Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbonate: an alternative dopant to stabilize new perovskite phases; synthesis and structure of Ba3Yb2O5CO3 and related isostructural phases Ba3Ln2O5CO3 (Ln = Y, Dy, Ho, Er, Tm and Lu)†
Joshua
Deakin
a,
Ivan
Trussov
 a,
Alexandra
Gibbs
a,
Alexandra
Gibbs
 b,
Emma
Kendrick
c and
Peter R.
Slater
b,
Emma
Kendrick
c and
Peter R.
Slater
 *a
*a
aSchool of Chemistry, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: p.r.slater@bham.ac.uk; Fax: +44 (0)1214 144403; Tel: +44 (0)1214 148906
bISIS Facility, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, OX11 0QX, UK
cWarwick Manufacturing Group, University of Warwick, Coventry, CV4 7AL, UK
First published on 13th August 2018
Abstract
In this paper we report the synthesis of the new layered perovskite oxide carbonate, Ba3Yb2O5CO3. This phase is formed when 3BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Yb2O3 mixtures are heated in air at temperatures ≤1000 °C, while above this temperature the carbonate is lost and the simple oxide phase Ba3Yb4O9 is observed. The structure of Ba3Yb2O5CO3 was determined from neutron diffraction studies and consists of a tripled perovskite with double Yb–O layers separated by carbonate layers, the first example of a material with such a structure. Further studies showed that analogous Ba3Ln2O5CO3 phases could be formed for other rare earths (Ln = Y, Dy, Ho, Er, Tm and Lu). The results highlight the ability of the perovskite structure to accommodate carbonate groups, and emphasise the need to consider their potential presence particularly for perovskite systems prepared in lower temperature synthesis routes.
1Yb2O3 mixtures are heated in air at temperatures ≤1000 °C, while above this temperature the carbonate is lost and the simple oxide phase Ba3Yb4O9 is observed. The structure of Ba3Yb2O5CO3 was determined from neutron diffraction studies and consists of a tripled perovskite with double Yb–O layers separated by carbonate layers, the first example of a material with such a structure. Further studies showed that analogous Ba3Ln2O5CO3 phases could be formed for other rare earths (Ln = Y, Dy, Ho, Er, Tm and Lu). The results highlight the ability of the perovskite structure to accommodate carbonate groups, and emphasise the need to consider their potential presence particularly for perovskite systems prepared in lower temperature synthesis routes.
Introduction
Perovskite materials have attracted considerable interest due to a wide range of technologically important properties displayed by materials with this structure-type, including superconductivity, ionic conductivity, colossal magnetoresistance, ferroelectric properties, and the ability to catalyse a range of reactions. In addition to this rich wealth of properties, perovskites also display a wealth of interesting and, at time, unexpected structural diversity. In particular, research on high Tc cuprate superconductors showed the ability of the perovskite structure to accommodate carbonate and other oxyanions (borate, nitrate, sulfate, phosphate).1–12 In these situations, the C, B, N, P, S of the oxyanion group resides on the perovskite B cation site, while the oxide ions of this group fill 3 (C, B, N)–4 (P, S) of the available 6 oxide ion positions around this site, albeit displaced so as to achieve the required geometry for the oxyanion. The incorporation of oxyanions into other perovskite transition metal containing systems has also subsequently been reported, e.g. Sr(Co/Fe/Mn)O3−δ, La1−xSrxCo0.8Fe0.2O3−δ, Ba1−xSrxCo0.8Fe0.2O3−δ, CaMnO3 and La1−xSrxMnO3-type materials.13–30 Recently the incorporation of sulfate and phosphate have also been reported in Ba2(In/Sc)2O5 leading to new cubic perovskites with high oxide ion conductivity/proton conductivity.31–33In terms of these doping studies, it has been shown that oxyanions such as sulfate, borate, and phosphate exhibit higher thermal stability in the structure than carbonate. Therefore, work on incorporating carbonate has commonly employed reaction in evacuated quartz tubes to achieve reaction while preventing carbonate loss.15–19 As such, other researchers may be viewing such carbonate containing systems as “exotic” compounds, which would not be formed under conventional synthesis conditions. This is a particularly worrying omission, since there is a growing trend in research to move to lower temperature synthesis (e.g. sol gel) routes where carbonate may be present. Indeed evidence for the importance of carbonate in materials is illustrated by recent work on other structure types; for example Li et al. have shown that hexagonal “YAlO3” is an oxide carbonate,34 with a true composition of Y3Al3O8CO3, while orthorhombic Ba2TiO4 has also been recently shown to contain carbonate.35
While prior carbonate doped perovskite work has focused on systems containing transition metals, such as Cu, here we extend such studies to investigate whether a perovskite phase could be formed for non-transition metal systems, with synthesis in air rather than evacuated quartz tubes. In particular, given that a cubic perovskite, Ba2Yb1.5P0.5O5.5 has been recently shown to be stabilised by phosphate incorporation, we therefore investigated the possibility that a perovskite containing carbonate could be formed in the BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb2O3 phase diagram at lower temperatures (≤1000 °C) to allow carbonate incorporation. This led to the identification of a new phase with BaCO3
Yb2O3 phase diagram at lower temperatures (≤1000 °C) to allow carbonate incorporation. This led to the identification of a new phase with BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb2O3 ratio of 3
Yb2O3 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and in this paper we report the synthesis and structural characterisation of this new carbonate containing perovskite system. In addition, in order to investigate the versatility for carbonate incorporation in perovskites, we also illustrate the formation of isostructural phases for a range of other rare earths.
