) ions in AgNP exposure conditions, specifically Ag(I) ions bound to media constituents or bacterial components. This development will allow for an accurate representation of the AgNP to be modeled with Ag(I) ion-only exposures by delivering a series of doses that mimic the total rate of AgNP dissolution – the preferred and suggested best practice for an ion control. These recommendations for minimal characterization and reporting guidelines in future studies are summarized in Table 2.
| Criteria to report | Possible methods | Rationale |
|---|---|---|
| a There is a need to develop methodology for quantifying the distribution of ions in all components of the experimental system. Abbreviations: DLS – dynamic light scattering; GF-AAS – graphite furnace atomic absorption spectroscopy; HDD – hydrodynamic diameter; ICP-MS or AES – inductively coupled plasma mass spectrometry or atomic emission spectroscopy; NMR – nuclear magnetic resonance; SEM – scanning electron microscopy; TEM – transmission electron microscopy; UV-vis – ultraviolet-visible spectroscopy; XPS – X-ray photoelectron spectroscopy; XRD – X-ray diffraction. | ||
| General NP characterization | ||
| Initial NP concentration | ICP-MS or AES, UV-vis, or GF-AAS | Critical for calculating theoretical ion release |
| NP size | TEM in exposure media, pre- and post-exposure | Necessary to quantify size effects and estimate surface area for calculating theoretical ion release |
| NP shape | TEM in exposure media, pre- and post-exposure | Necessary to quantify shape effects and estimate surface area for calculating theoretical ion release |
| NP surface chemistry (e.g., ligand identities, sign and magnitude of charge, ligand density) | Zeta potential for sign and magnitude ofcharge in the experimental media pre- and post-exposure | Necessary to quantify surface chemistry effects. Surface chemistry and changes in surface chemistry resulting from exposure to the experimental system will influence the magnitude of ion release and interaction with bacteria |
| NMR, TGA, IR-based methods (among others) for ligand density, pre- and post-exposure | ||
| Method of NP synthesis and purification | Several; unique to NP | Elucidate possible synthesis or purification artefacts causing different surface morphology and ion release |
| NP aggregation | Monitor hydrodynamic diameter and zeta potential over duration of experiment and in experimental media using DLS | Aggregation confounds ion and particle contributions leading to differing conclusions drawn in studies (since aggregation can lead to non-nano sized particles) |
| Monitor optical density over duration of experiment | ||
| Scaled ion control considerations | ||
| Bulk ion concentration | Quantify bulk ion concentration in broth and presence of organism over duration of experiment (e.g. using ion-selective electrodes, UV-vis spectroscopy, centrifugation and then quantification of ions in the filtrate using elemental analysis techniques (e.g., ICP-MS or AES or GF-AAS)) | Completes the mass balance of ions in the system to inform delivery of a scaled ion dose and enable attribution of observed endpoints to the true ion concentration to which the organism is exposed |
| Intracellular ion concentration | E.g. monitor intracellular ion concentration using a bioluminescent analogue of the model organism over the duration of the experiment | |
| Media-complexed and cell-bound ion concentration | Method needed. An ideal method would be a standard assay that can isolate and quantify ions bound to media constituents or extracellular bacteria components | |
| Ion concentration associated with laboratory materials used in experiments | Digest lab materials and measure ion content with ICP-MS/OES or GF-AAS | |
While this systematic review is limited to bacteria, the importance of elucidating the mechanism of action and contribution of the ion and particle is ubiquitous across other types of organisms (e.g., algae, fungi, viruses, mammalian cells). In fact, it is critical from a human and environmental health standpoint that AgNPs used as antimicrobial agents be designed to maximize efficacy while minimizing harm to organisms higher up on the trophic level. Elucidating the mechanism of action and contribution of the ion and particle across trophic levels is important and is likely to shift due to organism-specific responses. For example, endocytosis mechanisms of eukaryotic cells support potential particle-driven and combined ion-particle mechanisms of action. Our conclusions are not directly transferrable across different model organisms due to such phenotypic differences, yet the abovementioned best practices of AgNP characterization and use of scaled ion controls do translate across trophic levels and are critical to decoupling ion and particle contributions to the mechanism and magnitude of impact.
