Open Access Article
Open Access ArticleAntimicrobial glycoconjugate vaccines: an overview of classic and modern approaches for protein modification
Francesco
Berti
 * and
Roberto
Adamo
* and
Roberto
Adamo
 *
*
GSK, Via Fiorentina 1, 53100 Siena, Italy. E-mail: roberto.x.adamo@gsk.com; francesco.x.berti@gsk.com
First published on 2nd October 2018
Abstract
Glycoconjugate vaccines obtained by chemical linkage of a carbohydrate antigen to a protein are part of routine vaccinations in many countries. Licensed antimicrobial glycan–protein conjugate vaccines are obtained by random conjugation of native or sized polysaccharides to lysine, aspartic or glutamic amino acid residues that are generally abundantly exposed on the protein surface. In the last few years, the structural approaches for the definition of the polysaccharide portion (epitope) responsible for the immunological activity has shown potential to aid a deeper understanding of the mode of action of glycoconjugates and to lead to the rational design of more efficacious and safer vaccines. The combination of technologies to obtain more defined carbohydrate antigens of higher purity and novel approaches for protein modification has a fundamental role. In particular, methods for site selective glycoconjugation like chemical or enzymatic modification of specific amino acid residues, incorporation of unnatural amino acids and glycoengineering, are rapidly evolving. Here we discuss the state of the art of protein engineering with carbohydrates to obtain glycococonjugates vaccines and future perspectives.
Key learning points(a) The covalent linkage with proteins is fundamental to transform carbohydrates, which are per se T-cell independent antigens, in immunogens capable of evoking a long-lasting T-cell memory response.(b) Principal strategies for conjugation of bacterial polysaccharides with the protein carrier used in licensed glycoconjugate vaccines. (c) Modern methods for site selective glycoconjugation comprise chemical or enzymatic modification of specific amino acid residues, incorporation of unnatural amino acids and glycoengineering. (d) Importance of the definition of the polysaccharide portion (epitope) responsible for the immunological activity in the design of glycoconjugate vaccines. (e) How information from structural studies, strategies for carbohydrate production and protein modification can be combined in order to maximize the immunological activity of glycoconjugate vaccines. |
1. Introduction
Vaccination is an effective means to reduce death and morbidity caused by infectious diseases. Over the years, the practise of vaccination with live attenuated bacteria, as originally conceived by Jenner to treat smallpox in 1796 and later developed by Pasteur, has most often left the stage to safer subunit vaccines, which achieve protection by focusing the immune response on one or a few selected antigens. Among these, the structurally unique carbohydrates coating the surface of bacteria have become an optimal target for vaccine development.1 Since the 1970s, it has been evident that the purified capsular polysaccharide from Streptococcus pneumoniae, Haemophilus influenzae type b (Hib) and Neisseria meningitidis infections could be used for the prevention of infections in adults. However it was only in the 1980s that revisiting the concept of sugar conjugation to a carrier protein, first introduced by Avery and Goebel in the early 1930s,2 carbohydrate-based vaccines effective also in infants could be developed.Currently, glycoconjugate vaccines, as the product of chemical linkage of a carbohydrate antigen to a protein is typically termed, are part of routine vaccinations in many countries. The covalent linkage between sugars and proteins enables bacterial surface carbohydrates, which are per se T-cell independent antigens, to be capable of evoking a long-lasting T-cell memory response. This type of response is accompanied by the differentiation of polysaccharide-specific B cells to plasma cells. Reinfection with the pathogen or boosting, in the case of a vaccine results in proliferation of plasma cells and maturation of high-affinity antibodies that can eliminate both the disease and the carriage of bacteria in the immunized individuals.
Following the success of capsular polysaccharide derived vaccines, the practise of protein conjugation has been extented to other classes of carbohydrates, including O-antigens, exopolysaccharides and teichoic acids.1
Furthermore, emergence of bacterial species resistant to antibiotic treatment, as stressed out by recent WHO and CDC reports, is generating a call for novel, improved, fast acting vaccines.1
While the commercially available vaccines (Table 1) were obtained through isolation of the polysaccharides from pathogens and were developed primarily based on empiric evidence, in the last twenty years there has been a rapid evolution in the approaches to obtain more defined carbohydrate antigens of higher purity.
| Pathogen | Commercial trade name/manufacturer | Carrier protein | Saccharide chain length | Conjugation chemistry |
|---|---|---|---|---|
| a https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm and EMA http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 up to 31 May 2018. | ||||
| Haemophilus influenzae type B | ActHIB/Sanofi Pasteur (monovalent) | TT | Native polysaccharide | Information not available |
| (Multivalent formulations also containing Hib conjugate: pentacel, DTaP and inactivated poliovirus; Hexacima/Hexyon, DtaP, hepatitis B rDNA and inactivated poliomyelitis) | ||||
| Hiberix/GSK vaccines | TT | Size-reduced polysaccharide | Information not available | |
| Quinvaxem/GSK vaccines (multivalent formulation containing DTP, HepB and Hib conjugate) | CRM197 | Depolymerized polysaccharide | Active ester chemistry | |
| PedvaxHIB/Merck | OMPC | Native polysaccharide | Information not available | |
| Neisseria meningitidis serogroup C | NeisVac-C/Pfizer | TT | Native polysaccharide | Reductive amination |
| Meningitec/Nuron Biotech | CRM197 | Reductive amination | ||
| Menjugate/GSK vaccines | CRM197 | Depolymerized polysaccharide | Active ester chemistry | |
| Menitorix/GSK vaccines (with Hib) | TT | Size-reduced polysaccharide | Information not available | |
| Neisseria meningitidis serogroup CY | MenHibrix/GSK vaccines (with Hib) | TT | Information not available | |
| Neisseria meningitidis serogroup ACWY | Menactra/Sanofi Pasteur | DT | Depolymerized polysaccharide | Information not available |
| Menveo/GSK | CRM197 | Depolymerized polysaccharide | Active ester chemistry | |
| Nimenrix/Pfizer | TT | Size-reduced polysaccharide | Active ester chemistry | |
| Streptococcus pneumoniae serogroup 4, 6B, 9V, 14, 18C, 19F, 23F | Prevnar/Pfizer | CRM197 | Native polysaccharide | Reductive amination |
| Streptococcus pneumoniae serogroup 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F | Synflorix/GSK | NTHi PD, DT, TT | Size-reduced polysaccharide | Reductive amination |
| Streptococcus pneumoniae serogroup 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F | Prevnar13/Pfizer | CRM197 | Native polysaccharide | Information not available |
In parallel, novel technologies for protein modification with glycans have emerged, in some cases inspired by the conjugation methods of small molecules to therapeutic monoclonal antibodies.3 Combination of these techniques and structural approaches for the definition of the polysaccharide portion (epitope) responsible for the immunological activity has the potential to aid a deeper understanding of the mode of action of glycoconjugates and to lead to the rational design of more efficacious and safer vaccines.
