Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mismatch in cation size causes rapid anion dynamics in solid electrolytes: the role of the Arrhenius pre-factor†
Stefan
Breuer
 *a and
Martin
Wilkening
*a and
Martin
Wilkening
 ab
ab
aChristian Doppler Laboratory for Lithium Batteries and Institute for Chemistry and Technology of Materials, Graz University of Technology (NAWI Graz), Stremayrgasse 9, 8010 Graz, Austria
bALISTORE-ERI European Research Institute, 33 rue Saint Leu, 80039 Amiens, France
First published on 21st February 2018
Abstract
Crystalline ion conductors exhibiting fast ion dynamics are of utmost importance for the development of, e.g., sensors or rechargeable batteries. In some layer-structured or nanostructured compounds fluorine ions participate in remarkably fast self-diffusion processes. As has been shown earlier, F ion dynamics in nanocrystalline, defect-rich BaF2 is much higher than that in the coarse-grained counterpart BaF2. The thermally metastable fluoride (Ba,Ca)F2, which can be prepared by joint high-energy ball milling of the binary fluorides, exhibits even better ion transport properties. While long-range ion dynamics has been studied recently, less information is known about local ion hopping processes to which 19F nuclear magnetic resonance (NMR) spin–lattice relaxation is sensitive. The present paper aims at understanding ion dynamics in metastable, nanocrystalline (Ba,Ca)F2 by correlating short-range ion hopping with long-range transport properties. Variable-temperature NMR line shapes clearly indicate fast and slow F spin reservoirs. Surprisingly, from an atomic-scale point of view increased ion dynamics at intermediate values of composition is reflected by increased absolute spin–lattice relaxation rates rather than by a distinct minimum in activation energy. Hence, the pre-factor of the underlying Arrhenius relation, which is determined by the number of mobile spins, the attempt frequency and entropy effects, is identified as the parameter that directly enhances short-range ion dynamics in metastable (Ba,Ca)F2. Concerted ion migration could also play an important role to explain the anomalies seen in NMR spin–lattice relaxation.
Introduction
The irregular movement of small anions and cations in crystalline and amorphous solids is a natural phenomenon that plays a key role in many devices as well as technological and geological processes. Extracting general rules from the existing amount of experimental data that control ionic transport and ionic diffusivity is, thus, of importance if we want to optimize devices, such as batteries,1–4 relying on dynamic processes of ions. Nanostructured materials play a prominent role in these fields.1,5,6Although ion dynamics in a huge number of various nanomaterials have been studied over the last decades with a wide range of techniques,7–11 our picture about ionic diffusivity is far from being completely understood. The introduction of defects, that is, in a more general sense describable with structural disorder, represents a powerful instrument with which we can control the chemical and physical properties of a material.12–17 Ion dynamics sensitively depend on local arrangements. The present work is aimed at describing the interrelations between local disorder and short-range ion dynamics using a suitable model substance that may also be of interest as a fast ion conducting solid electrolyte.4,18–23
The metastable (quasi) solid-solution (Ba,Ca)F2 served as such model substance.24,25 Recently, it has also attracted groups from theory to model ion conductivities.26,27 Nanocrystalline Ba1−xCaxF2 (0 < x < 1) can easily be prepared by mechanochemical reaction at low temperatures from the binary fluorides BaF2 and CaF2.24,25 Joint milling the two fluorides forces the cations to mix at atomic scale while the symmetry of the educts remains the same; both BaF2 and CaF2 crystallize with antifluorite structure. Thus, over the entire compositional range the original crystal symmetry is preserved whereas local atomic arrangements do, however, differ.28 At x = 0.5, and after sufficiently long mechanical treatment, the majority of the F anions are directly surrounded by both Ba and Ca ions without any heavy clustering of Ba- and Ca-rich regions.28 These features, as well as the possibility to prepare incompletely mixed samples, render Ba1−xCaxF2 a complex model system to study the influence of structural disorder on local ion dynamics.
Although the mixing effect on (i) atomic structure and (ii) long-range ion transport in Ba1−xCaxF2 has recently been studied via DC conductivity measurements in detail,28 less is known about the change of local hopping processes. Starting from x = 0 the ionic conductivity increases and passes through a maximum at approximately x = 0.5 before it decreases and reaches the value of pure CaF2 (x = 1).28 In turn, the associated activation energy runs through a minimum at x = 0.5 being the composition with maximum structural disorder with regard to intimate cation mixing. The specific conductivity of the solid solution Ba0.5Ca0.5F2 prepared by ball milling exceeds even that of epitaxially grown BaF2–CaF2–BaF2 layers.28,29 This layered system29 is one of the most famous examples of an artificial ion conductor whose transport properties are explained by non-trivial size effects, i.e., by overlapping space charge zones.9,10,30,31 Such effects might also influence ion transport in mechanosynthesized (Ba,Ca)F2.28
While local strain, i.e., a strongly heterogeneous potential landscape, has been made responsible for the increase in conductivity of Ba0.5Ca0.5F2,28 short-range ion dynamics have so far not been probed. Shedding light on these hopping processes is expected to help identify the driving forces that control the increase in ionic diffusivity seen. Here, we took advantage of 19F NMR (spin-lock) relaxometry to extract mean activation barriers the ions are subjected to in Ba1−xCaxF2. For heterogeneous nanocrystalline materials with disordered interfacial regions and crystalline grains NMR can, in ideal cases, differentiate the mobile ions in or near the (percolating) interfaces from those in the defect-rich interior of the nm-sized grains.32–34 For Li2O and the dispersed, nanocrystalline ion conductors Li2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X2O3 (X = Al, B) it was possible to draw conclusions about the location of the mobile ions responsible for fast macroscopic ion transport.32,33
X2O3 (X = Al, B) it was possible to draw conclusions about the location of the mobile ions responsible for fast macroscopic ion transport.32,33
Experimental
Mechanochemical preparation and characterization of the samples
A series of nanocrystalline samples Ba1−xCaxF2 with the compositions of x = 0, 0.1, 0.2, 0.5, 0.8, 1.0 were prepared by high-energy ball milling. Pure, microcrystalline BaF2 (99.99%, Sigma-Aldrich) and CaF2 (99.9%, ABCR) served as starting materials. We used of a Fritsch Pulverisette 7 (premium line) planetary mill. Two 45 mL grinding beakers, filled with the reactants (the total mass was 4 g) and 180 ZrO2 milling balls (5 mm in diameter) in each case, were employed. The mixtures were mechanically treated for 10 h at a rotational speed of 600 rpm. To exclude contamination with moisture or oxygen during milling, the beakers were filled and emptied in a glovebox with dry argon atmosphere. The powders obtained were characterized by X-ray powder diffraction (XRPD) using a Bruker D8 Advance diffractometer that operates with Bragg Brentano geometry and Cu Kα radiation.Conductivity measurements