1, and in this paper we report the synthesis and structural characterisation of this new carbonate containing perovskite system. In addition, in order to investigate the versatility for carbonate incorporation in perovskites, we also illustrate the formation of isostructural phases for a range of other rare earths.
Experimental
High purity BaCO3, Yb2O3 were used to prepare Ba3Yb2O5CO3. The powders were intimately ground in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BaCO3, Yb2O3 ratio and heated initially to 900 °C for 12 h in air. They were then reground and reheated to 1000 °C in air for a further 24 h with regrinding every 12 hours. After the initial synthesis in air, we also investigated the formation of this compound under dry N2 in order to allow higher temperature heat treatment by reducing CO2 loss through reaction with moisture in the air. In this case, it was found that Ba3Yb2O5CO3 could be prepared in a shorter timescale (12 h) by employing a higher temperature (1050 °C). Note heat treatment in air at 1050 °C leads to large Ba3Yb4O9 impurities due to carbonate loss. These synthesis routes were then used to make a wider range of isostructural Ba3Ln2O5CO3 systems (Ln = Y, Dy, Ho, Er, Tm and Lu).
1 BaCO3, Yb2O3 ratio and heated initially to 900 °C for 12 h in air. They were then reground and reheated to 1000 °C in air for a further 24 h with regrinding every 12 hours. After the initial synthesis in air, we also investigated the formation of this compound under dry N2 in order to allow higher temperature heat treatment by reducing CO2 loss through reaction with moisture in the air. In this case, it was found that Ba3Yb2O5CO3 could be prepared in a shorter timescale (12 h) by employing a higher temperature (1050 °C). Note heat treatment in air at 1050 °C leads to large Ba3Yb4O9 impurities due to carbonate loss. These synthesis routes were then used to make a wider range of isostructural Ba3Ln2O5CO3 systems (Ln = Y, Dy, Ho, Er, Tm and Lu).
Phase identification and initial structure determination was carried out by Rietveld profile refinement using powder X-ray diffraction data (XRD) collected on a Panalytical Empyrean diffractometer (Cu Kα radiation) or a Bruker D2 diffractometer (Co Kα radiation).
Raman data for Ba3Yb2O5CO3 were collected on a Renishaw inVia Raman microscope using a 532 nm laser in order to confirm the presence of carbonate in the material.
For the detailed structure determination on Ba3Yb2O5CO3, time of flight powder neutron diffraction (NPD) data were recorded on the HRPD diffractometer at the ISIS pulsed spallation source (Rutherford Appleton Laboratory, UK). Structure refinements using the NPD data were performed using the Rietveld method with the General Structure Analysis System GSAS-II suite of programs.36
Results and discussion
Phase identification and structure determination of Ba3Yb2O5CO3
Initially a range of BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb2O3 mixtures with different ratios were investigated, and it was found that a new phase was observed with an optimum BaCO3
Yb2O3 mixtures with different ratios were investigated, and it was found that a new phase was observed with an optimum BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb2O3 ratio of 3
Yb2O3 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
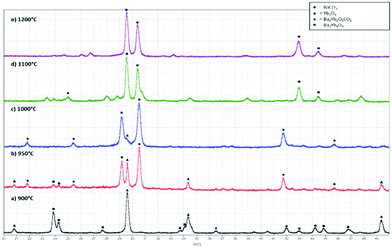
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The formation of this phase was shown to be very sensitive to the synthesis temperature (Fig. 1). At temperatures up to 900 °C, no reaction was observed, and the XRD pattern showed simply the presence of the starting materials, BaCO3 and Yb2O3. Heating to 950 °C led to the appearance of the new perovskite phase, with the purity improving by heating at 1000 °C. At temperatures above 1000 °C, this compound was shown to decompose, and the formation of the simple oxide Ba3Yb4O9 was observed, which suggested that the initial perovskite phase contained carbonate.