In addition to resolving the independent and synergistic contributions of the ion and particle, such research activities will inform the tuning of particle design specific to the intended application. Given the rising demand for effective antimicrobials, particularly in the wake of the global antimicrobial resistance crisis, there is a need to inform the use of AgNPs as antimicrobials. More generally, the mechanistic knowledge gained can be used to inform design of other effective nano- and non-nano alternatives, particularly the delivery of multiple mechanisms of inactivation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the generous support from the Department of Defense (DoD) through the National Defense Science and Engineering Graduate Fellowship (NDSEG) Program, the 3M Non-Tenured Faculty Award, the Research Corporation for Science Advancement, and the Departments of Chemistry and Civil and Environmental Engineering at the University of Pittsburgh. Additionally, the authors would like to thank Michael Hartmann for his development of the surface atom calculations.References
- A. J. Huh and Y. J. Kwon, Nanoantibiotics: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era, J. Controlled Release, 2011, 156, 128–145 CrossRef PubMed.
- G. Franci, A. Falanga, S. Galdiero, L. Palomba, M. Rai, G. Morelli and M. Galdiero, Silver nanoparticles as potential antibacterial agents, Molecules, 2015, 20, 8856–8874 CrossRef PubMed.
- M. L. W. Knetch and L. H. Koole, New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles, Polymer, 2011, 3, 340–366 Search PubMed.
- K. Mijnendonckx, N. Leys, J. Mahillon, S. Silver and R. Van Houdt, Antimicrobial silver: uses, toxicity and potential for resistance, BioMetals, 2013, 26, 609–621 CrossRef PubMed.
- M. Rai, A. Yadav and A. Gade, Silver nanoparticles as a new generation of antimicrobials, Biotechnol. Adv., 2009, 27, 76–83 CrossRef PubMed.
- B. Reidy, A. Haase, A. Luch, K. A. Dawson and I. Lynch, Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications, Materials, 2013, 6, 2295–2350 CrossRef PubMed.
- B. Simoncic and D. Klemencic, Preparation and perforamnce of silver as an antimicrobial agent for textiles: A review, Text. Res. J., 2016, 86, 210–223 CrossRef.
- X. Chen and H. J. Schluesener, Nanosilver: a nanoproduct in medical application, Toxicol. Lett., 2008, 176, 1–12 CrossRef PubMed.
- S. Silver, T. Phung le and G. Silver, Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds, J. Ind. Microbiol. Biotechnol., 2006, 33, 627–634 CrossRef PubMed.
- G. P. Gruere, Implications of nanotechnology growth in food and agriculture in OECD countries, Food Policy, 2012, 37, 191–198 CrossRef.
- S. Mishra and H. B. Singh, Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture, Appl. Microbiol. Biotechnol., 2015, 99, 1097–1107 CrossRef PubMed.
- T. V. Duncan, Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors, J. Colloid Interface Sci., 2011, 363, 1–24 CrossRef PubMed.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. Alvarez, Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications, Water Res., 2008, 42, 4591–4602 CrossRef PubMed.
- N. Duran, M. Duran, M. B. de Jesus, A. B. Seabra, W. J. Favaro and G. Nakazato, Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity, Nanomedicine, 2016, 12, 789–799 CrossRef PubMed.
- J. L. Graves, Jr., M. Tajkarimi, Q. Cunningham, A. Campbell, H. Nonga, S. H. Harrison and J. E. Barrick, Rapid evolution of silver nanoparticle resistance in Escherichia coli, Front. Genet., 2015, 6, 42 Search PubMed.
- I. Chopra, The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern?, J. Antimicrob. Chemother., 2007, 59, 587–590 CrossRef PubMed.
- M. K. Rai, S. D. Deshmukh, A. P. Ingle and A. K. Gade, Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria, J. Appl. Microbiol., 2012, 112, 841–852 CrossRef PubMed.
- T. C. Dakal, A. Kumar, R. S. Majumdar and V. Yadav, Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles, Front. Microbiol., 2016, 7, 1831 Search PubMed.
- J. W. Molling, J. W. Seezink, B. E. Teunissen, I. Muijrers-Chen and P. J. Borm, Comparative performance of a panel of commercially available antimicrobial nanocoatings in Europe, Nanotechnol., Sci. Appl., 2014, 7, 97–104 CrossRef PubMed.
