Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Water oxidation by a copper(II) complex: new findings, questions, challenges and a new hypothesis
Mohammad
Mahdi Najafpour
*abc,
Somayeh
Mehrabani
a,
Younes
Mousazade
a and
Małgorzata
Hołyńska
d
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: +9824 3315 3201
bCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran
cResearch Center for Basic Sciences & Modern Technologies (RBST), Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan 45137-66731, Iran
dFachbereich Chemie and Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Straße, D-35032 Marburg, Germany
First published on 16th August 2018
Abstract
Correction for ‘Water oxidation by a copper(II) complex: new findings, questions, challenges and a new hypothesis’ by Mohammad Mahdi Najafpour et al., Dalton Trans., 2018, 47, 9021–9029.
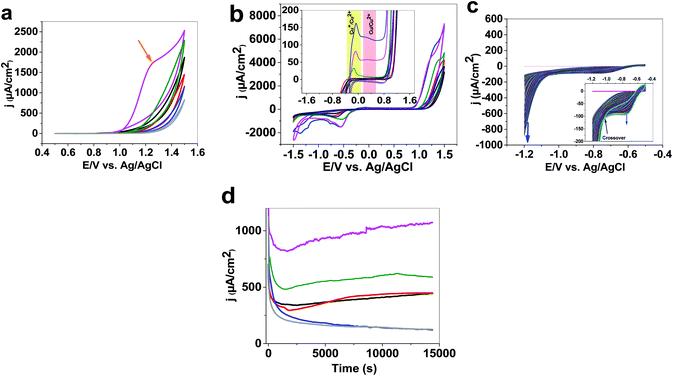
The authors would like to correct part b of Fig. 1, which, in the published manuscript, is the same as part a. The correct Fig. 1 is shown below:
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2018 |