For our conductivity measurements cylindrical pellets with 5 mm in diameter and ca. 1.5 mm in thickness were used. The pellets were uniaxially cold-pressed at a pressure of approximately 0.5 GPa. Blocking electrodes with a layer thickness of 100 nm were applied with a Leica EM SCD 050 evaporator. Impedance measurements were carried out with a Novocontrol Concept 80 broadband dielectric spectrometer. Our measurements covered a frequency range from 0.01 Hz to 10 MHz; temperatures (173 K–473 K) were adjusted with a QUATRO controller (Novocontrol). To avoid any contamination with water or oxygen during the measurements, the pellets were dried inside the impedance cell, which was permanently flushed with dry nitrogen gas.NMR measurements
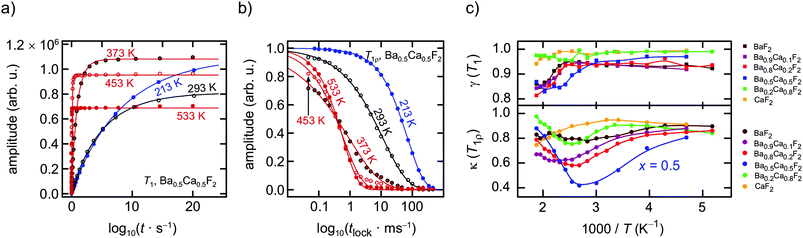
To assess the fluorine self-diffusion parameters 19F NMR spectroscopy and relaxometry were used to estimate jump rates and activation energies. Here, we took advantage of the saturation recovery and the spin-lock technique to record longitudinal (1/T1) as well as transversal (1/T1ρ) NMR spin–lattice relaxation (SLR) rates. For the NMR measurements the dry powders were fire-sealed in Duran glass tubes (ca. 4 cm in length and 3 mm in diameter) under vacuum to protect them from any impact of humidity. All measurements were carried out on a Bruker Avance III spectrometer that was connected to a shimmed cryomagnet with a nominal magnetic field of 7 T. This field corresponds to a 19F NMR Larmor frequency of ω0/2π = 282.0 MHz. A home-built NMR probe, which is suitable to record NMR signals under static conditions in a temperature range from 193 K to 533 K, was used to record the SLR rates and the 19F NMR spectra. At 200 W the π/2 pulse length was approximately 1 μs. A Eurotherm temperature controller in combination with a type-T thermocouple was utilized for both temperature controlling and monitoring. The saturation recovery pulse sequence consisted of ten succeeding pulses that destroy any longitudinal magnetization Mz, after a delay time td the reading pulse was sent to detect the recovery of Mz as a free induction decay (FID): 10 × π/2-td − π/2-acquisition (acq.). To construct the magnetization transients Mz(td) we plotted the area under the FIDs vs. td. Additionally, rotating-frame 19F NMR SLRρ rates 1/T1ρ were measured by means of the spin-lock technique, π/2 p(tlock)-acq., using a locking frequency ω1/2π of approximately 100 kHz.18,35 We varied the locking pulse length from tlock = 46 μs to tlock = 460 ms. To guarantee full longitudinal relaxation between each scan the recycle delay was set to at least 5 × T1. Both the 1/T1 and 1/T1ρ rates were obtained by parameterizing the resulting transients Mz(td) and Mρ(tlock) with stretched exponentials of the form Mz(td) ∝ 1 − exp(−(tw/T1)γ) and Mρ(tlock) ∝ exp(−(tlock/T1ρ)κ), respectively. While the stretching exponents γ varied from 1 to 0.8, the parameter κ ranged from 0.4 to 1.0 depending on temperature.Static 19F NMR spectra were obtained with a single 90° pulse experiment via Fourier transformation of the FID. We employed a 500 MHz Avance spectrometer (Bruker) to record the spectra; the 19F Larmor frequency was 470.4 MHz. The NMR lines shown represent Fourier transforms recorded under static, i.e., non-rotating, conditions. 19F NMR spectra under magic angle spinning (MAS) were measured at a rotation speed of 60 kHz using 1.3 mm rotors. Spectra were referenced to crystalline CaF2, which shows, as a secondary reference, an isotropic chemical shift δiso of 58 ppm; C6F6 served as primary reference.
Results and discussion
Characterization via X-ray powder diffraction and 19F MAS NMR
The phase purity of the samples and the formation of a mixed (Ba,Ca)F2 phase with retained cubic symmetry (see Fig. 1) was verified with the help of XRPD. We collected diffraction patterns at room temperature and in air atmosphere. Exemplarily, Fig. 1(a) shows the pattern of the mixture with the composition Ba0.2Ca0.8F2 which was milled for 10 h in the P7 planetary mill (see Fig. S1 in the ESI† for the characterization of the other samples studied). For comparison, in Fig. 1 the patterns of nanocrystalline BaF2 and CaF2 prepared under the same milling conditions are presented. The pure nanocrystalline samples feature reflections at exactly the same positions as their coarse-grained samples. A marked difference is, however, that the reflections of the powders ball-milled for 10 h show noticeable broadening. This broadening is attributed to the formation of nm-sized crystallites and the generation of strain upon mechanical treatment. As has been shown by earlier studies on nanocrystalline (Ba,Ca)F2 as well as other fluorides and oxides prepared by high-energy ball milling the final average crystallite size reached after many hours of mechanical treatment is in the order of 10 to 17 nm for the mixed samples.36 This result was obtained by evaluating the width of the reflections with the equation introduced by Scherrer37 (see ESI (Table S1†) for the exact crystallite sizes calculated). These values are supported by high-resolution transmission electron microscopy carried out in early studies.24,25 It is worth noting that abrasion of ZrO2 on ion conductivities turned out to be negligible in our case; such effects have been discussed by some of us in earlier studies.24,25,28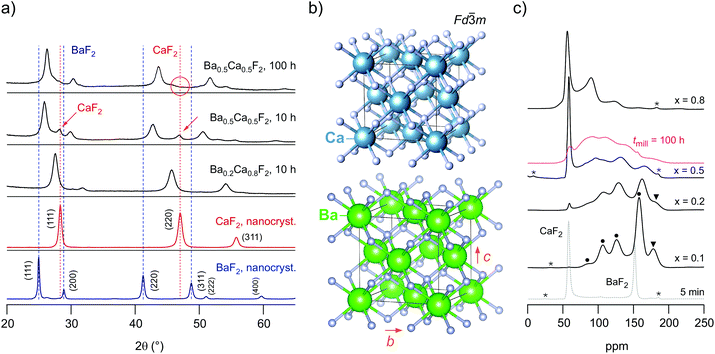 | ||
| Fig. 1 (a) X-ray powder patterns of high-energy ball milled CaF2, BaF2 and a mixtures Ba1−xCaxF2 with the compositions Ba0.2Ca0.8F2 and Ba0.5Ca0.5F2. From the point of view of XRPD, the pattern of the mixed phase with x = 0.8 reveals the formation of a nanocrystalline solid solution as all reflections refer to a cubic phase with shifts to lower diffraction angles. The same holds for x = 0.2. The samples were prepared in a planetary mill using a ZrO2 vial set; the rotational speed was 600 rpm, the milling time was set to 10 h. To prepare Ba0.5Ca0.5F2 free of any residuals of nanocrystalline Ca(Ba)F2 longer milling times are needed. The conductivity response of the two samples is the same. (b) Crystal structure of cubic CaF2 and BaF2; the two materials only differ by the lattice constant a. (c) 19F MAS NMR spectra of Ba1−xCaxF2 recorded at 470.4 MHz and a spinning frequency of 60 kHz. The asterisks mark spinning sidebands, the black dots (x = 0.1) indicate the different F environments. Note that signals arising from orthorhombic BaF2, as also seen in X-ray diffraction (see Fig. 1(a)), are marked by red dots; these partly overlap with those from the mixed phases. If not stated otherwise, the milling time was 10 h. The spectrum, referring to a milling time of 5 min, shows a stochiometric mixture of BaF2 and CaF2 to highlight the initial δiso values of the starting materials, 58 ppm (CaF2) and 152 ppm (BaF2), respectively. | ||
A close look at the pattern of the Ba0.2Ca0.8F2 mixture reveals that the reflections shifted to smaller diffraction angles if compared with those of pure (nanocrystalline) CaF2. The incorporation of the larger Ba2+ ions (ionic radius of 1.42) into the F-sublattice of CaF2, the ionic radius of Ca2+ is 1.12, causes the original lattice to expand. According to Bragg's law this mismatch in size results in lower diffraction angles. The reverse process is observed when some Ca2+ ions are introduced into BaF2. For Ba1−xCaxF2 with x = 0.5 the final reflections are characterized by almost the arithmetic average.28 The formation of mixed (Ba,Ca)F2 and the discussion of the corresponding XRPD patterns is already well documented in literature.24,25,28 Most important for us is the following aspect: after milling the educts for 10 h, the reflections originating from the non-mixed starting materials BaF2 are no longer visible for most compositions. As an example, for Ba0.2Ca0.8F2 the formation of a mixed phase with no significant amounts of crystalline educts is seen by XRPD.