- Silver Nanoparticles Market by Application (Electronics & Electrical, Healthcare, Food & Beverages, Textiles) and Segment Forecasts to 2022, Grand View Research, 2015 Search PubMed.
- J. W. Alexander, History of the medical use of silver, Surg. Infect., 2009, 10, 289–292 CrossRef PubMed.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. A. Radzig, V. A. Nadtochenko, O. A. Koksharova, J. Kiwi, V. A. Lipasova and I. A. Khmel, Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action, Colloids Surf., B, 2013, 102, 300–306 CrossRef PubMed.
- A. Ivask, A. Elbadawy, C. Kaweeteerawat, D. Boren, H. Fischer, Z. Ji, C. H. Chang, R. Liu, T. Tolaymat, D. Telesca, J. I. Zink, Y. Cohen, P. A. Holden and H. A. Godwin, Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver, ACS Nano, 2014, 8, 374–386 CrossRef PubMed.
- C. Marambio-Jones and E. M. V. Hoek, A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef.
- J. S. McQuillan, H. G. Infante, E. Stokes and A. M. Shaw, Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12, Nanotoxicology, 2012, 6, 857–866 CrossRef PubMed.
- Z. M. Xiu, Q. B. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Negligible particle-specific antibacterial activity of silver nanoparticles, Nano Lett., 2012, 12, 4271–4275 CrossRef PubMed.
- Z. M. Xiu, J. Ma and P. J. Alvarez, Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions, Environ. Sci. Technol., 2011, 45, 9003–9008 CrossRef PubMed.
- X. Yang, A. P. Gondikas, S. M. Marinakos, M. Auffan, J. Liu, H. Hsu-Kim and J. N. Meyer, Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans, Environ. Sci. Technol., 2012, 46, 1119–1127 CrossRef PubMed.
- S. Ahmed, M. Ahmad, B. L. Swami and S. Ikram, A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise, J. Adv. Res., 2016, 7, 17–28 CrossRef PubMed.
- A. M. El Badawy, R. G. Silva, B. Morris, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Surface Charge-Dependent Toxicity of Silver Nanoparticles, Environ. Sci. Technol., 2011, 45, 283–287 CrossRef PubMed.
- N. S. Wigginton, A. de Titta, F. Piccapietra, J. Dobias, V. J. Nesatyy, M. J. Suter and R. Bernier-Latmani, Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity, Environ. Sci. Technol., 2010, 44, 2163–2168 CrossRef PubMed.
- Y. M. Long, L. G. Hu, X. T. Yan, X. C. Zhao, Q. F. Zhou, Y. Cai and G. B. Jiang, Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli, Int. J. Nanomed., 2017, 12, 3193–3206 CrossRef PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, M. K. Ahmad, A. A. Mahdi, R. Pal and S. S. Cameotra, Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules, J. Basic Microbiol., 2014, 54, 905–915 CrossRef PubMed.
- K. K. Y. Wong and X. Liu, Silver nanoparticles - the real “silver bullet” in clinical medicine?, MedChemComm, 2010, 1, 125–131 RSC.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346–2353 CrossRef PubMed.
- I. L. Hsiao, Y. K. Hsieh, C. F. Wang, I. C. Chen and Y. J. Huang, Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis, Environ. Sci. Technol., 2015, 49, 3813–3821 CrossRef PubMed.
- K. A. Johnston, L. M. Stabryla, A. M. Smith, X. Y. Gan, L. M. Gilbertson and J. E. Millstone, Impacts of broth chemistry on silver ion release, surface chemistry composition, and bacterial cytotoxicity of silver nanoparticles, Environ. Sci.: Nano, 2018, 5, 304–312 RSC.
- G. A. Sotiriou, A. Meyer, J. T. Knijnenburg, S. Panke and S. E. Pratsinis, Quantifying the origin of released Ag+ ions from nanosilver, Langmuir, 2012, 28, 15929–15936 CrossRef PubMed.
- B. Molleman and T. Hiemstra, Surface Structure of Silver Nanoparticles as a Model for Understanding the Oxidative Dissolution of Silver Ions, Langmuir, 2015, 31, 13361–13372 CrossRef PubMed.