Here we discuss the state of the art of protein engineering with carbohydrates to obtain glycococonjugates vaccines and future perspectives.
2. The construction of glycoconjugate vaccines
2.1 Why conjugation is needed
Polysaccharides present on the surface of capsulated bacteria are composed of many identical repeating units. Each repeating unit can be a single monomer (homopolymer) or a combination of few monomers (heteropolymer). In adult, purified polysaccharides stimulate B-cell differentiation into plasma cells producing antibodies by cross-linking the B-cell receptor. In infant, this mechanism is not fully mature and polysaccharides fail to trigger a T cell memory response.4 However, when a polysaccharide is conjugated to a carrier protein, peptides deriving from their intracellular digestion enter the cavity of the Major Histocompatibility Complex Class II (MHCII) and are re-exposed for engagement of the T cell receptor.4 In one example T cell recognizing specifically the carbohydrate portion of a glycopeptide anchored to MHCII have been found.5 T cells in turn trigger B cells to specialized and produce highly specific antibodies that can activate cascade reactions for bacterial distruction.4While the necessity of a covalent linkage between carbohydrate and protein is challenged by some studies using alternative systems such as liposomes for co-delivery of the two components,6 licensed vaccines are all based on the concept of covalent conjugation. This tutorial focuses only on glycan–protein conjugates and methods for protein conjugation. For a broader overview on other types of glycoconjugates based on peptides and fully synthetic scaffolds we suggest reading ref. 7 and references therein.7
2.2 Carbohydrate production
For the manufacturing of licensed vaccines, purified polysaccharides are used natural or size-reduced through mechanical (i.e. microfluidization) or chemical (i.e. acidic hydrolysis, hydrogen peroxide) treatments. The size reduction of polysaccharide chains results in more defined oligosaccharide populations and reduce the density/viscosity of those compounds in aqueous solution facilitating the chemical coupling to the protein carrier.Chemical synthesis has been successful for large scale production of the Hib antigen, which was made by a one-step poly-condensation reaction based on the H-phosphonate chemistry.8
At preclinical and clinical level, there are a plethora of methods currently used to achieve oligosaccharide synthesis. The pentadecasaccharide of Shigella flexneri 2a has been led to Phase-1 clinical trial after intensive synthesis optimization.9 Approaches for speeding up the carbohydrate antigen production include the use of solid-phase automated synthesis, iterative protocols or combined chemo-enzymatic strategies.10–12 Typically the selection of the more suited approach for a target structure is still a result of extensive experience in the field. Among these technologies, the number of structures achieved by automated synthesis is rapidly growing. This method so far can deliver production of oligosaccharide at a scale sufficient for preclinical testing, while subsequent phases of vaccine manufacturing would require process development.
E. coli glycoengineering for in vivo expression of glycoproteins is a technology able to provide the oligosaccharide directly linked to the carrier protein and will discussed more in depth below.
2.3 Protein selection
Carrier proteins needs to provide T cell epitopes and present a sufficient number of surface exposed functional groups for conjugation. As shown in Table 1, five carrier proteins (TT, Tetanus Toxoid; DT, Diphtheria Toxoid; CRM197, Cross Reacting Material 197; NTHi PD, non typeable H. influenzae protein D; OMPC, outer membrane protein complex) have been used for all licensed conjugate vaccines.1 The toxoids DT and TT are obtained from the respective toxins by chemical detoxification with formaldehyde, while CRM197 is a nontoxic mutant of diphtheria toxin isolated from the supernatant of Corynebacterium diphtheriae C7(b197) tox(−) strain bacterial growths or produced recombinantly in E. coli. Also NTHi PD is expressed in E. coli as recombinant protein. Other proteins such as recombinant Pseudomonas aeruginosa exotoxin A (rEPA) have been used at preclinical and clinical level.132.4 Conjugation chemistry
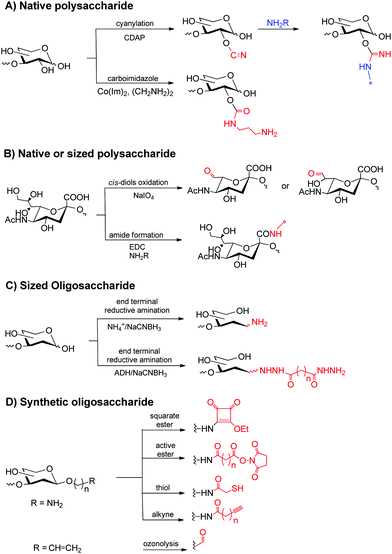
For conjugation, sugars and proteins are typically modified to achieve the chemoselective coupling reaction. Characteristics of the final conjugates as modification pattern of the sugar (e.g. O-acetylation), saccharide length, saccharide/protein ratio and type of carrier protein, conjugation chemistry and linkers eventually used for coupling are relevant for the immunogenicity. These features depend on the approaches used for the preparation of glycoconjugates. Below are discussed the most common methods used for this scope.More chemoselective methods are based on periodate oxidation of (i) cis-diols in the sugar ring or at the glycerol moiety of sialic acid residues to generate aldehydes or (ii) activation of carboxyl groups of sialic or acid uronic residues (Fig. 1B).14
Linkers can be used to insert chemical handles for conjugation, reducing steric hindrance between protein and saccharide. Generated aldehydes or end terminal aldehydes from oligosaccharides are typically reacted with the ε-amine of lysine residues by reductive amination. Alternative reductive amination can be performed with ammonium salts to provide an amine ready for coupling or derivatized with di-hydrazide spacers (Fig. 1C).14 Inserted amines can be reacted directly with carboxylic acids of the protein or coupled to a variety of bifunctional linkers to incorporate squaric ester, maleimide, thiol, azide or alkyne moieties to aid conjugation.
Synthetic sugars offer the advantage of bearing reactive groups such as amines (for further modification, vide supra), thiols or alkenes for aldehyde generation (Fig. 1D).