1. The formation of this phase was shown to be very sensitive to the synthesis temperature (Fig. 1). At temperatures up to 900 °C, no reaction was observed, and the XRD pattern showed simply the presence of the starting materials, BaCO3 and Yb2O3. Heating to 950 °C led to the appearance of the new perovskite phase, with the purity improving by heating at 1000 °C. At temperatures above 1000 °C, this compound was shown to decompose, and the formation of the simple oxide Ba3Yb4O9 was observed, which suggested that the initial perovskite phase contained carbonate.
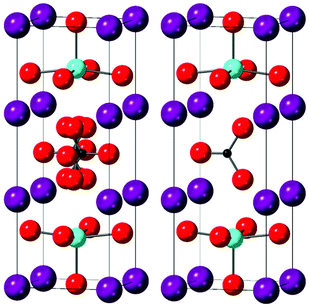
Indexing the pattern for this new phase gave a tetragonal perovskite-related cell which was tripled along the c direction. From the unit cell parameters obtained, a structure was then surmised based on the assumption that oxygen vacancies around the Yb sites would be less favourable. This predicted structure consisted of double Yb–O layers separated by carbonate layers.
This structure was then used as the starting point for the Rietveld refinement of the neutron diffraction data. The refinement was performed using the GSAS-II programme and constraints were initially placed upon the carbonate group within the system which included O–C–O angles constrained to 120° and C–O bond lengths constrained to 1.28 Å, in line with those expected for carbonate. In line with expectations that oxygen vacancies would be less favourable around the Yb site, the carbonate group was shown to be orientated with two of its oxygens directed towards the Yb sites, with the remaining oxygen equatorial to the carbonate layer. This gives an essentially square pyramidal Yb with a sixth longer bond to the carbonate oxygen.
There was no evidence for superlattice reflections/lowering of symmetry indicative of ordering of the orientation of the carbonate groups. In the final Rietveld refinement of the Ba3Yb2O5CO3 structure, the constraints on the O–C–O angles and C–O distances were removed (the only constraint included was that the sum of the occupancies of these O sites equal 3 in line with requirements for a carbonate group). This refinement led to a good fit to the data with the structural parameters and bond distances given in Tables 1 and 2, and the observed, calculated and difference neutron profiles shown in Fig. 2 (the data indicated a small amount (2.5 wt%) of Yb2O3 impurity, which was included in the final refinement). Given the presence of small Yb2O3 impurity, attempts were also made to refine the Yb site occupancy. This gave a value just above 1, and so in the final refinement was fixed at 1.0. This precludes both the presence of Yb vacancies or Ba on the Yb site (Ba has a significantly lower neutron scattering factor than Yb). The presence of this Yb2O3 impurity is most likely due to a small amount of amorphous BaCO3, or Ba volatility as has been observed for other Ba containing perovskite systems.31–33
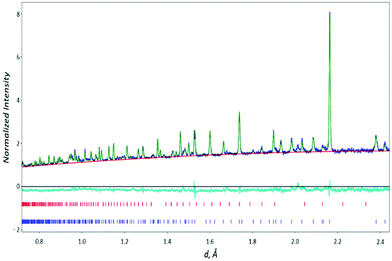 | ||
| Fig. 2 Observed, calculated and difference profiles for Ba3Yb2O5CO3 neutron data (upper tick marks – Yb2O3, lower tick marks Ba3Yb2O5CO3). | ||
| Atom | U 11 | U 22 | U 33 | U 12 | U 13 | U 23 |
|---|---|---|---|---|---|---|