- S. Kittler, C. Greulich, J. Diendorf, M. Koller and M. Epple, Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions, Chem. Mater., 2010, 22, 4548–4554 CrossRef.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Jr., Environmental transformations of silver nanoparticles: impact on stability and toxicity, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef PubMed.
- C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry and G. E. Brown, Jr., Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli, Environ. Sci. Technol., 2013, 47, 5738–5745 CrossRef PubMed.
- C. Levard, B. C. Reinsch, F. M. Michel, C. Oumahi, G. V. Lowry and G. E. Brown, Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate, Environ. Sci. Technol., 2011, 45, 5260–5266 CrossRef PubMed.
- B. Le Ouay and F. Stellacci, Antibacterial activity of silver nanoparticles: a surface science insight, Nano Today, 2015, 10, 339–354 CrossRef.
- J. Liu and R. H. Hurt, Ion release kinetics and particle persistence in aqueous nano-silver colloids, Environ. Sci. Technol., 2010, 44, 2169–2175 CrossRef PubMed.
- J. Liu, D. A. Sonshine, S. Shervani and R. H. Hurt, Controlled release of biologically active silver from nanosilver surfaces, ACS Nano, 2010, 4, 6903–6913 CrossRef PubMed.
- B. Molleman and T. Hiemstra, Time, pH, and size dependency of silver nanoparticle dissolution: the road to equilibrium, Environ. Sci.: Nano, 2017, 4, 1314–1327 RSC.
- W. Zhang, in Nanoparticle aggregation: principles and modeling, ed. D. G. Capco and Y. Chen, Springer, Dordrecht, 2014, ch. 2, vol. 811, pp. 19–43 Search PubMed.
- G. A. Sotiriou and S. E. Pratsinis, Antibacterial activity of nanosilver ions and particles, Environ. Sci. Technol., 2010, 44, 5649–5654 CrossRef PubMed.
- R. Ma, C. Levard, S. M. Marinakos, Y. Cheng, J. Liu, F. M. Michel, G. E. Brown and G. V. Lowry, Size-controlled dissolution of organic-coated silver nanoparticles, Environ. Sci. Technol., 2012, 46, 752–759 CrossRef PubMed.
- C. Gutierrez-Wing, M. Perez-Alvarez, G. Mondragon-Galicia, J. Arenas-Alatorre, M. T. Gutierrez-Wing, M. C. Henk, I. I. Negulescu and K. A. Rusch, Coalescence phenomena in 1D silver nanostructures, J. Phys.: Condens. Matter, 2009, 21, 295301 CrossRef PubMed.
- S. Pal, Y. K. Tak and J. M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef PubMed.
- D. W. Hatchett and H. S. White, Electrochemistry of sulfur adlayers on the low-index faces of silver, J. Phys. Chem., 1996, 100, 9854–9859 CrossRef.
- B. Wiley, Y. Sun and Y. Xia, Synthesis of silver nanostructures with controlled shapes and properties, Acc. Chem. Res., 2007, 40, 1067–1076 CrossRef PubMed.
- V. Sundaresan, J. W. Monaghan and K. A. Willets, Visualizing the effect of partial oxide formation on single silver nanoparticle electrodissolution, J. Phys. Chem. C, 2018, 122, 3138–3145 CrossRef.
- A. Georgantzopoulou, Y. L. Balachandran, P. Rosenkranz, M. Dusinska, A. Lankoff, M. Wojewodzka, M. Kruszewski, C. Guignard, J. N. Audinot, S. Girija, L. Hoffmann and A. C. Gutleb, Ag nanoparticles: size- and surface-dependent effects on model aquatic organisms and uptake evaluation with NanoSIMS, Nanotoxicology, 2013, 7, 1168–1178 CrossRef PubMed.
- S. Rajeshkumar and L. V. Bharath, Mechanism of plant-mediated synthesis of silver nanoparticles - A review on biomolecules involved, characterisation and antibacterial activity, Chem.-Biol. Interact., 2017, 273, 219–227 CrossRef PubMed.