3. Site-selective approaches in glycoconjugation
More defined glycoconjugates than those currently licensed are attractive candidates to have well characterized products and to correlate the immunogenicity to a precise attachment site.15 For a more extensive review of this topic we refer the readers to the ref. 16. Selective approaches appear more compatible with protein used with dual role of antigen and glycan carrier. Pathogen related carrier proteins could be a way to counteract possible reduced immune response against glycoconjugates due to repetitive administration of the same carrier protein.16Four major approaches for site selective protein engineering have found application to glycoconjugation: chemical modification of amino acid residues, enzyme catalysed conjugation, incorporation of unnatural amino acids and glycoengineering.3
Site selective reactions exploit the specific reactivity of certain amino acids residues in the context of the protein sequence.
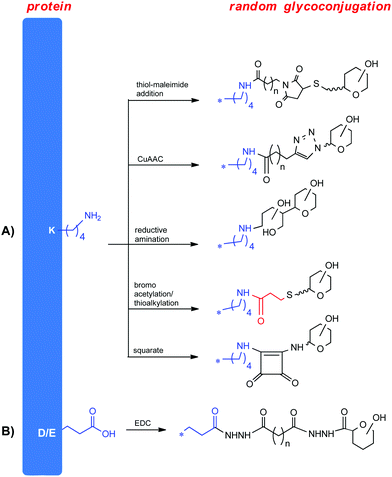
Traditional conjugation methods are addressed to the very abundant lysine or aspartatic/glutamic acid residues. Because of their ionic character these residues are well exposed on the protein surface. On the opposite, their high reactivity renders challenging the control of the conjugation site.
In particular, the high nucleophilicity of the amine in the K side chain is probably the most common feature employed for conjugation. The reactivity of lysine depends not only on solvent/reagent accessibility but also on the relative amino acid contour and pH. In some cases preferentiality for some lysine residues compared to others caused by the better exposition has been observed.17 Very recently it has been shown that at slightly basic pH the lysine with the lowest pKa is the kinetically favoured residue for reaction.18
3.1 Chemical or enzymatic modification of natural amino acids
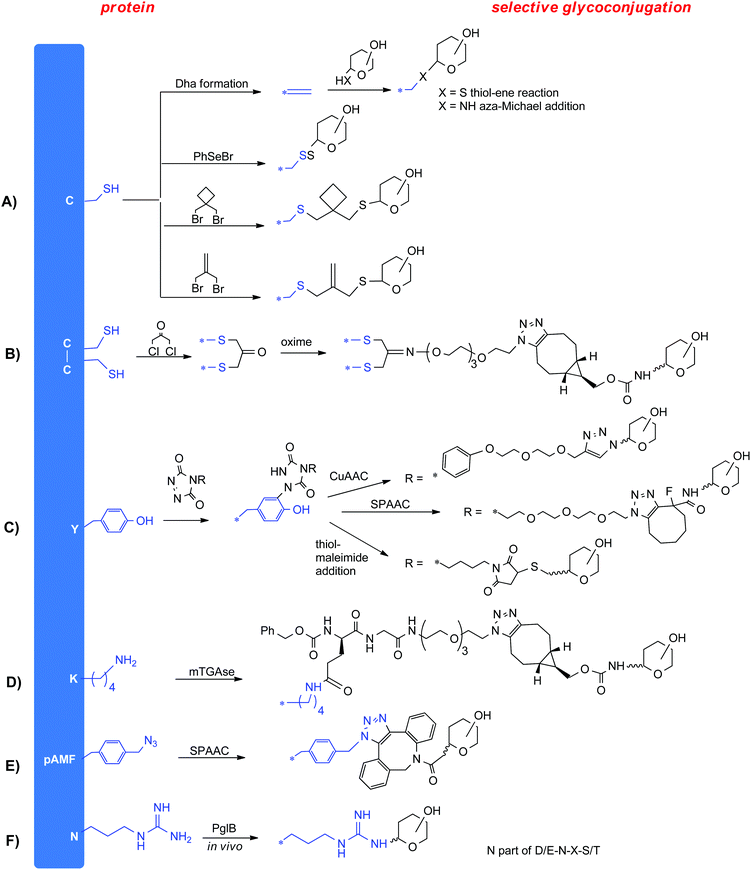
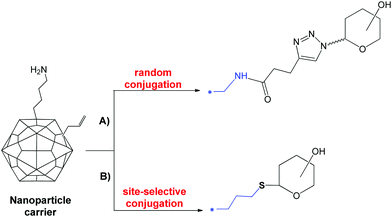
Site selective coupling is typically achieved by targeting highly nucleophilic cysteine residues either naturally present or genetically installed at the protein surface. Both native and engineered cysteine residues are known to react rapidly with a variety of electrophilic reagents, including sugars derivatives with thio or selenoether, maleimides and haloalkyl groups (Fig. 3A).19 Alternatively, cysteine can be elongated with an electrophilic chemical handle, like a bromooxetane20 or a bromoisobutylene21 for following halogen displacement with a thiosugar. Cysteine can also be converted into dehydroalanine to undergo Michael addition of thiosugars22 or aza-Michael ligation of a wide-range of N-nucleophiles, including carbohydrates bearing an alkylamine linker.23Cysteine residues involved in disulfide bond formation usually plays a role in stabilizing protein tertiary structure. It is possible to temporarily cleave the disulfide bond to then staple the two cysteines through a short covalent bridge (Fig. 3B). This bridge can be functionalized with a chemical group for glycan coupling, such as a ketone.24
Tyrosine residues are much less abundant and exposed than lysine residues so they can be more selectively targeted for glycoconjugation (Fig. 3C). To this end, triazolinediones has been exploited to react with the phenol ring of Y.25 This reaction requires the use of tris(hydroxymethyl)aminomethane (Tris) buffer as scavenger for the isocyanates formed by the in situ degradation of the triazolidinones in order to direct the reaction to Y rather than to K residues.26
Modification of K, Q and G residues can be also selectively achieved by enzymatic methods.27 In particular, enzymes can react with specific residues incorporated in a short amino acid tag which can be introduced either on the protein or on the sugar to be conjugated. Among these enzymes, transglutaminases (Tgases, Fig. 3D) are a family of widely expressed enzymes that have been used to label Q28 or K.29 Sortases from Staphylococcus aureus or Streptococcus pyogenes have been successfully applied for conjugation to an N-terminal (G)n (n ≥ 3) or C-terminal LPXTG tag.