| R wp = 2.93% GOF = 1.71 P4/mmm a = 4.3258(2) Å, c = 11.9036(5) Å.a Anisotropic atomic displacement parameters. | ||||||
| Ba1 | 0.034(1) | 0.034(1) | 0.015(3) | 0.000 | 0.000 | 0.000 |
| Ba2 | 0.024(1) | 0.024(1) | 0.030(2) | 0.000 | 0.000 | 0.000 |
| O1 | 0.008(1) | 0.026(1) | 0.071(1) | 0.000 | 0.000 | 0.000 |
| O2 | 0.088(1) | 0.088(1) | 0.004(2) | 0.000 | 0.000 | 0.000 |
| O3 | 0.020(5) | 0.020(5) | 0.008(5) | 0.000 | 0.000 | 0.000 |
| O4 | 0.043(2) | 0.052(2) | 0.052(2) | 0.000 | −0.013(1) | 0.000 |
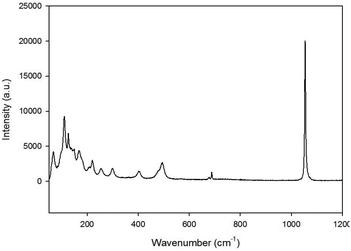
Raman studies of the Ba3Yb2O5CO3 material (Fig. 4) confirmed the presence of carbonate bands, with the most significant band (ν1 symmetric stretch) seen at 1054.51 cm−1. In comparison, BaCO3 was also analysed with the carbonate ν1 symmetric stretch observed at a higher energy of 1059.53 cm−1. This difference confirms that the CO32− peak was due to the Ba3Yb2O5CO3 material and not just small amounts of BaCO3 reagent.
The final structural model is shown in Fig. 3, which shows that the structure, consists of double Yb–O layers separated by carbonate layers, representing a new example of an ordered perovskite stabilised through carbonate incorporation. The anisotropic atomic displacement parameters for O1 and O2 suggest local tilting of the YbO6 octahedra to coincide with the adjacent carbonate orientation.
Synthesis of other Ba3Ln2O5CO3 (Ln = rare earth) phases
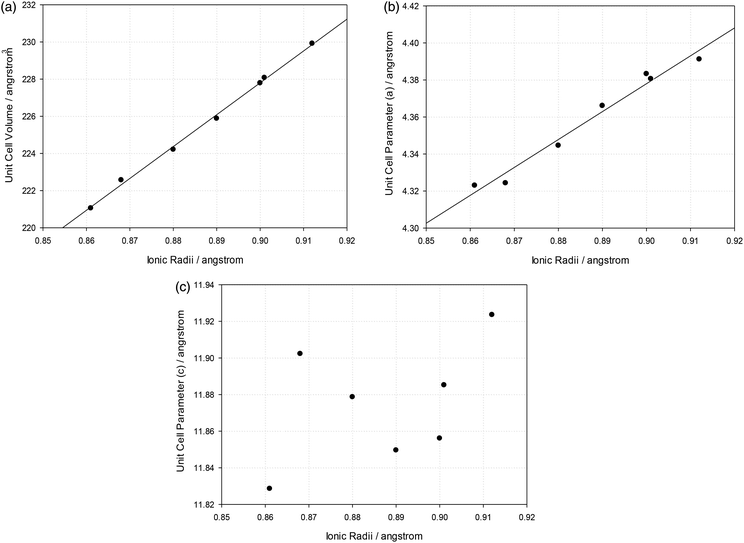
Following the successful synthesis and structure determination of Ba3Yb2O5CO3, the possible synthesis of analogous phases with different rare earths was investigated. Similar synthesis in air led to the successful formation of Ba3Ln2O5CO3 for Ln = Lu, Tm. For other rare earths (Ln = Y, Er, Ho and Dy) air synthesis led to samples with large Ba3Ln4O9 or BaLn2O4 impurities. For these systems, better quality samples could be prepared by utilising the dry N2 synthesis approach to limit loss of CO2, and hence maintain the presence of carbonate in the sample. Attempts to prepare these phases for larger rare earths, e.g. Sm, Nd, resulted in no presence of the perovskite oxide carbonate phase. The structure obtained for Ba3Yb2O5CO3 was used for preliminary structure refinement to obtain cell parameters for these Ba3Ln2O5CO3 systems. Each refinement showed a good fit to the data. However, through the refinements it became apparent that each still contained small amounts (∼3–4 wt%) of their respective Ln2O3 starting materials.The refined unit cell parameters are given in Table 3, with observed, calculated and difference profile fits shown in ESI.† As expected, there is a linear increase in unit cell volume with increasing rare earth size (Table 3, Fig. 5a). However, the variation in the individual cell parameters follow a less systematic trend. While the a parameter shows a similar general increase with rare earth size (Fig. 5b), c follows a non-systematic variation (Fig. 5c). More detailed neutron diffraction structure determination would be required to explain this, which may be related to changes in local carbonate orientations. For example, it is possible that there might be some carbonate groups rotated 90° so that two oxygens are in the equatorial positions.