Besides decreasing the crystallite size and introducing structural disorder, it cannot be ruled out that mechanical treatment also lead to the formation of some amorphous material. Although recent EXAFS studies by Chadwick and co-workers38 did not point to large fractions of amorphous regions in mechanosynthesized Ba1−xCaxF2, the XRPD pattern of Ba0.2Ca0.8F2 revealed a shallow hump at low diffraction angles. This feature is frequently assigned to amorphous material.39
For samples with x being close to 0.5, i.e., considering equimolar mixtures, longer milling times than 10 h are needed to ensure intimate mixing of the earth alkaline cations. As an example, in Fig. 1 the XRPD pattern of two samples of Ba0.5Ca0.5F2 are shown that were obtained after milling for tmill = 10 h and 100 h, respectively. After 10 h residual CaF2 is still present and not all Ca2+ ions have been fully incorporated into the BaF2 lattice, as can be seen by the small CaF2 reflections visible (see the red arrows in Fig. 1(a)). After milling the mixture for 100 h these reflections disappear. Additionally, the more Ca2+ ions are introduced into the BaF2 lattice the larger the lattice contraction of BaF2 which in turn causes a shift of the reflections toward larger diffraction angles. Hence, only very long milling times ensured the absence of any (nanocrystalline) starting materials.
The positions of the final reflections of the sample obtained after 100 h suggest the formation of an almost fully mixed sample. Of course, the existence of both Ba-rich and Ca-rich regions cannot be excluded on the basis of XRPD. As seen by (high-resolution) 19F MAS NMR, which is sensitive to the local, atomic environments of the F nuclei, heavy clustering, however, seems to be negligible for x = 0.5. This conclusion is at least valid for mixtures obtained after sufficiently long periods of milling with tmill = 100 h (see Fig. 1c). The 19F MAS NMR spectra of the fully mixed samples are composed of several overlapping lines revealing partly the magnetically inequivalent F anions in Ba0.5Ca0.5F2. In general, since each F anion is directly surrounded by 4 cations, in an ideal solid solution the environments are [Ba]3[Ca], [Ba]2[Ca]2, [Ba][Ca]3 as well as [Ba]4 and [Ca]4 (see Fig. S2 in the ESI† for the assignment of the different atomic F environments in Ba-rich Ba1−xCaxF2 including a deconvolution of the spectra).28
The signals of [Ba]4 and [Ca]4 are the weakest in intensity for x = 0.5 and tmill = 100 h. Keeping in mind that the NMR chemical shift for [Ba]4 in completely mixed Ba0.5Ca0.5F2 (182 ppm) is different compared to that in pure nanocrystalline BaF2 (152 ppm), the NMR spectra point to mixing of the earth alkaline ions on atomic scale. For the sample milled only for 10 h (x = 0.5) we see that the sharp signal around 52 ppm points to Ca-rich regions that only vanish if tmill is increased to 100 h. The reason for the disappearance of the [Ca]4 signal is, that more and more CaF2 gets incorporated into BaF2 the longer the milling time is chosen. After 100 h of milling, the equimolar mixture forms a disordered phase with a large amount of mixed F environments: [Ba]3[Ca], [Ba]2[Ca]2, [Ba][Ca]3. The more effective the mixing process is, the less the probability to find Ba- and Ca-rich species [Ba]4 and [Ca]4 originating from the educts (see also the corresponding XRPD pattern in Fig. 1(a)).
When comparing the samples with x = 0.8 and x = 0.1 it is, however, evident that after a milling period of 10 h Ca-rich and Ba-rich regions are clearly present. The Ca-rich sample (x = 0.8) reveals the fastest 19F SLR rates, see below. 19F MAS NMR reveals further that mixing of the earth-alkaline ions seems to be easier for compositions x < 0.5, i.e., for Ba-rich samples. Mechanical treatment of cubic-BaF2 always leads to the formation of some orthorhombic material (up to 20 wt% depending on the milling conditions). The orthorhombic form is a high-pressure phase of BaF2; it is also seen in 19F MAS NMR. With increasing x the amount of orthorhombic BaF2 decreases.
As outlined above, after the formation of the mixed phase, either completely or partially, the F anion conductivity σDC sharply increases when compared to the pure nanocrystalline starting materials. σDC passes through a maximum at intermediate compositions. Correspondingly, the activation energy takes a minimum at x = 0.5. While σDC refers to long-range ion transport, that is affected by grain boundaries, interfacial regions and porosity, NMR relaxometry, on the other hand, is primarily sensitive to bulk properties and, most importantly, to local F− hopping processes. In ideal cases, NMR was able to differentiate between ions in the interfacial regions and those in the bulk. This separation was possible for some single- and two-phase nanocrystalline oxides as well as binary fluorides with a large number fraction of ions residing in the structurally disordered interfacial regions.
19F NMR relaxometry to evaluate short-range F anion hopping
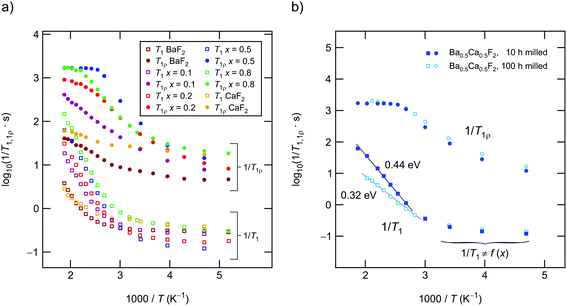
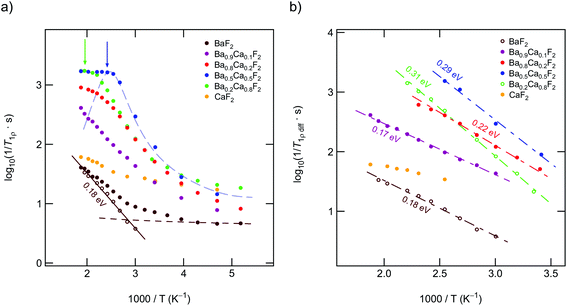
In Fig. 2 an overview of all variable-temperature 19F spin–lattice and spin-lock NMR relaxation rates is presented. To identify the thermally activated regions, from which hopping activation energies can be extracted, the rates were plotted in an Arrhenius representation. Stretched exponential functions served to parameterize the magnetization transients Mz(td) and Mρ(tlock), see Fig. 3(a) and (b) and section 2. The corresponding stretching exponents γ are illustrated in Fig. 3(c).The stretching factors of the Mz(td) transients, which contain the rate T1, are almost exponential, except for few compositions deviations from γ = 1 show up at temperatures above 393 K. The κ values of the spin-lock transients reveal a more complex behavior and pass through minima at temperatures higher than 333 K; this feature is especially seen for the mixed sample Ba0.5Ca0.5F2. The educts, which are characterized by lower ionic conductivities (see below), do not show such behavior. The Ba0.5Ca0.5F2 sample features the lowest minimum with values of approximately κ = 0.4 at T = 360 K.