- Y. Li, J. Zhao, E. Shang, X. Xia, J. Niu and J. Crittenden, Effects of chloride ions on dissolution, ROS generation, and toxicity of silver nanoparticles under UV irradiation, Environ. Sci. Technol., 2018, 52(8), 4842–4849 CrossRef PubMed.
- X. Li, J. J. Lenhart and H. W. Walker, Dissolution-accompanied aggregation kinetics of silver nanoparticles, Langmuir, 2010, 26, 16690–16698 CrossRef PubMed.
- A. M. El Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles, Environ. Sci. Technol., 2010, 44, 1260–1266 CrossRef PubMed.
- C. L. Arnaout and C. K. Gunsch, Impacts of silver nanoparticle coating on the nitrification potential of Nitrosomonas europaea, Environ. Sci. Technol., 2012, 46, 5387–5395 CrossRef PubMed.
- N. T. Giao, T. Limpiyakorn, P. Kunapongkiti, P. Thuptimdang and S. Siripattanakul-Ratpukdi, Influence of silver nanoparticles and liberated silver ions on nitrifying sludge: ammonia oxidation inhibitory kinetics and mechanism, Environ. Sci. Pollut. Res., 2017, 24, 9229–9240 CrossRef PubMed.
- N. Hachicho, P. Hoffmann, K. Ahlert and H. J. Heipieper, Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2, FEMS Microbiol. Lett., 2014, 355, 71–77 CrossRef PubMed.
- W. Hong, L. Li, J. Liang, J. Wang, X. Wang, S. Xu, L. Wu, G. Zhao, A. Xu and S. Chen, Investigating the environmental factors affecting the toxicity of silver nanoparticles in Escherichia coli with dual fluorescence analysis, Chemosphere, 2016, 155, 329–335 CrossRef PubMed.
- A. Ivask, I. Kurvet, K. Kasemets, I. Blinova, V. Aruoja, S. Suppi, H. Vija, A. Kakinen, T. Titma, M. Heinlaan, M. Visnapuu, D. Koller, V. Kisand and A. Kahru, Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro, PLoS One, 2014, 9, e102108 CrossRef PubMed.
- A. Masrahi, A. R. VandeVoort and Y. Arai, Effects of silver nanoparticle on soil-nitrification processes, Arch. Environ. Contam. Toxicol., 2014, 66, 504–513 CrossRef PubMed.
- M. A. Maurer-Jones, M. P. S. Mousavi, L. D. Chen, P. Buhlmann and C. L. Haynes, Characterization of silver ion dissolution from silver nanoparticles using fluorous-phase ion-seelctive electrodes and assessment of resultant toxicity to Shewanella oneidensis, Chem. Sci., 2013, 4, 2564–2572 RSC.
- T. S. Radniecki, D. P. Stankus, A. Neigh, J. A. Nason and L. Semprini, Influence of liberated silver from silver nanoparticles on nitrification inhibition of Nitrosomonas europaea, Chemosphere, 2011, 85, 43–49 CrossRef PubMed.
- T. Silva, L. R. Pokhrel, B. Dubey, T. M. Tolaymat, K. J. Maier and X. Liu, Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity, Sci. Total Environ., 2014, 468–469, 968–976 CrossRef PubMed.
- Y. Yang, J. Quensen, J. Mathieu, Q. Wang, J. Wang, M. Li, J. M. Tiedje and P. J. Alvarez, Pyrosequencing reveals higher impact of silver nanoparticles than Ag+ on the microbial community structure of activated sludge, Water Res., 2014, 48, 317–325 CrossRef PubMed.
- E. Amato, Y. A. Diaz-Fernandez, A. Taglietti, P. Pallavicini, L. Pasotti, L. Cucca, C. Milanese, P. Grisoli, C. Dacarro, J. M. Fernandez-Hechavarria and V. Necchi, Synthesis, characterization and antibacterial activity against Gram positive and Gram negative bacteria of biomimetically coated silver nanoparticles, Langmuir, 2011, 27, 9165–9173 CrossRef PubMed.
- A. D. Burchardt, R. N. Carvalho, A. Valente, P. Nativo, D. Gilliland, C. P. Garcia, R. Passarella, V. Pedroni, F. Rossi and T. Lettieri, Effects of silver nanoparticles in diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp, Environ. Sci. Technol., 2012, 46, 11336–11344 CrossRef PubMed.
