Living ring-opening polymerization of ε-caprolactone catalyzed by β-quinolyl-enamino aluminium complexes: ligand electronic effects†
Abstract
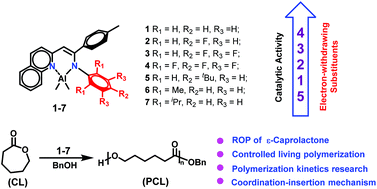
A series of β-quinolyl-enamino aluminium complexes [AlLMe2] 1–7 {L = [(2-C9H6N)-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.
C(4-MeC6H4)-N(Ar)–], Ar = C6H5 (1), 4-FC6H4 (2), 3,4,5-F3C6H2 (3), C6F5 (4), 5-tBuC6H4 (5), 2,6-Me2C6H3 (6), and 2,6-iPr2C6H3 (7)} were synthesized by the reaction of AlMe3 with the corresponding β-quinolyl-enamine ligands. All the complexes were characterized by 1H and 13C NMR spectroscopy and elemental analysis. The molecular structures of complexes 1–3 and 7 were confirmed by single-crystal X-ray diffraction, which revealed that all the Al atoms adopt a distorted tetrahedral geometry. These Al complexes were tested as the initiators for the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). The polymerization results showed that 1–5 in the presence of BnOH proceeded efficiently and their catalytic behaviors toward the ROP of ε-CL were significantly affected by the substituents of the aryl ring (Ar) on the end of the enamino framework, where the placement of electron-withdrawing substituents caused higher catalytic activity. However 6 and 7 displayed poor activity under the same conditions, suggesting that ortho-substituents on the Ar moieties hamper the coordination–insertion of the ε-CL monomer. The ROP of ε-CL by efficient binary catalytic systems proceeded in a living manner evidenced by the narrow PDIs and the linear nature of Mnversus conversion plots and monomer/catalyst ratios.


 Please wait while we load your content...
Please wait while we load your content...