For all samples the 19F NMR SLR rates, measured in the laboratory frame of reference (1/T1), show a non-diffusion induced regime below 330 K. In this T range the rates only marginally depend on temperature, see also Fig. 4(a). Background relaxation is in many cases driven by lattice vibrations or coupling of the spins to paramagnetic impurities.40
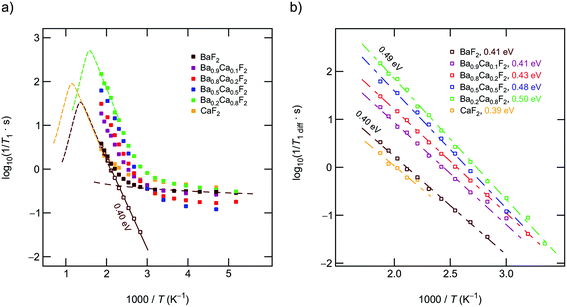
Above 330 K the rates start to increase because of F jumps on the time scale of the NMR experiment. We approximated the background rates with a power law, 1/T1,bgr ∝ Tβ, and extrapolated the rates to higher T to calculate background-free, diffusion-induced rates: 1/T1diff = 1/T1 − 1/T1,bgr.41β values ranged from 0.3 (x = 0) to 1.5 (x = 0.5). For intermediate values of x we obtained β ≈ 1. In Fig. 4(a) this procedure is exemplarily shown for nanocrystalline BaF2, see unfilled symbols. The rates 1/T1diff follow Arrhenius behavior characterized by an activation energy of 0.4 eV.
As x in Bax−1CaxF2 is increased, the beginning of the diffusion-induced flank is shifted toward lower T. This clearly indicates increased F self-diffusion in the partially or completely mixed phases. As can be already seen in Fig. 4(a), the slope of the different flanks do not vary much but run in parallel to each other. The diffusion induced rates, see Fig. 4(b), reveal measurable but by far not great differences in activation energies which range, disregarding the results of CaF2, from 0.41 eV to 0.50 eV. These values are in good agreement with results from recently performed molecular dynamics studies.27 For nanocrystalline CaF2 background effects are predominant in the T range investigated; these effects do not allow the proper determination of an activation energy in the T range covered. Here, we estimated Ea after background correction to be approximately 0.39 eV. The better the flank is accessible the more precise the activation energy can be determined.
Fig. 4(b) entails two important information. First, the absolute rates 1/T1diff increase with increasing x and sharply decrease if x exceeds 0.8. As a function of x, the diffusion-induced rates pass through a maximum that is similar, but not exactly the same, to that seen via DC conductivity spectroscopy. T1 is most effective for Ca-rich samples, see the rates for x = 0.8. Apart from this small but important distinctions, the rates support the mixed earthy-alkaline effect observed in our earlier study.28 Second, the activation energies from NMR turn out to be smaller than those seen by DC conductivity spectroscopy (see Table 1). Furthermore, their overall change with composition is less pronounced as seen for long-range ion transport.28 In contrast to conductivity measurements, Ea from NMR pass through a very shallow maximum (ca. 0.5 eV at x = 0.5) rather than a minimum.
| Conductivity spectroscopy | NMR relaxation | |||||||
|---|---|---|---|---|---|---|---|---|
| x | E a | σ DC (298 K) (S cm−1) | log(σ0/S cm−1 K) | E a (T1)a (eV) | E′a(T1)f (eV) | E a (T1ρ) (eV) | α | |
| a Corrected values that take into account a non-diffusive background; the error for all values is ±0.01 eV. b Activation energy read off at 1 MHz: 0.50 eV. c 0.58 eV (1 MHz). d 0.55 eV (1 MHz). e After 100 h of milling a value of 5.44 is obtained; the largest increase for the pre-factor is seen when comparing nanocrystalline CaF2 with nanocrystalline BaF2. f E′a denotes values corrected using a joint fit of both diffusion and non-diffusive effects. g Values refer to Ea(T1); α = 2 would result for 3D (isotropic) uncorrelated motion. h For x = 0.5 and tmill = 100 h we obtained E′a = Ea = 0.35 eV. After 100 h of milling we obtain α = 1.54. | ||||||||
| 0 | 0.78 | 1.6 × 10−11 | 5.17 | 0.41 | 0.43 | 0.19 | 1.51 | Low-T flank not completely reached |
| 0.1 | — | — | — | 0.41 | 0.45 | 0.17 | ||
| 0.2 | 0.64b | 4.6 × 10−9 | 5.06 | 0.43 | 0.43 | 0.22 | 1.66 | |
| 0.5 | 0.61c | 1.6 × 10−8 | 5.23e | 0.49 | 0.46 | 0.29 | 1.80 | E a(T1) = 0.35 eV,a,h for tmill = 100 h. |
| 0.8 | 0.71d | 8.7 × 10−10 | 5.52 | 0.50 | 0.51 | 0.32 | 1.70 | |
| 1.0 | 0.80 | 7.2 × 10−14 | 3.41 | 0.39 | 0.35 | 0.09 | 1.39 | Low-T flank only partly accessible below 500 K |
To further support the activation energies obtained according the correction procedure sketched above, we also analyzed our T1 data with an Arrhenius fit that directly takes into account the deviation at low temperatures with the help of a power law ansatz: 1/T1 = c·exp(−Ea/(kBT)) + a·(1/T)n. c and a denote fitting parameters. The exponent n (0.4 < n < 1.2) should agree with β. It characterizes the type of relaxation, e.g., driven by coupling of the spins to electrons or phonons, being responsible for the change in 1/T1 at low T. The fitting results are shown in Fig. 5; the activation energies E′a are listed also in Table 1. E′a is, for all samples, closely comparable with Ea.
Comparison with results from conductivity spectroscopy and 19F NMR line shapes
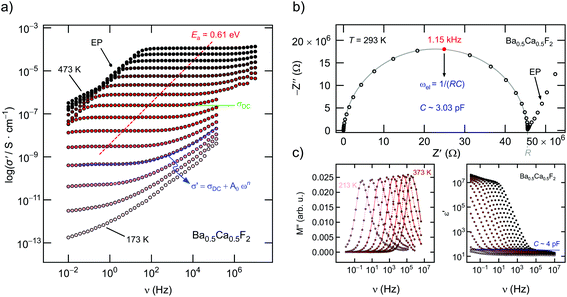
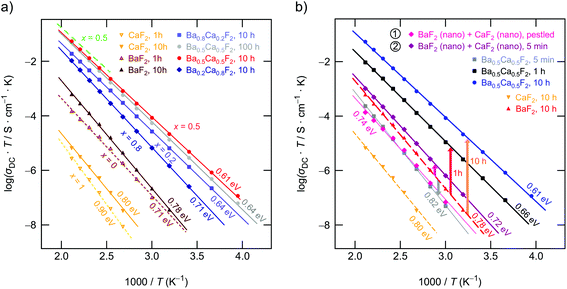
To characterize long-range F ion transport through impedance spectroscopy, in Fig. 6(a) the conductivity isotherms of Ba0.5Ca0.5F2 are shown. While at low frequencies and sufficiently high T electrode polarization effects show up, the main parts of the spectra are dominated by the so-called DC plateau (ν → 0) from which σDC can be read off. Pronounced dispersion regions are only visible at sufficiently low T. The DC plateau transforms into a semi-circle when the Nyquist representation is used to analyze the impedance data. The semicircle shown in Fig. 6(b) is characterized by a capacity C of 3 pF which unequivocally points to a bulk electrical relaxation process. Blocking grain boundary resistances seem to be negligible. A value of 3 pF is also in perfect agreement with permittivities well below 102, see Fig. 6(c). The modulus representation, M′′(ν), reveals single peaks with a width (full width at half maximum) larger than that expected for a Debye process, cf.Fig. 6(c). Hence, the F ions are subjected to correlated motion in Ba0.5Ca0.5F2 sensing a distribution of diffusion pathways in an irregularly shaped potential landscape. Such dynamics are usually described with non-exponential motional correlation functions.42Evaluating σDCT, see Fig. 7(a), illustrates that, depending on x, the activation energy for long-range ion transport varies from 0.80 eV (CaF2, ball milled for 10 h) to 0.61 eV (Ba0.5Ca0.5F2). Pure nanocrystalline BaF2 (10 h) has to be characterized by 0.78 eV. These values perfectly agree with those published earlier.28 In Fig. 7(b) we show the increase of σDCT for Ba0.5Ca0.5F2 with increasing milling time. While for a mixture of BaF2 and CaF2 milled for only 5 min σDC is still affected by the low-conducting CaF2, milling for 1 h already results in a significant increase of the DC ionic conductivity exceeding that of nanocrystalline BaF2 (10 h) by one order of magnitude. After 10 h of mechanical treatment the upper limit of σDC is reached. Further increase of tmill does not change σDC significantly.