- J. Yi and J. Cheng, Effects of water chemistry and surface contact on the toxicity of silver nanoparticles to Bacillus subtilis, Ecotoxicology, 2017, 26, 639–647 CrossRef PubMed.
- M. Visnapuu, U. Joost, K. Juganson, K. Kunnis-Beres, A. Kahru, V. Kisand and A. Ivask, Dissolution of silver nanowires and nanospheres dictates their toxicity to Escherichia coli, BioMed Res. Int., 2013, 2013, 819252 Search PubMed.
- M. A. Doody, D. Wang, H. P. Bais and Y. Jin, Differential antimicrobial activity of silver nanoparticles to bacteria Bacillus subtilis and Escherichia coli, and toxicity to crop plant Zea mays and beneficial B. subtilis-inoculated Z. mays, J. Nanopart. Res., 2016, 18, 290 CrossRef.
- A. Ivask, O. Bondarenko, N. Jepihhina and A. Kahru, Profiling of the reactive oxygen species-related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: differentiating the impact of particles and solubilised metals, Anal. Bioanal. Chem., 2010, 398, 701–716 CrossRef PubMed.
- M. J. Grant and A. Booth, A typology of reviews: an analysis of 14 review types and associated methodologies, Health Info. Libr. J., 2009, 26, 91–108 CrossRef PubMed.
- H. Hofmeister and G. L. Tan, Shape and internal structure of silver nanoparticles embedded in glass, J. Mater. Res., 2005, 20, 1551–1562 CrossRef.
- A. K. Singh, Introduction to nanoparticles and nanotoxicology, Engineered Nanoparticles: Structure, Properties and Mechanisms of Toxicity, 2016, pp. 1–18 Search PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions, Environ. Sci. Technol., 2013, 47, 10293–10301 Search PubMed.
- A. B. Smetana, K. J. Klabunde, G. R. Marchin and C. M. Sorensen, Biocidal activity of nanocrystalline silver powders and particles, Langmuir, 2008, 24, 7457–7464 CrossRef PubMed.
- C. Liu, W. Leng and P. J. Vikesland, Controlled Evaluation of the Impacts of Surface Coatings on Silver Nanoparticle Dissolution Rates, Environ. Sci. Technol., 2018, 52, 2726–2734 CrossRef PubMed.
- Y. Negishi, T. Nakazaki, S. Malola, S. Takano, Y. Niihori, W. Kurashige, S. Yamazoe, T. Tsukuda and H. Hakkinen, A critical size for emergence of nonbulk electronic and geometric structures in dodecanethiolate-protected Au clusters, J. Am. Chem. Soc., 2015, 137, 1206–1212 CrossRef PubMed.
- M. J. Hartmann, H. Hakkinen, J. E. Millstone and D. S. Lambrecht, Impacts of copper position on the electronic structure of [Au25-xCux(SH)18]- nanoclusters, J. Phys. Chem. C, 2015, 119, 8290–8298 CrossRef.
- J. J. Ortega-Calvo, R. Molina, C. Jimenez-Sanchez, P. J. Dobson and I. P. Thompson, Bacterial tactic response to silver nanoparticles, Environ. Microbiol. Rep., 2011, 3, 526–534 CrossRef PubMed.
- I. Sondi and B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef PubMed.
- A. K. Suresh, D. A. Pelletier, W. Wang, J. W. Moon, B. Gu, N. P. Mortensen, D. P. Allison, D. C. Joy, T. J. Phelps and M. J. Doktycz, Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria, Environ. Sci. Technol., 2010, 44, 5210–5215 CrossRef PubMed.
- C. R. Bowman, F. C. Bailey, M. Elrod-Erickson, A. M. Neigh and R. R. Otter, Effects of silver nanoparticles on zebrafish (Danio rerio) and Escherichia coli (ATCC 25922): a comparison of toxicity based on total surface area versus mass concentration of particles in a model eukaryotic and prokaryotic system, Environ. Toxicol. Chem., 2012, 31, 1793–1800 CrossRef PubMed.