It is worth considering that while tyrosine modification allows conjugation at more than one connectivity point selected among the naturally present residues, methods based on protein engineering with cysteine or incorporation of a tag for enzymatic transglutamination offer a larger flexibility in directing the conjugation to desired domains. However, a limitation often associated with the expression of proteins engineered with multiple cysteines is the tendency to aggregation which can dramatically decrease the production yield.
3.2 Unnatural amino acids
Proteins incorporating unnatural (i.e. not naturally found or encoded) amino acids (uAAs) in their sequence can be expressed through a modified translational machinery that include a specific codon, a tRNA recognizing the codon and a tRNA synthetase specific for the target uAA.30 Among other possible expression systems, E. coli can be engineered to increase the production of the mutated protein. This combined with a large variety of uAAs currently available can expand the tools for protein conjugation. Inverse demand Diels–Alder reaction between tetrazine and genetically encoded trans-cyclooctene (TCO) and bicyclononyne (BCN) modified uAAs has been harnessed for conjugation of sugar moieties directly in cellular medium or in lysates.31 Proteins engineered with uAAs can be produced also from E. coli derived cell free extract. Sutrovax has developed a proprietary system termed XtractCF+ for production of proteins modified with functional groups for click chemistry with carbohydrates (Fig. 3E).323.3 Glycoengineering
The in vivo production of glycoproteins through the so-called protein glycan coupling technology has recently found application for production of a variety of glycoconjugates.33 This approach is based on the N-linked glycosylation system from Campylobacter jejuni that can be functionally expressed in E. coli and the ability of E. coli to synthesize heterologous polysaccharides on its glycosyl carrier lipid. To deliver a target glycoprotein E. coli is engineering with (i) the genome clusters encoding for the desired bacterial polysaccharide, (ii) the oligosaccharyl transferase PglB from Campylobacter jejuni and (iii) a plasmid encoding for the carrier protein carrying the N-glycosylation consensus sequences D/E-N-X-S/T, where X can be any amino acid except proline (Fig. 3F).34 During the expression, pathogen glycans repeating units are first assembled in the cytoplasm anchored to a lipid carrier (undecaprenyl–pyrophosphate, Und–PP), and then flipped across the cytoplasmic membrane, typically by an ATP-binding cassette (ABC) transporter. In the periplasmic space, a polymerase (Wzy) builds up the sequence on the lipid carrier, and finally PglB transfers the resulting lipid linked oligosaccharides (LLO) to the asparagine residues of the N-glycosylation consensus sequence incorporated into the carrier protein. PglB is a promiscuous enzyme, but with higher preferentiality for hexosamines.4. Effect of the glycoconjugate design on the immunogenicity
4.1 Glycan epitopes
In general, minimal antigenic determinant of polysaccharides (meaning the portion which is interacting with antibodies) are defined oligosaccharides, which can structurally vary from a short linear disaccharide, as for Vibrio cholerae O1,35 up to a more complex hexasaccharide as observed for type III polysaccharide of Group B Streptococcus (GBS)36 or even a nonasaccharide, as in the case of S. flexneri serotype 2a O-antigen.37 Branching points determining structural rigidity seems relevant in these epitopes as observed for pneumococcal type 14 repeating unit and GBS type III polysaccharide.36,38How these glyco-epitopes influence stimulation of B cells is still not clear, however it is assumed that oligosaccharides long enough to fill antibody binding sites are more likely to elicit antibodies reactive with the corresponding polysaccharides. The binding pocket of the variable regions of antibodies can interact with an area that could differ depending on the tridimensional structure that polysaccharides can acquire (e.g. helical conformations).39
The shape of the glycoconjugates and the way the glyco-epitopes are presented to the immune system are influenced particularly by some of the glycoconjugates’ characteristics, specifically saccharide length, saccharide/protein ratio and conjugation chemistry. These factors interact in a strictly interconnected manner and affect the final immunological activity. This is well exemplified by an early study in infants where Hib polysaccharide fragments with average chain length 8 or 20 repeat units (average Degree of Polymerization, avDP8 and avDP20) were conjugated to DT via reductive amination by incorporating 2 and 3 sugar moieties per protein molecule, respectively. The avDP8 oligomer elicited poorer anti carbohydrate response than the avDP20.40 However, in a follow up study, ∼4.5 moieties of an end terminally activated DP7 oligosaccharide were conjugated to CRM197, resulting comparably immunogenic to the previously tested avDP20,41 indicating that a higher level of glycosylation could compensate the chain length. Years later, an avDP8 was chemically synthesized and conjugated to TT to obtain the first licensed glycoconjugate based on a synthetic antigen.8 More recently a Hib tetramer has been shown to possibly be a sufficient length for immunogenicity in the animal model which explains the immunological activity of DP7.42
4.2 Different types of site-selective glycoconjugates currently available
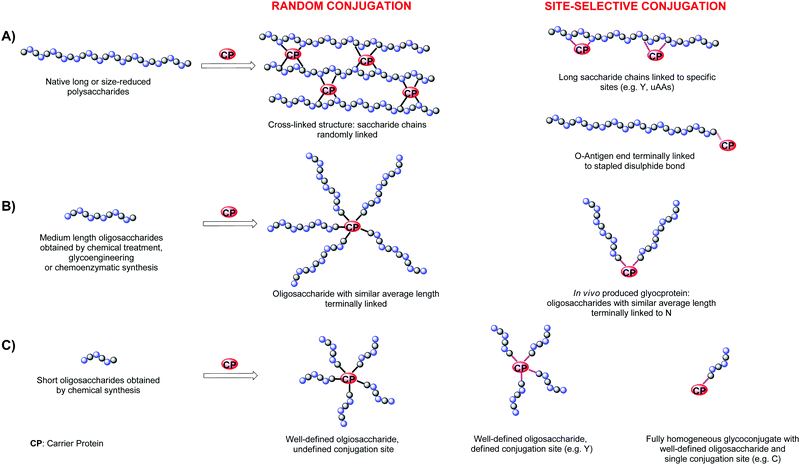
Heterogeneous polysaccharides generally ensure exposition of a sufficient number of sugar epitopes along the carbohydrate chain to trigger B cells and promote T cell activation. However, their characterization is not trivial. Saccharide/protein ratio is described in terms of w/w and it is challenging to define the number of carbohydrate moieties linked to each molecule of carrier protein, therefore structure–immunogenicity relationship is not straightforward.Different designs can help to better correlate the immunogenicity with glycoconjugates characteristics (Table 2).