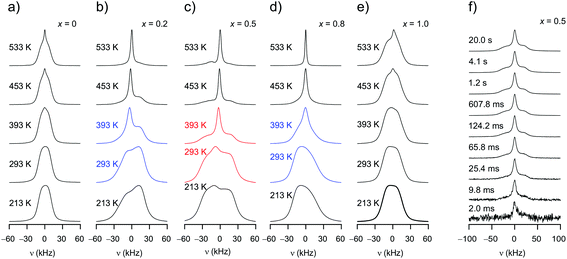
While for σDC the activation energy and the σ0 in σDCT = σ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea, DC/kBT) may vary with x, in 19F NMR relaxometry the change of the NMR pre-factor 1/τ0, NMR (see below) seems to play a more significant role. This idea is illustrated in Fig. 4(a). The higher F anion conductivity in mixed samples is a proven fact. Indeed, 19F NMR line shape measurements also show enhanced F anion hopping in the mixed samples as compared to the pure end members. In contrast to BaF2 and CaF2 the corresponding NMR lines of the samples with 0.2 < x < 0.8 are composed of a clearly visible motionally narrowed contribution that is attributed to F anions being fast enough to average (homonuclear) dipole–dipole couplings (Fig. 8). As the rigid lattice 19F NMR line width of BaF2 (and CaF2) is at least 30 kHz, full narrowing, which is already seen slightly above 400 K, corresponds to F jump rates 1/τNMR in the order of 106 s−1, see also the estimation below via spin-lock NMR. Such a value will definitely affect the low-T regime of SLR NMR. The peak maximum of the 1/T1 rate would be expected to show up if 1/τNMR reaches values in the order of 1.8 × 109 s−1.
exp(−Ea, DC/kBT) may vary with x, in 19F NMR relaxometry the change of the NMR pre-factor 1/τ0, NMR (see below) seems to play a more significant role. This idea is illustrated in Fig. 4(a). The higher F anion conductivity in mixed samples is a proven fact. Indeed, 19F NMR line shape measurements also show enhanced F anion hopping in the mixed samples as compared to the pure end members. In contrast to BaF2 and CaF2 the corresponding NMR lines of the samples with 0.2 < x < 0.8 are composed of a clearly visible motionally narrowed contribution that is attributed to F anions being fast enough to average (homonuclear) dipole–dipole couplings (Fig. 8). As the rigid lattice 19F NMR line width of BaF2 (and CaF2) is at least 30 kHz, full narrowing, which is already seen slightly above 400 K, corresponds to F jump rates 1/τNMR in the order of 106 s−1, see also the estimation below via spin-lock NMR. Such a value will definitely affect the low-T regime of SLR NMR. The peak maximum of the 1/T1 rate would be expected to show up if 1/τNMR reaches values in the order of 1.8 × 109 s−1.
As the rates of Ba0.5Ca0.5F2 reveal the onset of the diffusion-induced low-T flank at lower temperatures we expect the corresponding rate peak, see dashed lines in Fig. 4(a), to show up at lower T as compared to BaF2 and CaF2, respectively. With the low-T activation energies being rather similar, especially if we consider the flanks of the samples with x = 0.2, x = 0.5 and x = 0.8, the only parameter that can further increase 1/τNMR is the pre-factor in 1/τNMR = 1/τ0, NMR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea, high-T/kBT). Here, Ea, high-T denotes the activation energy of the high-T flank of the peaks indicated in Fig. 5(a). The flank is inaccessible as the nanocrystalline samples are sensitive to grain growth and decomposition if NMR experiments were performed at too high temperatures. Ea, high-T can be identified with Ea, DC. Although Ea, DC change from 0.61 eV to 0.71 eV for x ranging from 0.2 to 0.8 (cf.Table 1), the variation in Ea, DC alone could not explain the significant shift of the low-T flank. As for σ0, also 1/τ0 is, besides other factors, affected mainly by the attempt frequency v0, the activation entropy ΔS as well as the number of spins effectively taking part in 19F NMR spin–lattice relaxation. The latter number is strongly associated to the concentration of available sites where the mobile ions can jump to. As has been shown recently, the softness of a lattice may influence v0.43 In the present case, besides such an influence, effects of migration entropy, affected by both vibrational or configurational contributions, is expected to control the pre-factor mostly. Frenkel defects show a considerable high migration entropy that may especially affect the samples with x = 0.5 and x = 0.8.
exp(−Ea, high-T/kBT). Here, Ea, high-T denotes the activation energy of the high-T flank of the peaks indicated in Fig. 5(a). The flank is inaccessible as the nanocrystalline samples are sensitive to grain growth and decomposition if NMR experiments were performed at too high temperatures. Ea, high-T can be identified with Ea, DC. Although Ea, DC change from 0.61 eV to 0.71 eV for x ranging from 0.2 to 0.8 (cf.Table 1), the variation in Ea, DC alone could not explain the significant shift of the low-T flank. As for σ0, also 1/τ0 is, besides other factors, affected mainly by the attempt frequency v0, the activation entropy ΔS as well as the number of spins effectively taking part in 19F NMR spin–lattice relaxation. The latter number is strongly associated to the concentration of available sites where the mobile ions can jump to. As has been shown recently, the softness of a lattice may influence v0.43 In the present case, besides such an influence, effects of migration entropy, affected by both vibrational or configurational contributions, is expected to control the pre-factor mostly. Frenkel defects show a considerable high migration entropy that may especially affect the samples with x = 0.5 and x = 0.8.
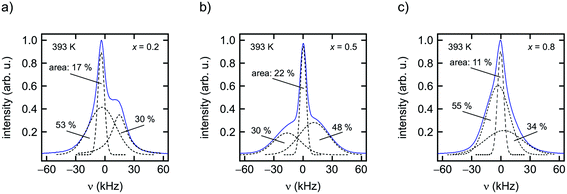
As can be clearly inferred from the 19F NMR spectra (Fig. 8(a)–(e)), the number fraction of mobile F anions, which are represented by the motionally narrowed lines for each sample, passes through a maximum at x = 0.5. This is best seen at T = 393 K. For the sake of clarity we deconvoluted the spectra with appropriate Voigt functions to estimate the area fraction Af of the motionally narrowed NMR component (see Fig. 9).12 The larger the number of mobile ions, performing net displacements as well as local jumps, the more effective spin fluctuations. Of course, the x-dependent number of effective charge carriers will, to some extent, also affect σ0, which is related to long-range ion transport. It turned out, however, that its influence on localized jump processes is much larger.