| Formula | Ionic radius of Ln3+ (6 coordinate)37 | Unit cell parameters | Unit cell volume | |
|---|---|---|---|---|
| (a) | (c) | |||
| Ba3Lu2O5CO3 | 0.861 Å | 4.3223(1) Å | 11.8311(4) Å | 221.03(1) Å3 |
| Ba3Yb2O5CO3 | 0.868 Å | 4.3258(2) Å | 11.9036(5) Å | 222.75(2) Å3 |
| Ba3Tm2O5CO3 | 0.880 Å | 4.3439(1) Å | 11.8795(4) Å | 224.16(2) Å3 |
| Ba3Er2O5CO3 | 0.890 Å | 4.3671(3) Å | 11.8623(7) Å | 226.24(4) Å3 |
| Ba3Y2O5CO3 | 0.900 Å | 4.3809(3) Å | 11.8514(7) Å | 227.46(4) Å3 |
| Ba3Ho2O5CO3 | 0.901 Å | 4.3813(2) Å | 11.8871(4) Å | 228.19(2) Å3 |
| Ba3Dy2O5CO3 | 0.912 Å | 4.3900(3) Å | 11.9244(8) Å | 229.81(4) Å3 |
Conclusions
In this work, we illustrate the formation of perovskite oxide carbonate phases in the BaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln2O3 phase diagram for a ratio of 3
Ln2O3 phase diagram for a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These Ba3Ln2O5CO3 phases are shown to be new layered perovskites, with double Ln–O layers separated by carbonate layers, the first example of a material with such a structure. The results highlight the need to consider the potential incorporation of carbonate in perovskite materials prepared at temperatures ≤1000 °C. Thus it raises questions whether many perovskite systems prepared at low temperature (e.g. via sol–gel routes) may contain carbonate, affecting properties rather than simply previously considered morphological effects.
1. These Ba3Ln2O5CO3 phases are shown to be new layered perovskites, with double Ln–O layers separated by carbonate layers, the first example of a material with such a structure. The results highlight the need to consider the potential incorporation of carbonate in perovskite materials prepared at temperatures ≤1000 °C. Thus it raises questions whether many perovskite systems prepared at low temperature (e.g. via sol–gel routes) may contain carbonate, affecting properties rather than simply previously considered morphological effects.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express thanks to the Leverhulme Trust (RPG-2017-011), and Bolashak International Scholarship (PhD scholarship for Ivan Trussov) for funding. We would also like to thank the STFC ISIS Facility for the provision of neutron diffraction time. The raw neutron diffraction data file can be found in ref. 38 (within files beginning hrp68277).Notes and references
- C. Greaves and P. R. Slater, J. Mater. Chem., 1991, 1, 17 RSC.
- C. Greaves and P. R. Slater, Phys. C, 1991, 175, 172 CrossRef.
- P. R. Slater, C. Greaves, M. Slaski and C. M. Muirhead, Phys. C, 1993, 208, 193 CrossRef.
- Y. Miyazaki, H. Yamane, N. Ohnishi, T. Kajitani, K. Hiraga, Y. Mori, S. Funahashi and T. Hirai, Phys. C, 1992, 198, 7 CrossRef.
- A. Maignan, M. Hervieu, C. Michel and B. Raveau, Phys. C, 1993, 208, 116 CrossRef.
- K. Kinochita and T. Yamada, Nature, 1992, 357, 313 CrossRef.
- M. G. Francesconi and C. Greaves, Supercond. Sci. Technol., 1997, 10, A29 CrossRef.
- M. Uehara, H. Nakata and J. Akimitsu, Phys. C, 1993, 216, 453 CrossRef.
- R. Li, R. K. Kremer and J. Maier, J. Solid State Chem., 1993, 105, 609 CrossRef.
- Y. Miyazaki, H. Yamane, T. Kajitani, N. Kobayashi, K. Hiraga, Y. Morii, S. Funahashi and T. Hirai, Phys. C, 1994, 230, 89 CrossRef.