| Carbohydrate source | Conjugation | Carbohydrate length | Saccharide moieties/protein | Conjug. site |
|---|---|---|---|---|
| Synthesis | Random | 1 (or subunit)-3 repeating units from heteroPS | Variable, generally 3–25 | K |
| Synthesis | Selective | Short oligosaccharide | 1 | C, pAMF |
| 4 | Y | |||
| Sized polysaccharide | Random | 5–50 repeating units | Variable | K |
| E. coli expression | Selective | 10–20 repeating units | 1–2 | N |
| Native polysaccharide | Random | 70–200 repeating units | Undefined | K, A/E |
| Native polysaccharide | Selective | 1 | K, C–C pAMF | |
| 4 | Y |
Polysaccharides linked to preactivated tyrosine had allowed directing the conjugation to few predetermined sites, generating more defined conjugates presenting multiple protein copies along the carbohydrate chain (Fig. 4A).
Capsular polysaccharides from GBS type II and V, a pathogen responsible for neonatal infections, have been coupled by strain promoted azide alkyne cycloaddition (SPAAC) to the three-four more exposed tyrosine residues of the pathogen related proteins GBS80 or GBS67, respectively.43,44 The immunogenicity of the tyrosine directed GBS type II polysaccharide-GBS80 and GBS type V polysaccharide-GBS67 conjugates was shown non inferior to the corresponding CRM197 conjugates.43,44
Anti-glycan and anti-protein antibodies were effective in inducing bacterial killing in vitro of strain expressing either the capsular polysaccharide or the pilus protein in comparable manner to random conjugation to the same proteins but with the advantage in terms of characterization of predetermined connectivity.44,45 For GBS type V polysaccharide conjugation, the thiol-malemide addition was compared to SPAAC showing to provide an efficacious vaccine, without production of anti-linker antibodies that are conversely induced by the linkers for click chemistry.
Expression in cell free derived E. coli extract of the carrier proteins modified with uAAs is being used by Sutrovax for the development of a conjugate vaccine against multiple pneumococcal strains.
End terminal linkage of semisynthetic or synthetic carbohydrates allows for a radial exposition of the glycan epitopes. In this case, the number of conjugated carbohydrates can be more accurately determined.
Conjugation of large polysaccharides to one connectivity has been achieved by microbial transglutaminase (mTGase) catalysed lysine modification and disulfide stapling.24,44 In the first case basic pH was key to achieve selectivity at K37/39 of CRM197 with the Salmonella O-antigen modified with a ZQG azido spacer (with Z = Cbz). At acidic pH the additional site K33 was modified.
Disulfide rebridging was successful to modify selectively C186-C201, the more exposed of the two S–S bonds of CRM197. This disulfide was reduced by TCEP for incorporation of 1,3-dichloroacetone which was then extended with a bifunctional aminooxy–azide linker to form an oxime. The azide was then clicked with the Salmonella O-antigen modified with a cyclooctene–cylopropane spacer.24 Interestingly the stapled conjugate showed superior immunological activity than the one at K37/39, suggesting a possible role of the conjugation site in the vaccine efficacy which deserves further elucidation.
Following studies on the carrier protein CRM197 showed that the selective installation of an oxetane motif on the disulfide bridge enabled stabilization of folded structures and resulted in enhanced immunogenicity of the protein as antigen.46
Glycoengineering has been used by LimmaTech Biologicals (former GlycoVaxyn) to access to a number of structurally different glycoproteins, termed as bioconjugates to differ from chemical conjugates. The biosynthesized bacterial oligosaccharides vary from O-antigens (Salmonella enterica, Shigella spp, E. Coli LPS) or capsular polysaccharides (S. aureus serotype 5 or 8 CPS and S. pneumoniae).1 Typically, these conjugates are characterized by medium length heterogeneous oligosaccharides linked to one or two tags genetically inserted in the protein carrier (Fig. 4B). For example, the conjugate against Shigella dysenteriae was composed by an O-antigen with a length of 13–20 repeating.13 For the expression of the E. coli O121 O-antigen, which was obtained with the aim of mimicking the structurally similar polysaccharide from Salmonella typhi, two N-glycosylation sites were inserted.47 Glycosylation occurred preferentially at one site with an average of 12 repeating units. To date the most used protein carrier is rEPA, although pathogen related carrier proteins have been also employed, such as S. aureus α toxin Hla which was conjugated to capsular oligosaccharides from the same bacterium.48
Bioconjugate vaccine candidates are at a more advanced stage of development compared to other approaches. Vaccines against S. dysenteriae O1 and S. flexneri 2a,49 and extra intestinal pathogenic E. coli have completed Phase-1 clinical trials showing to be safe and promising immunogenicity.50
Glycoconjugates with defined sugars at the more exposed tyrosines of the carrier protein have produced by GSK vaccines (former Novartis Vaccines & Diagnostics) and Novartis Institutes for Biomedical Research (NIBR) by tyrosine ligation (Fig. 4C).26 The genetically detoxified diphtheria toxin mutant CRM197, a protein component of numerous licensed vaccines, was first modified primarily at Y27, Y46, Y358 and Y380, with an alkyne linker for CuAAC of a β-(1,3)-glucan hexasaccharide bearing an azide spacer. Insertion of double copies of the β-(1,3)-glucan antigen for each targeted tyrosine residues was also achieved.51
These two Y-directed conjugates constructs induced in mice comparable immune response than a CRM197 conjugate of laminarin composed of a long heterogeneous β-(1,3)-(1,6)-glucan attached randomly to K residues, known to be highly protective against systemic and mucosal C. albicans infections.
Fully homogeneous products with one saccharide chain and connectivity to the protein (Fig. 4C) have been made by Davis and coworkers through coordinated carbohydrate synthesis of a thiol polyrhamnoside from Klebsiella pneumoniae O-antigen and following addition to the selenenylsulfide preactivated cysteine of the subtilisin mutant protein S156C.19
Production of the malaria antigen Pfs25 bearing the uAA p-azidomethyl phenylalanine (pAMF) at the C-terminus through the Sutrovax platform has recently been used for generating a conjugate with dibenzocyclooctyne (DBCO) derivatized glycan core of malaria GPI using strain promoted click chemistry. The Pfs25–GPI conjugate induced in mice significantly higher titers compared to the unconjugated protein, and purified anti Pfs25–GPI IgGs were able to block transmission of parasites to mosquitoes.32
While fully homogeneous conjugates would allow precise correlation of immunogenicity to length and attachment site, only few studies are available and the optimal carbohydrate size for these constructs is still unknown.