Although F anion dynamics is clearly heterogeneous in Bax−1CaxF2, effective spin-diffusion, i.e., flip-flop processes without any mass transfer, ensures homogeneous relaxation of the spin system. Accordingly, the 19F NMR transients do not feature a two-step decay of fast and slowly relaxing components as it has been seen for other nanocrystalline core–shell model systems.32–34 In the frame of a saturation recovery experiment 19F NMR spectra uniformly buildup with increasing waiting time (see Fig. 8(f)). Thus, there is fast communication between all 19F spins in mixed Ba0.5Ca0.5F2.
Influence of correlated motion on activation energies
Understanding the slight increase in Ea, low-T when going from x = 0.2 (0.43 eV) to x = 0.5 (0.48 eV) and further to x = 0.8 (0.50 eV) also needs our attention. Although perhaps not applicable to our disordered system with relatively low barriers, the trend of both pre-factor and Ea from NMR resembles that of the Meyer–Neldel rule after which a higher activation energy is associated with a higher pre-factor.44 In our case, however, we have to consider the following. In contrast to Ea, high-T, Ea, low-T is a so-called apparent activation energy that is additionally affected by correlation effects.45 It is comparable to AC conductivity activation energies read off in the dispersive regions of the conductivity isotherms (see also Table 1).16 The larger the impact of correlation effects, such as structural disorder and Coulomb interactions of F− interstitials and vacancies, the larger the difference between Ea, high-T and Ea, low-T. The two activation energies are linked according to the following relation Ea, low-T = (α − 1)Ea, high-T.46 For 3D uncorrelated motion the parameter α equals 2. Correlated motion results in α < 2 and, thus, yields asymmetric NMR rate peaks.45 Here it turned out that α pass through a maximum located near x = 0.5 (see Table 1). This result means less correlated motion for the samples with the highest diffusion-induced relaxation rates. To be clear, in all samples correlated motion is sensed by NMR; its extent, however, seems to be less for local ion dynamics in samples milled just for 10 h and x = 0.5 or x = 0.8. It is evident from XRPD and 19F MAS NMR that under these conditions mixing at atomic scale is incomplete; 19F MAS NMR revealed that the same is true for the sample with x = 0.8. Therefore, fast F anion diffusion might occur along or near Ca- and Ba-rich regions as mentioned above. Precisely speaking, diffusion induced T1 relaxation is more effective for x = 0.8, i.e., for mixed but Ca-rich environments..Recently, we probed a very similar trend of α for Li ion diffusivity in layer-structured 2H-LiyNbS2.47 Although in 2H-LiyNbS2 Li diffusivity decreases when y approaches y = 1, which is the maximum Li load, the low-T activation energy in deduced from T1(T) measurements steadily decreases when going from y = 0.3 to y = 1.0. This effect has been ascribed to an increasing effect of correlated ion motion which is maximal when the van-der-Waals gap in NbS2 is completely filled with Li ions. At the same time, also dipolar magnetic and electric quadrupolar interactions increase the larger the Li concentration.
Here, the origin of correlated motion in mixed Bax−1CaxF2 is, however, definitely different from that seen for 2H-LiyNbS2. An increasing number fraction of mobile F spins, activation entropy contributions and lower long-range activation energies cause a sharp increase in Li diffusivity and ionic conductivity when mixed phases are formed. For all samples correlated motion is expected because the ions are subjected to a highly irregular potential landscape, including also metastable states. The extent and origins of correlation effects seem, however, change with composition. According to the NMR factor α the Ba-rich phases (x = 0.1, x = 0.2) provide an additional source of correlated motion leading to lower apparent activation energies than expected. Zahn et al. pointed out differences in the concentration of mobile charge carriers when Ca or Ba ions are introduced in BaF2 and CaF2, respectively. At very low levels of doping, the incorporation of Ca2+ ions resulted in trapped F interstitial sites and mobile F vacancies as the opponent defect.26 The formation of such interstitial site next to a Ca2+ dopant in BaF2 was promoted by 0.08 eV. On the other hand, the generation of Frenkel defects in Ba-doped CaF2 is energetically disfavored by 0.14 eV.26
In our case substitutional disorder is much larger than dopant concentrations and a direct comparison with results for extremely low levels of doping turns out to be difficult. At least we can say the following. Even if we consider complete and uniform mixing at atomic scale, we deal with samples that naturally have larger regions being rich in Ba2+. Segregation effects in interfacial regions or clustering on the nm scale will lead to even larger non-uniform areas. As has been shown recently for several types of ion conductors, concerted ion movements always cause the mean activation barrier to decrease.48 Thus, concerted and, thus, highly correlated ion movements in the vicinity of Ca-doped Ba-rich regions or at the interface of Ba-rich and Ca-rich regions in non-intimately mixed samples could serve as an explanation for the unusual 19F NMR SLR behavior and the origin of additional correlation effects seen for the Ba-rich samples.
Obviously, on the angstrom length scale, to which 19F SLR NMR is sensitive, this effect levels off when the region 0.5 ≤ x ≤ 0.8 is reached. Note that also the activation energy of Ba0.5Ca0.5F2 (0.58 eV, see Table 1), when determined from AC impedance spectroscopy at 1 MHz, is higher than that of Ba0.2Ca0.8F2 (0.55 eV) and Ba0.8Ca0.2F2 (0.50 eV). In this compositional range, the increase in mobile spins near non-uniformly mixed interfaces is more significant and leads to a further increase in diffusivity. Simultaneously, the impact of correlation effects on the low-T19F NMR T1 measurements decreases, at least for samples milled for 10 h.