- C. Greulich, D. Braun, A. Peetsch, J. Diendorf, B. Siebers, M. Epple and M. Koller, The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range, RSC Adv., 2012, 2, 6981–6987 RSC.
- A. P. Zarubina, L. I. Deev, I. M. Parkhomenko, E. Y. Parshina, A. S. Sarycheva, L. A. Novoselova, E. P. Lukashev, A. I. Netrusov and A. B. Rubin, Evaluation of Toxicity of Silver Ions and Nanoparticles using Model Bacteria with Luminescent Phenotype, Nanotechnol. Russ., 2015, 10, 475–483 CrossRef.
- J. S. McQuillan and A. M. Shaw, Differential gene regulation in the Ag nanoparticle and Ag(+)-induced silver stress response in Escherichia coli: a full transcriptomic profile, Nanotoxicology, 2014, 8(Suppl 1), 177–184 CrossRef PubMed.
- A. M. Smith, K. A. Johnston, S. E. Crawford, L. E. Marbella and J. E. Millstone, Ligand density quantification on colloidal inorganic nanoparticles, Analyst, 2017, 142, 11–29 RSC.
- M. A. Boles, D. Ling, T. Hyeon and D. V. Talapin, The surface science of nanocrystals, Nat. Mater., 2016, 15, 141–153 CrossRef PubMed.
- T. L. Cheng, K. H. Chuang, B. M. Chen and S. R. Roffler, Analytical measurement of PEGylated molecules, Bioconjugate Chem., 2012, 23, 881–899 CrossRef PubMed.
- D. J. Gavia and Y. S. Shon, Controlling surface ligand density and core size of alkanethiolate-capped Pd nanoparticles and their effects on catalysis, Langmuir, 2012, 28, 14502–14508 CrossRef PubMed.
- G. He, Y. Song, X. Kang and S. Chen, Alkyne-functionalized palladium nanoparticles: synthesis, characterization, and electrocatalytic activity in ethylene glycol oxidation, Electrochim. Acta, 2013, 94, 98–103 CrossRef.
- J. V. Jokerst, T. Lobovkina, R. N. Zare and S. S. Gambhir, Nanoparticle PEGylation for imaging and therapy, Nanomedicine, 2011, 6, 715–728 CrossRef PubMed.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Self-assembled monolayers of thiolates on metals as a form of nanotechnology, Chem. Rev., 2005, 105, 1103–1169 CrossRef PubMed.
- C. Beer, R. Foldbjerg, Y. Hayashi, D. S. Sutherland and H. Autrup, Toxicity of silver nanoparticles - nanoparticle or silver ion?, Toxicol. Lett., 2012, 208, 286–292 CrossRef PubMed.
- A. Ivask, T. Rolova and A. Kahru, A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing, BMC Biotechnol., 2009, 9, 41 CrossRef PubMed.
- Z. Chen, P. Yang, Z. Yuan and J. Guo, Aerobic condition enhances bacteriostatic effects of silver nanoparticles in aquatic environment: an antimicrobial study on Pseudomonas aeruginosa, Sci. Rep., 2017, 7, 7398 CrossRef PubMed.
- C. N. Lok, C. M. Ho, R. Chen, Q. Y. He, W. Y. Yu, H. Sun, P. K. Tam, J. F. Chiu and C. M. Che, Silver nanoparticles: partial oxidation and antibacterial activities, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef PubMed.
- H. Arai, J. H. Roh, J. M. Eraso and S. Kaplan, Transcriptome response to nitrosative stress in Rhodobacter sphaeroides 2.4.1, Biosci., Biotechnol., Biochem., 2013, 77, 111–118 CrossRef PubMed.
- R. Behra, L. Sigg, M. J. Clift, F. Herzog, M. Minghetti, B. Johnston, A. Petri-Fink and B. Rothen-Rutishauser, Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective, J. R. Soc., Interface, 2013, 10, 20130396 CrossRef PubMed.
- D. A. Mosselhy, M. A. El-Aziz, M. Hanna, M. A. Ahmed, M. M. Husien and Q. Feng, Comparative synthesis and antimicrobial activity of nanoparticles and silver nitrate, J. Nanopart. Res., 2015, 17, 473 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00429c |
| This journal is © The Royal Society of Chemistry 2018 |