There is no simple general rule to predict the ideal saccharide/protein molar ratio in these vaccines. It has been postulated that a high level of protein modification could be needed for conjugation of short oligomers in order to elicit an optimal immune response, while a lower modification level could be sufficient for longer oligosaccharides.52 Indeed glycosylation degrees of 8–10 sugar moieties have been shown sufficient to provide robust immunes response to single repeating units32 or even subunit epitopes, as recently shown for a S. pneumoniae type 1 trisaccharide epitope.53
In a recent work, glycosylation degrees varying from 5 to 26 were tested for the random conjugation of a pentadecasaccharides from S. flexneri 2a to TT, and a trend of increased anti-carbohydrate antibody levels was observed going up from 5 to 17 carbohydrate moieties, while titers declined for the highest glycosylation degree.9 This led to selection of the conjugate with 17 carbohydrate moieties for Phase-1 clinical trials.
Of note, the above mentioned conjugates of β-(1,3)-glucans to 4 tyrosine residues of CRM197 gave statistically similar antibody titers compared to higher levels of glycosylation at K,51 although a certain trend of increased IgG titers was observed for a random conjugate with 17 glycan moieties. This suggests that in some cases few defined conjugation sites could be sufficient to raise an adequate immunogenicity.
The Salmonella O-antigen (composed of approximately 50 repeating units) has been able to provide robust immunogenicity even with a single attachment sites, depending on the targeted amino acid. The same applies to bioconjugates from Shigella or other bacterial O-antigens which contains oligosaccharide chains of ∼10–20 repeating units. We can reason that lower degree of glycan incorporation might be compensated by the use of longer oligosaccharides, which express multiple copies of the minimal epitope. Therefore a balance of defined attachment sites and optimized saccharide length could give rise to highly immunogenic homogenous vaccines.
4.3 Alternative approaches
On the opposite direction go other recent strategies aiming at maximizing the exposition of short defined glycans by conjugation of sugar clusters or multivalent display around a variety of organic or inorganic nanoparticle cores. This approach, which has been proven fundamental to overcome the inherent poor immunogenicity of mammalian glycans as those decorating cancer cells or the viral surface7,54 has been recently applied also for the improvement of antimicrobial glycoconjugates. Random conjugation via CuAAC of 360 copies of a synthetic tetrasaccharide epitope at the surface K residues of Qb virus-like bacteriophage particles has allowed achieving dramatic increase in the affinity of antibodies generated towards pneumococcal type 14 capsule (Fig. 5A).55 Site-selective glycosylation of Qb virus-like bacteriophage particles has also been shown feasible by incorporation of the uAA homoallylglycine followed by thiol–ene addition of 180 copies of thioglycosides (Fig. 5B).19 Nanoparticle systems could provide efficient presentation of small synthetic glycan epitopes.5. Conclusions
Glycoconjugation has provided a key tool for the preparation of efficacious vaccines. New methods for producing the carbohydrate component have matured in the last decades, along with selective conjugation methods. Chemical synthesis combined to structural studies for the identification of the minimal antigenic polysaccharide portion responsible for the interaction with protective antibodies is providing relevant information for carbohydrate selection.Combination of production of rationally defined carbohydrates either in vitro chemo-enzymatic or in vivo glycoengineering methods and selective conjugation holds the potential to design glycoconjugates with higher quality control standard, process reproducibility and pathogen free production. In addition to those features, synthetic carbohydrates specifically lack of bacterial contaminants.
A lot needs to be unravelled on the mechanism of action of glycoconjugates, particularly on the impact of the conjugation site on the protein peptides responsible for the T cell help. Therefore, selective conjugation can contribute also to a better understanding of the mode of action of this class of vaccines. Recently different novel designs have been allowed by progress in chemical modification of proteins, incorporation of unnatural amino acids and glycoengineering. Particularly, the latter approach has led different novel glycoconjugates to clinical trials. Synthetic methods for carbohydrate production are also maturing and a vaccine against S. flexneri 2a has recently completed a Phase-1 study.
While methods for selective conjugation of polysaccharides appear timely more feasible, factors effecting the immunogenic activity of fully homogeneous conjugates with defined glycan and attachment site (e.g. optimal sugar length and number of attachment sites) still need more profound studies.
We expect that the new designs based on selective glycoconjugation will become more and more employed for vaccine production leading to a new generation of innovative and improved vaccines.
Conflicts of interest
FB and RA are employees of GSK group of companies.Acknowledgements
We acknowledge the European Union's Horizon 2020 research and innovation programme for funding work on this topic under the Marie Skłodowska-Curie grant agreement Glycovax No. 675671.Notes and references
- F. Micoli, P. Costantino and R. Adamo, FEMS Microbiol. Rev., 2018, 42, 388–423 CrossRef PubMed.
- O. T. Avery and W. F. Goebel, J. Exp. Med., 1931, 54, 437–447 CrossRef CAS PubMed.
- Q.-Y. Hu, F. Berti and R. Adamo, Chem. Soc. Rev., 2016, 45, 1691–1719 RSC.
- E. De Gregorio and R. Rappuoli, Nat. Rev. Immunol., 2014, 14, 505–514 CrossRef CAS PubMed.
- F. Y. Avci, X. Li, M. Tsuji and D. L. Kasper, Nat. Med., 2011, 17, 1602–1609 CrossRef CAS PubMed.
- C. H. Jones, G. Zhang, R. Nayerhoda, M. Beitelshees, A. Hill, P. Rostami, Y. Li, B. A. Davidson, P. I. Knight and B. A. Pfeifer, Sci. Adv., 2017, 3, e1701797 CrossRef PubMed.
- M. Marradi, F. Chiodo, I. Garcia and S. Penades, Chem. Soc. Rev., 2013, 42, 4728–4745 RSC.
- V. Verez-Bencomo, V. Fernandez-Santana, E. Hardy, M. E. Toledo, M. C. Rodriguez, L. Heynngnezz, A. Rodriguez, A. Baly, L. Herrera, M. Izquierdo, A. Villar, Y. Valdes, K. Cosme, M. L. Deler, M. Montane, E. Garcia, A. Ramos, A. Aguilar, E. Medina, G. Torano, I. Sosa, I. Hernandez, R. Martinez, A. Muzachio, A. Carmenates, L. Costa, F. Cardoso, C. Campa, M. Diaz and R. Roy, Science, 2004, 305, 522–525 CrossRef CAS PubMed.