19F NMR spin-lock NMR and the effect of long-term milling
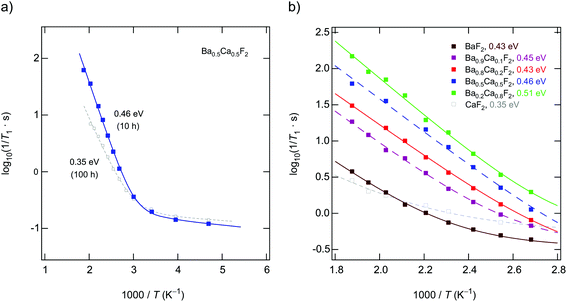
If we consider the corresponding rates measured in the kHz range through spin-lock NMR, see Fig. 10(a) and (b), the activation energies seem to reflect the same behavior. Regarding the absolute values for Ea suggests, however, that the temperature dependence of the 19F NMR SLR rates, see data for x = 0.1 and x = 0.2, are additionally influenced by other effects. Besides changes in the diffusion mechanisms, non-diffusive effects, such as spin-diffusion, presumably play the most important role. The characteristic spin-diffusion correlation times are expected to take values in the s to ms range, which fall into the time window of spin-lock NMR. Dipolarly coupled 19F spins with short distances to each other are prone to be affected by flip-flop effects. Hence, 1/T1ρ NMR rates are expected to be much larger influenced by spin-diffusion as laboratory-frame SLR measurements performed in the MHz to GHz range. Apart from such interfering effects, spin-lock NMR relaxation is most effective for the sample with x = 0.5. This finding mirrors the change of long-range ion dynamics as seen by DC conductivity measurements.Up to here, we discussed results from samples prepared by milling the starting materials for 10 h. Except for Ba0.5Ca0.5F2 our results from XRPD do not reveal any substantial amounts of the nanocrystalline binary parents. Increasing tmill up to 100 h forces the earth alkaline ions to mix almost completely, as is also verified by 19F MAS NMR. Importantly, the 1/T1ρ rates, as well as the DC conductivity values, do not change when tmill is increased from 10 to 100 h. Hence, bulk long-range ion transport and spin-diffusion effects are already fully developed at shorter periods of mechanical treatment. In contrast, 19F NMR spectra and 1/T1 do depend on tmill. Although static line shapes reveal an increase of the number of mobile spins on the NMR time scale, this change does not cause an increase of σDC, see above (Table 1 and Fig. 7(a)). While the onset of the low-T19F NMR 1/T1 flank remains unchanged (see Fig. 4(b)), the corresponding activation energy decreases from Ea = 0.49 eV to only 0.35 eV. We attribute this unusual change in Ea(T1) to enhanced localized ion jump processes. Localized or caged jumps, including unsuccessful highly correlated forward–backward processes, will definitely affect the low-T 1/T1diff rates but do not contribute to net charge transport, which is sensed by σ′ in the limit of low frequencies. This is the classical way to interpret differences between diffusion parameters derived from NMR and those from conductivity spectroscopy. In general, the two methods are sensitive to quite different kinds of motional (auto-)correlation functions, see above. The relationship between parameters from NMR and conductivity spectroscopy in disordered media, such as glasses, has been discussed in detail by Ngai and co-workers.49–54
Careful inspection of Fig. 7(a) shows that the sample milled for 100 h shows even lower conductivities as compared to that milled for 10 h. We also see a slight increase of the corresponding activation energy Ea (0.64 eV vs. 0.61 eV). This finding supports our idea that long-time milling, i.e., the formation of solid-solution like environments, mainly lead to an increase of localized correlated motional processes. The corresponding factor α turned out to be 1.54. This value is lower than α ≈ 1.8 found for Ba0.5Ca0.5F2, which was prepared using tmill = 10 h. On the other hand, it resembles the behavior of nanocrystalline BaF2. Obviously, the most favorable configurations for long-range ion transport are internal, heterogeneously intermixed interfaces between Ca-rich and Ba-rich regions, as discussed above. Complete mixing, or the formation of clusters with, e.g., diameters of 1 nm, as suggested recently,26 seems to be not needed to ensure effective long-range ion transport. Here, we assume that these heterogeneous regions act as non-stopping or percolating pathways similar to those in dispersed ion conductors.32,33,55–58 Interestingly, these regions, acting as hosts for fast F anion diffusivity, have partly been formed already at shorter milling times of tmill = 1 h (see Fig. 7(b)). As compared to nanocrystalline BaF2 we see a considerable increase in σDC for such a sample. Even if we mix nanocrystalline BaF2 and CaF2, each separately milled for 10 h, for just 5 min in a planetary mill, we found a measurable increase in ionic conductivity. For these samples 19F MAS NMR also points to a very low, but detectable fraction of F anions residing in or near the earth-alkaline mixed environments.
Self-diffusion coefficient
In contrast to the T1 measurements, the spin-lock data (see Fig. 10(a)) reveal the beginning of a peak maximum from which we can finally deduce an upper limit of a diffusion coefficient. Interestingly, at the temperature where 1/T1ρ passes through a maximum, in many cases the corresponding stretching factor, κ, takes its minimal value (see Fig. 3(c)). At the 1/T1ρ peak maximum the F anion jump rate τNMR should be in the order of the (effective) locking frequency. Using ω1/2π = 100 kHz as a lower limit, via ω1τ = 0.5 we estimate 1/τNMR to be in the order of 1.3 × 106 s−1 at 410 K (x = 0.5). This value translates into a self-diffusion coefficient of D410 K = a2/(6τNMR) of 1.8 × 10−14 m2 s−1. Here, a denotes the average jump distance; for Ba0.5Ca0.5F2 we used a = 2.92 Å as a good approximation of the mean F–F distance in Ba0.5Ca0.5F2 (10 h). D410 K is a typical value for a moderate ionic conductor. The diffusion coefficient determined at the rate maximum of a spin-lock experiment carried out at 100 kHz should be comparable to that which we obtain when DC conductivities are analyzed that probe successful ion displacements. With the ionic conductivity at 410 K we estimated a solid-state diffusion coefficient via the Nernst–Einstein relation, Dσ = σDCkBT/(q2N) with q being the charge of the F anions and N the number density of charge carriers. From 19F NMR we know that approximately 20% of the ions take part in ionic conduction. In this case Dσ, 410 K amounts to 1.7 × 10−14 m2 s−1, which is very close to the Einstein–Smoluchowski diffusion coefficient. For x = 0.8 the corresponding 1/T1ρ peak is shifted toward higher T, directly reflecting the decrease in σDC as seen in Fig. 7(a). For x = 0.2, i.e., Ba-rich samples, strongly correlated motions (see above) and spin-diffusion effects govern the low-T19F NMR spin-lock rates.Summary and conclusions
Mechanical treatment of CaF2 and BaF2 in high-energy ball mills leads to the formation of metastable Bax−1CaxF2. Long-range ion transport is maximized for equimolar compositions and Ea, determined from DC conductivity measurements, passes through a minimum value (0.61 eV) at this composition. Through 19F NMR relaxometry measurements, being in our case sensitive to short-range F anion dynamics, we tried to sharpen this picture. Here, we showed that ion dynamics on the angstrom length scale is characterized by very similar activation energies if x varies from 0.2 (0.43 eV) to 0.5 (0.50 eV). The increase of the absolute 19F SLR NMR rates can be explained by an increase of the pre-factor of the underlying Arrhenius equation, which is, besides other factors, governed by the activation entropy. For all samples we observed highly correlated ionic motion. While macroscopic properties, such as σDC, do not depend much on milling time; short-range order and motional correlation effects do. By comparing the various samples prepared we conclude that F anion diffusivity in earth-alkaline mixed interfacial regions, either rich in Ba or Ca, mainly boost long-range ion transport. Extensive mixing of the cations during long milling periods, on the other hand, primarily influences short-range ion dynamics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Veronika Pregartner for her help with sample preparation and characterization. Financial support by the Deutsche Forschungsgemeinschaft (DFG Research Unit 1277, grant no. WI3600/2-1(4-1) and WI3600/5-2 (SPP 1415)) as well as by the Austrian Federal Ministry of Science, Research and Economy, and the Austrian National Foundation for Research, Technology and Development (Christian Doppler Laboratory of Lithium Batteries: Ageing Effects, Technology and New Materials) is greatly appreciated.References
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- J. C. Bachman, S. Muy, A. Grimaud, H. H. Chang, N. Pour, S. F. Lux, O. Paschos, F. Maglia, S. Lupart, P. Lamp, L. Giordano and Y. Shao-Horn, Chem. Rev., 2016, 116, 140–162 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- M. Anji Reddy and M. Fichtner, J. Mater. Chem., 2011, 21, 17059–17062 RSC.
- P. Heitjans and S. Indris, J. Phys.: Condens. Matter, 2003, 15, R1257–R1289 CrossRef CAS.
- A. Chadwick and S. Savin, Solid State Ionics, 2006, 177, 3001–3008 CrossRef CAS.
- J. Maier, Chem. Mater., 2014, 26, 348–360 CrossRef CAS.
- J. Maier, Phys. Chem. Chem. Phys., 2009, 11, 3011–3022 RSC.
- J. Maier, Z. Phys. Chem., 2003, 217, 415–436 CrossRef CAS.
- P. Heitjans, M. Masoud, A. Feldhoff and M. Wilkening, Faraday Discuss., 2007, 134, 67–82 RSC.
- F. Preishuber-Pflügl, V. Epp, S. Nakhal, M. Lerch and M. Wilkening, Phys. Status Solidi C, 2015, 12, 10–14 CrossRef.
- D. Wohlmuth, V. Epp, P. Bottke, I. Hanzu, B. Bitschnau, I. Letofsky-Papst, M. Kriechbaum, H. Amenitsch, F. Hofer and M. Wilkening, J. Mater. Chem. A, 2014, 2, 20295–20306 CAS.
- V. Epp and M. Wilkening, ChemPhysChem, 2013, 14, 3706–3713 CrossRef CAS PubMed.
- M. Wilkening, V. Epp, A. Feldhoff and P. Heitjans, J. Phys. Chem. C, 2008, 112, 9291–9300 CAS.
- F. Preishuber-Pflügl, P. Bottke, V. Pregartner, B. Bitschnau and M. Wilkening, Phys. Chem. Chem. Phys., 2014, 16, 9580–9590 RSC.
- V. Epp and M. Wilkening, in Handbook of Solid State Batteries, World Scientific, Singapore, 2015, ch. Li dynamics in solids as seen via NMR relaxometry Search PubMed.
- M. Heise, G. Scholz, A. Düvel, P. Heitjans and E. Kemnitz, Solid State Sci., 2016, 60, 65–74 CrossRef CAS.
- G. Scholz, S. Breitfeld, T. Krahl, A. Düvel, P. Heitjans and E. Kemnitz, Solid State Sci., 2015, 50, 32–41 CrossRef CAS.
- H. Bhatia, D. T. Thieu, A. H. Pohl, V. S. K. Chakravadhanula, M. H. Fawey, C. Kubel and M. Fichtner, ACS Appl. Mater. Interfaces, 2017, 9, 23707–23715 CAS.
- C. Rongeat, M. A. Reddy, R. Witter and M. Fichtner, ACS Appl. Mater. Interfaces, 2014, 6, 2103–2110 CAS.
- C. Rongeat, M. A. Reddy, R. Witter and M. Fichtner, J. Phys. Chem. C, 2013, 117, 4943–4950 CAS.
- B. Ruprecht, M. Wilkening, A. Feldhoff, S. Steuernagel and P. Heitjans, Phys. Chem. Chem. Phys., 2009, 11, 3071–3081 RSC.
- B. Ruprecht, M. Wilkening, S. Steuernagel and P. Heitjans, J. Mater. Chem., 2008, 18, 5412–5416 RSC.
- D. Zahn, P. Heitjans and J. Maier, Chem. – Eur. J., 2012, 18, 6225–6229 CrossRef CAS PubMed.
- A. Düvel, P. Heitjans, P. Fedorov, G. Scholz, G. Cibin, A. V. Chadwick, D. M. Pickup, S. Ramos, L. W. L. Sayle, E. K. L. Sayle, T. X. T. Sayle and D. C. Sayle, J. Am. Chem. Soc., 2017, 139, 5842–5848 CrossRef PubMed.
- A. Düvel, B. Ruprecht, P. Heitjans and M. Wilkening, J. Phys. Chem. C, 2011, 115, 23784–23789 Search PubMed.
- N. Sata, K. Eberman, K. Eberl and J. Maier, Nature, 2000, 408, 946–949 CrossRef CAS PubMed.
- J. Maier, Prog. Solid State Chem., 1995, 23, 171–263 CrossRef CAS.
- J. Maier, J. Phys. Chem. Solids, 1985, 46, 309–320 CrossRef CAS.
- M. Wilkening, S. Indris and P. Heitjans, Phys. Chem. Chem. Phys., 2003, 5, 2225–2231 RSC.
- S. Indris and P. Heitjans, J. Non-Cryst. Solids, 2002, 307, 555–564 CrossRef.
- P. Heitjans and M. Wilkening, Mater. Res. Bull., 2009, 34, 915–922 CrossRef CAS.
- D. C. Look and I. J. Lowe, J. Chem. Phys., 1966, 44, 2995–3000 CrossRef CAS.
- S. Indris, D. Bork and P. Heitjans, J. Mater. Synth. Process., 2000, 8, 245–250 CrossRef CAS.
- A. L. Patterson, Phys. Rev., 1939, 56, 978–982 CrossRef CAS.
- A. V. Chadwick, A. Düvel, P. Heitjans, D. M. Pickup, S. Ramos, D. C. Sayle and T. X. T. Sayle, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 80 CAS , 012005.
- D. Wohlmuth, V. Epp, B. Stanje, A. M. Welsch, H. Behrens and M. Wilkening, J. Am. Ceram. Soc., 2016, 99, 1687–1693 CrossRef CAS.
- M. Wilkening and P. Heitjans, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77 CrossRef CAS , 024311.
- M. Wilkening, D. Bork, S. Indris and P. Heitjans, Phys. Chem. Chem. Phys., 2002, 4, 3246–3251 RSC.
- B. Ruprecht, H. Billetter, U. Ruschewitz and M. Wilkening, J. Phys.: Condens. Matter, 2010, 22 CrossRef CAS PubMed , 245901.
- M. A. Kraft, S. P. Culver, M. Calderon, F. Bocher, T. Krauskopf, A. Senyshyn, C. Dietrich, A. Zevalkink, J. Janek and W. G. Zeier, J. Am. Chem. Soc., 2017, 139, 10909–10918 CrossRef CAS PubMed.
- W. Meyer and H. Neldel, Phys. Z., 1937, 38, 1014–1019 CAS.
- P. Heitjans, A. Schirmer and S. Indris, in Diffusion in Condensed Matter – Methods, Materials, Models, ed. P. Heitjans and J. Kärger, Springer, Berlin, 2005, pp. 367–415 Search PubMed.
- M. Uitz, V. Epp, P. Bottke and M. Wilkening, J. Electroceram., 2017, 38, 142 CrossRef CAS.
- B. Stanje, V. Epp, S. Nakhal, M. Lerch and M. Wilkening, ACS Appl. Mater. Interfaces, 2015, 7, 4089–4099 CAS.
- X. F. He, Y. Z. Zhu and Y. F. Mo, Nat. Commun., 2017, 8 Search PubMed , 15893.
- K. L. Ngai, Solid State Ionics, 1998, 105, 225–230 CrossRef CAS.
- K. L. Ngai, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 13481–13485 CrossRef CAS.
- V. Blache, J. Forster, H. Jain, O. Kanert, R. Kuchler and K. L. Ngai, Solid State Ionics, 1998, 113, 723–731 CrossRef.
- O. Kanert, R. Kuchler, K. L. Ngai and H. Jain, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 76–82 CrossRef CAS.
- K. L. Ngai and O. Kanert, Solid State Ionics, 1992, 53, 936–946 CrossRef.
- O. Kanert, J. Steinert, H. Jain and K. L. Ngai, J. Non-Cryst. Solids, 1991, 131, 1001–1010 CrossRef.
- C. C. Liang, J. Electrochem. Soc., 1973, 120, 1289–1292 CrossRef CAS.
- S. Indris, P. Heitjans, M. Ulrich and A. Bunde, Z. Phys. Chem., 2005, 219, 89–103 CrossRef CAS.
- M. Ulrich, A. Bunde, S. Indris and P. Heitjans, Phys. Chem. Chem. Phys., 2004, 6, 3680–3683 RSC.
- S. Indris, P. Heitjans, H. E. Roman and A. Bunde, Phys. Rev. Lett., 2000, 84, 2889–2892 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRPD patterns, crystallite sizes, deconvoluted 19F MAS NMR spectra. See DOI: 10.1039/c7dt04487a |
| This journal is © The Royal Society of Chemistry 2018 |