- J. Akimitsu, M. Uehara, M. Ogawa, H. Nakata, K. Tomimoto, Y. Miyazaki, H. Yamane, T. Hirai, K. Kinoshita and Y. Matsui, Phys. C, 1992, 201, 320 CrossRef.
- B. Domenges, M. Hervieu and B. Raveau, Phys. C, 1993, 207, 65 CrossRef.
- Y. Shimakawa, J. D. Jorgensen, D. G. Hinks, H. Shaked and R. L. Hitterman, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 16008 CrossRef.
- C. A. Hancock, J. M. Porras-Vazquez, P. A. Keenan and P. R. Slater, Dalton Trans., 2015, 44, 10559 RSC.
- V. Caignaert, B. Domenges and B. Raveau, J. Solid State Chem., 1995, 120, 279 CrossRef.
- D. Pelloquin, M. Hervieu, C. Michel, N. Nguyen and B. Raveau, J. Solid State Chem., 1997, 134, 395 CrossRef.
- M. Hervieu, C. Michel, D. Pelloquin, A. Maignan and B. Raveau, J. Solid State Chem., 2000, 149, 226 CrossRef.
- Y. Breard, C. Michel, M. Hervieu, N. Nguyen, A. Ducouret, V. Hardy, A. Maignan, B. Raveau, F. Bouree and G. Andre, Chem. Mater., 2004, 16, 2895–2905 CrossRef.
- B. Raveau, M. Hervieu, D. Pelloquin, C. Michel and R. Retoux, Z. Anorg. Allg. Chem., 2005, 631, 1831 CrossRef.
- J. F. Shin, L. Hussey, A. Orera and P. R. Slater, Chem. Commun., 2010, 46, 4613 RSC.
- J. F. Shin, D. C. Apperley and P. R. Slater, Chem. Mater., 2010, 22, 5945 CrossRef.
- J. F. Shin and P. R. Slater, J. Power Sources, 2011, 196, 8539 CrossRef.
- C. A. Hancock, R. C. T. Slade, J. R. Varcoe and P. R. Slater, J. Solid State Chem., 2011, 184, 2972 CrossRef.
- J. M. Porras-Vazquez and P. R. Slater, J. Power Sources, 2012, 209, 180 CrossRef.
- J. M. Porras-Vazquez, T. F. Kemp, J. V. Hanna and P. R. Slater, J. Mater. Chem., 2012, 22, 8287 RSC.
- J. M. Porras-Vazquez and P. R. Slater, Fuel Cells, 2012, 12, 1056 CrossRef.
- C. A. Hancock and P. R. Slater, Dalton Trans., 2011, 40, 5599 RSC.
- J. M. Porras-Vazquez, E. R. Losilla, P. J. Keenan, C. A. Hancock, T. F. Kemp, J. V. Hanna and P. R. Slater, Dalton Trans., 2013, 42, 5421 RSC.
- J. M. Porras-Vazquez, T. Pike, C. A. Hancock, J. F. Marco, F. J. Berry and P. R. Slater, J. Mater. Chem. A, 2013, 1, 11834 RSC.
- J. M. Porras-Vazquez, R. I. Smith and P. R. Slater, J. Solid State Chem., 2014, 1, 132 CrossRef.
- J. F. Shin, K. Joubel, D. C. Apperley and P. R. Slater, Dalton Trans., 2012, 41, 261–266 RSC.
- J. F. Shin, A. Orera, D. C. Apperley and P. R. Slater, J. Mater. Chem., 2011, 21, 874 RSC.
- A. D. Smith and P. R. Slater, Inorganics, 2014, 2, 16 CrossRef.
- J. Li, A. E. Smith, P. Jiang, J. K. Stalick, A. W. Sleight and M. A. Subramanian, Inorg. Chem., 2015, 54, 837 CrossRef PubMed.
- A. J. McSloy, I. Trussov, A. Jarvis, D. J. Cooke, P. R. Slater and P. M. Panchmatia;, J. Phys. Chem. C, 2018, 122, 1061 CrossRef.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751 CrossRef.
- A. Gibbs, et al., STFC ISIS Facility, 2017, 1720038, DOI: DOI:10.5286/ISIS.E.87846860.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8dt02691b |
| This journal is © The Royal Society of Chemistry 2018 |