- R. M. van der Put, T. H. Kim, C. Guerreiro, F. Thouron, P. Hoogerhout, P. J. Sansonetti, J. Westdijk, M. Stork, A. Phalipon and L. A. Mulard, Bioconjugate Chem., 2016, 27, 883–892 CrossRef CAS PubMed.
- H. S. Hahm, M. K. Schlegel, M. Hurevich, S. Eller, F. Schuhmacher, J. Hofmann, K. Pagel and P. H. Seeberger, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E3385–E3389 CrossRef CAS PubMed.
- D. Oldrini, T. Fiebig, M. R. Romano, D. Proietti, M. Berger, M. Tontini, R. De Ricco, L. Santini, L. Morelli, L. Lay, R. Gerardy-Schahn, F. Berti and R. Adamo, ACS Chem. Biol., 2018, 13, 984–994 CrossRef CAS PubMed.
- Z. Hu, A. F. Bongat White and L. A. Mulard, Chem. – Asian J., 2017, 12, 419–439 CrossRef CAS PubMed.
- N. Ravenscroft, M. A. Haeuptle, M. Kowarik, F. S. Fernandez, P. Carranza, A. Brunner, M. Steffen, M. Wetter, S. Keller, C. Ruch and M. Wacker, Glycobiology, 2016, 26, 51–62 CAS.
- N. Ravenscroft, P. Costantino, P. Talaga, R. Rodriguez and W. Egan, Vaccine Analysis: Strategies, Principles, and Control, Springer Nature, Switzerland AG, 2015, pp. 301–381 Search PubMed.
- R. Adamo, A. Nilo, B. Castagner, O. Boutureira, F. Berti and G. J. Bernardes, Chem. Sci., 2013, 4, 2995–3008 RSC.
- R. Dagan, J. Poolman and C. A. Siegrist, Vaccine, 2010, 28, 5513–5523 CrossRef CAS PubMed.
- S. Crotti, H. Zhai, J. Zhou, M. Allan, D. Proietti, W. Pansegrau, Q. Y. Hu, F. Berti and R. Adamo, ChemBioChem, 2014, 15, 836–843 CrossRef CAS PubMed.
- M. J. Matos, B. L. Oliveira, N. Martínez-Sáez, A. Guerreiro, P. M. S. D. Cal, J. Bertoldo, M. Maneiro, E. Perkins, J. Howard, M. J. Deery, J. M. Chalker, F. Corzana, G. Jiménez-Osés and G. J. L. Bernardes, J. Am. Chem. Soc., 2018, 140, 4004–4017 CrossRef CAS PubMed.
- E. J. Grayson, G. J. Bernardes, J. M. Chalker, O. Boutureira, J. R. Koeppe and B. G. Davis, Angew. Chem., Int. Ed., 2011, 50, 4127–4132 CrossRef CAS PubMed.
- O. Boutureira, N. Martinez-Saez, K. M. Brindle, A. A. Neves, F. Corzana and G. J. L. Bernardes, Chem. – Eur. J., 2017, 23, 6483–6489 CrossRef CAS PubMed.
- B. Lee, S. Sun, E. Jimenez-Moreno, A. A. Neves and G. J. L. Bernardes, Bioorg. Med. Chem., 2018, 28, 2403–2407 CrossRef PubMed.
- J. M. Chalker, S. B. Gunnoo, O. Boutureira, S. C. Gerstberger, M. Fernández-González, G. J. L. Bernardes, L. Griffin, H. Hailu, C. J. Schofield and B. G. Davis, Chem. Sci., 2011, 2, 1666 RSC.
- A. M. Freedy, M. J. Matos, O. Boutureira, F. Corzana, A. Guerreiro, P. Akkapeddi, V. J. Somovilla, T. Rodrigues, K. Nicholls, B. Xie, G. Jimenez-Oses, K. M. Brindle, A. A. Neves and G. J. L. Bernardes, J. Am. Chem. Soc., 2017, 139, 18365–18375 CrossRef CAS PubMed.
- G. Stefanetti, Q. Y. Hu, A. Usera, Z. Robinson, M. Allan, A. Singh, H. Imase, J. Cobb, H. Zhai, D. Quinn, M. Lei, A. Saul, R. Adamo, C. A. MacLennan and F. Micoli, Angew. Chem., Int. Ed., 2015, 54, 13198–13203 CrossRef CAS PubMed.
- H. Ban, J. Gavrilyuk and C. F. Barbas, J. Am. Chem. Soc., 2010, 132, 1523–1525 CrossRef CAS PubMed.
- Q.-Y. Hu, M. Allan, R. Adamo, D. Quinn, H. Zhai, G. Wu, K. Clark, J. Zhou, S. Ortiz, B. Wang, E. Danieli, S. Crotti, M. Tontini, G. Brogioni and F. Berti, Chem. Sci., 2013, 4, 3827–3832 RSC.
- D. Rabuka, Curr. Opin. Chem. Biol., 2010, 14, 790–796 CrossRef CAS PubMed.
- C. W. Lin and A. Y. Ting, J. Am. Chem. Soc., 2006, 128, 4542–4543 CrossRef CAS PubMed.
- S. Jeger, K. Zimmermann, A. Blanc, J. Grunberg, M. Honer, P. Hunziker, H. Struthers and R. Schibli, Angew. Chem., Int. Ed., 2010, 49, 9995–9997 CrossRef CAS PubMed.
- K. Wals and H. Ovaa, Front. Chem., 2014, 2 DOI:10.3389/fchem.2014.00015.
- T. Machida, K. Lang, L. Xue, J. W. Chin and N. Winssinger, Bioconjugate Chem., 2015, 26, 802–806 CrossRef CAS PubMed.
- J. F. Zawada, G. Yin, A. R. Steiner, J. Yang, A. Naresh, S. M. Roy, D. S. Gold, H. G. Heinsohn and C. J. Murray, Biotechnol. Bioeng., 2011, 108, 1570–1578 CrossRef CAS PubMed.
- M. Wacker, D. Linton, P. G. Hitchen, M. Nita-Lazar, S. M. Haslam, S. J. North, M. Panico, H. R. Morris, A. Dell, B. W. Wren and A. Markus, Science, 2002, 298, 1790–1793 CrossRef CAS PubMed.
- M. Kowarik, N. M. Young, S. Numao, B. L. Schulz, I. Hug, N. Callewaert, D. C. Mills, D. C. Watson, M. Hernandez, J. F. Kelly, M. Wacker and M. Aebi, EMBO J., 2006, 25, 1957–1966 CrossRef CAS PubMed.
- S. Villeneuve, H. Souchon, M. M. Riottot, J. C. Mazie, P. Lei, C. P. Glaudemans, P. Kovac, J. M. Fournier and P. M. Alzari, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 8433–8438 CrossRef CAS PubMed.
- F. Carboni, R. Adamo, M. Fabbrini, R. De Ricco, V. Cattaneo, B. Brogioni, D. Veggi, V. Pinto, I. Passalacqua, D. Oldrini, R. Rappuoli, E. Malito, I. Y. R. Margarit and F. Berti, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5017–5022 CrossRef CAS PubMed.
- B. Vulliez-Le Normand, F. A. Saul, A. Phalipon, F. Belot, C. Guerreiro, L. A. Mulard and G. A. Bentley, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9976–9981 CrossRef CAS PubMed.
- D. Safari, H. A. Dekker, J. A. Joosten, D. Michalik, A. C. de Souza, R. Adamo, M. Lahmann, A. Sundgren, S. Oscarson, J. P. Kamerling and H. Snippe, Infect. Immun., 2008, 76, 4615–4623 CrossRef CAS PubMed.
- C. Anish, B. Schumann, C. L. Pereira and P. H. Seeberger, Chem. Biol., 2014, 21, 38–50 CrossRef CAS PubMed.
- P. W. Anderson, M. E. Pichichero, R. A. Insel, R. Betts, R. Eby and D. H. Smith, J. Immunol., 1986, 137, 1181–1186 CAS.
- P. W. Anderson, M. E. Pichichero, E. C. Stein, S. Porcelli, R. F. Betts, D. Connuck, D. Korones, R. A. Insel, J. M. Zahradnik and R. Eby, J. Immunol., 1989, 142, 2464–2468 CAS.
- J. Y. Baek, A. Geissner, D. C. K. Rathwell, D. Meierhofer, C. L. Pereira and P. H. Seeberger, Chem. Sci., 2017, 9, 1279–1288 RSC.
- A. Nilo, L. Morelli, I. Passalacqua, B. Brogioni, M. Allan, F. Carboni, A. Pezzicoli, F. Zerbini, D. Maione, M. Fabbrini, M. R. Romano, Q. Y. Hu, I. Margarit, F. Berti and R. Adamo, ACS Chem. Biol., 2015, 10, 1737–1746 CrossRef CAS PubMed.
- A. Nilo, I. Passalacqua, M. Fabbrini, M. Allan, A. Usera, F. Carboni, B. Brogioni, A. Pezzicoli, J. Cobb, M. R. Romano, I. Margarit, Q. Y. Hu, F. Berti and R. Adamo, Bioconjugate Chem., 2015, 26, 1839–1849 CrossRef CAS PubMed.
- A. Nilo, M. Allan, B. Brogioni, D. Proietti, V. Cattaneo, S. Crotti, S. Sokup, H. Zhai, I. Margarit, F. Berti, Q. Hu and R. Adamo, Bioconjugate Chem., 2014, 25, 2105–2111 CrossRef CAS PubMed.
- N. Martinez-Saez, S. Sun, D. Oldrini, P. Sormanni, O. Boutureira, F. Carboni, I. Companon, M. Deery, M. Vendruscolo, F. Corzana, R. Adamo and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2017, 56, 14963–14967 CrossRef CAS PubMed.
- M. Wetter, M. Kowarik, M. Steffen, P. Carranza, G. Corradin and M. Wacker, Glycoconjugate J., 2013, 30, 511–522 CrossRef CAS PubMed.
- M. Wacker, L. Wang, M. Kowarik, M. Dowd, G. Lipowsky, A. Faridmoayer, K. Shields, S. Park, C. Alaimo, K. A. Kelley, M. Braun, J. Quebatte, V. Gambillara, P. Carranza, M. Steffen and J. C. Lee, J. Infect. Dis., 2014, 209, 1551–1561 CrossRef CAS PubMed.
- C. F. Hatz, B. Bally, S. Rohrer, R. Steffen, S. Kramme, C. A. Siegrist, M. Wacker, C. Alaimo and V. G. Fonck, Vaccine, 2015, 33, 4594–4601 CrossRef CAS PubMed.
- A. Huttner, C. Hatz, G. van den Dobbelsteen, D. Abbanat, A. Hornacek, R. Frölich, A. M. Dreyer, P. Martin, T. Davies, K. Fae, I. van den Nieuwenhof, S. Thoelen, S. de Vallière, A. Kuhn, E. Bernasconi, V. Viereck, T. Kavvadias, K. Kling, G. Ryu, T. Hülder, S. Gröger, D. Scheiner, C. Alaimo, S. Harbarth, J. Poolman and V. G. Fonck, Lancet Infect. Dis., 2017, 17, 528–537 CrossRef CAS PubMed.
- R. Adamo, Q.-Y. Hu, A. Torosantucci, S. Crotti, G. Brogioni, M. Allan, P. Chiani, C. Bromuro, D. Quinn, M. Tontini and B. Berti, Chem. Sci., 2014, 5, 4302–4311 RSC.
- V. Pozsgay, C. Chu, L. Pannell, J. Wolfe, J. B. Robbins and R. Schneerson, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5194–5197 CrossRef CAS.
- B. Schumann, K. Reppe, P. Kaplonek, A. Wahlbrink, C. Anish, M. Witzenrath, C. L. Pereira and P. H. Seeberger, ACS Cent. Sci., 2018, 4, 357–361 CrossRef CAS PubMed.
- F. Peri, Chem. Soc. Rev., 2013, 42, 4543–4556 RSC.
- Z. Polonskaya, S. Deng, A. Sarkar, L. Kain, M. Comellas-Aragones, C. S. McKay, K. Kaczanowska, M. Holt, R. McBride, V. Palomo, K. M. Self, S. Taylor, A. Irimia, S. R. Mehta, J. M. Dan, M. Brigger, S. Crotty, S. P. Schoenberger, J. C. Paulson, I. A. Wilson, P. B. Savage, M. G. Finn and L. Teyton, J. Clin. Invest., 2017, 127, 1491–1504 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |